Abstract
Background
In people with acute ischaemic stroke, platelets become activated and can cause blood clots to form and block an artery in the brain, resulting in damage to part of the brain. Such damage gives rise to the symptoms of stroke. Antiplatelet therapy might reduce the volume of brain damaged by ischaemia and also reduce the risk of early recurrent ischaemic stroke, thereby reducing the risk of early death and improving long‐term outcomes in survivors. However, antiplatelet therapy might also increase the risk of fatal or disabling intracranial haemorrhage.
Objectives
To assess the efficacy and safety of immediate oral antiplatelet therapy (that is started as soon as possible and no later than two weeks after stroke onset) in people with acute presumed ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched 16 October 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2013), MEDLINE (June 1998 to May 2013), and EMBASE (June 1998 to May 2013). In 1998, for a previous version of this review, we searched the register of the Antiplatelet Trialists' Collaboration, MedStrategy and contacted relevant drug companies.
Selection criteria
Randomised trials comparing oral antiplatelet therapy (started within 14 days of the stroke) with control in people with definite or presumed ischaemic stroke.
Data collection and analysis
Two review authors independently applied the inclusion criteria and assessed trial quality. For the included trials, they extracted and cross‐checked the data.
Main results
We included eight trials involving 41,483 participants. No new trials have been added since the last update.Two trials testing aspirin 160 mg to 300 mg once daily, started within 48 hours of onset, contributed 98% of the data. The risk of bias was low. The maximum follow‐up was six months. With treatment, there was a significant decrease in death or dependency at the end of follow‐up (odds ratio (OR) 0.95, 95% confidence interval (CI) 0.91 to 0.99). For every 1000 people treated with aspirin, 13 people would avoid death or dependency (number needed to treat 79). Antiplatelet therapy was associated with a small but definite excess of symptomatic intracranial haemorrhages, but this small hazard was significantly outnumbered by the benefit, the reduction in recurrent ischaemic stroke and pulmonary embolus.
Authors' conclusions
Antiplatelet therapy with aspirin 160 mg to 300 mg daily, given orally (or by nasogastric tube or per rectum in people who cannot swallow) and started within 48 hours of onset of presumed ischaemic stroke, reduced the risk of early recurrent ischaemic stroke without a major risk of early haemorrhagic complications; long‐term outcomes were improved.
Plain language summary
Oral antiplatelet therapy for acute ischaemic stroke
Question
We wanted to compare the safety and effectiveness of oral antiplatelet therapy versus placebo or no treatment in people with acute ischaemic stroke to see if oral antiplatelet drugs reduced the number of deaths and improved the long‐term outcomes in survivors.
Background
Most strokes are caused by a sudden blockage of an artery in the brain that is usually due to a blood clot (called an ischaemic stroke). Immediate treatment with antiplatelet drugs such as aspirin may prevent new clots from forming and hence improve recovery after stroke. However, antiplatelet drugs may also cause bleeding in the brain, which could offset any benefits.
Study characteristics
We identified eight studies, up to October 2013, for inclusion in the review. These studies included a total of 41,483 participants. Two of the studies contributed 98% of the data. Four studies tested aspirin, three studies tested ticlopidine and one study tested aspirin plus dipyridamole. The majority of participants in the review were elderly, with a significant proportion over 70 years of age. Males and females were almost equally represented in the trials. There appeared to be some variation in stroke severity among the included trials. The scheduled duration of treatment varied from five days to three months and the scheduled follow‐up period varied from 10 days to six months.
Key results
Aspirin, at a dose of 160 mg to 300 mg daily started within 48 hours of the onset of stroke symptoms, saved lives and reduced the risk of further stroke occurring in the first two weeks. If treatment was started more than 48 hours after onset but within 14 days, the limited evidence from this review and other external data suggests that aspirin is of benefit even starting at this late stage. Aspirin also increased the chances of being alive and independent and improved the chances of making a complete recovery from the stroke. The risk of serious bleeding was very low. Almost all the evidence in this review comes from trials of aspirin. There is no reliable evidence on the effects of the other oral antiplatelet drugs in acute stroke that were assessed in this review (clopidogrel, ticlopidine, cilostazol, satigrel, sarpolgrelate, KBT 3022, iisbogrel).
Quality of the evidence
The quality of the evidence contributing to these results was generally good.
Background
Description of the condition
Stroke is an enormous and serious public health problem. According to the World Health Organization, 15 million people suffer stroke worldwide each year. It is also a major cause of death and disability worldwide. Approximately 80% to 87% of all strokes are ischaemic (that is due to a blockage of an artery in the brain) in white populations (Jauch 2013; Warlow 2001) and about 67% in Asian populations (Tsai 2013).
Description of the intervention
Platelets become activated in people with acute ischaemic stroke. This review is focused on oral antiplatelet agents. Oral antiplatelet agents work via different mechanisms to inhibit platelet adhesion and aggregation. The types of drugs include cyclo‐oxygenase inhibitors (for example acetylsalicylic acid (ASA)), thienopyridine derivatives (for example ticlopidine, clopidogrel), phosphodiesterase inhibitors (for example dipyridamole, cilostazol) and thromboxane A2 antagonists (for example ozagrel).
How the intervention might work
Antiplatelet therapy is effective for long‐term secondary prevention of serious vascular events in people at high risk of vascular disease (ATC 1994a; ATC 2002; ATC 2009). In people with acute myocardial infarction, starting antiplatelet therapy immediately after the event, and continuing it for a month, avoids about 38 vascular events for every 1000 people treated (ATC 1994a; ATC 2002). In individuals with ischaemic stroke or transient ischaemic attack (TIA), being on long‐term antiplatelet therapy avoids about 36 serious vascular events for every 1000 people treated for three years (ATC 1994a; ATC 2002). Platelets are activated in the acute phase of ischaemic stroke, releasing neurotoxic and thrombogenic eicosanoids including thromboxane B2 (van Kooten 1994).
Antiplatelet therapy is, therefore, a logical treatment to evaluate in acute ischaemic stroke. It might reduce early deaths and improve outcomes in survivors by reducing the volume of brain damaged by ischaemia and reducing the risk of early recurrent ischaemic stroke and pulmonary embolism (ATC 1994a; ATC 1994b). However, antiplatelet therapy could also increase the risk of fatal or disabling intracranial haemorrhage, thus offsetting any benefits (ATC 1994a). The initial data were sufficiently promising that two large‐scale trials were undertaken, the International Stroke Trial (IST 1997) and the Chinese Acute Stroke Trial (CAST 1997), which together included over 40,000 participants. These trials provided reliable evidence of the net benefit from aspirin in this setting. As a result, evidence‐based guidelines in Europe, Canada and USA now recommend aspirin as the standard antithrombotic treatment for acute ischaemic stroke (CSS 2010; ESO 2008; Jauch 2013; RCP Guideline 2012).
The majority of the data relating to orally active antiplatelet agents is derived from trials of aspirin. Data regarding the utility of other single oral antiplatelet agents, including clopidogrel, dipyridamole or cilostazol, for the treatment of acute stroke are limited (CAIST 2011; Chairangsarit 2005; Suri 2008). Overall, the data do not provide solid evidence about the utility of these antiplatelet agents in the management of people with acute ischaemic stroke. There has been limited experience and no evidence to support the use of ozagrel in the setting of acute stroke (Zhang 2012). Recent trials have investigated the early use of multiple antiplatelet agents in addition to aspirin in the acute phase of stroke. Early initiation of aspirin plus extended‐release dipyridamole seem to be as safe and effective in preventing disability as later initiation after conventional aspirin monotherapy (EARLY 2010). Other trials have examined aspirin and clopidogrel combination therapy. The combination was found to be only significantly effective in the immediate high‐risk interval after stroke or TIA (CHANCE 2013; FASTER 2007;Hankey 2010). Ongoing trials continue to seek alternative regimes in dual or triple antiplatelet therapy (POINT; TARDIS).
Why it is important to do this review
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2008, Issue 3) of 'Antiplatelet therapy for acute ischaemic stroke'. The previous version of this Cochrane review was published in 2008 and stated that antiplatelet therapy with aspirin is safe and effective when started within 48 hours after stroke. Since then more trials have been published. For this update we did not include parenterally administrated antiplatelet agents. Platelet glycoprotein (GP) IIb/IIIa receptor inhibitors are the subject of a separate review (Ciccone 2006). Therefore, we conducted this updated review to assess the efficacy and safety of oral antiplatelet therapy when administered to people with acute ischaemic stroke to provide more up‐to‐date evidence for clinical practice and to identify trials of newer agents.
Objectives
To assess the efficacy and safety of immediate oral antiplatelet therapy (that is started as soon as possible and no later than two weeks after stroke onset) in people with acute presumed ischaemic stroke.
We wished to test the hypotheses that oral antiplatelet therapy:
reduces the risk of a poor outcome (that is the risk of being dead or dependent on others for activities of daily living) several months after the stroke;
reduces the risk of death several months after ischaemic stroke;
reduces the risk of deep vein thrombosis (DVT) and pulmonary embolism (PE) following ischaemic stroke;
reduces the risk of recurrent ischaemic stroke during the scheduled treatment period;
may increase the risk of bleeding, and that the incidence of both intracranial haemorrhage (ICH) and major extracranial haemorrhage may be increased during the scheduled treatment period.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all randomised unconfounded trials of early treatment with oral antiplatelet therapy in which treatment allocation was adequately concealed from doctors entering people into the trials. We did not include trials that were not truly random (for example alternating or based on date of birth, day of the week, hospital number) or in which allocation to the treatment or control group was not adequately concealed (such as an open random number list) since foreknowledge of treatment allocation might lead to non‐random treatment allocation and consequent bias in the estimation of treatment effects (Odgaard‐Jensen 2011).
Types of participants
We included all trials that recruited people of any age or sex within two weeks of onset of presumed ischaemic stroke. We excluded trials of antiplatelet therapy after known primary intracerebral or subarachnoid haemorrhage, but included trials that did not adequately differentiate between ischaemic and haemorrhagic stroke by computed tomography (CT) or magnetic resonance (MR) scans prior to randomisation on the basis that 80% to 87% of strokes are ischaemic in predominantly white populations (Jauch 2013; Warlow 2001).
Types of interventions
We considered all unconfounded trials that compared either a single oral antiplatelet agent or a combination of oral antiplatelet agents with control (placebo or no treatment) as eligible. We excluded studies either involving 'head‐to‐head' direct comparisons of one agent versus another or comparison of one multiple agent regimen versus a different multi‐agent regimen (the latter is the subject of a separate review (Kamal 2012)). We broadly defined oral antiplatelet agents as any agents whose principal effects were to inhibit platelet adhesion and aggregation. These included:
cyclo‐oxygenase inhibitors (e.g. acetylsalicylic acid (ASA));
thienopyridine derivatives inhibiting adenosine diphosphate (ADP) receptors (e.g. ticlopidine, clopidogrel);
phosphodiesterase inhibitors (e.g. dipyridamole, cilostazol);
thromboxane A2 antagonists (e.g. ozagrel).
For this update we excluded parenterally administrated antiplatelet agents. GP IIb/IIIa receptor inhibitors are the focus of a separate review (Ciccone 2006), which is currently being updated.
We did not include agents with multiple modes of action including some antiplatelet activity (for example piracetam, prostacyclin, oxpentifylline), and some of these agents have been evaluated in other Cochrane systematic reviews (Bath 2004a; Bath 2004b; Ricci 2012).
Types of outcome measures
Primary outcomes
For each treatment group we sought the number of participants who were either dead or dependent on help from other people for their activities of daily living at least one month after their stroke. Many people regard this as the most important outcome since the aim of treatment should not only be to prevent death but also to prevent serious disability in survivors. The minimum interval of one month was used to allow time for recovery from the initial stroke.
Secondary outcomes
For each treatment group we sought the number of participants who:
died from any cause during the scheduled treatment period (generally shorter than the scheduled follow‐up period);
died from any cause during the scheduled follow‐up period (generally considered to be greater than one month after the stroke);
had objective evidence of deep vein thrombosis (DVT) detected by the systematic use of imaging techniques such as iodine 125 fibrinogen scanning (I‐125 scan), ultrasound of the leg, plethysmography, or X‐ray contrast venography in all participants during the scheduled treatment period (these methods detected both clinically suspected and silent DVTs, the outcome was therefore 'symptomatic or asymptomatic DVT'. Screening of participants by clinical observation alone was not considered adequate);
had at least one confirmed symptomatic pulmonary embolism (PE) diagnosed during life, or at autopsy (symptomatic or not) within the scheduled treatment period;
had a recurrent stroke during the treatment period that was either definitely ischaemic (haemorrhage excluded by CT or MR scan or autopsy) or of unknown type (no CT or MR scan or autopsy performed);
had a symptomatic intracranial (intracerebral and extracerebral) haemorrhage, including symptomatic haemorrhagic transformation of the cerebral infarct, during the scheduled treatment period (the haemorrhage must have been confirmed by CT (or MR) scanning after clinical deterioration, or by autopsy);
had any recurrent stroke (either of ischaemic or unknown type) or symptomatic intracranial haemorrhage during the treatment period;
had any major extracranial haemorrhage during the scheduled treatment period (the definition of major haemorrhage was usually taken from the original article but if none was given it was defined as any fatal bleed, or bleeding severe enough to require transfusion or operation);
made a complete recovery from their stroke.
Please note, the last outcome is a post hoc analysis for this outcome only, and we acknowledge that the addition of post hoc data is subject to selection bias. However, at the time we assessed the data for this review we realised that two trials (CAST 1997; IST 1997) reported data on the number of participants who had made a complete recovery from their stroke, an important functional outcome that was not a widely reported outcome in stroke trials when the protocol for the earlier version of this review was written. With the recently reported effects of thrombolysis on increasing the proportion of people who recover completely from their stroke (for example, the NINDS trial of tissue plasminogen activator (NINDS 1995)), it seems reasonable to include this outcome here with due allowance for its post hoc nature.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers published in languages other than English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in October 2013. In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4) (searched May 2013) (Appendix 1), MEDLINE (Ovid) (June 1998 to May 2013) (Appendix 2), and EMBASE (Ovid) (June 1998 to May 2013) (Appendix 3).
We developed the search strategies for CENTRAL, MEDLINE and EMBASE with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and we updated the search strategies to include any new vocabulary terms and drug names.
Searching other resources
In 1998, we searched the registers of the Antiplatelet Trialists' Collaboration (ATC 1994a; ATC 1994b) and MedStrategy (MedStrategy 1995). We contacted the following pharmaceutical companies who marketed antiplatelet agents for details of any trials, particularly unpublished ones: Roussel‐Uclaf (defibrotide), Syntex and Sanofi Winthrop (ticlopidine), Otsuka (cilostazol), Eisai (satigrel), Tokyo Tanebe Seiyaku (sarpolgrelate), Kanebo (KBT 3022), and Takeda Chemical Company (isbogrel). For this version of the review, we did not update the previous searches of the Antiplatelet Trialists' Collaboration register (no longer available), MedStrategy or make any further contact with pharmaceutical companies.
Data collection and analysis
Selection of studies
Two authors (for this update, PS and EC; for previous versions, PS and CC, PS and GG, PS and PF, PS and MT operating in pairs) read the titles, abstracts and keywords of all records identified from the searches of the electronic bibliographic databases and excluded studies that were clearly irrelevant. We obtained the full texts of the remaining studies and the same two authors selected trials for inclusion based on our defined criteria. The two review authors resolved any disagreements by discussion.
Data extraction and management
For each version of the review, two review authors independently extracted the data on methods, participants, interventions, outcomes and results, and recorded the information on a data extraction form. We sought data on the number of participants with each outcome event by allocated treatment group, irrespective of compliance and whether or not the participant was subsequently deemed ineligible or otherwise excluded from treatment or follow‐up, to allow an intention‐to‐treat analysis. We also sought data on whether CT or MR scanning was performed prior to randomisation. The same two review authors cross‐checked all extracted data and resolved any disagreements by discussion. If any of the above data were unavailable from the publications, we sought further information by contacting the study authors.
Assessment of risk of bias in included studies
The same review authors planned to assess the methodological quality of any new trials. We decided not to use a scoring system to assess quality but simply to record details of the following domains: random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessment; incomplete outcome data; and selective outcome reporting. We planned to classify the risk of bias in any new trials as 'low risk', 'high risk' or 'unclear risk' according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. However, since no new trials were identified we have retained the original assessment of risk of bias.
Measures of treatment effect
We calculated odds ratios (ORs), that is the ratio of the odds of an outcome among treatment‐allocated participants to the corresponding odds amongst controls) with 95% confidence intervals (CI), which we calculated using RevMan 5.2 (RevMan 2012).
Dealing with missing data
If any data were not available from the publications, we sought further information from the authors or the relevant pharmaceutical company. When data were missing and could not be derived, we used the published analysis.
Assessment of heterogeneity
We assessed the extent of heterogeneity between trial results by using the I2 statistic, which measures the percentage of the variability in effect estimates attributable to heterogeneity rather than sampling error (Higgins 2011). We considered a value greater than 50% as substantial heterogeneity.
Assessment of reporting biases
We planned to assess trials for selective outcome reporting and for the assessment of each new trial to be reported in the 'Risk of bias' tables. We sought evidence of publication bias in the assessment of the primary outcome and for death within the scheduled treatment period with funnel plots.
Data synthesis
We calculated ORs with 95% CIs, which were calculated using the Peto fixed‐effect method (ATC 1994a) using RevMan 5.2 (RevMan 2012). We also calculated the number needed to treat to benefit (NNTB) and the number of events avoided per 1000 people treated for each outcome if the result was statistically significant. We performed these calculations with the on‐line calculator provided by the Cochrane Stroke Group at http://www.dcn.ed.ac.uk/csrg/entity/entity_NNT2.asp. The control event rate applied was based on the average of the relevant control event rates in CAST 1997 and IST 1997.
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses for the major outcomes of the review:
the type of oral antiplatelet agent used;
trials in which all participants had intracerebral haemorrhage excluded by CT or MR scanning prior to trial randomisation;
trials in which participants were recruited within 48 hours of their stroke.
We performed an additional post hoc subgroup analysis among participants with intracerebral haemorrhages inadvertently randomised in the trials. Two trials (CAST 1997; IST 1997) included a number of participants who did not have a CT scan until after randomisation. Data on those participants in whom the diagnosis of the initial event leading to randomisation was haemorrhagic stroke and who were dead or dependent at follow‐up were reported in the IST publication (IST 1997) and were kindly supplied on request from the CAST trial (CAST 1997).
We assessed heterogeneity by the I2 statistic.
Sensitivity analysis
We planned several pre‐specified sensitivity analyses limited to the major outcomes (for example death or dependency, death from all causes) of the review, including:
only trials with adequate concealment of randomisation;
only trials with blinding of participants, personnel and outcome assessment;
only trials at low risk of bias due to completeness of follow‐up.
In the sensitivity analysis, we compared two ORs by assessing whether the difference in the natural logarithms of the two ORs (lnOR) was significantly different from zero using a normal approximation. The variance of each lnOR was estimated as the reciprocal of the variance of the O‐E statistic given in RevMan 2012.
However, following an analysis of the available randomised evidence it became apparent that three of the pre‐specified analyses (trials in which participants were randomised within 48 hours, trials with adequate concealment of randomisation and trials with CT scans prior to randomisation) were inappropriate; this is explained in the Results section.
Results
Description of studies
Results of the search
We identified a total of 6544 records from the electronic searches. After eliminating duplicates and non‐relevant studies from the titles and abstracts, we selected 88 possibly relevant studies. After examining the abstracts, or in some cases the full papers, we excluded 78 records: 12 records relating to previously identified studies and 66 records because they were non‐randomised, not of an oral antiplatelet therapy, not acute stroke therapy, had no control group or involved regimens with multiple antiplatelet agents (the latter are reviewed in a separate Cochrane review (Kamal 2012)). This process left 10 potentially relevant studies. Of these, six did not fulfil all the inclusion criteria of this review and are listed in the Characteristics of excluded studies table, two are ongoing (CAIST‐J 2006; CAPS 2009) and two are awaiting assessment (Fujimoto 2010; Wang 2000). Fujimoto 2010 has only been published in abstract form so far and further information is required, particularly regarding the method of randomisation; this study is therefore classified as awaiting classification. So, no new studies fulfilled all the inclusion criteria of this review. We excluded four studies included in the previous review because they assessed only parenterally administrated antiplatelet agents (Abciximab 2000; AbESTT 2005; AbESTT‐II (that contained three study cohorts: AbESTT‐II/P 2008, AbESTT‐II/C 2008, and AbESTT‐II/W 2008); Ohtomo 1991). A PRISMA flowchart of study selection is shown in Figure 1.
1.
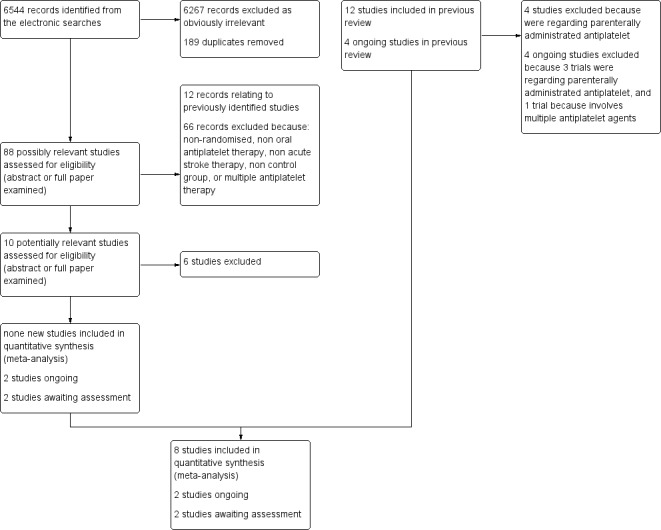
Flow diagram.
Included studies
We included eight studies involving a total of 41,483 participants (see Characteristics of included studies). Three of the included trials (162 participants) remain unpublished (Pince 1981; Turpie 1983; Utsumi 1988). The majority of participants in the review were elderly, with a significant proportion of participants over 70 years of age. For example, 61% of participants enrolled in the International Stroke Trial (IST 1997) were aged 70 years or older. Males and females were almost equally represented in the trials.
Antiplatelet regimens tested
Two of the studies (CAST 1997; IST 1997) contributed 98% of the data. IST 1997 was an open‐treatment, blinded outcome study with a factorial design; participants were allocated to 14 days of treatment with 300 mg aspirin, heparin, both, or neither; that is the trial tested the effects of aspirin in the presence and absence of heparin (and vice versa). CAST 1997 was a double‐blind randomised trial of one month's treatment with either aspirin 160 mg or matching placebo. In MAST‐I 1995 (a factorial trial of streptokinase and aspirin involving 309 participants) only those participants randomised to aspirin alone and the no treatment group were included in this review as there was a significant interaction between aspirin and streptokinase which invalidated the aspirin plus streptokinase versus streptokinase alone comparison. Other antiplatelet regimens that were compared with control were: aspirin (Rödén‐Jüllig 2003), ticlopidine (Ciufetti 1990; Turpie 1983; Utsumi 1988), and aspirin plus dipyridamole (Pince 1981).
Time window for inclusion
Trials included participants randomised within six hours (MAST‐I 1995), 12 hours (Ciufetti 1990), 48 hours (CAST 1997; IST 1997), 72 hours (Rödén‐Jüllig 2003) or six days (Pince 1981) of stroke onset. In two trials, the formal entry criterion was a stroke within the previous four weeks (Turpie 1983; Utsumi 1988) but as most participants were entered within two weeks they were included in this review. Data on only those participants entered within two weeks were not available from the authors or the pharmaceutical company.
Exclusion criteria
The two main trials in the review (CAST 1997; IST 1997) did not precisely specify exclusion criteria as they used the uncertainty principle, but suggested that these might include participants thought to be at high risk of adverse effects (for example clotting disorders, hepatic or renal failure) or those with a small likelihood of worthwhile benefit.
CT scanning
Three trials adequately excluded people with intracerebral haemorrhage by CT scanning all possible participants before entry into the trial (Ciufetti 1990; MAST‐I 1995; Rödén‐Jüllig 2003). Two trials (CAST 1997; IST 1997) performed a CT scan in almost all participants; in these trials clinicians had to have a low suspicion of intracranial haemorrhage prior to randomisation. In CAST 1997 87% had a CT prior to randomisation; by discharge this number had risen to 94%. In IST 1997 67% were scanned before randomisation and 29% after randomisation, so that overall 96% of participants were scanned. Two trials performed no CT scans (Pince 1981; Turpie 1983) and in Utsumi 1988 the use of CT scanning was uncertain. As a result of the variable use of pre‐randomisation CT scanning, some people with intracerebral haemorrhage were inadvertently entered in the trials and these were included in the main analyses of this review. This may have biased the results against antiplatelet agents although this is unlikely given the relatively small numbers of participants involved. Furthermore, the inclusion of these people may actually make the conclusions of the review more broadly applicable, since many hospitals admitting people with acute stroke do not have immediate access to CT scanning and so acute treatment may have to be started without definite knowledge of the pathological type of stroke.
Stroke severity at entry
There appeared to be some variation in stroke severity among the included trials. For example, in the control group of IST 1997 early death was recorded as 9%, but was only 4% in CAST 1997, even though CAST 1997 assessed participants at four weeks versus two weeks for IST 1997. Rödén‐Jüllig 2003 used a Scandinavian Stroke Supervision Scale score of one point or more as the inclusion criterion.
Scheduled duration of trial treatment
The scheduled duration of treatment varied from five days (Rödén‐Jüllig 2003) to three months (Utsumi 1988). The scheduled follow‐up period varied from 10 days (Pince 1981) to six months ( IST 1997; MAST‐I 1995).
Measures of outcome
Clinically important outcomes were poorly reported in the smaller trials. All trials evaluated death during the treatment period. Comparable definitions of dependence were used in most of the trials. They included: the modified Rankin disability scale (greater than or equal to three) (CAST 1997; MAST‐I 1995); and needing help from another person with daily activities (IST 1997). For Rödén‐Jüllig 2003 we used 'living in an institution' as equivalent to being dependent. Three trials (CAST 1997; IST 1997; MAST‐I 1995) used validated scales (Bamford 1989; Dennis 1997; Candelise 1994, respectively). In two trials the primary outcome was DVT (Pince 1981; Turpie 1983); these trials did not formally evaluate survival free of dependency for activities of daily living. Progression of stroke symptoms measured by the Scandinavian Stroke Supervision Scale was the primary outcome event of Rödén‐Jüllig 2003. Two trials (CAST 1997; IST 1997) recorded information on participants making a complete recovery from their stroke, and this information has been included in a post hoc analysis as discussed in the Types of outcome measures section of this review. No trials systematically assessed quality of life.
Excluded studies
We excluded 39 trials for a variety of reasons (see Characteristics of excluded studies). In the process of doing this update we excluded four studies that were included in the previous review because they assessed parenterally administrated antiplatelet agents (Abciximab 2000; AbESTT 2005; AbESTT‐II (that contained three study cohorts: AbESTT‐II/P 2008, AbESTT‐II/C 2008, AbESTT‐II/W 2008); Ohtomo 1991). We have now excluded four trials that were ongoing. Of these, we excluded three trials because they were assessing parenterally administrated antiplatelet agents (ARTIS 2012; Cheung 2000; SaTIS 2011), and one trial because it assessed multiple antiplatelet agents (Matsumoto 2005).
Risk of bias in included studies
Baseline characteristics
The large numbers of participants randomised in CAST 1997 and IST 1997 resulted in an equal distribution of baseline patient characteristics between the treatment and control groups. In two smaller trials (MAST‐I 1995; Utsumi 1988) there were significant imbalances between the treatment and control groups in potentially important baseline factors (level of consciousness in Utsumi 1988, and time to treatment in MAST‐I 1995) but these differences cannot bias the overall results due to the small numbers of participants involved.
Allocation
The method of randomisation provided adequate concealment of allocation in five trials (CAST 1997; IST 1997; MAST‐I 1995; Rödén‐Jüllig 2003; Turpie 1983). Sealed envelopes were used in Pince 1981 but it was unclear if the envelopes were opaque. In Ciufetti 1990 a random table was used but it was not clear if this was open to the investigators. The detail of the method of randomisation remains unknown in Utsumi 1988.
Blinding
Five trials used placebo as control (CAST 1997; Ciufetti 1990; Rödén‐Jüllig 2003; Pince 1981; Turpie 1983). Two trials did not use placebo but did use methods to obtain outcome data in a masked fashion (IST 1997; MAST‐I 1995). In IST 1997 the follow‐up data were collected by self‐completed questionnaires mailed to the participants six months after randomisation or by telephone interview by a person blinded to treatment allocation. An analysis of 207 participants from the UK who were enrolled in the IST pilot study showed that at the six month follow‐up the majority of participants could not remember what they had been treated with, and so these participants were effectively blinded (IST 1996). Utsumi 1988 did not appear to use any form of blinded outcome assessment.
Incomplete outcome data
Three trials (Pince 1981; Turpie 1983; Utsumi 1988) excluded a total of 14 participants (seven in the antiplatelet group, seven in the control group) after randomisation. In addition, two trials (CAST 1997; IST 1997) lost a total of 601 participants (300 antiplatelet group, 301 control group) to follow‐up (see Characteristics of included studies). An intention‐to‐treat analysis, which included the results on all participants randomised, was therefore only possible for three trials (Ciufetti 1990; MAST‐I 1995; Rödén‐Jüllig 2003). In the remaining trials we assumed that all participants who were randomised but excluded or lost from follow‐up did not have an outcome event of interest for the primary analyses. Given the very small numbers of participants lost or excluded (1.5% of all participants randomised), the results did not change if these participants were excluded from the denominators.
Selective reporting
Funnel plots did not suggest substantial publication bias in respect of the primary outcome (Figure 2) or death at the scheduled end of follow‐up (Figure 3).
2.
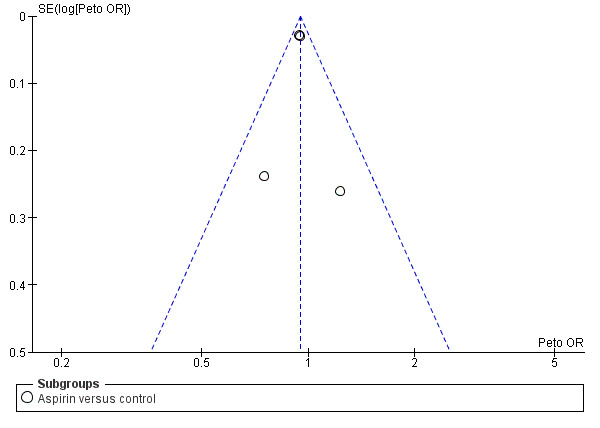
Funnel plot of comparison: 1 Antiplatelet agent versus control in acute presumed ischaemic stroke, outcome: 1.1 Death or dependence at end of follow‐up.
3.
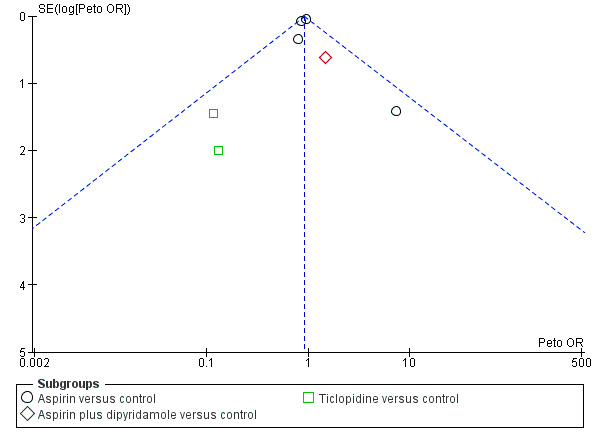
Funnel plot of comparison: 1 Antiplatelet agent versus control in acute presumed ischaemic stroke, outcome: 1.2 Deaths from all causes during treatment period.
Effects of interventions
Comparison 1.1: death or dependence at end of follow‐up
Data from four trials with 41,291 participants were available. Antiplatelet therapy was associated with a significant reduction in the odds of being dead or dependent at final follow‐up (OR 0.95, 95% CI 0.91 to 0.99; P = 0.01) (Analysis 1.1). For aspirin, for every 1000 people treated 13 people would avoid death or dependency (NNTB 79) (Table 1). A pre‐specified sensitivity analysis (data not shown in forest plots) showed no statistically significant difference in the effect of treatment on death or dependence at final follow‐up between trials that were double‐blind (OR 0.95, 95% CI 0.90 to 1.01) or not (OR 0.94, 95% CI 0.89 to 1.00). A post hoc subgroup analysis (data not shown in forest plots) restricted to the subset of participants in whom the initial stroke was due to intracerebral haemorrhages and who had been inadvertently randomised in the trials (597 in IST 1997 and 174 in CAST 1997) showed that the odds of a poor outcome were significantly lower among those allocated to aspirin (OR 0.68, 95% CI 0.49 to 0.94), although the CIs were wide (Keir 2002). In CAST 1997 11 participants in the aspirin‐allocated group and five participants in the control group were not accounted for in this analysis. Assuming a worst‐case scenario (that is where all participants in the experimental group were assumed to be either dead or dependent, and all participants in the control group recovered fully), the trend toward a better outcome for the aspirin‐treated group continued but was no longer statistically significant (OR 0.74, 95% CI 0.53 to 1.03) (data not shown in forest plots). Thus, these data do not provide clear evidence of any harm to people with haemorrhagic stroke inadvertently treated with aspirin.
1.1. Analysis.
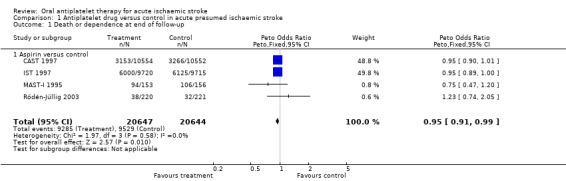
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 1 Death or dependence at end of follow‐up.
1. Absolute risk reductions of aspirin treatment in acute stroke.
| Outcome | Control event rate | No of events avoided | NNTB or NNTH |
| Per 1000 people treated (95% CI) | Data are number needed to treat to benefit (NNTB) (95% CI) unless otherwise indicated. NNTH = number needed to treat to harm | ||
| Estimated from the average of the control event rate in the 2 largest trials (CAST 1997 and IST 1997) | Estimated by applying the odds ratio for the outcome for studies of aspirin. Calculator is available at: http://www.dcn.ed.ac.uk/csrg/entity/entity_NNT2.asp | Estimated by applying the odds ratio for the outcome for studies of aspirin. Calculator is available at: http://www.dcn.ed.ac.uk/csrg/entity/entity_NNT2.asp | |
| Death or dependence at end of follow‐up | 0.47 | 13 (3 to 23) | 79 (43 to 400) |
| Deaths from all causes during follow‐up | 0.13 | 9 (2 to 15) | 108 (66 to 436) |
| Pulmonary embolism during treatment period | 0.01 | 1 (0 to 2) | 693 (427 to 6700) |
| Recurrent ischaemic/unknown stroke during treatment period | 0.03 | 7 (4 to 10) | 140 (104 to 248) |
| Symptomatic intracranial haemorrhage during treatment period | 0.01 | ‐2 (i.e. 2 extra) (‐4 to 0) | NNTH 574 (254 to 126 010) |
| Any recurrent stroke/intracranial haemorrhage during treatment | 0.04 | 5 (1 to 8) | 200 (123 to 868) |
| Major extracranial haemorrhage during treatment period | 0.01 | ‐4 (i.e. 4 extra) (‐7 to ‐2) | NNTH 245 (153 to 481) |
| Complete recovery from stroke (post hoc) | 0.26 | 11 (2 to 21) | 89 (49 to 523) |
Comparison 1.2: deaths from all causes during treatment period
Data were available from eight trials with 41,483 participants. Antiplatelet therapy was associated with a nominally significant reduction in death at the end of the treatment period (OR 0.92, 95% CI 0.85 to 1.00; P = 0.05) (Analysis 1.2).
1.2. Analysis.
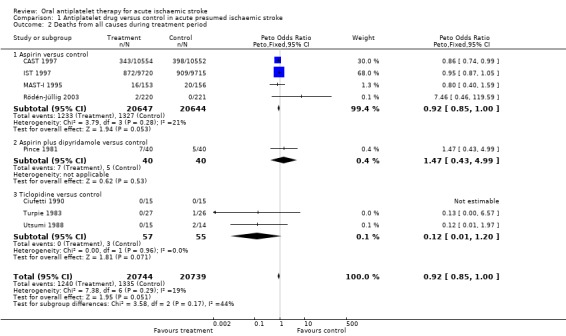
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 2 Deaths from all causes during treatment period.
Comparison 1.3: deaths from all causes during follow‐up
Data were available for eight trials including 41,483 participants. Antiplatelet therapy was associated with a significant reduction in the odds of death at a final follow‐up of greater than one month (OR 0.92, 95% CI 0.87 to 0.98; P = 0.01) (Analysis 1.3). For aspirin, for every 1000 people treated nine people would avoid death (NNTB 108) (Table 1). A pre‐specified sensitivity analysis showed no statistically significant difference in the effect of treatment on death at final follow‐up between trials which were of a double‐blind design (OR 0.87, 95% CI 0.76 to 1.00) or not (OR 0.94, 95% CI 0.87 to 1.00).
1.3. Analysis.
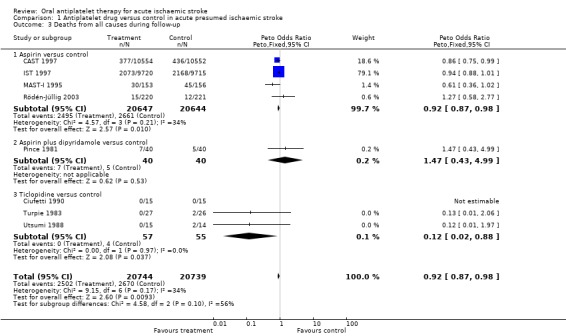
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 3 Deaths from all causes during follow‐up.
Comparison 1.4: deep venous thrombosis (DVT) during treatment period
Two trials (Pince 1981; Turpie 1983) that included randomised data from 133 participants (less than 0.3% of participants included in the overall review) sought to systematically determine the effect of antiplatelet agents on the occurrence of 'symptomatic or asymptomatic DVT' at the end of the treatment period, as detected by I‐125 fibrinogen scanning. DVT was observed in 16/67 (23.9%) of those allocated to antiplatelet treatment and 19/66 (28.8%) of those allocated to control, a non‐significant result (OR 0.78, 95% CI 0.36 to 1.67; P = 0.52) (Analysis 1.4). There was substantial heterogeneity between these two trials (I2 = 82.9%), one of which involved ticlopidine and one a combination of aspirin and dipyridamole. This heterogeneity may have been due to chance or to the fact that the time between stroke onset and starting treatment varied in the two trials: less than six days in one (Pince 1981) and less than four weeks in the other (Turpie 1983). Sensitivity analyses were not possible for this outcome due to the limited amount of data.
1.4. Analysis.
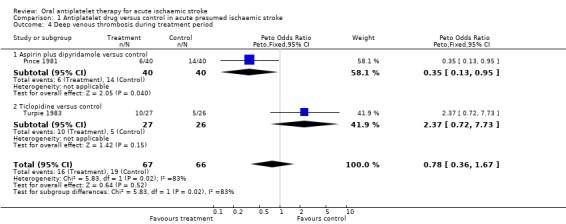
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 4 Deep venous thrombosis during treatment period.
Comparison 1.5: pulmonary embolism (PE) during treatment period
Data were available from seven trials, including data from 41,042 participants. Importantly, no trial systematically sought asymptomatic PE by performing ventilation‐perfusion scans in all participants at the end of the treatment period. Antiplatelet therapy was associated with a significant reduction in the odds of PE (OR 0.71, 95% CI 0.53 to 0.96; P = 0.03) (Analysis 1.5). For aspirin, for every 1000 people treated one person would avoid PE (NNTB 693) (Table 1). This may be an underestimate if antiplatelet treatment prevented both major and minor PE since minor PE was not sought systematically in any trial. Sensitivity analysis showed no statistically significant difference in this outcome between trials of a double‐blind design (OR 0.60, 95% CI 0.31 to 1.16) or not (OR 0.74, 95% CI 0.53 to 1.04).
1.5. Analysis.
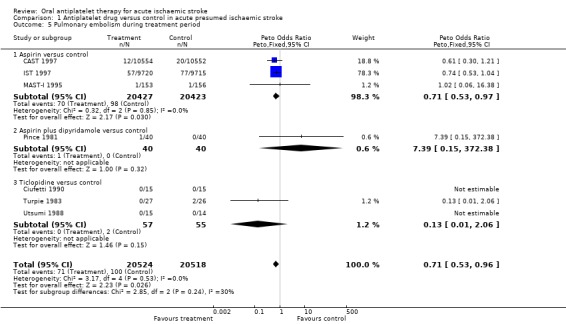
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 5 Pulmonary embolism during treatment period.
Comparison 1.6: recurrent ischaemic or unknown stroke during treatment period
Data on recurrent stroke were available from seven trials with 41,042 participants, which systematically sought to record early recurrent strokes that were definitely ischaemic (CT scan excluded haemorrhage) or probably ischaemic, that is in which the cerebral pathology was unknown because brain imaging had not been performed. A total of 495 of the 551 reported recurrent ischaemic strokes took place in CAST 1997 and IST 1997. The use of antiplatelet agents (chiefly aspirin) was associated with a statistically significant reduction in recurrent ischaemic strokes (OR 0.77, 95% CI 0.69 to 0.87; P < 0.00001) (Analysis 1.6). For every 1000 people treated with aspirin, seven people would avoid recurrent ischaemic stroke (NNTB 140) (Table 1). Sensitivity analysis (data not shown in forest plots) showed no statistically significant difference in this outcome between trials of a double‐blind design (OR 0.85, 95% CI 0.71 to 1.02) or not (OR 0.72, 95% CI 0.62 to 0.85).
1.6. Analysis.
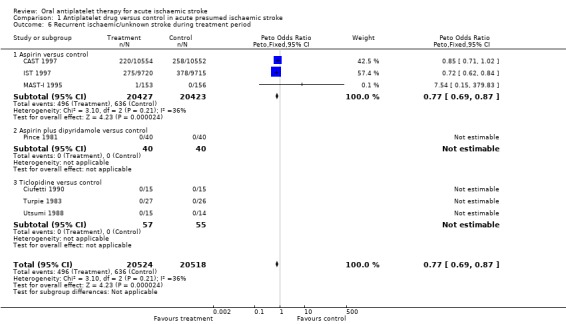
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 6 Recurrent ischaemic/unknown stroke during treatment period.
Comparison 1.7: symptomatic intracranial haemorrhage during treatment period
Data were available for seven trials including 41,042 participants. It remained unclear (even after correspondence) how haemorrhages were detected in three small trials (Pince 1981; Turpie 1983; Utsumi 1988). However, it was likely that participants who deteriorated neurologically were scanned using CT, or that haemorrhages were found at autopsy. In one trial (Pince 1981) four participants, two in each group, were excluded after randomisation because they were found to have intracerebral haemorrhage. These participants were included as having symptomatic intracranial haemorrhage in this analysis. In the trials where participants did not have a CT scan before randomisation, it was difficult to determine whether any intracranial haemorrhage first identified after treatment had been started was new or had been present before randomisation. For the purposes of this analysis, we assumed that all such haemorrhages were new. Antiplatelet therapy significantly increased the odds of symptomatic intracranial haemorrhage (OR 1.23, 95% CI 1.00 to 1.50; P = 0.04) (Analysis 1.7). For every 1000 people treated with aspirin, two people would have a symptomatic intracranial haemorrhage; the number needed to treat to harm (NNTH) was 574 (Table 1). There was the possibility of some bias within these data as there may have been a lower threshold for re‐scanning participants who had deteriorated clinically if they were known to be receiving antithrombotic treatment (for example in IST 1997, which was not blinded). However, this was not demonstrated in the sensitivity analysis (data not shown in forest plots), which found no statistically significant difference in the effect of treatment on symptomatic intracranial haemorrhage during the treatment period between trials which were of a double‐blind design (OR 1.24, 95% CI 0.95 to 1.63) or not (OR 1.21, 95% CI 0.89 to 1.64).
1.7. Analysis.
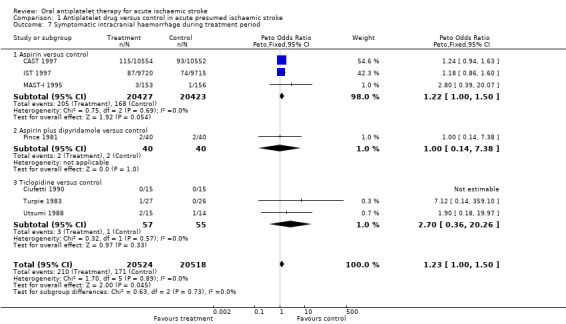
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 7 Symptomatic intracranial haemorrhage during treatment period.
Comparison 1.8: any recurrent stroke or intracranial haemorrhage during treatment period
Immediate use of antiplatelet agents reduced the odds of ischaemic stroke but also appeared to increase the odds of symptomatic intracranial haemorrhage. An outcome which combines these two (without double counting, that is participant allowed only one of ischaemic stroke or intracranial haemorrhage with the first event being the one which was included) was useful for assessing the net short‐term effects of antiplatelet agents. However, symptomatic intracranial haemorrhages are more likely to cause death or disability than ischaemic recurrences, and so the severity of the recurrence also needed to be considered. No trial reported the severity of recurrences. Data were available from seven trials that included 41,042 participants. Antiplatelet therapy was associated with a net reduction in the odds of this outcome (OR 0.88, 95% CI 0.79 to 0.97; P = 0.01) (Analysis 1.8). For every 1000 people treated with aspirin, five people would avoid recurrent ischaemic stroke or symptomatic intracranial haemorrhage (NNTB 200) (Table 1). Sensitivity analysis showed that although the effect was somewhat smaller in trials of double‐blind design (OR 0.96, 95% CI 0.82 to 1.11) compared with non‐double blind trials (OR 0.81, 95% CI 0.71 to 0.93) the difference (test for interaction) was not significant.
1.8. Analysis.
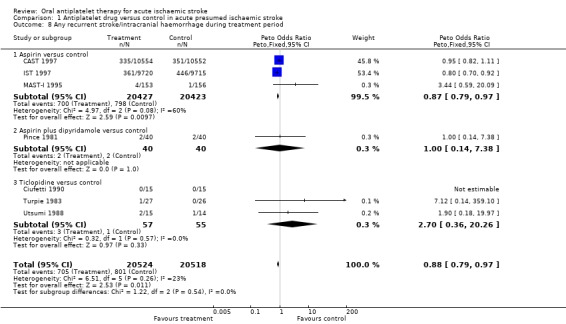
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 8 Any recurrent stroke/intracranial haemorrhage during treatment period.
Comparison 1.9: major extracranial haemorrhage during treatment period
Data were available for seven trials including 41,042 participants. Allocation to antiplatelet agents was associated with a significant increase in major extracranial haemorrhage (OR 1.69, 95% CI 1.35 to 2.11; P < 0.00001) (Analysis 1.9). For every 1000 people treated with aspirin, four people would have a symptomatic extracranial haemorrhage (NNTH 245) (Table 1). Sensitivity analysis (data not shown in forest plots) showed no statistically significant difference in this outcome between trials of a double‐blind design (OR 1.48, 95% CI 1.07 to 2.05) or not (OR 1.90, 95% CI 1.40 to 2.57).
1.9. Analysis.
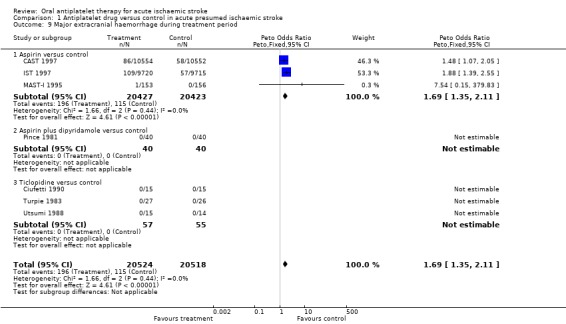
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 9 Major extracranial haemorrhage during treatment period.
Comparison 1.10: complete recovery from stroke (post hoc)
Two trials (CAST 1997; IST 1997) including randomised data on 40,541 participants (98% of participants included in the overall review) reported data on this outcome. Allocation to antiplatelet therapy significantly increased the odds of a complete recovery (OR 1.06, 95% CI 1.01 to 1.11; P = 0.02) (Analysis 1.10). For every 1000 people treated with aspirin, an extra 11 people would make a complete recovery (NNTB 89) (Table 1).
1.10. Analysis.
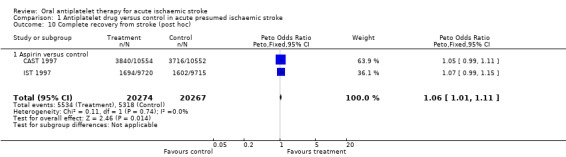
Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 10 Complete recovery from stroke (post hoc).
Re‐evaluation of the planned sensitivity and subgroup analyses in light of the available data
After an evaluation of the available evidence from the randomised trials, it became apparent that three of the planned sensitivity analyses were inappropriate. These were the analyses restricted to trials: (1) in which participants were randomised within 48 hours of the stroke; (2) with adequate concealment of randomisation; and (3) with 100% CT scans prior to randomisation. As the overwhelming majority of the data came from trials that randomised participants within 48 hours of stroke onset, the planned sensitivity analysis evaluating the effects of treatment beyond 48 hours was largely uninformative. Similarly, the analyses based on the concealment of treatment allocation were uninformative about trials with inadequate concealment as these trials contributed so few data. An analysis based on whether or not all participants had brain imaging prior to randomisation to rule out haemorrhage would exclude CAST 1997 and IST 1997, and would therefore disregard 98% of the data. An individual patient data analysis has examined the effect subdivided by whether participants had CT prior to randomisation or not; there was no clear heterogeneity of effect (Chen 2000).
Discussion
Strength of evidence of benefit on major outcomes
This systematic review has reliably established that antiplatelet therapy is safe and effective in the acute phase of ischaemic stroke. The conclusion is based on data from over 40,000 participants. Ninety‐eight per cent of the data came from two trials of medium dose aspirin, that is 160 mg to 300 mg daily (CAST 1997; IST 1997). Overall, antiplatelet therapy with aspirin started within 48 hours of onset of presumed ischaemic stroke was beneficial. Although associated with a small but definite risk of bleeding, this hazard was more than offset by the reduction in recurrent ischaemic stroke. The analysis of the effects of aspirin among participants who were first scanned after randomisation and who turned out to have had a haemorrhagic stroke was reassuring.
The benefits of a short course of antiplatelet therapy in acute ischaemic stroke compare very favourably with longer‐term antiplatelet therapy for secondary prevention in vascular disease. Two to four weeks of treatment in IST 1997 and CAST 1997 resulted in about eight fewer deaths per 1000 participants treated, whereas in long‐term secondary prevention a month of antiplatelet therapy typically avoids less than one death per 1000 (ATC 2002). Similarly, long‐term antiplatelet use prevents about one recurrent stroke per 1000 people per month (ATC 1994a; ATC 2002), whereas in the acute phase of ischaemic stroke one month of antiplatelet therapy prevents about four recurrent strokes per 1000 people (seven fewer ischaemic strokes and three extra haemorrhagic strokes).
The main conclusions of this review have not changed from the last update, despite the exclusion of the trials of intravenous antiplatelet agents.
Effects on venous thromboembolism
Aspirin reduced the odds of PE by 29%, but since the reported rate of pulmonary emboli was low the absolute benefit, one event prevented per 1000 participants treated, is very small. However, if there was a substantial under‐ascertainment of pulmonary emboli in the trials included in this systematic review, then the absolute benefits of aspirin may have been underestimated. Clinical series report a range of 0% to 3% for symptomatic PE (Davenport 1996). If the observed 29% reduction in the odds of PE was applied to a population with a 3% risk of pulmonary embolism the absolute reduction would increase to eight for every 1000 people treated. It seems reasonable to conclude that routine use of aspirin alone in people with acute ischaemic stroke will reduce the risk of DVT and PE somewhat yet not be associated with any substantial excess of intracerebral haemorrhages. It remains unclear whether aspirin alone is as good as heparin alone at preventing venous thromboembolism in acute ischaemic stroke, but data from IST 1997 suggest no statistically or clinically significant difference between aspirin and heparin in the prevention of PE.
Robustness of the findings
The sensitivity analyses have shown that the conclusions about the benefits of antiplatelet therapy are robust. A meta‐analysis based on individual patient data from CAST 1997 and IST 1997 confirmed this (Chen 2000). It showed no clear heterogeneity of treatment effect with delay in starting aspirin, age, gender, stroke type, infarct subtype, the presence of impaired consciousness or the presence or absence of atrial fibrillation. These results suggest that a wide variety of people with ischaemic stroke are likely to benefit from antiplatelet therapy with aspirin (Chen 2000). As a result of the strength of evidence, aspirin is now recommended as a standard therapy. Four major recent evidence‐based guidelines make strong recommendations for the routine use of aspirin for all people with acute ischaemic stroke (CSS 2010; ESO 2008; Jauch 2013; RCP Guideline 2012).
Public health impact
It can be argued that, although effective, the net benefits of aspirin are rather small when compared with the effects of stroke unit care (the OR of death or dependency was 0.79, 95% CI 0.68 to 0.90) (SUTC 2013), and thrombolysis with tissue plasminogen activator within three hours of stroke (the OR of death or dependency was 0.71, 95% CI 0.52 to 0.96) (Wardlaw 2009). However, aspirin is inexpensive, easy to administer and safe, which increases its potential public health impact worldwide and especially in developing countries. In a study published in 1997, the World Health Organization estimated that there were 4.6 million cerebrovascular deaths in the world in 1990 (Murray 1997). The global disease burden of stroke increased by 19% between 1990 and 2010 (Murray 2012) and current projections estimate the number of deaths worldwide will rise to 6.5 million in 2015 (Strong 2007). It is reasonable to estimate that about 80% of the deaths (5.2 million) are due to ischaemic stroke. If treatment with aspirin prevents seven deaths per 1000, then an additional 36,400 lives could be saved worldwide per year with substantial numbers of survivors avoiding long‐term disability.
Aspirin dose and route of administration
The benefits of aspirin in acute stroke are drawn from trials that tested a dose of aspirin between 160 mg and 330 mg daily. In acute myocardial infarction, 160 mg is the lowest dose that has been shown to be effective (ATC 1994a; ATC 2002; Dalen 2006; Patrono 1998). Lower doses of aspirin are effective for long‐term secondary stroke prevention but have not been evaluated in acute stroke. There is some (but not abundant) evidence that at least 120 mg of aspirin is needed to acetylate all circulating platelets within a short period of time (Patrono 1994; van Gijn 1992). There is also some experimental evidence that a dose of 160 mg to 300 mg of aspirin daily is required in the acute phase of an ischaemic cerebral or cardiac event in order to achieve rapid inhibition of thromboxane biosynthesis (Patrono 1998; van Kooten 1994; van Kooten 1997). For people who can swallow, aspirin can be given by mouth. However, as many people with stroke are unable to swallow another route may need to be used on occasions. In CAST 1997 aspirin was given via a nasogastric tube, and in IST 1997 as a rectal suppository or intravenously as 100 mg of the lysine salt of acetylsalicylic acid.
Other antiplatelet agents
The indirect comparisons of different agents in this review showed no evidence of significant heterogeneity of effect between the different agents tested, aspirin alone, ticlopidine alone, the combination of aspirin and dipyridamole. However, the data from the non‐aspirin regimens were extremely limited and such indirect comparisons are unreliable (ATC 1994a). The focus of current research is on comparing the effects of short‐term (30 days to three months) combination therapy with single agents, chiefly in people with TIA and minor stroke (CHANCE 2013; POINT; TARDIS); these regimes are the subject of a separate review (Kamal 2012).
Combination of aspirin with anticoagulants
Another question that remains unanswered is whether the addition of low‐dose subcutaneous heparin to aspirin could further reduce the risk of DVT and PE without unduly increasing the risk of intracranial and extracranial haemorrhage. A systematic review of the randomised trials of anticoagulants in acute myocardial infarction showed that the addition of intravenous or subcutaneous heparin did not add worthwhile extra benefit to the use of antiplatelet therapy alone (Collins 1996). However, the addition of low‐dose subcutaneous heparin to aspirin might be more effective in acute stroke. The only trial that provided a direct randomised comparison of aspirin with aspirin plus low‐dose heparin was IST 1997, yet the available data did not provide conclusive evidence that the combination was more effective than aspirin alone. The question of whether or not to add low‐dose heparin to aspirin can, therefore, only be answered reliably by a further, much larger trial that randomly allocates participants to aspirin or aspirin plus low‐dose subcutaneous heparin. Symptomatic PE (and intracranial haemorrhage) are infrequent in people with ischaemic stroke. Therefore, any proposed trial would need to include several 10s of 1000s of participants in order to provide reliable evidence on the size of any difference in the effects of these two antithrombotic regimens.
Time window for benefit from aspirin
There was clear evidence of net benefit when aspirin therapy was started within 48 hours of stroke onset. A more detailed meta‐analysis based on individual patient data from IST 1997 and CAST 1997 showed no clear evidence that the benefit declined with increasing time from stroke onset up to 48 hours (Chen 2000). The evidence on the effects of starting treatment at more that 48 hours and within 14 days of onset was extremely limited in this review. However, taken with the data from the Antithrombotic Trialists Collaboration the evidence is very strongly suggestive that starting after 48 hours but within 14 days of onset and continuing long‐term is highly likely to be of net benefit (ATC 2002).
Interaction with thrombolytic therapy
Thrombolytic therapy for acute ischaemic stroke within 4.5 hours of symptom onset has received regulatory approval (or is recommended in guidelines) in many places, and is the subject of ongoing research. Since antiplatelet and thrombolytic therapy can cause serious bleeding, it is important to assess the evidence for any interaction between the two agents. The subject is dealt with in some detail in the Cochrane systematic review of thrombolytic therapy (Wardlaw 2009). The interaction between thrombolytic drugs and antithrombotic drugs given simultaneously (or the latter very soon after the former) was only tested by random allocation in MAST‐I 1995, which therefore provides the only truly valid evidence. In MAST‐I 1995 there was a clinically important adverse interaction between aspirin and streptokinase when given simultaneously, resulting in a substantial increase in case fatality (early and late), which was not offset by a reduction in the number of dead or dependent participants at the end of follow‐up; 28% of those allocated to streptokinase alone versus 43% of those allocated to streptokinase plus aspirin were dead by the end of follow‐up (P < 0.001), and 62% and 63% were dead or dependent, respectively (versus 68% in the control group). The actual cause of the increase in early and total deaths with streptokinase and aspirin appeared largely to be due to neurological events. Aspirin with streptokinase significantly increased the number of deaths in hospital from all causes (OR 2.2, 95% CI 1.3 to 3.8), neurological causes (OR 2.0, 95% CI 1.1 to 3.7), and intracranial haemorrhage on CT scan or at autopsy (OR 2.2, 95% CI 1.0 to 5.0) when compared with the group who received streptokinase alone. There was no difference in deaths from neurological causes without intracranial haemorrhage, but note that more participants in the streptokinase plus aspirin group died of neurological causes without a CT scan or autopsy so they could also have had an intracranial haemorrhage. That is, the increase in intracranial haemorrhage with aspirin and streptokinase may be even greater. Information is also available on antithrombotic drug use in 12 other trials. There was a trend towards increased case fatality, which was more frequent the nearer to the administration of thrombolysis the concomitant antithrombotic drug use was (OR 1.95 when all participants received antithrombotic drugs within 24 hours of thrombolysis; 1.27 when some participants received antithrombotic drugs within 24 hours; 1.21 when no participants received antithrombotic drugs within 24 hours but some thereafter; and 0.89 for no antithrombotic drugs within the first 10 to 14 days). Although these data are based mainly on non‐randomised comparisons, they do support the evidence of a clinically significant adverse interaction between the concurrent use of thrombolysis and antithrombotic drugs as found in MAST‐I 1995. This is confirmed by the recent ARTIS 2012 trial comparing recombinant tissue plasminogen activator (rTPA) plus intravenous aspirin with rTPA alone.
New developments
The concept of acute stroke (and the differentiation of stroke from TIA) is changing as people with acute cerebral ischaemia are assessed and treated increasingly earlier and more aggressively. The boundary between acute treatment and very early initiation of secondary prevention is becoming blurred, as evidenced by the FASTER 2007, CHANCE 2013, TARDIS and POINT trials. There is clearly a place for further trials of more intensive antiplatelet regimens, started as soon after symptom onset as possible. However, since aspirin will remain the comparator treatment, such trials will need to be even larger than current trials if they are to produce reliable results.
Authors' conclusions
Implications for practice.
The review provided strong evidence for the benefits of aspirin 160 to 300 mg, given as soon as is practicable (and continued as a once daily dose), in people with suspected acute ischaemic stroke. This evidence applied chiefly to people seen within 48 hours of stroke onset and in whom intracranial haemorrhage had been excluded, or was thought to be clinically unlikely, and had no definite contraindications to aspirin. In people who are unable to swallow safely, aspirin may be given per rectum as a suppository, via a nasogastric tube or intravenously (as 100 mg of the lysine salt of acetylsalicylic acid).
The assessment of the safety and efficacy of antiplatelet agents in people with primary intracranial haemorrhage was not the aim of this review. However, it did provide limited evidence on the effects of aspirin in people in whom intracranial haemorrhage had not been ruled out by brain scanning before treatment was started and who subsequently were shown to have had an intracranial haemorrhage. There was no evidence of net harm in such people. Thus, if there is likely to be a delay before CT or MR brain scanning can be performed to exclude intracranial haemorrhage it may be reasonable to give aspirin until the scan result is known. If the scan shows intracranial haemorrhage then aspirin should probably be discontinued.
In people who cannot tolerate aspirin an alternative antiplatelet agent should be considered, although the evidence for other agents is inadequate at present.
In view of the potential interaction, people who have been treated with thrombolytic therapy should not be started on aspirin for 24 to 48 hours.
Although this review provided very limited evidence on the effects of starting aspirin in people who first present with stroke at between 48 hours and 14 days of onset, what little evidence there was, supported by strong external evidence, suggested net benefit from starting aspirin even at this late stage.
This review confirmed the benefit of continuing treatment in hospital, and external evidence supports its continuation after hospital discharge.
Implications for research.
The overall treatment effect of antiplatelet agents in acute ischaemic stroke is not large and better acute therapies are therefore necessary. The question of whether any particular antiplatelet agent is superior to aspirin 160 mg to 300 mg in the treatment of acute ischaemic stroke remains to be determined, and would require a very large randomised trial to be answered reliably.
In people with unstable coronary artery disease, trials have evaluated the addition of low molecular‐weight heparin or another antiplatelet agent (such as a GP IIb/IIIa inhibitor or clopidogrel) to aspirin. There is a case for such trials to be undertaken in acute ischaemic stroke. There is also a case for further trials of low‐dose subcutaneous heparin (or low‐dose low molecular‐weight heparin) plus aspirin versus aspirin alone in the prevention of post‐stroke deep vein thrombosis and pulmonary embolism, and in reducing neurological disability from the original or recurrent strokes. Such trials would need to include several 10s of 1000s of participants.
Future trials comparing more intense antiplatelet or antithrombotic regimens with aspirin in acute ischaemic stroke will need to include several 10s of 1000s of participants.
Feedback
Are trials of anticoagulant therapy for acute ischaemic stroke ethical?, 26 June 2007
Summary
The Implications for research section states: 'There is also a case for further trials of low‐dose subcutaneous heparin (or low‐dose low‐molecular‐weight heparin) plus aspirin versus aspirin alone in the prevention of post‐stroke deep vein thrombosis and pulmonary embolism, and in reducing neurological disability from the original or recurrent strokes. Such trials would need to include several tens of thousands of patients'. This review should be updated to reflect that, given the lack of efficacy of anticoagulants in multiple trials and high bleeding risk in stroke patients, further trials with low‐dose heparin or low‐dose low‐molecular‐weight heparin would be unethical.
Reply
This comment was submitted in response to the previous version of this review (this response to feedback was delayed by a number of unavoidable administrative factors). We do not agree that the data are sufficiently robust to support a statement that 'further trials with low‐dose heparin or low‐dose low‐molecular‐weight heparin in acute ischemic stroke would be unethical.' Such decisions should rest with the relevant research ethics committees and the trialists (advised by their steering and data monitoring and safety committees).
Contributors
Commenter: David A Cundiff MD
Reply: Peter Sandercock
Feedback and response 2014, 24 June 2014
Summary
I read with interest the Cochrane Review by Sandercock et al which reviewed the use of oral antiplatelet therapy for acute ischemic stroke, and commend the authors on doing an excellent job filtering through the literature and analyzing all available data1. While I do agree with the majority of the authors' conclusions and the overall trend of their therapeutic recommendations, I believe that some conclusion statements regarding use of aspirin post‐stroke are stronger than the data supporting them.
The majority of data in this review (98%) were drawn from two large trials in 1997, CAST2 and IST3. The authors acknowledge that 601 total participants from these trials were lost to follow‐up (300 treatment and 301 control), with the majority coming from CAST (219 treatment and 232 control), but claim that due to the relatively small percentage of patients missing, they can safely assume that these patients did not have an outcome event for the primary analysis. Unfortunately, using Review Manager 5.2 to run sensitivity analyses on several comparisons revealed that results found to be statistically significant in this review became non‐statistically significant when only a few events were added to a group. The CAST trial was used for my analyses due to the larger amount of missing data, and I specifically analyzed the comparisons of death or dependence at the end of follow‐up, all‐cause death during treatment, all‐cause death at follow‐up, pulmonary embolism, and symptomatic intracranial hemorrhage. The primary comparison of death or dependence at end of follow‐up was relatively robust, and required an almost worst‐case scenario of all 219 missing patients in the aspirin group to have had an event to push the result to statistical non‐significance. On the other hand, only five more deaths in the aspirin group for the comparison of all‐cause death during treatment period pushed the upper end of the CI to 1.01. All‐cause death at follow‐up became non‐significant when 50 deaths were added to the treatment group. The finding of reduced pulmonary embolism became non‐significant when only two events were added to the treatment group. Adding 27 events to the aspirin group for the outcome of any recurrent stroke during treatment period similarly results in non‐significance. Interestingly, adding only one symptomatic intracranial hemorrhage to the control group for the outcome looking at this event during treatment period pushed the confidence interval to non‐significance. I believe the results of this review will be viewed in a more accurate light if a discussion of the potential confounding effect of the missing data was included, especially for the outcomes where only a handful of missing events could result in non‐significant data.
There also appears to be an underlying issue with the methodology of the IST study. The IST study was deemed to be at "low risk of bias" by the Cochrane group, even though there was no use of placebo, and trial participants/assessors were not blinded during treatment. They do mention the fact that at follow‐up, the majority of patients appear to not remember what treatment they received and are therefore effectively blinded towards the primary endpoint of death and disability at six months; however, there is no mention of the fact that the open design could have introduced significant bias. It is feasible that clinicians may have been more likely to conduct CT scans on patients who deteriorate clinically while on an antiplatelet, increasing the likelihood of detecting intracranial hemorrhages. On the other hand, clinicians who knew their patient was not receiving an antiplatelet may have tended to follow these patients more closely or found other ways to counterbalance what they may have believed was suboptimal therapy. Sensitivity analyses were done for each comparison, including only double‐blinded studies which essentially excluded IST data and reported CAST results in isolation. This resulted in six comparisons (including the primary) to become statistically non‐significant. I believe it is important to mention how the open design of the IST trial may have affected results in either a positive to negative direction in either the section discussing blinding of trials or in a separate limitations section.
To summarize, I appreciate the effort and dedication put into this periodically updated Cochrane review, but feel that there should be more discussion on the limitations inherent to the two main studies which this review is based on to avoid overestimation of the resulting data.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Harrison Jefferey Lee, B.Sc(Pharm); Joan Chung Yan Ng, B.Sc(Pharm)
References:
Sandercock PAG, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No.: CD000029. DOI: 10.1002/14651858.CD000029.pub3.
CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997; 7;349(9066):1641‐9.
Sandercock P, Collins R, Counsell C, Farrell B, Peto R, Slattery J, et al. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet 1997;349(9065):1569‐81.
Reply
Thank you for these comments on the review. Lee and Ng's comment focus on two points.
1. That the estimates of effect of aspirin are not robust in various sensitivity analyses, applying 'worst case scenario methods' to examine the effect of missing data on some of the outcomes. While this is one approach to assessing the plausibility of the effects of aspirin observed in patients with acute ischaemic stroke, it is important to view the estimates in the context of the effects of aspirin in other patients with acute vascular events (e.g. acute MI) and in secondary prevention after an ischaemic event of the brain heart or peripheral circulation. From that perspective, the effects observed in acute stroke are consistent with those observed in other categories of patients at high risk, which we mention in the discussion.
2. The lack of blinding in IST is a potential source of bias that was not sufficiently discussed in the review. The lack of blinding is discussed, but not exhaustively, partly because, as set out in the primary report of the IST trial, the impact on the primary outcome was likely to be small and the estimates of effect on the secondary outcomes were remarkably similar in the unblinded (IST) and blinded (CAST) studies. The 1997 report of the IST in the Lancet stated: "To minimise bias in the assessment of the 6 month outcome the assessors in most countries were blind to treatment allocation. Moreover, the pilot phase of the study indicated that most patients could not recall their treatment allocation at 6 months,3 so they too were effectively blinded. Estimates of treatment effects among those with central follow‐up (which is likely to be largely blinded) and those without were not significantly different. Thus, lack of blinding probably did not materially affect the main findings for the primary outcomes. Clinicians might, however, have been more likely to arrange repeat CT scanning in patients on active treatment who worsened clinically, detecting more intracranial haemorrhages, so the open design may have introduced some bias in the assessment of the secondary outcomes. However, the apparent effects of aspirin in the open IST were similar to those in CAST, which was placebo‐controlled."
Contributors
Feedback: Harrison Jefferey Lee, B.Sc(Pharm); Joan Chung Yan Ng, B.Sc(Pharm)
Response: Peter Sandercock
What's new
| Date | Event | Description |
|---|---|---|
| 27 September 2019 | Amended | Revised 'Declarations of Interest' statement added |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 25 June 2014 | Feedback has been incorporated | User feedback and authors' responses incorporated. |
| 24 October 2013 | New citation required but conclusions have not changed | Title and inclusion criteria changed. Change to authorship: a new co‐author, Emanuela Cecconi, has replaced Gordon Gubitz. |
| 22 October 2013 | New search has been performed | The searches of CENTRAL, MEDLINE and EMBASE have been updated to May 2013, and the search of the Cochrane Stroke Group Trials Register to October 2013. No new studies have been added. Four studies included in the previous review have been excluded for this update because they assessed parenterally administrated antiplatelet agents, which are now the subject of a separate review. The title of this review was therefore changed to 'Oral antiplatelet agents for acute ischaemic stroke'. There are now eight included trials with 41,483 participants. |
Acknowledgements
We would like to thank Professor Bernard Boneu for providing us with Dr Pince's thesis; Dr Helen Massey (Syntex, UK) for providing us with unpublished data from the ticlopidine trials; Boehringer Ingelheim for providing information on the Kaye 1989 trial; Professor Livia Candelise for providing additional data from MAST‐I 1995; Dr Zheng‐Ming Chen and Dr Hongchao Pan for providing additional data from CAST 1997; Dr Colin Baigent and Dr Cathie Sudlow at the Clinical Trials Service Unit in Oxford for providing data from the Antiplatelet Trialists' Collaboration; Hazel Fraser for sending us regular lists of trials identified by the Cochrane Stroke Review Group's search strategy, and Brenda Thomas for help with trial searching.
Ongoing trials
Any clinician who knows of additional trials that we have omitted please write to Peter Sandercock.
Appendices
Appendix 1. CENTRAL search strategy
#1 [mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh ^"brain ischemia"] or [mh "brain infarction"] or [mh ^"hypoxia‐ischemia, brain"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"intracranial arterial diseases"] or [mh ^"cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh stroke] or [mh ^"vertebral artery dissection"]
#2 isch*mi* near/6 (stroke* or apoplex* or cerebral next vasc* or cerebrovasc* or cva or attack*):ti,ab,kw (Word variations have been searched)
#3 (brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle next cerebr* or mca* or "anterior circulation") near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 [mh "Platelet aggregation inhibitors"]
#6 [mh "Cyclooxygenase Inhibitors"] or [mh Thienopyridines] or [mh "Phosphodiesterase Inhibitors"] or [mh "Thromboxane A2"/AI] or [mh "Purinergic P2Y Receptor Antagonists"]
#7 [mh "Platelet activation"/DE]
#8 [mh "Blood platelets"/DE]
#9 antiplatelet* or anti‐platelet* or antithrombocytic or "anti‐thrombocytic":ti,ab,kw (Word variations have been searched)
#10 (platelet* or thrombocyte*) near/5 (inhibit* or antagonist* or antiaggreg* or anti‐aggreg*):ti,ab,kw (Word variations have been searched)
#11 cyclooxygenase next inhibitor* or thienopyridine* or phosphodiesterase next inhibitor*:ti,ab,kw (Word variations have been searched)
#12 "thromboxane A2" near/3 (inhib* or antag*):ti,ab,kw (Word variations have been searched)
#13 aspirin* or "acetyl salicylic acid" or "acetylsalicylic acid":ti,ab,kw (Word variations have been searched)
#14 ARC1779 or AZD6140 or alprostadil or asasantin or carnitine or cilostazol or clopidogrel or cloricromene or cv4151 or "cv‐4151" or defibrotide or dilazep or dipyridamol* or disintegrin* or ditazol or E5880 or E5510 or epoprostenol* or fluribrofen or "fut‐175" or iloprost* or indobufen or isbogrel or kbt3022 or "kbt‐3022" or ketanserin* or ketoprofen or ketorolac or levamisol* or ligustrazine* or tromethamine* or milrinone* or mopidamol* or naudicelle or nimesulide or ozagrel* or oky046 or "oky‐046" or "oky‐1581" or phthalzinol or picotamide or policosanol or prasugrel or procainamide or sarpogrelate or satigrel or sulphinpyrazone or sulfinpyrazone or suloctadil or terutroban or ticagrelor or ticlopidine or trapidil or triflusal or vorapaxar:ti,ab,kw (Word variations have been searched)
#15 {or #5‐#14}
#16 #4 and #15
#17 cardiac or aneurysm* or angina or "atrial fibrillation" or cancer or athritis or diabetes or coronary or myocardial:ti (Word variations have been searched)
#18 #16 not #17
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or hypoxia‐ischemia, brain/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid artery, internal, dissection/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ or vertebral artery dissection/
2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. 1 or 2 or 3
5. exp Platelet aggregation inhibitors/
6. exp Cyclooxygenase Inhibitors/ or exp Thienopyridines/ or exp Phosphodiesterase Inhibitors/ or Thromboxane A2/ai or exp Purinergic P2Y Receptor Antagonists/
7. exp Platelet activation/de
8. exp Blood platelets/de
9. (antiplatelet$ or anti‐platelet$ or antithrombocytic or anti‐thrombocytic).tw.
10. ((platelet$ or thrombocyte$) adj5 (inhibit$ or antagonist$ or antiaggreg$ or anti‐aggreg$)).tw.
11. (cyclooxygenase inhibitor$ or thienopyridine$ or phosphodiesterase inhibitor$).tw.
12. (thromboxane A2 adj3 (inhib$ or antag$)).tw.
13. (aspirin$ or acetyl salicylic acid$ or acetyl?salicylic acid$).tw,nm.
14. (ARC1779 or AZD6140 or alprostadil or asasantin or carnitine or cilostazol or clopidogrel or cloricromene or cv4151 or cv‐4151 or defibrotide or dilazep or dipyridamol$ or disintegrin$ or ditazol or E5880 or E5510 or epoprostenol$ or fluribrofen or fut‐175 or iloprost$ or indobufen or isbogrel or kbt3022 or kbt‐3022 or ketanserin$ or ketoprofen or ketorolac or levamisol$ or ligustrazine$ or tromethamine$ or milrinone$ or mopidamol$ or naudicelle or nimesulide or ozagrel$ or oky046 or oky‐046 or oky‐1581 or phthalzinol or picotamide or policosanol or prasugrel or procainamide or sarpogrelate or satigrel or sulphinpyrazone or sulfinpyrazone or suloctadil or terutroban or ticagrelor or ticlopidine or trapidil or triflusal or vorapaxar).tw,nm.
15. or/5‐14
16. Randomized Controlled Trials as Topic/
17. random allocation/
18. Controlled Clinical Trials as Topic/
19. control groups/
20. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
21. double‐blind method/
22. single‐blind method/
23. Placebos/
24. placebo effect/
25. Therapies, Investigational/
26. Drug Evaluation/
27. Research Design/
28. randomized controlled trial.pt.
29. controlled clinical trial.pt.
30. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
31. (random$ or RCT or RCTs).tw.
32. (controlled adj5 (trial$ or stud$)).tw.
33. (clinical$ adj5 trial$).tw.
34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
35. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
36. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
37. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
38. (placebo$ or sham).tw.
39. trial.ti.
40. (assign$ or allocate$).tw.
41. or/16‐40
42. 4 and 15 and 41
43. exp animals/ not humans.sh.
44. 42 not 43
Appendix 3. EMBASE search strategy
1. cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or exp brain ischemia/ or carotid artery disease/ or exp carotid artery obstruction/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke patient/
2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. 1 or 2 or 3
5. exp Antithrombocytic agent/
6. thienopyridine derivative/ or exp phosphodiesterase inhibitor/ or thromboxane A2 receptor blocking agent/ or exp purinergic receptor blocking agent/
7. (antiplatelet$ or anti‐platelet$ or antithrombocytic or anti‐thrombocytic).tw.
8. ((platelet$ or thrombocyte$) adj5 (inhibit$ or antagonist$ or antiaggreg$ or anti‐aggreg$)).tw.
9. (cyclooxygenase inhibitor$ or thienopyridine$ or phosphodiesterase inhibitor$).tw.
10. (thromboxane A2 adj3 (inhib$ or antag$)).tw.
11. (aspirin$ or acetyl salicylic acid$ or acetyl?salicylic acid$).tw.
12. (ARC1779 or AZD6140 or alprostadil or asasantin or carnitine or cilostazol or clopidogrel or cloricromene or cv4151 or cv‐4151 or defibrotide or dilazep or dipyridamol$ or disintegrin$ or ditazol or E5880 or E5510 or epoprostenol$ or fluribrofen or fut‐175 or iloprost$ or indobufen or isbogrel or kbt3022 or kbt‐3022 or ketanserin$ or ketoprofen or ketorolac or levamisol$ or ligustrazine$ or tromethamine$ or milrinone$ or mopidamol$ or naudicelle or nimesulide or ozagrel$ or oky046 or oky‐046 or oky‐1581 or phthalzinol or picotamide or policosanol or prasugrel or procainamide or sarpogrelate or satigrel or sulphinpyrazone or sulfinpyrazone or suloctadil or terutroban or ticagrelor or ticlopidine or trapidil or triflusal or vorapaxar).tw.
13. or/5‐12
14. Randomized Controlled Trial/
15. Randomization/
16. Controlled Study/
17. control group/
18. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
19. Double Blind Procedure/
20. Single Blind Procedure/ or triple blind procedure/
21. placebo/
22. drug comparison/ or drug dose comparison/
23. "types of study"/
24. random$.tw.
25. (controlled adj5 (trial$ or stud$)).tw.
26. (clinical$ adj5 trial$).tw.
27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
31. (placebo$ or sham).tw.
32. trial.ti.
33. (assign$ or allocat$).tw.
34. (RCT or RCTs).tw.
35. or/14‐34
36. 4 and 13 and 35
37. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
38. human/ or normal human/ or human cell/
39. 37 not 38
40. 36 not 39
41. (cardiac or aneurysm$ or angina or atrial fibrillation or cancer or athritis or diabetes or coronary or myocardial).ti.
42. 40 not 41
Data and analyses
Comparison 1. Antiplatelet drug versus control in acute presumed ischaemic stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependence at end of follow‐up | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| 1.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| 2 Deaths from all causes during treatment period | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 2.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 2.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 2.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.20] |
| 3 Deaths from all causes during follow‐up | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 3.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 3.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 3.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.88] |
| 4 Deep venous thrombosis during treatment period | 2 | 133 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.36, 1.67] |
| 4.1 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.13, 0.95] |
| 4.2 Ticlopidine versus control | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.37 [0.72, 7.73] |
| 5 Pulmonary embolism during treatment period | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.96] |
| 5.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.97] |
| 5.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 5.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.06] |
| 6 Recurrent ischaemic/unknown stroke during treatment period | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 6.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 6.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Symptomatic intracranial haemorrhage during treatment period | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [1.00, 1.50] |
| 7.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [1.00, 1.50] |
| 7.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 7.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 8 Any recurrent stroke/intracranial haemorrhage during treatment period | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| 8.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.79, 0.97] |
| 8.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 8.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 9 Major extracranial haemorrhage during treatment period | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| 9.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| 9.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Complete recovery from stroke (post hoc) | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
| 10.1 Aspirin versus control | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CAST 1997.
| Methods | C = centrally produced, prepacked, sequentially numbered envelopes Double blind Exclusions during trial: none Losses to follow‐up: 451 (219 Rx, 232 control) at 4 weeks | |
| Participants | China 21,106 participants 63% male 28% were more than 70 years old 87% had CT before entry Ischaemic stroke less than 48 hours since stroke onset | |
| Interventions | Rx: aspirin 160 mg once daily orally or via nasogastric tube Control: placebo Duration: 4 weeks, or until death or earlier discharge | |
| Outcomes | Death Recurrent stroke Functional outcome (modified Rankin disability scale less than 3 = independent) Pulmonary embolism (symptomatic) Extracranial haemorrhage | |
| Notes | Ex: not specified by protocol but by responsible physician, possibly including increased risk of adverse effects, or little likelihood of any worthwhile benefit in hospital FU: 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ciufetti 1990.
| Methods | R = random number list Double blind Exclusions during trial: none Losses to follow‐up: none | |
| Participants | Italy 30 participants 14 (47%) male Mean age: 73 years (all more than 65 years) 100% CT before entry Hemiparetic ischaemic stroke less than 12 hours since stroke onset | |
| Interventions | Rx: ticlopidine 250 mg orally 12 hourly Control: placebo Duration: 3 weeks | |
| Outcomes | Death Pulmonary embolism (symptomatic) Recurrent stroke | |
| Notes | Ex: cerebral oedema FU: 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
IST 1997.
| Methods | C = telephone randomisation Unblinded; dependency assessment mainly blinded Exclusions during trial: none Losses to follow‐up: 2 at 14 days (1 Rx, 1 control); 150 at 6 months (81 Rx, 69 control) | |
| Participants | International 19,435 participants 54% male 61% more than 70 years 67% CT prior to randomisation, 29% CT after randomisation Ischaemic stroke less than 48 hours since stroke onset | |
| Interventions | Rx: subcutaneous heparin (5000 IU or 12 500 IU 12 hourly), aspirin 300 mg, both, or neither (factorial design) Duration: 14 days or until discharge from hospital Aspirin by mouth if able to swallow, if not then by rectal suppository or by injection of 100 mg of the lysine salt of aspirin | |
| Outcomes | Death Functional outcome (validated simple questions) Recurrent stroke Pulmonary embolus (symptomatic) Intracranial haemorrhage (symptomatic CT) Extracranial haemorrhage | |
| Notes | Ex: small likelihood of worthwhile benefit; high risk of adverse effect (e.g. hypersensitivity of aspirin, recent GI bleed or peptic ulcer disease, already on long‐term anticoagulation) FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
MAST‐I 1995.
| Methods | C = telephone central office Assessor blind (telephone follow‐up) Exclusions during trial: none Losses to follow‐up: none | |
| Participants | Europe 309 participants 163 (53%) male 22% less than 60 years, 46% 61 to 75 years 100% CT before entry Ischaemic stroke Less than 6 hours since stroke onset | |
| Interventions | Factorial design of streptokinase and aspirin versus no treatment; only aspirin alone versus no aspirin included to prevent confounding influence of streptokinase Rx: aspirin 300 mg oral (or iv/rectal) 24 hourly Control: no treatment Duration: 10 days | |
| Outcomes | Death plus cause of death Functional outcome at 6 months (modified Rankin less than 3 = independent) Intracranial haemorrhage (symptomatic plus systematic) Extracranial haemorrhage Pulmonary embolism (symptomatic) Recurrent stroke Myocardial infarction | |
| Notes | Ex: coma, bleeding risk FU: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Pince 1981.
| Methods | R = sealed envelope Double blind Exclusions during trial: 9 (5 Rx, 4 control) Losses to follow‐up: none | |
| Participants | France 80 participants 50 (62%) male Mean age: 66 years No CT before entry; 100% had lumbar puncture Presumed ischaemic stroke Less than 6 days since stroke onset | |
| Interventions | Rx: aspirin 330 mg 8 hourly (oral) plus dipyridamole 75 mg 8 hourly (oral) Control: placebo Duration: 1 week | |
| Outcomes | Death DVT (systematic I125 scan) Pulmonary embolism (symptomatic) Intracranial haemorrhage (symptomatic) Extracranial haemorrhage | |
| Notes | Ex: bleeding risk, aspirin allergy FU: 10 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rödén‐Jüllig 2003.
| Methods | R = randomisation tables, stratified for gender C = sequentially numbered containers from pharmacy Double blind Exclusions during trials: none Losses to follow‐up: none | |
| Participants | Sweden 441 participants 226 (51%) male 100% CT before entry Ischaemic stroke less than 72 hours since stroke onset SSSS 1 point or more | |
| Interventions | Rx: aspirin 325 mg orally once daily Control: placebo Duration: 5 days | |
| Outcomes | Death 2 points or more (may be in different items) worsening on SSSS at 5 days Ability to walk unaided, increased need for ADL help at 3 months | |
| Notes | Ex: specified by protocol ‐ includes severe concomitant medical conditions or pre‐existing neurological illness, bleeding risk, blood pressure above 240/140 mmHg FU: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Turpie 1983.
| Methods | R = identical, sequentially numbered bottles from pharmacy Double blind Exclusions during trial: 4 (2 Rx, 2 control) Losses to follow‐up: none | |
| Participants | Canada 53 participants 21 (40%) male Age range 33 to 92 years No CT before entry Any stroke with leg paresis Less than 4 weeks since stroke onset (most less than 2 weeks) | |
| Interventions | Rx: ticlopidine 250 mg orally 12 hourly Control: placebo Duration: 10 to 21 days | |
| Outcomes | Death DVT (systematic I125 scan/plethysmography and venography) Pulmonary embolism (symptomatic) Intracranial haemorrhage (symptomatic) Extracranial haemorrhage | |
| Notes | Ex: unknown FU: 21 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Utsumi 1988.
| Methods | R = unknown Not blind Exclusions during trial: 1 (control) Losses to follow‐up: none | |
| Participants | Japan 29 participants 5 (17%) male Mean age: 63 years CT scanning was not stated Ischaemic stroke less than 4 weeks since stroke onset (only 1 more than 2 weeks) | |
| Interventions | Rx: ticlopidine 100 mg orally 8 to 12 hourly Control: no treatment Duration: up to 3 months (median 1 month) | |
| Outcomes | Death Pulmonary embolism (symptomatic) Intracranial haemorrhage Extracranial haemorrhage | |
| Notes | Ex: unknown FU: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ADL: activities of daily living C: concealment CT: computerised tomography DVT: deep venous thrombosis Ex: exclusions FU: follow‐up GI: gastrointestinal iv: intravenous R: randomisation Rx: treatment SSSS: Scandinavian Stroke Supervision Scale Systematic: outcome sought by systematically scanning all patients at a predefined time, irrespective of presence or absence of symptoms
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abciximab 2000 | Intravenous antiplatelet therapy |
| AbESTT 2005 | Intravenous antiplatelet therapy |
| AbESTT‐II/C 2008 | Intravenous antiplatelet therapy |
| AbESTT‐II/P 2008 | Intravenous antiplatelet therapy |
| AbESTT‐II/W 2008 | Intravenous antiplatelet therapy |
| Aoki 2000 | Not adequately randomised; participants allocated on alternating basis Not acute therapy |
| APLAUD 2000 | Not acute stroke therapy Randomisation up to 6 months post‐stroke |
| Apollonia 1989 | Not adequately randomised; confounded; people with acute and non‐acute stroke included |
| ARTIS 2012 | Intravenous antiplatelet therapy |
| Balkuv‐Ulutin 1989 | Not randomised |
| BRAVO 2000 | Not acute stroke therapy; people included up to 30 days post‐stroke Trial halted prematurely because of bleeding risk, but trial data remain unpublished |
| Chen 1988 | Not adequately randomised |
| Cheung 2000 | Intravenous antiplatelet therapy |
| CLEAR trial | Confounded trial |
| D'Andrea 1993 | No control group which did not receive antiplatelet therapy (picotamide versus aspirin versus aspirin and picotamide) |
| Fan 2003 | Ligustrazine, a kind of Chinese herbal medicine, is not an antiplatelet agent |
| FASTER 2007 | No control group (clopidogrel plus aspirin versus aspirin alone in minor stroke and TIA within 24 hours of onset) |
| Gao 1989 | No control group (aspirin versus ligustrazine versus betahistine) |
| Garcia Tigera 1994 | No control group (aspirin versus levamisol) |
| Hakim 1984 | Not randomised |
| Iida 1984 | Not acute stroke therapy |
| JASAP 2011 | No control group (extended‐release dipyridamole 200 mg plus acetylsalicylic acid 50 mg in a capsule, 2 capsules twice daily versus acetylsalicylic acid 81 mg,1 tablet once daily) Not acute stroke therapy; participants included between 1 week and 6 months after stroke |
| Joseph 1989 | Not adequately randomised |
| Junghans 2002 | Not randomised |
| Kamath 2001 | No results published, described as 'discontinued' |
| Kaye 1989 | No control group which did not receive antiplatelet therapy (aspirin and dipyridamole combined versus aspirin alone) |
| Lecrubier 1977 | Not acute stroke therapy |
| Li 1999 | Non‐randomised, dose escalation study |
| Li 2005 | Intravenous antiplatelet therapy |
| Liu 1994 | Method of allocation unclear, no relevant clinical outcomes reported |
| Matsumoto 2005 | Multiple antiplatelet agents |
| Monreal 1987 | No relevant clinical outcomes reported |
| Ohtomo 1991 | Intravenous antiplatelet therapy |
| SaTIS 2011 | Intravenous antiplatelet therapy |
| Seitz 2004 | No relevant clinical outcomes reported |
| Siebler 2003 | No relevant clinical outcomes reported |
| TACS 2000 | Not acute stroke therapy; participants included up to 6 months after stroke |
| TARDIS | Multiple antiplatelet agents. Ongoing trial |
| Wu 2005 | Ligustrazine, a kind of Chinese herbal medicine, is not an antiplatelet agent |
| Yan 1998 | Not adequately randomised |
| Zhang 2005 | Method of allocation unclear, no relevant clinical outcomes reported |
TIA: transient ischaemic attack
Characteristics of studies awaiting assessment [ordered by study ID]
Fujimoto 2010.
| Methods | |
| Participants | People with non‐cardioembolic ischaemic stroke within 72 hours of the onset and NIHSS score ≤ 7 |
| Interventions | Cilostazol (200 mg/d) plus intravenous antithrombotic agents (heparin, ozagrel sodium or argatroban) versus intravenous antithrombotic agents (heparin, ozagrel sodium or argatroban) |
| Outcomes | Neurological deterioration and stroke recurrence at 14 days, and mRS at 3 months |
| Notes |
Wang 2000.
| Methods | Randomised, placebo‐controlled trial |
| Participants | People with ischaemic stroke within 48 hours of the onset and demonstrated by CT |
| Interventions | Aspirin (160 mg/d for 4 weeks) versus placebo |
| Outcomes | |
| Notes |
CT: computerised tomography mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Characteristics of ongoing studies [ordered by study ID]
CAIST‐J 2006.
| Trial name or title | Cilostazol in Acute Ischemic Stroke Trial |
| Methods | Randomised, single‐blind ‐ participants are blinded |
| Participants | People with ischaemic stroke within 72 hours after the onset and NIHSS score ≥ 14 |
| Interventions | Cilostazol versus conventional therapy |
| Outcomes | Primary outcome: mRS 0 to 2 at 3 months Secondary outcome: recurrence of stroke, NIHSS, JSS, Barthel Indes, mRS, MMSE, neurological deterioration, pneumonia |
| Starting date | 2006 |
| Contact information | Norihiro Suzuki Keio University School of Medicine Department of Neurology 35 Shinanomachi Shinjuku‐ku Tokyo 165‐8582 Japan |
| Notes |
CAPS 2009.
| Trial name or title | A randomised clinical trial of an antiplatelet agent in the treatment of acute stroke: cilostazol in the prevention of acute progressing stroke (CAPS) study |
| Methods | Randomised, open‐label |
| Participants | People with non‐cardioembolic cerebral infarction within 24 hours after the onset, aged 20 to 80 years |
| Interventions | Cilostazol (200 mg/d) plus standard therapy versus standard therapy |
| Outcomes | mRS at 3 months, progressing stroke, incidence of cardiovascular events |
| Starting date | 2009 |
| Contact information | Teiji Tominaga Tohoku University Graduate School of Medicine Department of Neurosurgery 1‐1, Seiryo‐machi, Aoba‐ku, SENDAI 980‐8574 |
| Notes |
JSS: Japan Stroke Scale MMSE: Mini Mental State Exmanination mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Differences between protocol and review
For this update we changed the title of the review to 'Oral antiplatelet therapy for acute ischaemic stroke'. We decided to exclude parenterally administrated antiplatelet agents since some of them, GP IIb/IIIa receptor inhibitors, are the focus of a separate review (Ciccone 2006) which is being updated.
Moreover, we planned to assess the methodological quality of any new trials in the following domains: random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessment; incomplete outcome data; and selective outcome reporting. However, since no new trials were identified we have retained the original assessment of risk of bias and no new sensitivity analyses were performed.
Contributions of authors
Carl Counsell prepared the original version of this Cochrane review, and has checked the analyses and commented on each subsequent revision. Gord Gubitz performed the literature searches, extracted the data, performed the statistical analyses and re‐redrafted the text for the 1999 update. Mei‐Chiun Tseng did the searches, extracted data, updated the analyses and commented on the text for the 2007 update. Peter Sandercock designed and performed a pre‐Cochrane systematic review in 1993 of trials of antiplatelet agents versus control, which became the basis for this review; for each subsequent update he helped with searches, data extraction, data checking, analysis and drafting of the text, and is the guarantor of the review. For this update Emanuela Cecconi did the searches, updated the text and analyses, and commented on the review.
Sources of support
Internal sources
University of Edinburgh, UK.
University of Aberdeen, UK.
External sources
Wellcome Trust, UK.
Medical Research Council, UK.
Declarations of interest
Peter Sandercock was the principal investigator of the International Stroke Trial (IST 1997).
Carl Counsell was on the Steering Committee of IST. He has received from a variety of manufacturers of antiplatelet and anticoagulant drugs (Sanofi, Bristol Meyer Squibb, Sanofi‐Synthelabo, Organon, Boehringer Ingelheim, Janssen) lecture fees and travel expenses for lectures delivered at conferences; consultancy fees; has in the past received research grants from Glaxo‐Wellcome and Boehringer Ingelheim; and the drug supply for the start‐up phase of the IST‐3 trial was donated to his department by Boehringer Ingelheim. However, he does not have any contractual consultancy arrangements with any company, or any current research grants from any company, nor does he hold stock (or hold any other financial interests) in any pharmaceutical company.
Gordon Gubitz: randomised participants in the IST.
Mei‐Chiun Tseng: none known.
Edited (no change to conclusions)
References
References to studies included in this review
CAST 1997 {published and unpublished data}
- CAST (Chinese Acute Stroke Trial) Collaborative Group. A randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641‐9. [PubMed] [Google Scholar]
Ciufetti 1990 {published and unpublished data}
- Ciuffetti G, Aisa G, Mercuri M, Lombardini R, Paltriccia R, Neri C, et al. Effects of ticlopidine on the neurologic outcome and the hemorheologic pattern in the postacute phase of ischemic stroke: a pilot study. Angiology 1990;41:505‐11. [DOI] [PubMed] [Google Scholar]
IST 1997 {published and unpublished data}
- International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569‐81. [PubMed] [Google Scholar]
MAST‐I 1995 {published and unpublished data}
- Multicentre Acute Stroke Trial‐Italy (MAST‐I) Group. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Lancet 1995;346:1509‐14. [PubMed] [Google Scholar]
Pince 1981 {unpublished data only}
- Pince J. Thromboses veineuses des membres inferieures et embolies pulmonaires au cours des accidents vasculaires cerebraux. A propos d'un essai comparatif de traitment preventif. (These pour le doctorat d'etat en medicine). Toulouse: Universite Paul Sabatier, 1981. [Google Scholar]
Rödén‐Jüllig 2003 {published data only}
- Rödén‐Jüllig Å, Britton M, Malmkvist K, Leijd B. Aspirin in the prevention of progressing stroke: a randomized controlled study. Journal of Internal Medicine 2003;254:584‐90. [DOI] [PubMed] [Google Scholar]
Turpie 1983 {unpublished data only}
- Turpie AGG, Dobkin B, McKenna R. A trial of ticlopidine, an antiplatelet agent, for acute cerebral infarction. Guildford: Sanofi Winthrop (Sanofi internal report 1983.001.6.188) 1983.
Utsumi 1988 {unpublished data only}
- Utsumi H. Evaluation of utility of ticlopidine, an antiplatelet agent, for acute cerebral infarction. Guildford: Sanofi Winthrop (Sanofi internal report 001.6.128) 1984.
References to studies excluded from this review
Abciximab 2000 {published data only}
- The Abciximab in Ischemic Stroke Investigators. Abciximab in acute ischemic stroke. A randomized, double‐blind, placebo‐controlled, dose escalation study. Stroke 2000;31:601‐9. [DOI] [PubMed] [Google Scholar]
AbESTT 2005 {published data only}
- Abciximab Emergent Stroke Treatment Trial (AbESTT) Investigators. Emergency administration of abciximab for treatment of patients with acute ischemic stroke. Results of a randomized phase 2 trial. Stroke 2005;36:880‐90. [DOI] [PubMed] [Google Scholar]
AbESTT‐II/C 2008 {published data only}
- Adams HP Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT‐II). Stroke 2008;39:87‐99. [DOI] [PubMed] [Google Scholar]
AbESTT‐II/P 2008 {published data only}
- Adams HP Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT‐II). Stroke 2008;39:87‐99. [DOI] [PubMed] [Google Scholar]
AbESTT‐II/W 2008 {published data only}
- Adams HP Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT‐II). Stroke 2008;39:87‐99. [DOI] [PubMed] [Google Scholar]
Aoki 2000 {unpublished data only}
- Aoki T. Prolonged administration of ticlopidine to cerebral infarction. Unpublished. [Cochrane Stroke Group Trials Register ID 3461]
APLAUD 2000 {published data only}
- Harrington RA, Armstrong PW, et al. for the Anti‐PLAtelet Useful Dose (APLAUD) Study Investigators. Dose‐finding, safety, and tolerability study of an oral platelet glycoprotein IIb/IIIa inhibitor, lotrafiban, in patients with coronary or cerebral atherosclerotic disease. Circulation 2000;102:728‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Apollonia 1989 {published data only}
- Apollonia A, Castignani P, Magrini L, Angeletti R. Ticlopidine‐pentoxiphylline combination in the treatment of atherosclerosis and the prevention of cerebrovascular accidents. Journal of International Medical Research 1989;17:28‐35. [DOI] [PubMed] [Google Scholar]
ARTIS 2012 {published data only}
- Zinkstok SM, Roos YB, ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet 2012;380(9843):731‐7. [DOI: 10.1016/S0140-6736(12)60949-0] [DOI] [PubMed] [Google Scholar]
Balkuv‐Ulutin 1989 {published data only}
- Balkuv‐Ulutin S, Hacihanifioglu M, Ulutin UN. Clinical and laboratory results obtained with defibrotide in the case of acute stroke. Thrombosis and Haemostasis 1989;62:585. [Google Scholar]
BRAVO 2000 {published data only}
- Topol EJ, Easton JD, Amarenco P, Califf R, Harrington R, Graffagnino C, et al. Design of the blockade of the glycoprotein IIb/IIIa receptor to avoid vascular occlusion (BRAVO) trial. American Heart Journal 2000;139:927‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 1988 {published data only}
- Chen J, Ye M, Wei J. Hemorrheological study on the effect of acupuncture in treating cerebral infarction. Journal of Traditional Chinese Medicine 1988;8:167‐72. [Cochrane Stroke Group Trials Register id 4731] [PubMed] [Google Scholar]
Cheung 2000 {published data only}
- Cheung RTF, Ho DSW. Fatal hemorrhagic transformation of acute cerebral infarction after the use of abciximab. Stroke 2000;31:2518‐9. [DOI] [PubMed] [Google Scholar]
CLEAR trial {published data only}
- Pancioli A, for the CLEAR Trial Investigators. The combined approach to lysis utilizing eptifibatide and rt‐PA in acute ischemic stroke (the CLEAR stroke trial): blinded results from tier I and II. Proceedings of the International Stroke Conference; 2006 February 16‐18; Kissimmee (FL), USA. 2006:(Abstract CTP17). [DOI] [PMC free article] [PubMed]
- Pancioli AM, Broderick JP, the CLEAR Stroke Trial Investigators. The combined approach to lysis utilizing eptifibatide and rt‐PA in acute ischemic stroke (The CLEAR Stroke Trial). Proceedings of the 29th International Stroke Conference; 2004 February 5‐7; San Diego (CA), USA. 2004:(Abstract CTP16). [DOI] [PMC free article] [PubMed]
D'Andrea 1993 {published data only}
- D'Andrea G, Cananzi AR, Alecci M, Perini F, Zamberlan F, Milone FF. Study of effect of picotamide, ASA, and picotamide plus ASA on platelet aggregation in ischemic stroke. Canadian Journal of Neurological Sciences 1993;20 Suppl 4:s23. [Google Scholar]
- D'Andrea G, Perini F, Hasselmark L, Alecci M, Cananzi AR. Effect of picotamide and aspirin, combined or alone, on platelet aggregation in patients with cerebral infarction. Functional Neurology 1995;10:91‐8. [PubMed] [Google Scholar]
Fan 2003 {published data only}
- Fan DL. Clinical research of ligustrazine for acute cerebral infarction. Chinese Journal of Hemorheology 2003;13:250. [Google Scholar]
FASTER 2007 {published data only}
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurology 2007;6:961‐9. [DOI] [PubMed] [Google Scholar]
Gao 1989 {published data only}
- Gao B, Hu ZX, Li CM. The effect of ligustrazine, aspirin and betahistine on the platelet aggregation of ischemic stroke patients. Chinese Journal of Neurology and Psychiatry 1989;22:148‐51. [Cn‐00280553] [PubMed] [Google Scholar]
Garcia Tigera 1994 {published data only}
- Garcia Tigera J, Martinez Dreke R. Levamisol, a new treatment for cerebral infarction [Abstract]. Journal of Neurology 1994;241 Suppl 1:S162. [Google Scholar]
Hakim 1984 {published data only}
- Hakim AM, Pokrupa RP, Wolfe LS. Preliminary report on the effectiveness of prostacyclin in stroke. Canadian Journal of Neurological Sciences 1984;11:409. [Google Scholar]
Iida 1984 {unpublished data only}
- Iida N. Clinical experience with ticlopidine in patients with cerebral infarction. Unpublished data 1984.
JASAP 2011 {published data only}
- Uchiyama S, Ikeda Y, Urano Y, Horie Y, Yamaguchi T. The Japanese aggrenox (extended‐release dipyridamole plus aspirin) stroke prevention versus aspirin programme (JASAP) study: a randomized, double‐blind, controlled trial. Cerebrovascular Diseases 2011;31:601‐13. [DOI: 10.1159/000327035] [DOI] [PubMed] [Google Scholar]
Joseph 1989 {published data only}
- Joseph R, Welch KM, D'Andrea G. Effect of therapy on platelet activation factor induced aggregation in acute stroke. Stroke 1989;20:609‐11. [DOI] [PubMed] [Google Scholar]
Junghans 2002 {published data only}
- Junghans U, Seitz R, Wittsack H, Fink GR, Freund H, Siebler M. Ischaemic brain tissue salvaged by infarction by the GP IIb/IIa platelet antagonist tirofiban. Neurology 2002;58:474‐6. [DOI] [PubMed] [Google Scholar]
Kamath 2001 {published data only}
- Kamath S, Lip GY. YM‐337 Yamanouchi. Current Opinion in Investigational Drugs 2001;2:1093‐6. [PubMed] [Google Scholar]
Kaye 1989 {unpublished data only}
- Kaye J. A trial to evaluate the relative roles of dipyridamole and aspirin in the prevention of deep vein thrombosis in stroke patients. Brackness (UK): Boehringer Ingelheim (Internal report trial no. CT 330/189) 1989.
Lecrubier 1977 {published data only}
- Lecrubier C, Conard J, Samama M, Bousser M‐G. Randomised trial of a new platelet anti‐aggregant agent: ticlopidine. Therapie 1977;32:189‐94. [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li SH, Li DY, Wang YJ, Guo H, Cheng CY, Zhao J. Study of optimal aspirin loading and maintaining dosage for cerebral infarction at acute stage. Chinese Journal of Neurology 1999;32:302. [Cn‐00282304] [Google Scholar]
Li 2005 {published data only}
- Li GY, Lin YX, Zhou Y, Song LF, Li BC. Effect of ozagrel sodium in ameliorating the motor function of patients with acute lacunar infarct of dyskinesias: A randomized, double‐blind, placebo‐controlled trial. Chinese Journal of Clinical Rehabilitation 2005;9:4‐5. [Google Scholar]
Liu 1994 {published data only}
- Liu X, Li X, Miao L, Wang Q, Bao L. Therapeutic observation and biochemical study of calcium antagonist and anti‐thromboxane A2 activity in acute ischaemic stroke. Journal of Apoplexy and Nervous Diseases 1994;11:72‐4. [Google Scholar]
Matsumoto 2005 {published data only}
- Matsumoto Y. Comparison of the effects between ozagrel Na combined with cilostazol and ozagrel Na alone for acute phase ischemic stroke in the territory of perforating arteries of the MCA: a randomized open trial. UMIN Clinical Trials Registry. center.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi (accessed 8 August 2007).
Monreal 1987 {published data only}
- Monreal M, Lafoz E, Foz M, Monasterio J. Aspirin and the kidney in patients with cerebral ischemia. Thrombosis and Haemostasis 1987;58:548. [Google Scholar]
Ohtomo 1991 {published data only}
- Ohtomo E, Kutsuzawa T, Kogure K, Hirai S, Goto F, Terashi Am Tazaki Y, et al. Clinical usefulness of OKY‐046 on the acute stage of cerebral thrombosis ‐ double blind trial in comparison with placebo. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1991;7:353‐88. [Google Scholar]
SaTIS 2011 {published data only}
- Siebler M, Hennerici MG, Schneider D, Reutern GM, Seitz RJ, Röther J, et al. Safety of Tirofiban in acute Ischemic Stroke: the SaTIS trial. Stroke 2011;42:2388‐92. [DOI: 10.1161/STROKEAHA.110.599662] [DOI] [PubMed] [Google Scholar]
Seitz 2004 {published data only}
- Seitz RJ, Meisel S, Moll M, Wittsack H‐J, Junghans U, Siebler M. The effect of combined thrombolysis with rtPA and tirofiban on ischemic brain lesions. Neurology 2004;62:2110‐2. [DOI] [PubMed] [Google Scholar]
Siebler 2003 {published data only}
- Siebler M. Cerebral microembolism is blocked by tirofiban, a selective nonpeptide platelet glycoprotein IIb/IIIa receptor antagonist. Circulation 2003;107:2717‐21. [DOI] [PubMed] [Google Scholar]
TACS 2000 {unpublished data only}
- Marini C, Rothwell P, Orio F, Maragliano E. Efficacy and safety of ticlopidine and aspirin versus aspirin alone in the prevention of vascular events after cerebral ischaemia. Unpublished 2000.
TARDIS {published data only}
- Bath P. Triple Antiplatelets for Reducing Dependency After Ischaemic Stroke (TARDIS) http://www.controlled‐trials.com/ISRCTN47823388. http://www.tardistrial.org/. [DOI] [PMC free article] [PubMed]
Wu 2005 {published and unpublished data}
- Bo W, Ming L, Song T. A randomized controlled trial of ligustrazine for acute ischemic stroke. Journal of the Neurological Sciences 2005;238 Suppl 1:S415. [Google Scholar]
Yan 1998 {published data only}
- Yan FL, Xu YM, Ma JM. Clinical investigation of ticlopidine hydrochloride in the treatment of cerebral infarction. Chinese Journal of Practical Internal Medicine 1998;18:657. [Google Scholar]
Zhang 2005 {published data only}
- Zhang D, Zhu S, Cui G, Li Y, Zhang H, Xia Z, Ren W. Clinical study of low‐molecular‐weight heparin calcium and aspirin therapy on acute cerebral infarction. Chinese Pharmaceutical Journal 2005;40:634‐6. [Google Scholar]
References to studies awaiting assessment
Fujimoto 2010 {published data only}
- Fujimoto S, Jinnouchi J, Inoue T, Suzuki S, Sadoshima S. Effect of combined antithrombotic treatment with cilostazol for progressive stroke. International Journal of Stroke 2010;5 Suppl 2:87‐8. [Google Scholar]
Wang 2000 {published data only}
- Wang C, Wang Y, Gao Y. Randomized placebo‐controlled trial of early aspirin used in 180 patients with acute ischaemic stroke in the elderly people. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2000;8:19‐21. [Google Scholar]
References to ongoing studies
CAIST‐J 2006 {published data only}
- Suzuki N. Cilostazol in Acute Ischemic Stroke Trial. UMIN Clinical Trials Registry (UMIN‐CTR). (http://www.umin.ac.jp/ctr/) 2006.
CAPS 2009 {published data only}
- Tominaga T. A randomized clinical trial of an antiplatelet agent in the treatment of acute stroke: cilostazol in the prevention of acute progressing stroke (CAPS) study. UMIN Clinical Trials Registry (UMIN‐CTR) (http://www.umin.ac.jp/ctr/) 2009.
Additional references
AbESTT‐II
- Adams HP Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT‐II). Stroke 2008;39:87‐99. [DOI] [PubMed] [Google Scholar]
AHA 2013
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐ 2013 update: a report from the American Heart Association. Circulation 2013;127::e6‐e245. [DOI: 10.1161/CIR.0b013e31828124ad] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATC 1994a
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy ‐ I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
ATC 1994b
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy ‐ III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ 1994;308:235‐46. [PMC free article] [PubMed] [Google Scholar]
ATC 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of antiplatelet therapy for prevention of death, myocardial infarction and stroke in high‐risk patients. BMJ 2002;324:71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATC 2009
- Antithrombotic Trialists’ Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet 2009;373:1849‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bamford 1989
- Bamford J, Sandercock P, Warlow C, Slattery J. Inter‐observer agreement for the assessment of handicap in stroke patients. Stroke 1989;20:8. [DOI] [PubMed] [Google Scholar]
Bath 2004a
- Bath PMW. Prostacyclin and analogues for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2004b
- Bath PMW, Bath‐Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000162.pub2] [DOI] [PubMed] [Google Scholar]
CAIST 2011
- Lee YS, Bae HJ, Kang DW, Lee SH, Yu K, Park JM, et al. Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double‐blind non‐inferiority trial. Cerebrovascular Diseases 2011;32:65‐71. [DOI: 10.1159/000327036] [DOI] [PubMed] [Google Scholar]
Candelise 1994
- Candelise L, Pinardi G, Aritzu E, Musicco M. Telephone interview for stroke outcome assessment. Cerebrovascular Diseases 1994;4:341‐3. [Google Scholar]
Chairangsarit 2005
- Chairangsarit P, Sithinamsuwan P, Niyasom S, Udommongkol C, Nidhinandana S, Suwantamee J. Comparison between aspirin combined with dipyridamole versus aspirin alone within 48 hours after ischemic stroke event for prevention of recurrent stroke and improvement of neurological function: a preliminary study. Journal of the Medical Association of Thailand 2005;88:S148–S154. [PubMed] [Google Scholar]
CHANCE 2013
- Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New England Journal of Medicine 2013;369:11‐9. [DOI: 10.1056/NEJMoa1215340] [DOI] [PubMed] [Google Scholar]
Chen 2000
- Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. On behalf of the CAST and IST Collaborative Groups. Stroke 2000;31:1240‐9. [DOI] [PubMed] [Google Scholar]
Ciccone 2006
- Ciccone A, Abraha I, Santilli I. Glycoprotein IIb‐IIIa inhibitors for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005208.pub2] [DOI] [PubMed] [Google Scholar]
Collins 1996
- Collins R, MacMahon S, Flather M, Baigent C, Remviq L, Mortensen S, et al. Clinical effects of anticoagulant therapy in suspected acute myocardial infarction: systematic review of randomised trials. BMJ 1996;313:652‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
CSS 2010
- Canadian Stroke Network and Heart and Stroke Foundation of Canada. Canadian Best Practice Recommendations for Stroke Care: Update 2010. http://www.canadianstrokenetwork.ca/ December 2010.
Dalen 2006
- Dalen JE. Aspirin to prevent heart attack and stroke: what's the right dose?. American Journal of Medicine 2006;119:198‐202. [DOI] [PubMed] [Google Scholar]
Davenport 1996
- Davenport R, Dennis M, Wellwood I, Warlow C. Complications after acute stroke. Stroke 1996;27:415‐20. [DOI] [PubMed] [Google Scholar]
Dennis 1997
- Dennis M, Wellwood I, Warlow C. Are simple questions a valid measure of outcome after stroke?. Cerebrovascular Diseases 1997;7:22‐7. [Google Scholar]
EARLY 2010
- Dengler R, Diener HC, Schwartz A, Grond M, Schumacher H, Machnig T, EARLY Investigators. Early treatment with aspirin plus extended‐release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open‐label, blinded‐endpoint trial. Lancet Neurology 2010;9:159‐66. [DOI: 10.1016/S1474-4422(09)70361-8] [DOI] [PubMed] [Google Scholar]
ESO 2008
- The European Stroke Organisation Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular Diseases 2008;25::457–507. [DOI: 10.1159/000131083] [DOI] [PubMed] [Google Scholar]
Hankey 2010
- Hankey GJ, Hacke W, Easton JD, Johnston SC, Mas JL, Brennan DM, CHARISMA Trial Investigators. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. International Journal of Stroke 2010;41:1679‐83. [DOI: 10.1161/STROKEAHA.110.586727] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
IST 1996
- International Stroke Trial Pilot Study Collaborative Group. Study design of the International Stroke Trial (IST), baseline data and outcome in 984 randomised patients in the pilot study. Journal of Neurology, Neurosurgery, and Psychiatry 1996;60:371‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jauch 2013
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870‐947. [DOI: 10.1161/STR.0b013e318284056a] [DOI] [PubMed] [Google Scholar]
Kamal 2012
- Kamal AK, Siddiqi SA, Naqvi I, Khan M, Majeed F, Ahmed B. Multiple versus one or more antiplatelet agents for preventing early recurrence after ischaemic stroke or transient ischaemic attack. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2002
- Keir S, Wardlaw J, Sandercock PAG, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovascular Diseases 2002;14(3‐4):197‐206. [DOI] [PubMed] [Google Scholar]
MedStrategy 1995
- Dick E. Stroke: A focus of opportunity. St. Louis, USA: MedStrategy, 1995. [Google Scholar]
Murray 1997
- Murray C, Lopez A. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269‐76. [DOI] [PubMed] [Google Scholar]
Murray 2012
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197‐223. [DOI] [PubMed] [Google Scholar]
NINDS 1995
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. New England Journal of Medicine 1995;333:1581‐7. [DOI] [PubMed] [Google Scholar]
Odgaard‐Jensen 2011
- Odgaard‐Jensen J, Vist GE, Timmer A, Kunz R, Akl EA, Schünemann H, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.MR000012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patrono 1994
- Patrono C. Aspirin as an antiplatelet drug. New England Journal of Medicine 1994;330:1287‐94. [DOI] [PubMed] [Google Scholar]
Patrono 1998
- Patrono C, Coller B, Dalen JE, Fuster V, Gent M, Harker LA, et al. Platelet‐active drugs: the relationships among dose, effectiveness, and side effects. Chest 1998;114:470S‐88S. [DOI] [PubMed] [Google Scholar]
POINT
- POINT Investigators. Platelet‐Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial. http://www.pointtrial.org. [DOI] [PMC free article] [PubMed]
RCP Guideline 2012
- Royal College of Physicians. National Clinical Guidelines for Stroke, Fourth edition 2012. http://www.rcplondon.ac.uk/resources/stroke‐guidelines September 2012.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ricci 2012
- Ricci S, Celani MG, Cantisani TA, Righetti E. Piracetam for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000419.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strong 2007
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6:182‐7. [DOI] [PubMed] [Google Scholar]
Suri 2008
- Suri MF, Hussein HM, Abdelmoula MM, Divani AA, Qureshi AI. Safety and tolerability of 600 mg clopidogrel bolus in patients with acute ischemic stroke: preliminary experience. Medical Science Monitor 2008;14:I39–44. [PubMed] [Google Scholar]
SUTC 2013
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000197.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2013
- Tsai CF, Thomas B, Sudlow CLM. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013;81(3):264‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Gijn 1992
- Gijn J. Aspirin: dose and indications in modern stroke prevention. Neurologic Clinics 1992;10:193‐209. [PubMed] [Google Scholar]
van Kooten 1994
- Kooten F, Ciabattone G, Patrono C, Schmitz PI, Gijn J, Koudstaal PJ. Evidence for episodic platelet activation in acute ischaemic stroke. Stroke 1994;25:278‐81. [DOI] [PubMed] [Google Scholar]
van Kooten 1997
- Kooten F, Ciabattoni G, Patrono C, Dippel DW, Koudstaal PJ. Platelet activation and lipid peroxidation in patients with acute ischaemic stroke. Stroke 1997;28:1557‐63. [DOI] [PubMed] [Google Scholar]
Wardlaw 2009
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000213.pub2] [DOI] [PubMed] [Google Scholar]
Warlow 2001
- Warlow C. Stroke, transient ischaemic attacks, and intracranial venous thrombosis. In: Donaghy M editor(s). Brain's Diseases of the Nervous System. 11th Edition. Oxford University Press, 2001:775‐896. [Google Scholar]
Zhang 2012
- Zhang J, Yang J, Chang X, Zhang C, Zhou H, Liu M. Ozagrel for acute ischemic stroke: a meta‐analysis of data from randomized controlled trials. Neurological Research 2012;34:346‐53. [DOI: 10.1179/1743132812Y.0000000022] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sandercock 2003
- Sandercock P, Gubitz G, Foley P, Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000029] [DOI] [PubMed] [Google Scholar]


