Abstract
Background
Noninfectious comorbid diseases (NCDs) contribute to morbidity and mortality in human immunodeficiency virus (HIV)–infected populations in resource-rich countries. With antiretroviral therapy (ART) scale-up in Africa, understanding burden NCD informs public health strategy.
Methods
At enrollment, participants at 11 HIV clinics in Kenya, Uganda, Tanzania, and Nigeria underwent medical history, physical, laboratory, and neuropsychological assessments to identify elevated blood pressure, hypercholesterolemia, dysglycemia, renal insufficiency, and cognitive impairment. Poisson regression models estimated adjusted relative risks (ARRs) and 95% confidence intervals (CIs) for the number of NCDs associated with factors of interest. Logistic regression was used to evaluate each NCD separately among HIV-infected participants.
Results
Among 2720 participants with complete NCD data, 2159 (79.4%) were HIV-infected. Of those, 1426 (66.0%) were taking ART and 813 (37.7%) had at least 1 NCD. HIV infection was associated with more NCDs, especially with ART (ARR, 1.42; 95% CI, 1.22–1.66). In addition to age, body mass index, and program site, ART usage was associated with more NCDs (ARR, 1.50; 95% CI, 1.27–1.78 for virologically suppressed and ARR, 1.38; 95% CI, 1.13–1.68 for viremic) among HIV-infected participants. In participants taking ART, CD4 nadir below 200 cells/mm3 was associated with more NCDs (ARR, 1.43; 95% CI, 1.06–1.93). ART use was independently associated with hypercholesterolemia and dysglycemia. Program site was significantly associated with all comorbidities except renal insufficiency.
Conclusions
HIV infection was a risk for NCDs, which were common in HIV-infected participants, geographically variable, and largely consistent with metabolic complications of first-line ART.
Keywords: HIV, comorbidity, noninfectious, Africa
In this cross-sectional evaluation of noninfectious diseases (NCDs) in Kenya, Uganda, Tanzania, and Nigeria, human immunodeficiency virus (HIV) infection was associated with more NCDs. In HIV-infected participants, NCDs were common, geographically variable, and associated with Antiretroviral Therapy use and CD4 nadir.
Long-term cohort studies have made important contributions to the understanding of the natural history and pathogenesis of human immunodeficiency virus (HIV) [1–3]. In resource-rich settings, cohorts have demonstrated that antiretroviral therapy (ART) has decreased HIV-associated morbidity and mortality [4–6]. Despite this, multiple cohorts in these settings have revealed that HIV-infected individuals have a shorter life expectancy than the general population [7–9] and may be at increased risk for noninfectious comorbidities [6, 10] including, but not limited to, cardiovascular disease, hypertension, renal failure, and diabetes [10–15]. Potential contributors may include ART exposure and toxicity, chronic immune activation and inflammation, and concomitant risk factors such as substance abuse [11, 16, 17].
The extent to which HIV and its treatment interact with noninfectious comorbidities in sub-Saharan Africa is largely unknown. The impact of these noninfectious comorbidities in the African setting is potentially distinct given a higher burden of endemic coinfections that impact both HIV-infected and HIV-uninfected populations [18]. Social and behavioral differences may play a role as well.
We established a large clinic-based cohort in 4 African countries (Kenya, Uganda, Tanzania, and Nigeria) to study HIV disease characteristics and long-term outcomes in a multinational, multi-subtype African context. The African Cohort Study (AFRICOS) has a planned 15-year duration and provides an opportunity to investigate the extent and consequences of noninfectious comorbidities in HIV-infected individuals, initially in cross-sectional fashion but ultimately with longitudinal analysis utilizing the cohort’s prospective repository of data and specimens.
METHODS
Study Design and Participants
AFRICOS, established in 2013, is a systematic longitudinal cohort study enrolling HIV-infected and HIV-uninfected adults at 11 clinics across 5 geographically distinct programs supported by the president’s Emergency Plan for AIDS Relief (PEPFAR) in Kenya, Tanzania, Uganda, and Nigeria (Figure 1). Most HIV-infected study participants were invited to the study based on random selection from existing clinic patient lists (stratified by gender and ART status) or new enrollees to the clinic, while a minority (less than 5%) are recruited from other HIV studies performed by our group locally to facilitate long-term follow-up. HIV-uninfected participants are recruited from individuals undergoing HIV counseling and testing at the clinics.
Figure 1.
Map of African Cohort Study sites. Kayunga, Uganda: Kayunga District Hospital; South Rift Valley, Kenya: Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital; Kisumu, Kenya: Kisumu West District Hospital; Mbeya, Tanzania: Mbeya Zonal Referral Hospital; Abuja, Nigeria: Defence Headquarters Medical Center; Lagos, Nigeria: 68th Nigerian Army Reference Hospital.
Recruitment and enrollment began 21 January 2013 and is ongoing up to a maximum of 3600 participants (3000 HIV infected and 600 HIV uninfected). Individuals were eligible if they were aged ≥18 years and consented to data and specimen collection. An additional inclusion criterion for HIV-infected individuals was the ongoing receipt of HIV care at the enrolling clinic. We excluded individuals who were pregnant at enrollment.
All participants provided written informed consent. The institutional review boards of the Walter Reed Army Institute of Research, Makerere University School of Public Health, Kenya Medical Research Institute, Tanzania National Institute of Medical Research, and Nigerian Ministry of Defence approved the study.
Procedures
On enrollment, participants were administered a medical history and physical exam, completed a broad demographic and behavioral questionnaire, and underwent phlebotomy. For HIV-infected participants, serum chemistry and blood cell counts were performed, CD4 T-lymphocyte count and HIV RNA (“viral load”) were enumerated, and fasting glucose and cholesterol levels were ascertained. All participants underwent a brief battery of neuropsychological tests including the International HIV Dementia Scale (IHDS), which includes evaluation of memory (registration and recall), motor speed (finger tapping), and manual dexterity (Luria sequence) [19].
To ensure comparability of data across sites, standard operating procedures for vital signs were followed, and laboratory measures were performed at laboratories that were accredited by the College of American Pathologists or had successfully completed external quality assurance. Study staff who performed neuropsychological testing underwent twice-yearly recertification through quality assurance visits after competency testing with initial certification.
Data Collection and Definitions
Data were entered and verified in the Clinplus platform (DZS Software Solutions, Bound Brock, NJ). Only baseline enrollment visit data were included in this cross-sectional analysis. Study participants enrolled as of 3 August 2016 were eligible for inclusion in these analyses.
The study database was used to identify participants with noninfectious diseases (NCDs) according to prespecified definitions. Elevated blood pressure was defined as a systolic blood pressure measurement >139 mm Hg, a diastolic blood pressure measurement >89 mm Hg, or receipt of antihypertensive medications, with abnormal blood pressures repeated for confirmation during the study visit. Hypercholesterolemia was defined as a total fasting cholesterol >199 mg/dL or receipt of lipid-lowering medications. Dysglycemia was defined as a fasting glucose >99 mg/dL, nonfasting glucose >199 mg/dL, or receipt of hypoglycemic medications. Renal insufficiency was defined as an estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2, calculated using the Modification of Diet in Renal Disease equation based on serum creatinine captured at entry [20]. Cognitive impairment was defined as having an IHDS score that was at least 2 standard deviations lower than the mean score of HIV-uninfected participants in the region. Since educational attainment was substantially different between Nigeria and East African countries, we examined them separately, yielding the 2 standard deviation cutpoint of <6 in East Africa and <7 in Nigeria.
Demographic variables, including sex, age, education level, and clinical care site, were collected upon enrollment. Body mass index (BMI) was calculated using participants’ height and weight, then categorized as underweight (<18.5), normal (18.5–24.9), or overweight (≥25). Anemia was defined as hemoglobin <13 g/dL for males or <12 g/dL for females [21]. CD4 count at enrollment was categorized as <200, 200–349, 350–499, or ≥500 cells/mm3. Antiretroviral medication use and viral load (VL) stratum were combined into the following categories: not on ART, on ART and VL <50 copies/mL, and on ART and VL ≥50 copies/mL. The 11 enrolling clinics were analyzed by program, with 1 clinic in the Kenya Kisumu West, Uganda, and Tanzania programs; 2 clinics in the Nigeria program; and 6 clinics in the Kenya South Rift Valley program (Figure 1).
Statistical Analyses
Only participants with the clinical and laboratory assessments required to assess all 5 NCDs of interest were included in these cross-sectional analyses. Participants were categorized based on the number of NCDs diagnosed (0, 1, or ≥2). Participant characteristics at study enrollment were compared across these 3 groups using the Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Univariate and multivariate Poisson regression models were used to estimate unadjusted and adjusted relative risks (ARRs) and 95% confidence intervals (CIs) for number of NCDs associated with prespecified demographic and clinical factors of interest. A secondary analysis was conducted comparing HIV-uninfected participants to HIV-infected participants on ART and HIV-infected participants not on ART. Each of the 5 NCDs were also evaluated separately, using multivariable logistic regression models to estimate adjusted odds ratios and 95% CIs for associations between the same prespecified factors and each NCD. Subgroup analyses were conducted in which the study population was restricted to participants prescribed ART. Due to the smaller sample size of HIV-uninfected participants, only descriptive statistics and the Poisson model examining total NCD count were performed. All analyses were performed using SAS 9.4 (Cary, NC).
Sensitivity Analyses
To assess for the effect of missingness on our results, all analyses were rerun, including those participants with missing values for any NCD. We also assessed the effect of enrollment in prior studies rerunning analyses restricting to participants who were not included in prior studies.
RESULTS
From 21 January 2013 to 3 August 2016, 2727 volunteers were enrolled, including 463 HIV-uninfected participants. A total of 2264 HIV-infected participants were enrolled from the general clinic population with the exception of 124 participants recruited for follow-up from other studies. A total of 379 participants from the HIV-uninfected group and 2159 participants from the HIV-infected group had complete data on NCDs and were included in these analyses. Participants included in these analyses had a median age of 38.5 (interquartile range, 31.6, 46.0) years and 1501 (59%) were female (Supplementary Table 1). Across the entire analyzed population, 1414 (56%) had zero NCDs, 786 (31%) had 1 NCD, and 338 (13%) had 2 or more (Supplementary Figure 1). Adjusting for program site, age, gender, BMI, anemia, and education, we found HIV infection to be associated with more NCDs (ARR, 1.42; 95% CI ,1.22–1.66 for individuals on ART and ARR, 1.14; 95% CI, 0.95–1.36 for untreated individuals; Supplementary Table 2).
Among HIV-infected participants, median CD4 count was 384.0 cells/mm3 and 1426 (66.0%) were taking ART at the time of enrollment (Table 1). Of those taking ART, 1245 (87.3%) had a viral load less than 1000 copies/mL and 1004 (70.4%) were suppressed below 50 copies/mL. Most ART-treated volunteers were on standard first-line therapy including a nonnucleotide reverse transcriptase inhibitor (NNRTI), with only 7.4% taking a protease inhibitor.
Table 1.
Study Population Characteristics at Enrollment
| Characteristic | All HIV+ (N = 2159) | No NCDs (N = 1346) | 1 NCD (N = 598) | 2+ NCDs (N = 215) | P Valuea |
|---|---|---|---|---|---|
| Program site | <.0001 | ||||
| Kayunga, Uganda | 402 (18.6%) | 320 (23.8%) | 70 (11.7%) | 12 (5.6%) | … |
| South Rift Valley, Kenya | 802 (37.1%) | 457 (34.0%) | 256 (42.8%) | 89 (41.4%) | … |
| Kisumu West, Kenya | 361 (16.7%) | 250 (18.6%) | 80 (13.4%) | 31 (14.4%) | … |
| Mbeya, Tanzania | 354 (16.4%) | 205 (15.2%) | 108 (18.1%) | 41 (19.1%) | … |
| Abuja and Lagos, Nigeria | 240 (11.1%) | 114 (8.5%) | 84 (14.0%) | 42 (19.5%) | … |
| Gender | .7727 | ||||
| Male | 887 (41.1%) | 552 (41.0%) | 242 (40.5%) | 93 (43.3%) | … |
| Female | 1272 (58.9%) | 794 (59.0%) | 356 (59.5%) | 122 (56.7%) | … |
| Age (years) | <.0001 | ||||
| 18–24 | 164 (7.6%) | 123 (9.1%) | 34 (5.7%) | 7 (3.3%) | … |
| 25–39 | 1013 (46.9%) | 722 (53.6%) | 239 (40.0%) | 52 (24.2%) | … |
| 40–49 | 612 (28.3%) | 341 (25.3%) | 194 (32.4%) | 77 (35.8%) | … |
| 50+ | 370 (17.1%) | 160 (11.9%) | 131 (21.9%) | 79 (36.7%) | … |
| Education | .0002 | ||||
| Less than primary school | 751 (34.8%) | 476 (35.4%) | 212 (35.5%) | 63 (29.3%) | … |
| Primary school | 829 (38.4%) | 549 (40.8%) | 208 (34.8%) | 72 (33.5%) | … |
| Secondary school or above | 577 (26.7%) | 320 (23.8%) | 177 (29.6%) | 80 (37.2%) | … |
| Missing/Unknown | 2 (0.1%) | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | … |
|
Body mass index (kg/m2) |
<.0001 | ||||
| 18.5–24.99 | 1357 (62.9%) | 912 (67.8%) | 340 (56.9%) | 105 (48.8%) | … |
| <18.5 | 235 (10.9%) | 160 (11.9%) | 63 (10.5%) | 12 (5.6%) | … |
| >25 | 567 (26.3%) | 274 (20.4%) | 195 (32.6%) | 98 (45.6%) | … |
| Anemia | <.0001 | ||||
| No | 1451 (68.5%) | 854 (64.6%) | 433 (74.1%) | 164 (77.7%) | … |
| Yes | 666 (31.5%) | 468 (35.4%) | 151 (25.9%) | 47 (22.3%) | … |
| Missing/Unknown | 42 (2.0%) | 24 (1.8%) | 14 (2.4%) | 4 (1.9%) | … |
| Current alcohol use | .3671 | ||||
| No | 1755 (81.3%) | 1106 (82.2%) | 476 (79.6%) | 173 (80.5%) | … |
| Yes | 403 (18.7%) | 239 (17.8%) | 122 (20.4%) | 42 (19.5%) | … |
| Missing/Unknown | 1 (0.0%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | … |
| Lowest CD4 (cells/mm3) | .0001 | ||||
| 500+ | 249 (11.5%) | 174 (12.9%) | 58 (9.7%) | 17 (7.9%) | … |
| 350–499 | 265 (12.3%) | 182 (13.5%) | 69 (11.5%) | 14 (6.5%) | … |
| 200–349 | 605 (28.0%) | 390 (29.0%) | 159 (26.6%) | 56 (26.0%) | … |
| <200 | 1038 (48.1%) | 598 (44.4%) | 312 (52.2%) | 128 (59.5%) | … |
| Missing/Unknown | 2 (0.1%) | 2 (0.1%) | … | … | … |
| Enrollment CD4 (cells/mm3) | .0408 | ||||
| 500+ | 691 (32.0%) | 412 (30.6%) | 195 (32.6%) | 84 (39.1%) | … |
| 350–499 | 486 (22.5%) | 309 (23.0%) | 138 (23.1%) | 39 (18.1%) | … |
| 200–349 | 536 (24.8%) | 334 (24.8%) | 141 (23.6%) | 61 (28.4%) | … |
| <200 | 404 (18.7%) | 271 (20.1%) | 105 (17.6%) | 28 (13.0%) | … |
| Missing/Unknown | 42 (1.9%) | 20 (1.5%) | 19 (3.2%) | 3 (1.4%) | … |
| Viral load (copies/mL) | <.0001 | ||||
| <50 | 1033 (47.8%) | 568 (42.2%) | 326 (54.5%) | 139 (64.7%) | … |
| 50+ | 1058 (49.0%) | 735 (54.6%) | 253 (42.3%) | 70 (32.6%) | … |
| Missing/Unknown | 68 (3.1%) | 43 (3.2%) | 19 (3.2%) | 6 (2.8%) | … |
| Years since HIV diagnosis, mean (SD) | |||||
| (n = 2139)b | 3.7 (3.6) | 3.4 (3.5) | 4.1 (3.7) | 5.0 (3.7) | <.0001 |
| ART status | <.0001 | ||||
| No | 733 (34.0%) | 542 (40.3%) | 161 (26.9%) | 30 (14.0%) | … |
| Yes | 1426 (66.0%) | 804 (59.7%) | 437 (73.1%) | 185 (86.0%) | … |
| Among participants on ARTc | |||||
| On ART containing tenofovir | 794 (55.7%) | 466 (57.9%) | 240 (54.9%) | 88 (47.6%) | .0364 |
| On ART containing efavirenz | 729 (51.0%) | 420 (52.2%) | 216 (49.4%) | 93 (50.3%) | .7388 |
| On ART containing nevirapine | 580 (40.7%) | 319 (39.7%) | 178 (40.7%) | 83 (44.9%) | .3574 |
| On ART containing a protease inhibitor | 105 (7.4%) | 62 (7.7%) | 37 (8.5%) | 6 (3.2%) | .0837 |
| Duration of ART (years), mean (SD) | 3.8 (3.0) | 3.6 (2.9) | 4.0 (2.9) | 4.4 (3.2) | .0011 |
| Nadir CD4 (cells/mm3), mean (SD) | 184.4 (138.5) | 195.0 (148.1) | 177.6 (132.3) | 155.1 (100.3) | .0117 |
All data presented as n (%) unless otherwise indicated. Bold indicates P value < 05. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NCD, noninfectious comorbid disease; SD, standard deviation.
a P values were calculated using Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables.
bTwenty participants were missing information on date of HIV diagnosis.
cEleven participants were on ART containing rilpivirine, 1 participant was only on tenofovir and lamivudine.
NCDs were common in the HIV-infected group, with 37.7% having at least 1 comorbidity and 10.0% having 2 or more. The most common comorbidity was hypercholesterolemia (19.1%) followed by elevated blood pressure (13.0%), dysglycemia (9.9%), cognitive impairment (5.7%), and renal insufficiency (1.3%).
We noted differences across program sites (P < .001; Table 1), with the programs in Kayunga, Uganda, and Kisumu West, Kenya, having the lowest prevalence of NCDs and Nigeria the highest (Figure 2). In unadjusted comparisons, we noted associations between a higher number of comorbidities and older age (RR, 1.97; 95% CI, 1.47–2.66 for 40–49 years and RR, 2.74; 95% CI, 2.03–3.71 for >50 years), higher BMI (RR, 1.72; 95% CI, 1.52–1.96), more years of education (RR, 1.30; 95% CI, 1.12–1.51), and taking ART (RR, 1.97; 95% CI, 1.68–2.29 for those virologically suppressed and RR, 1.74; 95% CI, 1.44–2.11 for viremic individuals). Lower category of CD4 T-lymphocyte count (RR, 0.78; 95% CI, 0.65–0.93) and anemia (RR, 0.69; 95% CI, 0.60–0.80) were associated with a lower number of comorbidities.
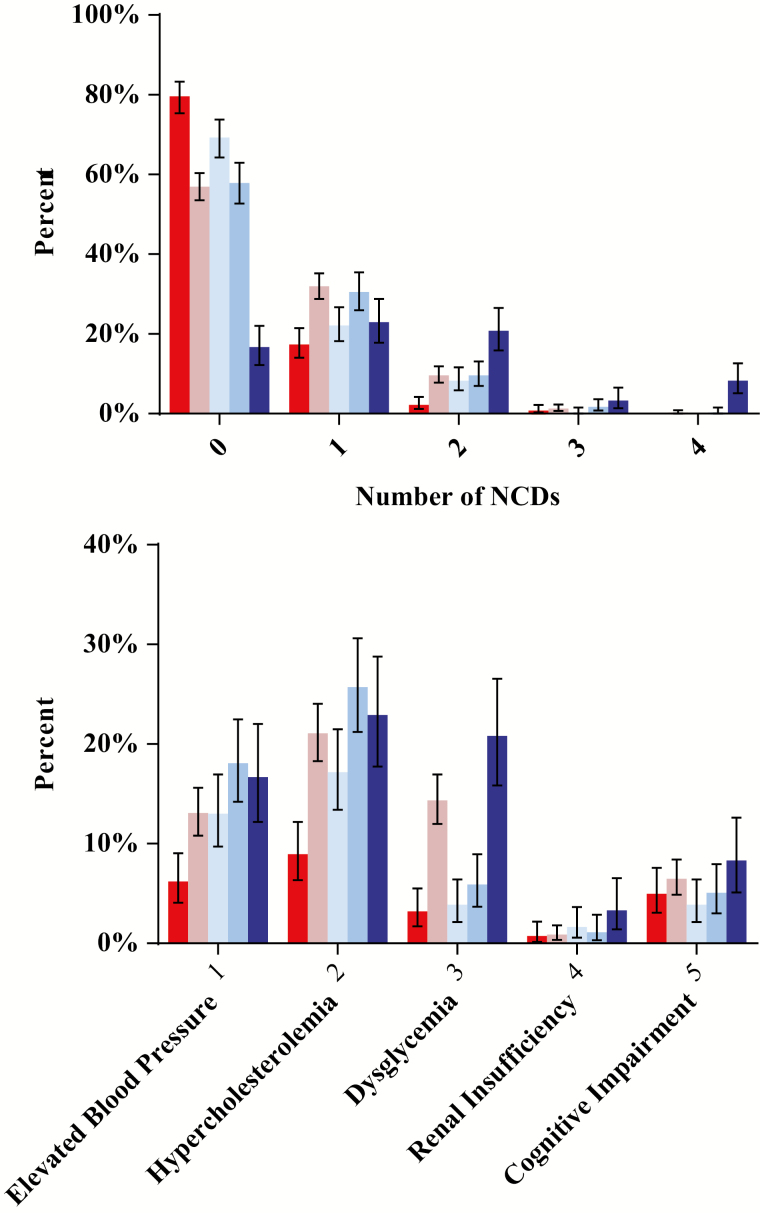
Figure 2.
(A) Percentage of participants by number of noninfectious comorbid diseases (NCDs) and site. Shown is the distribution of NCDs by site, with the majority of participants with 0 NCDs, although this varies by site. (B) Percentage of participants with select NCDs by site . Similar to overall count of NCDs, prevalence of individual NCDs varies by site:  Kayunga, Uganda;
Kayunga, Uganda;  South Rift Valley, Kenya;
South Rift Valley, Kenya;  Kisumu West, Kenya;
Kisumu West, Kenya;  Mbeya, Tanzania;
Mbeya, Tanzania;  Abuja and Lagos, Nigeria. 1: elevated blood pressure (BP); systolic BP >139 or diastolic BP >89 or on hypertension medications. 2: hypercholesterolemia; cholesterol >199 or on cholesterol medications. 3: dysglycemia; fasting glucose >99 or any glucose >199 or on glucose medications. 4; renal insufficiency, glomerular filtration rate <60 by Modification of Diet in Renal Disease Study equation. 5; cognitive impairment, International HIV (human immunodeficiency virus) Dementia Scale <6 for East Africa or <7 for Nigeria. Abbreviation: NCD, noninfectious comorbid disease.
Abuja and Lagos, Nigeria. 1: elevated blood pressure (BP); systolic BP >139 or diastolic BP >89 or on hypertension medications. 2: hypercholesterolemia; cholesterol >199 or on cholesterol medications. 3: dysglycemia; fasting glucose >99 or any glucose >199 or on glucose medications. 4; renal insufficiency, glomerular filtration rate <60 by Modification of Diet in Renal Disease Study equation. 5; cognitive impairment, International HIV (human immunodeficiency virus) Dementia Scale <6 for East Africa or <7 for Nigeria. Abbreviation: NCD, noninfectious comorbid disease.
In multivariable models adjusting for age, gender, BMI, and program site (Table 2), ART usage persisted in its association with more NCDs (ARR, 1.50; 95% CI, 1.27–1.78 for those virologically suppressed and ARR, 1.38; 95% CI, 1.13–1.68 for viremic individuals). In this adjusted model, anemia continued to be associated with fewer comorbidities (ARR, 0.78; 95% CI, 0.67–0.91). Evaluating only those taking ART (n = 1426; Supplementary Table 3) and adjusting for age, sex, BMI, and program, CD4 nadir less than 200 cells/mm3 prior to ART initiation was found to be associated with more NCDs (ARR, 1.43; 95% CI, 1.06–1.93).
Table 2.
Unadjusted and Adjusted Relative Risk for Select Factors and Number of Noninfectious Comorbid Diseases
| Factor | Unadjusted Relative Risk (95% CI) | Adjusted Relative Risk (95% CI) |
|---|---|---|
| Program site | ||
| Kayunga, Uganda | Reference group | … |
| South Rift Valley, Kenya | 2.31 (1.86–2.88)a | 1.98 (1.57–2.50)a |
| Kisumu West, Kenya | 1.64 (1.27–2.12)b | 1.38 (1.06–1.80)b |
| Mbeya, Tanzania | 2.32 (1.82–2.96)a | 2.04 (1.57–2.65)a |
| Abuja and Lagos, Nigeria | 2.99 (2.33–3.83)a | 3.16 (2.39–4.18)a |
| Gender | ||
| Female | Reference group | … |
| Male | 0.96 (0.85–1.09) | 0.98 (0.85–1.12) |
| Age (years) | ||
| 18–24 | Reference Group | … |
| 25–39 | 1.14 (0.85–1.54) | 0.98 (0.72–1.33) |
| 40–49 | 1.97 (1.47–2.66)a | 1.60 (1.18–2.18)b |
| 50+ | 2.74 (2.03–3.71)a | 2.31 (1.68–3.16)a |
| Education | ||
| Less than primary school | Reference group | … |
| Primary school | 0.95 (0.82–1.10) | 0.93 (0.79–1.09) |
| Secondary school or above | 1.30 (1.12–1.51)a | 0.92 (0.78–1.10) |
| Body mass index (kg/m2) | ||
| 18.5–24.99 | Reference group | … |
| <18.5 | 0.91 (0.73–1.14) | 0.97 (0.77–1.21) |
| >25 | 1.72 (1.52–1.96)a | 1.48 (1.29–1.70)a |
| Anemia | ||
| No | Reference group | … |
| Yes | 0.69 (0.60–0.80)a | 0.78 (0.67–0.91)b |
| Missing/Unknown | 0.97 (0.63–1.48) | 1.03 (0.66–1.59) |
| Enrollment CD4 (cells/mm3) | ||
| 500+ | Reference group | … |
| 350–499 | 0.85 (0.72–1.01) | 0.89 (0.75–1.05) |
| 200–349 | 0.94 (0.81–1.10) | 0.94 (0.80–1.11) |
| <200 | 0.78 (0.65–0.93)b | 0.87 (0.72–1.06) |
| Missing/Unknown | 1.11 (0.74–1.66) | 1.00 (0.66–1.52) |
| Viral load group | ||
| Not on ART | Reference group | … |
| On ART, virally suppressed (<50 copies/mL) | 1.97 (1.68–2.29)a | 1.50 (1.27–1.78)a |
| On ART, viremic (>50 copies/mL) | 1.74 (1.44–2.11)a | 1.38 (1.13–1.68)b |
| Missing/Unknown | 1.48 (1.02–2.15)b | 1.26 (0.85–1.87) |
N = 2159. Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
a P value <.001.
b P value <.05.
Among factors associated with each individual comorbidity, program site was significantly associated with all comorbidities except renal insufficiency (n = 28; Table 3). Male gender demonstrated an association with less hypercholesterolemia. Age was associated with elevated blood pressure, hypercholesterolemia, and meeting criteria for cognitive impairment, whereas more years of education was associated with less cognitive impairment. Anemia was associated with less elevated blood pressure and hypercholesterolemia, and we found an association or trend toward less hypercholesterolemia in CD4 groups less than 500 cell/mm3. ART use was associated with hypercholesterolemia and dysglycemia.
Table 3.
Adjusted Analyses of Factors Associated With Specific Noninfectious Comorbid Disease
| Factor | Elevated Blood Pressure N = 281 OR (95% CI) |
Hypercholesterolemia N = 413 OR (95% CI) |
Dysglycemia N = 213 OR (95% CI) |
Renal Insufficiency N = 28 OR (95% CI) |
Cognitive Impairment N = 124 OR (95% CI) |
|---|---|---|---|---|---|
| Program site | |||||
| Kayunga, Uganda | Reference group | … | … | … | … |
| South Rift Valley, Kenya | 2.05 (1.25–3.37)a | 1.93 (1.27–2.92)a | 4.25 (2.29–7.88)b | 1.09 (0.25–4.69) | 1.78 (1.00–3.16) |
| Kisumu West, Kenya | 2.22 (1.29–3.81)a | 1.44 (0.91–2.29) | 0.93 (0.42–2.05) | 2.09 (0.47–9.29) | 0.84 (0.40–1.76) |
| Mbeya, Tanzania | 3.15 (1.83–5.42)b | 2.86 (1.80–4.57)b | 1.52 (0.71–3.24) | 0.89 (0.18–4.54) | 1.55 (0.75–3.18) |
| Abuja and Lagos, Nigeria | 3.57 (1.93–6.63)b | 2.80 (1.65–4.76)b | 9.40 (4.61–19.16)b | 2.68 (0.56–12.89) | 3.55 (1.65–7.61)a |
| Gender | |||||
| Female | Reference group | … | … | … | … |
| Male | 1.28 (0.95–1.73) | 0.70 (0.54–0.90)a | 1.28 (0.92–1.79) | 1.40 (0.61–3.22) | 0.78 (0.52–1.18) |
| Age (years) | |||||
| 18–24 | Reference group | … | … | … | … |
| 25–39 | 1.05 (0.53–2.06) | 1.06 (0.63–1.79) | 0.64 (0.32–1.28) | 1.44 (0.17–11.78) | 1.03 (0.39–2.71) |
| 40–49 | 1.71 (0.86–3.38) | 1.89 (1.11–3.20)a | 1.00 (0.49–2.02) | 3.27 (0.40–26.90) | 2.76 (1.06–7.22)a |
| 50+ | 4.29 (2.15–8.57)b | 2.91 (1.68–5.05)b | 1.67 (0.80–3.46) | 3.23 (0.35–29.66) | 2.19 (0.80–6.02) |
| Educationc | |||||
| Less than primary school | Reference group | … | … | … | … |
| Primary school | 1.05 (0.75–1.47) | 1.21 (0.90–1.62) | 0.79 (0.53–1.17) | 1.58 (0.54–4.65) | 0.42 (0.26–0.67)b |
| Secondary school or higher | 0.95 (0.65–1.39) | 1.30 (0.94–1.81) | 0.78 (0.52–1.18) | 1.94 (0.61–6.19) | 0.38 (0.26–0.65)b |
| BMI (kg/m2)d | |||||
| 18.5–24.99 | Reference group | … | … | … | … |
| <18.5 | 0.83 (0.49–1.41) | 0.84 (0.54–1.29) | 1.15 (0.69–1.93) | … | 1.54 (0.89–2.65) |
| >25 | 2.57 (1.90–3.46)b | 1.43 (1.10–1.85)a | 1.70 (1.21–2.39)a | 1.51 (0.64–3.59) | 0.93 (0.59–1.48) |
| Anemia | |||||
| No | Reference group | … | … | … | … |
| Yes | 0.64 (0.45–0.89)a | 0.62 (0.46–0.83)a | 0.77 (0.53–1.12) | 1.35 (0.57–3.21) | 1.09 (0.70–1.68) |
| Missing/ Unknown | 0.63 (0.21–1.91) | 1.07 (0.49–2.33) | 0.90 (0.26–3.17) | 3.84 (0.68–21.80) | 1.54 (0.43–5.49) |
| Enrollment CD4 (cells/mm3) | |||||
| 500+ | Reference group | … | … | … | … |
| 350–499 | 0.99 (0.69–1.42) | 0.72 (0.52–0.98)a | 0.84 (0.54–1.31) | 0.54 (0.14–2.08) | 1.14 (0.67–1.93) |
| 200–349 | 0.91 (0.63–1.31) | 0.87 (0.64–1.18) | 1.01 (0.67–1.53) | 1.13 (0.40–3.23) | 0.91 (0.53–1.57) |
| <200 | 0.67 (0.43–1.05) | 0.52 (0.35–0.78)a | 1.40 (0.89–2.20) | 1.05 (0.34–3.25) | 1.43 (0.82–2.51) |
| Missing/Unknown | 0.61 (0.20–1.83) | 0.67 (0.31–1.46) | 2.22 (0.88–5.62) | 3.53 (0.61–20.41) | 0.93 (0.21–4.19) |
| Viral load group | |||||
| Not on ART | Reference group | … | … | … | … |
| On ART, virally suppressed (<50 copies/mL) | 1.15 (0.81–1.62) | 2.62 (1.91–3.61)b | 1.94 (1.29–2.93)a | 0.40 (0.14–1.12) | 1.08 (0.67–1.75) |
| On ART, viremic (>50 copies/mL) | 1.08 (0.71–1.63) | 2.05 (1.40–3.00)b | 1.56 (0.97–2.53) | 1.11 (0.42–2.95) | 1.21 (0.69–2.10) |
| Missing/Unknown | 0.38 (0.13–1.13) | 2.58 (1.34–4.97)a | 2.18 (0.83–5.73) | 0.53 (0.06–4.90) | 1.14 (0.32–4.07) |
N = 2159. Elevated blood pressure (BP): systolic BP >139 or diastolic BP >89 or on hypertension medications. Hypercholesterolemia: cholesterol >199 or on cholesterol medications. Dysglycemia: fasting glucose >99 or any glucose >199 or on glucose medications. Renal insufficiency: glomerular filtration rate <60 by Modification of Diet in Renal Disease Study equation. Cognitive impairment, International HIV (human immunodeficiency virus) Dementia Scale <6 for East Africa or <7 for Nigeria.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; OR, odds ratio.
a P value <.05.
b P value <.001.
cTwo participants with Missing/Unknown Education data were included in the “Less than Primary School” reference group.
dThere were 235 participants with BMI <18.5, of which none had renal insufficiency and were regrouped to the reference group BMI 18.5–24.99.
Evaluating factors associated with individual NCDs among ART-treated participants only (Supplementary Table 4), we observed a trend toward increased cognitive impairment in the group with CD4 nadir below 200 cells/ mm3 (P = .06) and an adjusted odds ratio of 4.64 (CI, 1.06–20.35; P = .04) for developing cognitive impairment with CD4 nadir 200–350 cells/mm3 compared to a nadir greater than 350 cells/mm3.
There were no significant differences in results when allowing participants with missing NCD values (n = 104) or participants enrolled in prior studies into the analyses.
DISCUSSION
This study characterized key chronic noninfectious comorbidities across 4 countries in sub-Saharan Africa, with a large number of HIV-infected participants represented. The enrollment was majority female, reflective of the overall site clinic populations. Also, the attainment of viral suppression to less than 1000 copies/mL by 87% of those on therapy reflects moderately successful ART implementation at the study sites, although it falls short of the 90% UNAIDS goal [22].
As with studies performed in resource-rich settings, we found HIV infection to be associated with more total NCDs, especially among those taking ART. The study was largely underpowered to detect differences between HIV-infected and HIV-uninfected groups for specific NCDs. Substantial differences in the frequency of NCDs were observed across program sites, even after adjustment for demographic and HIV characteristics in the multivariate model. We cannot entirely rule out variation in site performance of the assessments despite standardization measures taken, but we would highlight that the programs with the least comorbidity in Uganda and Kisumu are the most rural programs in the cohort and the sites in Nigeria are the most urban. These site contrasts may reflect dietary and lifestyle differences. Host genetic differences between east and west African populations should also be considered.
Compared to those not taking ART, positive ART status was associated with increased number of comorbidities, both among participants who were virally suppressed and as well as those failing therapy. This increase was largely mediated by higher rates of hyperglycemia and hyperlipidemia, which are known side effects of NNRTIs [23–26]. However, we are unable to make that specific attribution in this group because few participants were taking non-NNRTI–based regimens. This association between ART and more comorbidities may be modified in the future as ARTs thought to have more favorable metabolic profiles, such as integrase inhibitors [27, 28], are made more widely available in the resource-limited setting.
In this analysis, concurrent anemia emerged as independently associated with fewer NCDs. This may reflect poor underlying nutritional status that is not otherwise captured by other covariates but that may lessen propensity to develop vascular or metabolic comorbidities.
Lower CD4 nadir among those treated with ART was associated with more noninfectious comorbidities, specifically cognitive impairment. Low CD4 nadir is a well-described risk factor for HIV-associated cognitive impairment in resource-rich settings [29, 30], and this association is now demonstrated in this African cohort as well. This observation suggests cognitive benefits for test and start recommendations [31], particularly for programs able to find cases prior to advanced HIV progression.
These analyses leveraged a diverse cohort recruited from PEPFAR-supported clinics in 4 countries to characterize the burden of NCDs and factors associated with their frequency in sub-Saharan Africa, primarily focusing on a large number of HIV-infected participants. We were able to make comparisons in NCDs between HIV-infected and HIV-uninfected study participants; however, the small size and convenience-based enrollment strategy for HIV-uninfected individuals into the cohort is a limitation of that analysis. However, where relevant comparisons are available, the frequencies of NCDs observed in this study’s HIV-uninfected comparison group are generally consistent with published estimates from other HIV-uninfected populations in sub-Saharan Africa. We found elevated blood pressure in 19.3% of HIV-uninfected AFRICOS participants, and studies in Malawi, South Africa, Tanzania, and Nigeria have reported a prevalence of hypertension in HIV-uninfected populations of 8.0%–27. 9% [32–35]. Maganga and colleagues reported glucose metabolism disorders in 7.2% of an HIV-uninfected population in Tanzania [36], similar to our estimate of 7.1% with dysglycemia among uninfected AFRICOS enrollees. Renal insufficiency with GFR <60 mL/min/1.73 m2 was seen in 0.3% of uninfected AFRICOS participants [37, 38].
Prospective enrollment likely introduced some selection bias in the HIV-infected group despite high rates of participation in the study. Comparing basic demographics, we do see a higher proportion of males in our analysis than that in the overall clinic population sampled (41.1% vs 35.1%, P < .0001), and the cohort was on average slightly younger, with a mean age of 40 years (range, 18 – 76) in the cohort vs 41 years (range, 16–94) in the overall clinic population. We adjusted for age and gender in these analyses; however, these populations may also differ in unmeasured ways that limit the generalizability of our findings. The small number of study participants who were recruited from prior studies may be particularly more prone to bias; however, excluding them in the multiple regression analyses did not change our findings.
A further limitation is that these cross-sectional analyses did not evaluate important long-term outcomes of comorbid disease processes, such as myocardial infarction, heart failure, and cerebrovascular accident. However, ongoing prospective data collection will enable future analyses of such events. Some comorbidity definitions were adapted to suit the cross-sectional nature of these analyses, such as elevated blood pressure defined by a single-day measurement as opposed to hypertension that requires serial blood pressure measurements for diagnosis. Our definition for cognitive impairment likely selected only the most severe cases due to the use of a 2-standard deviation threshold and comparison to co-enrolled HIV-uninfected participants rather than normative data typically acquired from a healthy cognitively asymptomatic population. It is also possible that some members of our comparative cohort experienced cognitive impairment from head injuries, substance use, or other factors. Additionally, statistical power to evaluate for risk factors for renal insufficiency was limited since few participants experienced renal dysfunction defined by a GFR <60 mL/min/1.73 m2; however, the observed associations were robust to analyses that used a higher threshold. Finally, the overall model for comorbidities includes somewhat heterogeneous diseases, and underlying biological mechanisms likely vary.
Our data add substantially to the field by documenting a substantial frequency of noninfectious comorbidities in a large cohort across 4 African countries, with more than 37% of HIV-infected study participants meeting criteria for at least 1 NCD. Among HIV-infected participants, AFRICOS cohort prevalence of NCDs is generally less than older, highly treated HIV cohorts in resource-rich settings [39, 40]. However, as the HIV-infected population in Africa ages and ART increasingly scales up across the continent, it will be increasingly important for HIV treatment models to address evaluation and treatment of common NCDs in the comprehensive care of HIV-infected individuals. Future comparison of noninfectious comorbidity to a larger HIV-uninfected control population will enrich the understanding of HIV-associated noninfectious risks in Africa. Additional work in this cohort includes direct comparison of NCD data with a large Western HIV cohort and assessment of markers of immune inflammation to elucidate pathogenesis pathways in the African context. Pursuit of this work will be further advanced by evaluation of accumulating longitudinal data on the incidence of definitive clinical outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, local implementing partners, and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Defence Headquarters Medical Center, and 68th Nigerian Army Reference Hospital. The authors thank Dr. Peter Dawson of the EMMES Corporation for expert statistical review. The authors also thank the African Cohort Study Study Team: US Military human immunodeficiency virus (HIV) Research Program Data Coordination and Analysis Center: O. Falodun, K. Song, M. Milazzo, C. Zhang, R. Deshano. MHRP Clinical Operations Office: C. Thompson, G. Smith, T. Mebrahtu. MHRP Department of International HIV Prevention Treatment: P. Coakley. MHRP International Laboratory Program: K. Lombardi, M. Imbach. Walter Reed Army Institute of Research HIV Diagnostics Reference Lab: S. Peel, J. Malia. Ludwig Maximilian University of Munich, Germany: A. Kroidl, I. Kroidl, C. Geldmacher. Kayunga District Hospital, Uganda: C. Kafeero, A. Nambuya, J. Tegamanyi, H. Birungi, O. Mugagga, G. Nassali, P. Wangiri, M. Nantabo, P. Nambulondo, B. Atwijuka, A. Asiimwe, C. T. Nabanoba, M. Semwogerere, R. Mwesigwa, S. Jjuuko, R. Namagembe, E. Bagyendagye, A. Tindikahwa, I. Rwomushana, F. Ssentongo, H. Kibuuka, M. Millard. Kericho District Hospital, Kenya: J. Kapkiai, S. Wangare, R. Mangesoi, P. Chepkwony, L. Bor, E. Maera, A. Kasembeli. AIC Litein Hospital, Kericho, Kenya: J. Rotich, C. Kipkoech, W. Chepkemoi, A. Rono. Kapkatet District Hospital, Kericho, Kenya: Z. Kesi, J. Ngeno, E. Langat, K. Labosso, K. Langat, R. Kirui. Tenwek Mission Hospital, Bomet, Kenya: L. Rotich, M. Mabwai, E. Chelangat, J. Agutu, C. Tonui. Nandi Hills District Hospital, Kenya: E. Changwony, M. Bii, E. Chumba, J. Korir. Kapsabet District Hospital, Nandi, Kenya: J. Sugut, D. Gitonga, R. Ngetich, S. Kiprotich. Kenya Medical Research Institute, Kisumu: W. Rehema, C. Ogari, I. Ouma, O. Adimo, S. Ogai, C. Okwaro, E. Maranga, J. Ochola, K. Obambo, V. Sing’oei, L. Otieno, O. Nyapiedho, N. Sande, E. Odemba, F. Wanjiru. Walter Reed Program-Tanzania, Mbeya: S. Khamadi, E. Chiweka, A. Lwilla, D. Mkondoo, N. Somi, P. Kiliba, M. Mwaipopo, G. Mwaisanga, J. Muhumuza. Mbeya Zonal Referral Hospital, Tanzania: N. Mkingule, O. Mwasulama, A. Sanagare, P. Kishimbo. National Institute of Medical Research, Mbeya Medical Research Centre, Tanzania: G. David, F. Mbwayu, J. Mwamwaja, J. Likiliwike, J. Muhumuza, R. Mcharo, N. Mkingule, O. Mwasulama, B. Mtafya, C. Lueer, A. Kisinda, T. Mbena, H. Mfumbulwa, L. Mwandumbya, P. Edwin, W. Olomi. Walter Reed Program-Nigeria, Abuja: Y. Adamu, A. Akintunde, A. B. Tiamiyu, K. Afoke, M. Shehu. 68th Nigerian Army Reference Hospital, Yaba, Lagos: N. E. Harrison, U. C. Agbaim, O. A. Adegbite, R. M. Eluwa, G. A. Adelakun, A. U. Ikegbunam, J. C. Mbibi, F. O. Oni, R. O. Ndbuisi, J. Elemere. Defense Headquarters Medical Center, Abuja, Nigeria: N. Azuakola, T. T. Williams, M. Ayogu, O. Enameguono, A. F. Odo, I. C. Ukaegbu, O. Ugwuezumba . Defense Reference Lab, Abuja, Nigeria: S. O. Odeyemi, N. C. Okeke, L. Umeji, A. Rose, H. Daniel, H. Nwando, E. I. Nicholas, T. Iyanda, C. Okolo, V. Y. Mene, B. Dogonyaro, O. Olabulo, O. Akinseli, F. Onukun, G. Knopp.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the US Department of Defense (DoD).
Financial support. This work was supported by the Military Infectious Disease Research Program and also conducted in collaboration with a President’s Emergency Plan for AIDS Relief–supported basic program evaluation through the US DoD and funded via a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the US DoD. V. G.V.’s contributions were supported by the National Institute of Mental Health (K24MH098759) and the Global Brain Health Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
African Cohort Study Team:
O Falodun, K Song, M Milazzo, C Zhang, R Deshano, C Thompson, G Smith, T Mebrahtu, P Coakley, K Lombardi, M Imbach, S Peel, J Malia, A Kroidl, I Kroidl, C Geldmacher, C Kafeero, A Nambuya, J Tegamanyi, H Birungi, O Mugagga, G Nassali, P Wangiri, M Nantabo, P Nambulondo, B Atwijuka, A Asiimwe, C T Nabanoba, M Semwogerere, R Mwesigwa, S Jjuuko, R Namagembe, E Bagyendagye, A Tindikahwa, I Rwomushana, F Ssentongo, H Kibuuka, M Millard, J Kapkiai, S Wangare, R Mangesoi, P Chepkwony, L Bor, E Maera, A Kasembeli, J Rotich, C Kipkoech, W Chepkemoi, A Rono, Z Kesi, J Ngeno, E Langat, K Labosso, K Langat, R Kirui, L Rotich, M Mabwai, E Chelangat, J Agutu, C Tonui, E Changwony, M Bii, E Chumba, J Korir, J Sugut, D Gitonga, R Ngetich, S Kiprotich, W Rehema, C Ogari, I Ouma, O Adimo, S Ogai, C Okwaro, E Maranga, J Ochola, K Obambo, V Sing’oei, L Otieno, O Nyapiedho, N Sande, E Odemba, F Wanjiru, S Khamadi, E Chiweka, A Lwilla, D Mkondoo, N Somi, P Kiliba, M Mwaipopo, G Mwaisanga, J Muhumuza, N Mkingule, O Mwasulama, A Sanagare, P Kishimbo, G David, F Mbwayu, J Mwamwaja, J Likiliwike, J Muhumuza, R Mcharo, N Mkingule, O Mwasulama, B Mtafya, C Lueer, A Kisinda, T Mbena, H Mfumbulwa, L Mwandumbya, P Edwin, W Olomi, Y Adamu, A Akintunde, A B Tiamiyu, K Afoke, M Shehu, N E Harrison, U C Agbaim, O A Adegbite, R M Eluwa, G A Adelakun, A U Ikegbunam, J C Mbibi, F O Oni, R O Ndbuisi, J Elemere, N Azuakola, T T Williams, M Ayogu, O Enameguono, A F Odo, I C Ukaegbu, O Ugwuezumba, S O Odeyemi, N C Okeke, L Umeji, A Rose, H Daniel, H Nwando, E I Nicholas, T Iyanda, C Okolo, V Y Mene, B Dogonyaro, O Olabulo, O Akinseli, F Onukun, and G Knopp
References
- 1. Schoeni-Affolter F , Ledergerber B , Rickenbach M , et al. ; Swiss HIV Cohort Study Cohort profile: the Swiss HIV Cohort Study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 2. Egger M , Ekouevi DK , Williams C , et al. . Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaslow RA , Ostrow DG , Detels R , Phair JP , Polk BF , Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 4. Palella FJ Jr , Delaney KM , Moorman AC , et al. . Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 5. Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010;50:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasse B , Ledergerber B , Furrer H , et al. ; Swiss HIV Cohort Study. Morbidity and aging in HIV-infected persons: the Swiss HIV Cohort Study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 7. Wada N , Jacobson LP , Cohen M , French A , Phair J , Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984-2008. Am J Epidemiol 2013; 177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schouten J , Wit FW , Stolte IG , et al. ; AGEhIV Cohort Study Group. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 9. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV. 2017;4:e349–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guaraldi G , Orlando G , Zona S , et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 11. Deeks SG , Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 12. Goulet JL , Fultz SL , Rimland D , et al. . Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007; 45:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Achhra AC , Mocroft A , Reiss P , et al. ; D:A:D Study Group. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2016; 17:255–68. [DOI] [PubMed] [Google Scholar]
- 14. Mocroft A , Lundgren JD , Ross M , et al. ; D:A:D Study Group; Royal Free Hospital Clinic Cohort; INSIGHT Study Group; SMART Study Group; ESPRIT Study Group. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med 2015; 12:e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petoumenos K , Worm SW , Fontas E , et al. ; D:A:D Study Group. Predicting the short-term risk of diabetes in HIV-positive patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. J Int AIDS Soc 2012; 15:17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deeks SG , Tracy R , Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armah KA , McGinnis K , Baker J , et al. . HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendenhall E , Kohrt BA , Norris SA , Ndetei D , Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet 2017; 389:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sacktor NC , Wong M , Nakasujja N , et al. . The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS 2005; 19:1367–74. [PubMed] [Google Scholar]
- 20. Levey AS , Bosch JP , Lewis JB , Greene T , Rogers N , Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999; 130:461–70. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 22. UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2014. [Google Scholar]
- 23. Erlandson KM , Kitch D , Tierney C , et al. . Impact of randomized antiretroviral therapy initiation on glucose metabolism. AIDS 2014; 28:1451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dave JA , Lambert EV , Badri M , West S , Maartens G , Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr 2011; 57:284–9. [DOI] [PubMed] [Google Scholar]
- 25. Rosenkranz SL , Yarasheski KE , Para MF , Reichman RC , Morse GD. Antiretroviral drug levels and interactions affect lipid, lipoprotein, and glucose metabolism in HIV-1 seronegative subjects: a pharmacokinetic-pharmacodynamic analysis. Metab Syndr Relat Disord 2007; 5:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maggi P , Bellacosa C , Carito V , et al. . Cardiovascular risk factors in patients on long-term treatment with nevirapine- or efavirenz-based regimens. J Antimicrob Chemother 2011; 66:896–900. [DOI] [PubMed] [Google Scholar]
- 27. Lee FJ , Carr A. Tolerability of HIV integrase inhibitors. Curr Opin HIV AIDS 2012; 7:422–8. [DOI] [PubMed] [Google Scholar]
- 28. Quercia R , Roberts J , Martin-Carpenter L , Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015; 35:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valcour V , Yee P , Williams AE , et al. . Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–The Hawaii Aging with HIV Cohort. J Neurovirol 2006; 12:387–91. [DOI] [PubMed] [Google Scholar]
- 30. Ellis RJ , Badiee J , Vaida F , et al. ; CHARTER Group. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. WHO Guidelines Approved by the Guidelines Review Committee. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization;2016. [PubMed] [Google Scholar]
- 32. Malaza A , Mossong J , Bärnighausen T , Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One 2012; 7:e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathabire Rücker SC , Tayea A , Bitilinyu-Bangoh J , et al. . High rates of hypertension, diabetes, elevated low-density lipoprotein cholesterol, and cardiovascular disease risk factors in HIV-infected patients in Malawi. AIDS 2018; 32:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosha NR , Mahande M , Juma A , et al. . Prevalence, awareness and factors associated with hypertension in North West Tanzania. Glob Health Action 2017; 10:1321279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogunmola OJ , Oladosu OY , Olamoyegun AM. Association of hypertension and obesity with HIV and antiretroviral therapy in a rural tertiary health center in Nigeria: a cross-sectional cohort study. Vasc Health Risk Manag 2014; 10:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maganga E , Smart LR , Kalluvya S , et al. . Glucose metabolism disorders, HIV and antiretroviral therapy among Tanzanian adults. PLoS One 2015; 10:e0134410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lucas GM , Clarke W , Kagaayi J , et al. . Decreased kidney function in a community-based cohort of HIV-infected and HIV-negative individuals in Rakai, Uganda. J Acquir Immune Defic Syndr 2010; 55:491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wyatt CM , Shi Q , Novak JE , et al. . Prevalence of kidney disease in HIV-infected and uninfected Rwandan women. PLoS One 2011; 6:e18352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy ME , Greenberg AE , Hart R , Powers Happ L , Hadigan C , Castel A; DC Cohort Executive Committee. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington, DC. HIV Med 2017; 18:724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelchen-Matthews A , Ryom L , Borges ÁH , et al. ; EuroSIDA study. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS 2018; 32:2405–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.