Abstract
Omadacycline is a semisynthetic tetracycline antibiotic. Phase III clinical trial results have shown that omadacycline has an acceptable safety profile in the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. Similar to most tetracyclines, transient nausea and vomiting and low-magnitude increases in liver aminotransferases were the most frequent treatment-emergent adverse events in phase III studies but were not treatment limiting. Package insert warnings and precautions for omadacycline include tooth discoloration; enamel hypoplasia; inhibition of bone growth following use in late pregnancy, infancy, or childhood up to 8 years of age; an imbalance in mortality (2%, compared with 1% in moxifloxacin-treated patients) was observed in the phase III study in patients with community-acquired bacterial pneumonia. Omadacycline has no effect on the QT interval, and its affinity for muscarinic M2 receptors resulted in transient heart rate increases following dosing.
Keywords: omadacycline, safety, community-acquired bacterial pneumonia, skin and skin structure infections
Omadacycline is an aminomethylcycline, a semisynthetic tetracycline-class antibiotic [1]. Modifications to the tetracycline molecule protect omadacycline from tetracycline-specific active efflux and ribosomal modifications that lead to resistance to the historical tetracyclines [2]. Omadacycline has in vitro activity against gram-positive pathogens, many gram-negative pathogens, atypical pathogens, and some anaerobic pathogens [1]. However, no activity has been observed against Proteus, Providencia, Pseudomonas, and Morganella species [1]. In phase III studies, omadacycline was demonstrated to be noninferior to linezolid in acute bacterial skin and skin structure infections (ABSSSI) and to moxifloxacin in community-acquired bacterial pneumonia (CABP).
While bacterial resistance to the historic tetracyclines decreased their use several decades ago, tetracycline-class antibiotics have been in use for >70 years and have an established safety profile [3, 4]. Here, the relevant safety information on omadacycline is presented, with a focus on the safety profile observed from 3 pivotal, randomized, double-blind, multicenter, phase III clinical trials in ABSSSI and CABP: Omadacycline in Acute Skin and Skin Structure Infections Study (OASIS-1: NCT02378480; OASIS-2: NCT02877927) [5, 6], and Omadacycline for Pneumonia Treatment In the Community (OPTIC: NCT02531438) [7].
SAFETY ANALYSIS OF OMADACYCLINE IN CLINICAL TRIALS
Overview of the Collected Safety Data
Omadacycline has been studied in 27 phase I–III studies and has safety data from 3315 patients including 1947 treated with the active drug. The pivotal phase III registration studies included 2150 patients, including 1073 treated with omadacycline.
For the pivotal phase III trials, the integrated analysis of omadacycline safety data includes 2 phase III studies in ABSSSI (A3 pool: ABSI-1108 [OASIS-1] and ABSI-16301 [OASIS-2]), the phase III study in CABP (C3 pool: CABP-1200 [OPTIC]), and the combination of these 3 phase III studies (AC3 pool). Two trials focused on ABSSSI with linezolid as the comparator: OASIS-1 (intravenous [IV] to oral) and OASIS-2 (oral only). One trial was conducted for CABP, using moxifloxacin as the comparator drug: OPTIC (IV to oral administration) (Table 1). These studies included a total of 705 omadacycline patients who received the combined IV and oral omadacycline regimen, in both targeted indications, as well as 368 patients who received the oral-only omadacycline regimen for the treatment of ABSSSI. In all of the studies, safety data were collected from the time of informed consent until 30–37 days after the first dose of therapy. The evaluated safety parameters include treatment-emergent adverse events (TEAEs), clinical laboratory evaluations, vital signs, and electrocardiographic findings.
Table 1.
Patient Pooling From Phase III Acute Bacterial Skin and Skin Structure Infection (ABSI-1108 and ABSI-16301) and Community-acquired Bacterial Pneumonia (CABP-1200) Studies
| Patient Pool | Omadacycline All Doses (IV + Oral) | Linezolid 600 mg (IV + Oral) | Moxifloxacin 400 mg (IV + Oral) | Total |
|---|---|---|---|---|
| Phase III ABSSSI studies (A3 pool) | 691 | 689 | … | 1380 |
| ABSI-1108 | 323 | 322 | … | 645 |
| ABSI-16301 | 368 | 367 | … | 735 |
| Phase III CABP study (C3 pool) | 382 | … | 388 | 770 |
| CABP-1200 | 382 | … | 388 | 770 |
| Phase III ABSSSI and CABP studies (AC3 pool) | 1073 | 689 | 388 | 2150 |
| ABSI-1108 | 323 | 322 | … | 645 |
| ABSI-16301 | 368 | 367 | … | 735 |
| CABP-1200 | 382 | … | 388 | 770 |
Data are presented as No.
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; CABP, community-acquired bacterial pneumonia; IV, intravenous.
Mean Exposure to Treatment and Study Completion
In all studies, treatment was prescribed for 7–14 days. Mean duration of total therapy varied from 8 to 10 days in the pivotal phase III studies; exposure (standard deviation) was similar between omadacycline and both comparators (9.0 [2.87] days for omadacycline, 8.5 [2.96] days for linezolid, and 9.6 [2.95] days for moxifloxacin), with approximately 90% of dosing completed for all drugs. Regardless of the route of administration or indication, 34% of omadacycline patients received between 4 and 8 days of treatment, and 59.3% of omadacycline patients received between 9 and 14 days of treatment.
Completion of the study treatment in the phase III ABSSSI and CABP studies was accomplished by approximately 90% of patients (treatment group range, 87–91%). Completion of the study (ie, patients who received at least 1 dose of the study drug, and completed end of treatment [EOT], posttreatment evaluation/test of cure, and follow-up visits) was accomplished by approximately 90% of patients (treatment group range, 88–93%).
Demographics and Common Comorbidities
Overall, 60.6% of patients in the phase III ABSSSI and CABP studies were male, and 91.6% were white, and not Hispanic or Latino (75.8%). Across all the phase III studies, similar numbers of patients were in body mass index categories <25 (37.1%), 25–30 (34.0%), and >30 (28.9%) kg/m2. In the phase III ABSSSI studies, mean age was 45.1 years; mean age in the phase III CABP study was 61.5 years. The most common comorbidities (≥10% of patients) for patients in the ABSSSI studies included drug abuse, tobacco use, hepatitis C infection, anxiety, depression, and other wound and skin infections. In the CABP study, common comorbid conditions included hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and atrial fibrillation.
Most Frequent TEAEs, Severe TEAEs, and Serious TEAEs
An overview of adverse events (AEs) across all phase III studies (AC3 pool) is provided in Table 2. Omadacycline compared favorably with the comparators in the pivotal phase III studies. TEAEs from the pivotal phase III studies occurred in similar proportions of omadacycline (47.5%), linezolid (41.2%), and moxifloxacin (48.5%) patients. Within each indication, TEAEs graded as severe were reported in 1.7% of patients in the omadacycline group and 2.5% of patients in the linezolid group (OASIS studies), and in 6.5% of patients in the omadacycline group and 6.7% of patients in the moxifloxacin group (OPTIC study).
Table 2.
Overview of Adverse Events in Phase III Acute Bacterial Skin and Skin Structure Infection and Community-acquired Bacterial Pneumonia Studies (AC3 Pool)
| Adverse Event | Omadacycline (n = 1073) | Linezolid (n = 689) | Moxifloxacin (n = 388) |
|---|---|---|---|
| Any TEAE | 510 (47.5) | 284 (41.2) | 188 (48.5) |
| Drug-related TEAE | 236 (22.0) | 111 (16.1) | 69 (17.8) |
| Serious TEAE | 39 (3.6) | 13 (1.9) | 26 (6.7) |
| Drug-related serious TEAE | 2 (0.2) | 1 (0.1) | 2 (0.5) |
| TEAE leading to deatha | 9 (0.8) | 3 (0.4) | 4 (1.0) |
| TEAE leading to premature discontinuation of study drug | 33 (3.1) | 10 (1.5) | 27 (7.0) |
| TEAE leading to dose interruption of study drug | 2 (0.2) | 0 | 0 |
| Serious TEAE leading to premature discontinuation of study drug | 16 (1.5) | 5 (0.7) | 11 (2.8) |
Data are presented as No. (%).
Abbreviation: TEAE, treatment-emergent adverse event.
aCauses of death by preferred term in the omadacycline group were pleural effusion and metastatic lung cancer, overdose, cerebrovascular accident, aortic aneurysm rupture, septic shock, pneumonia and acute respiratory distress syndrome, cardiogenic shock, cardiorespiratory arrest, acute respiratory failure and multiorgan failure, and acute myocardial infarction; in the linezolid group: cardiac failure, cardiac arrest, and unknown; and in the moxifloxacin group: cardiac failure, acute respiratory failure, lung neoplasm, and pancreatic carcinoma.
Rates of serious TEAEs and discontinuations due to TEAEs and serious TEAEs were infrequent and similar in the omadacycline and comparator groups. Across all phase III studies, serious TEAEs occurred in 3.6% of omadacycline patients, 1.9% of linezolid patients, and 6.7% of moxifloxacin patients (AC3 pool; Table 2).There was a higher percentage of serious TEAEs reported in the OPTIC study (6.0% of patients in the omadacycline group and 6.7% of patients in the moxifloxacin group) than in the OASIS studies (2.3% of patients in the omadacycline group and 1.9% of patients in the linezolid group). The higher number of serious TEAEs in CABP was due to infection and respiratory AEs. TEAEs with an outcome of death occurred in ≤1.0% in all treatment groups. In the ABSSSI studies, there was 1 death (0.1%) in omadacycline patients vs 3 deaths (0.4%) in linezolid patients. In the OPTIC trial, there were 8 deaths (2.1%) in omadacycline patients and 4 deaths (1.0%) in moxifloxacin patients. The deaths in OPTIC were related to infection progression and underlying conditions [7].
Gastrointestinal AEs were the most frequent events in all treatment groups. Nausea (14.9% omadacycline, 8.7% linezolid, 5.4% moxifloxacin) and vomiting (8.3% omadacycline, 3.9% linezolid, 1.5% moxifloxacin) were the most frequently reported TEAEs that occurred at a greater frequency in the omadacycline group compared with the linezolid and moxifloxacin groups (Table 3). While these events occurred consistently across studies, low rates of nausea and vomiting were observed with the IV and oral formulations of omadacycline during the OASIS-1 and OPTIC trials, and higher rates of nausea and vomiting events occurred in the oral-only OASIS-2 study (Table 4). These higher percentages of nausea and vomiting were associated with the 450 mg loading dose during the first 2 days of the OASIS-2 study. During the oral-only OASIS-2 study, 30.2% and 7.6% of patients experienced at least 1 nausea TEAE, and 16.8% and 3.0% of patients experienced at least 1 vomiting TEAE in the omadacycline and linezolid groups, respectively. Onset of nausea and vomiting occurred primarily during the loading dose period on day 1 and day 2 in 25.3% and 12.5% of patients receiving omadacycline. After day 2 through EOT, onset of nausea and vomiting each occurred in 4.1% of omadacycline patients. While the reason for the increased nausea and vomiting is uncertain, it was observed that across the OASIS ABSSSI studies, the rate of nausea and vomiting in omadacycline patients was higher in those who were IV drug users (IVDUs; 26.3% and 14.1%) than in non-IVDUs (15.4% and 7.5%).
Table 3.
Most Frequent Treatment-emergent Adverse Events (≥2% for Any Group) in Phase III Acute Bacterial Skin and Skin Structure Infection and Community Acquired Bacterial Pneumonia Studies (AC3 Pool)
| Adverse Event | Omadacycline (n = 1073) | Linezolid (n = 689) | Moxifloxacin (n = 388) |
|---|---|---|---|
| Patients with ≥1 TEAE | 510 (47.5) | 284 (41.2) | 188 (48.5) |
| Gastrointestinal disorders | 241 (22.5) | 103 (14.9) | 70 (18.0) |
| Nausea | 160 (14.9) | 60 (8.7) | 21 (5.4) |
| Vomiting | 89 (8.3) | 27 (3.9) | 6 (1.5) |
| Diarrhea | 26 (2.4) | 20 (2.9) | 31 (8.0) |
| General disorders and administration-site conditions | 70 (6.5) | 42 (6.1) | 18 (4.6) |
| Infusion-site extravasation | 28 (2.6) | 19 (2.8) | 0 |
| Infections | 132 (12.3) | 92 (13.4) | 41 (10.6) |
| Wound infection | 30 (2.8) | 22 (3.2) | 0 |
| Cellulitis | 28 (2.6) | 24 (3.5) | 0 |
| Subcutaneous abscess | 23 (2.1) | 27 (3.9) | 0 |
| Investigations | 93 (8.7) | 56 (8.1) | 46 (11.9) |
| ALT increased | 42 (3.9) | 25 (3.6) | 18 (4.6) |
| AST increased | 33 (3.1) | 24 (3.5) | 14 (3.6) |
| GGT increased | 15 (1.4) | 8 (1.2) | 8 (2.1) |
| Nervous system disorders | 49 (4.6) | 32 (4.6) | 12 (3.1) |
| Headache | 31 (2.9) | 21 (3.0) | 5 (1.3) |
| Psychiatric disorders | 23 (2.1) | 13 (1.9) | 17 (4.4) |
| Insomnia | 14 (1.3) | 6 (0.9) | 8 (2.1) |
| Vascular disorders | 36 (3.4) | 8 (1.2) | 16 (4.1) |
| Hypertension | 19 (1.8) | 5 (0.7) | 11 (2.8) |
Data are presented as No. (%). A TEAE was defined as an AE with a start date/time on or after the date/time of the first dose of active study drug. Percentages were based on the number of patients in each treatment group. Patients may have been counted in >1 row.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; TEAE, treatment-emergent adverse event.
Table 4.
Rates of Nausea and Vomiting Associated With Omadacycline During Intravenous and Oral Phases of the Phase III OPTIC, OASIS-1, and OASIS-2 Studies
| OPTIC (IV/Oral) | OASIS-1 (IV/Oral) | OASIS-2 (Oral Only) | ||||
|---|---|---|---|---|---|---|
| Event | IV (n = 382) | Oral (n = 295) | IV (n = 323) | Oral (n = 286) | Oral Day 1 to Day 2 (n = 368) | Oral Day 3 to EOT (n = 368) |
| Nausea | 0.5 (2) | 2.4 (7) | 4.3 (14) | 9.1 (26) | 25.3 (93) | 4.1 (15) |
| Vomiting | 1.8 (7) | 1.0 (3) | 1.2 (4) | 4.5 (13) | 12.5 (46) | 4.1 (15) |
Data are presented as No. (%).
Abbreviations: EOT, end of treatment; IV, intravenous; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; OPTIC, Omadacycline for Pneumonia Treatment In the Community.
The nausea and vomiting due to omadacycline identified in the pivotal phase III studies were not treatment limiting. Except for 1 episode, all events of nausea and vomiting were of mild or moderate intensity, and most nausea and vomiting resolved on treatment with or without antiemetic therapy. For example, in the OASIS-2 study, concomitant antiemetic medications were used in 60 of 120 (50%) patients receiving omadacycline, and in 12 of 30 (40%) patients receiving linezolid who reported nausea or vomiting. Only 4 patients receiving omadacycline (0.4%), including 1 patient in the OASIS-2 study, discontinued treatment due to nausea and vomiting. Diarrhea occurred in lower numbers of omadacycline (2.4%) and linezolid patients (2.9%) compared with moxifloxacin patients (8.0%) (Table 3). No cases of Clostridioides difficile infection were reported in omadacycline and linezolid patients, but the infection was reported in 8 (2.1%) moxifloxacin patients. These results are consistent with C. difficile preclinical data for omadacycline and the lower propensity of C. difficile infection observed with the tetracycline class [8–12]. Although omadacycline shows in vitro activity against C. difficile, it has not been studied as a treatment option for C. difficile infection.
Infection-associated AEs were among the TEAEs reported at ≥2% (Table 3) in all phase III patients (AC3 pool). In the OASIS ABSSSI studies (A3 pool only), events of cellulitis (3.9% vs 3.5%), abscess (3.3% vs 3.9%), and wound infection (4.3% vs 3.2%) were similar among the omadacycline and linezolid treatment groups. All 3 AE terms included AEs associated with the primary skin infection under study as well as secondary skin infections. This was not unexpected given the large number of IVDU patients enrolled. Overall rates of serious TEAEs were low (AC3 pool; Table 5); serious infection-associated TEAEs (cellulitis, abscess, and wound infection) each occurred in <0.5% of omadacycline-treated ABSSSI patients. In the OPTIC CABP study, infection-associated (eg, pneumonia) and respiratory-associated (eg, pleural effusion, respiratory failure) TEAEs and serious TEAEs each occurred in ≤1.0% of omadacycline-treated patients, with a similar rate in moxifloxacin-treated patients [7].
Table 5.
Serious Treatment-emergent Adverse Events in Phase III Acute Bacterial Skin and Skin Structure Infection and Community-acquired Bacterial Pneumonia Studies (AC3 Pool by System Organ Class)
| Adverse Event | Omadacycline (n = 1073) | Linezolid (n = 689) | Moxifloxacin (n = 388) |
|---|---|---|---|
| Patients with ≥1 serious TEAE | 39 (3.6) | 13 (1.9) | 26 (6.7) |
| Infections and infestations | 20 (1.9) | 5 (0.7) | 16 (4.1) |
| Respiratory and thoracic disorders | 9 (0.8) | 2 (0.3) | 3 (0.8) |
| Cardiac disorders | 5 (0.5) | 2 (0.3) | 2 (0.5) |
| Hepatobiliary disorders | 3 (0.3) | 0 | 1 (0.3) |
| Nervous system disorders | 3 (0.3) | 0 | 0 |
| Neoplasms | 2 (0.2) | 0 | 6 (1.5) |
| Musculoskeletal and connective tissue disorders | 1 (0.1) | 0 | 0 |
| Psychiatric disorders | 1 (0.1) | 1 (0.1) | 0 |
| Vascular disorders | 1 (0.1) | 0 | 1 (0.3) |
| Renal and urinary disorders | 0 | 0 | 2 (0.5) |
| Gastrointestinal disorders | 0 | 0 | 1 (0.3) |
| Skin and subcutaneous disorders | 0 | 1 (0.1) | 0 |
| General disorders and administration-site conditions | 3 (0.3) | 1 (0.1) | 0 |
| Injury, poisoning, and procedural complications | 2 (0.2) | 1 (0.1) | 1 (0.3) |
Data are presented as No. (%).
Abbreviation: TEAE, treatment-emergent adverse event.
Infusion-site extravasation occurred at similar rates to linezolid when using all of the pivotal phase III data (AC3 pool); however, higher rates of infusion-site extravasation with omadacycline were observed in the ABSSSI studies (A3 pool). The difference between omadacycline and linezolid (4.1% vs 2.8%) was due to a greater number of events in the OASIS-1 study associated with difficult venous access in IVDU patients. When considering all infusion-site reactions (infusion-site extravasation, pain, irritation, erythema, inflammation and swelling, skin induration, and peripheral swelling), the difference between omadacycline and linezolid persisted (5.2% vs 3.6%). However, the rates from the OPTIC CABP study support the conclusion that these AEs were due to the population under study (ABSSSI and IVDU patients) as opposed to omadacycline properties. In the CABP study, infusion-site extravasation did not occur in either treatment group, and the rate of all infusion-site reactions was similar between omadacycline and moxifloxacin (0.5% vs 1.3%).
SUMMARY OF HEPATIC SAFETY
Tetracycline use is often associated with changes in liver function tests, in particular elevations in liver aminotransferases [13]. Phase I study results have shown transient serum alanine aminotransferase (ALT) elevations for IV omadacycline doses ≥300 mg, and dose-limiting changes at an oral dose of 600 mg [14]. In the phase III studies, patients with elevated liver enzymes at screening were excluded from all 3 studies (>2 × upper limit of normal [ULN] in OASIS-1 and OPTIC and >3 × ULN in OASIS-2). The majority of patients had normal liver enzymes at baseline.
Liver-associated TEAEs occurred in 5.4%, 4.9%, and 7.2% of omadacycline, linezolid, and moxifloxacin patients, respectively. The incidences of all hepatic AEs of interest, including increased ALT and increased aspartate aminotransferase (AST), were similar between the omadacycline and comparator groups. TEAEs of increased ALT and AST were the only liver AEs that occurred, at an incidence of ≥2% (Table 3). A single omadacycline patient had a liver-associated serious TEAE. The patient had a reversible AE of hepatic failure following a cardiac arrest. All hepatic AEs were either resolving or resolved without sequelae during or following completion of treatment, except in 2 omadacycline patients (who had no additional AEs of sequelae reported). Four patients (2 receiving omadacycline, 2 receiving moxifloxacin) had increases in ALT or AST, resulting in discontinuation of the study drug.
The results showing changes in liver enzymes from baseline revealed no clinically meaningful changes. Postbaseline ALT levels >3 × ULN occurred in 4.3%, 4.1%, and 4.5% of patients receiving omadacycline, linezolid, and moxifloxacin, respectively. Smaller numbers of patients had postbaseline ALT levels >5 × and >10 × ULN: these small between-group differences in postbaseline ALT were observed in omadacycline patients with abnormal baseline liver aminotransferase levels. Postbaseline changes in AST and total bilirubin were similar for all treatment groups (Table 6). Elevations in ALT and AST were mostly asymptomatic, low magnitude, and transient, with most patients having resolution on therapy or following the completion of therapy. Mean EOT postbaseline change in ALT was similar for omadacycline (6.89 U/L), linezolid (4.19 U/L), and moxifloxacin (7.30 U/L). No cases of Hy’s law (ALT >3 × ULN, total bilirubin >2 × ULN, alkaline phosphatase <2 × ULN, and no other reason to explain the elevated ALT or total bilirubin) occurred in any treatment group.
Table 6.
Summary of Liver Chemistry Elevation for Patients With Any Baseline Values in Phase III OASIS-1, OASIS-2, and OPTIC Studies (AC3 Pool)
| Parameter | Omadacycline (n = 1073) | Linezolid (n = 689) | Moxifloxacin (n = 388) |
|---|---|---|---|
| ALT (U/L), any value at baseline | n = 1031 | n = 659 | n = 381 |
| >3 × ULN | 44 (4.3) | 27 (4.1) | 17 (4.5) |
| >5 × ULN | 22 (2.1) | 5 (0.8) | 4 (1.0) |
| >10 × ULN | 9 (0.9) | 3 (0.5) | 1 (0.3) |
| AST (U/L), any value at baseline | n = 1040 | n = 661 | n = 379 |
| >3 × ULN | 38 (3.7) | 27 (4.1) | 12 (3.2) |
| >5 × ULN | 20 (1.9) | 7 (1.1) | 4 (1.1) |
| >10 × ULN | 6 (0.6) | 1 (0.2) | 2 (0.5) |
| Total bilirubin (µmol/L), any value at baseline | n = 1041 | n = 666 | n = 381 |
| >1.5 × ULN | 11 (1.1) | 3 (0.5) | 7 (1.8) |
| >2 × ULN | 6 (0.6) | 1 (0.2) | 4 (1.0) |
Data are presented as No. (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; OPTIC, Omadacycline for Pneumonia Treatment In the Community; ULN, upper limit of normal.
SUMMARY OF CARDIAC SAFETY
Nonclinical studies have shown that omadacycline inhibits carbamylcholine binding to the M2 subtype of the muscarinic acetylcholine receptor, but does not interact with other subtype muscarinic acetylcholine receptors or adrenergic receptors [15]. This is a seemingly unique effect of omadacycline and has not been observed among other in vitro studies with tetracyclines. In healthy volunteers, a maximum plasma concentration (Cmax)–related vagolytic effect results in a transient, generally asymptomatic increase in heart rate (HR; 12–17 beats per minute [bpm]) for 100 to 200 mg IV doses [16]. Preclinical studies also demonstrated no effect of omadacycline on hERG channel activity and no QTc prolongation in monkeys [15]. In a thorough QT (TQT) study in healthy adults, individualized HR-corrected QT interval (QTcS) was utilized as the primary endpoint to analyze changes following single 100 mg and 300 mg IV doses of omadacycline. The largest least-squares mean placebo-corrected ΔQTcS was 2.6 msec (90% confidence interval 0.55–4.67). Assay sensitivity was demonstrated with the 400 mg single dose of moxifloxacin. The TQT results are consistent with the results seen in the OPTIC CABP trial where 30–90 minutes after dose 1 and dose 3, omadacycline had mean increases in QTcF of 0.8 and 1.8 msec, respectively, compared with the 5.8 and 11.1 msec increases for moxifloxacin.
As a result of these HR findings, electrocardiography (30 minutes before and 30–90 minutes after omadacycline infusion) was used to measure HR changes before and after the day 1 (ie, dose 1) and day 2 (ie, dose 3) IV dosing time points in the OASIS-1 and OPTIC phase III studies. The timing postdose best represents time points that would most closely represent Cmax exposure to omadacycline. In addition, cardiac AEs were examined to look specifically at incidence rates of myocardial ischemia, heart failure, cardiac arrest, and tachyarrhythmias.
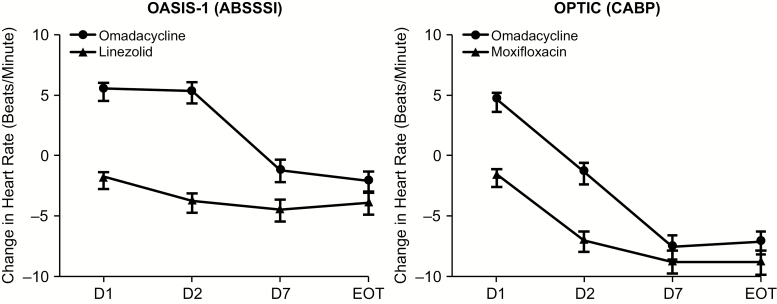
Mean HR changes (± standard error of the mean) from baseline on day 1 and day 2 are shown for OASIS-1 and OPTIC in Figure 1. In OASIS-1, the mean postdose difference was approximately +5 bpm on day 1 and day 2 for omadacycline vs a decrease of approximately 2–4 bpm for linezolid. In OPTIC, mean postdose changes in HR were similarly small in magnitude on day 1 (4.3 bpm) and day 2 (–1.1 bpm) for omadacycline-treated patients compared with moxifloxacin-treated patients (–1.5 and –6.8 bpm, respectively) on day 1 and day 2. In both studies, the mean HR declined from baseline to the EOT in all treatment groups; however, this decline was less rapid for omadacycline patients than for those on comparator drugs. The trend toward a reduction in the mean HR was concordant with the clinical improvement in all treatment groups. Postbaseline HR increases to ≥120 bpm occurred in 3.1%, 2.5%, and 5.7% of omadacycline, linezolid, and moxifloxacin patients, respectively. Systolic and diastolic blood pressure measurements after treatment initiation revealed no clinically meaningful differences between treatment groups.
Figure 1.
Mean changes in heart rate (beats per minute [bpm] ± standard error of the mean) from baseline. The mean heart rate changes from baseline on day 1 and day 2 for OASIS-1 and OPTIC demonstrate an approximate mean change in heart rate of 5 bpm after the first dose on day 1 for omadacycline. Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; CABP, community-acquired bacterial pneumonia; D, day; EOT, end of treatment; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; OPTIC, Omadacycline for Pneumonia Treatment In the Community.
Cardiac TEAEs occurred in 2.1%, 0.4%, and 5.2% of patients in the omadacycline, linezolid, and moxifloxacin groups, respectively. The majority of these events were mild or moderate in severity and most events resolved with no sequelae. Serious cardiac TEAEs occurred in 0.5%, 0.3%, and 0.5% of patients in the omadacycline, linezolid, and moxifloxacin groups, respectively. In omadacycline, linezolid, and moxifloxacin patients, TEAEs for ischemic heart disease (0.3%, 0.1%, 1.0%, respectively), heart failure (0.6%, 0.3%, 1.3%), and cardiac arrest (0.4%, 0.1%, and 0.3%) were similar. The most frequently observed tachyarrhythmias were atrial fibrillation and tachycardia, which occurred in 0.5% and 0.0%, 0.3% and 0.5%, and 0.0% and 0.8% of omadacycline, linezolid, and moxifloxacin patients, respectively. Most patients had underlying heart disease or cardiac risk factors, and events were typically transient.
OTHER KNOWN TETRACYCLINE EFFECTS
Since omadacycline is structurally similar to tetracyclines, potential risks associated with the tetracycline class were examined, including anti-anabolic events, as represented by blood urea nitrogen increases and azotemia; central nervous system side effects including light-headedness, vertigo or dizziness, hypersensitivity, photosensitivity, and pseudotumor cerebri; acute pancreatitis; and fungal infections, in particular vulvovaginal fungal infections. Pill esophagitis is noted with tetracyclines, in particular doxycycline [17, 18].
Specific examination of known tetracycline-class events occurring with omadacycline treatment suggests that patients receiving omadacycline showed infrequent TEAEs related to the tetracycline class, similar to non-tetracycline comparators (Table 7). No events of phototoxicity or pseudotumor cerebri were recorded. Vestibular disorder TEAEs, including vertigo and dizziness, were observed in 0.8%, 0.9%, and 1.0% of patients receiving omadacycline, linezolid, and moxifloxacin, respectively. One patient had an unrelated episode of esophagitis that occurred while taking IV omadacycline. No omadacycline patient had an AE of acute pancreatitis; 1 omadacycline patient had an AE of mild chronic pancreatitis that was reported concomitantly with a chronic cholecystitis AE. Across the phase III pivotal studies (AC3 pool), postbaseline lipase values did not suggest differences between omadacycline and comparator drugs. The mean highest postbaseline changes in lipase were similar between omadacycline (17.16 U/L), linezolid (14.71 U/L), and moxifloxacin (20.59 U/L).
Table 7.
Occurrence of Other Tetracycline-class Events of Interest in Phase III OASIS-1, OASIS-2, and OPTIC Studies (AC3 Pool)
| TEAE | Omadacycline (n = 1073) | Linezolid (n = 689) | Moxifloxacin (n = 388) |
|---|---|---|---|
| Hypersensitivity reactions | 20 (1.9) | 12 (1.7) | 10 (2.6) |
| Fungal infections | 11 (1.0) | 6 (0.9) | 5 (1.3) |
| Vestibular disorders | 9 (0.8) | 6 (0.9) | 4 (1.0) |
| Blood urea increased | 1 (0.1) | 0 | 0 |
| Esophageal disorders | 1 (0.1) | 0 | 0 |
| Pancreatitis | 1 (0.1) | 0 | 1 (0.3) |
Data are presented as No. (%). A TEAE was defined as an adverse event with a start date/time on or after the date/time of the first dose of active study drug. If a patient had >1 TEAE with the same category, the patient was counted only once for that category.
Abbreviations: OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; OPTIC, Omadacycline for Pneumonia Treatment In the Community; TEAE, treatment-emergent adverse event.
Hypersensitivity events were similar between treatment groups, and all omadacycline cases were considered mild or moderate in severity. No anaphylactic reactions have been observed in omadacycline patients; however, 3 patients experienced urticaria. Caution should be observed in prescribing omadacycline to any individual with a known serious hypersensitivity reaction to a tetracycline-class antibiotic. The incidence of fungal infections was low and similar across all treatment groups (1.0%, 0.9%, and 1.3% of patients in the omadacycline, linezolid, and moxifloxacin groups, respectively). These events were considered to be mild or moderate in severity and did not lead to treatment discontinuation or dose interruption.
In animal models, a reversible decrease in fibula growth rate has been observed in the fetus, and permanent tooth discoloration may occur if omadacycline is taken during tooth development (last half of pregnancy through 8 years of age). Omadacycline is not recommended for use in pregnant or lactating women, or in infants or children <8 years of age; no pediatric clinical studies have been performed to date. Additionally, there are currently no clinical data in patients treated with omadacycline for >14 days.
CONCLUSIONS
This integrated analysis of the 3 pivotal ABSSSI and CABP phase III studies demonstrates that omadacycline is safe and well tolerated. The majority of TEAEs in all treatment groups were considered mild or moderate in intensity, and there was a low rate of study drug discontinuation. The observed AEs were consistent with the known AE profile of the tetracycline class. Nausea and vomiting were the most frequent AEs, but were transient and not treatment limiting in the vast majority of patients. The diarrhea rate was lower in omadacycline-treated patients than in those on comparator drugs. A low propensity for C. difficile infection is suggested by the nonclinical and clinical data. Low-magnitude transient increases in liver aminotransferases occurred. Omadacycline had no effect on the QTc interval, and the observed small increases in HR had no clinical relevance.
Notes
Author contributions. All authors provided critical revisions to the manuscript for intellectual content and approved the final version for publication.
Acknowledgments. Editorial support was provided by Megan E. Breuer, PhD, and Samantha Scott, PhD, of Innovative Strategic Communications, LLC, Milford, Pennsylvania.
Financial support. This work was supported by Paratek Pharmaceuticals, Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Supplement sponsorship. This article appears as part of the supplement “Omadacycline: A New Option in an Era of Increasing Antibiotic Resistance,” sponsored by Paratek Pharmaceuticals, Inc., King of Prussia, Pennsylvania.
Potential conflicts of interest. S. O. has received grants from Asahi Kasei Pharma and MedImmune, outside the submitted work. T. M. F. has received personal fees from Shionogi, outside the submitted work. T. v. d. P. was on the data monitoring board and scientific advisory board at Paratek Pharmaceuticals during the conduct of the study. S. C. is a consultant to Paratek Pharmaceuticals. E. T. and P. M. are employees and shareholders of Paratek Pharmaceuticals. The authors received no financial compensation for the preparation of this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the article have been disclosed.
References
- 1. Villano S, Steenbergen J, Loh E. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol 2016; 11:1421–34. [DOI] [PubMed] [Google Scholar]
- 2. Draper MP, Weir S, Macone A, et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 2014; 58:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson ML, Levy SB. The history of the tetracyclines. Ann N Y Acad Sci 2011; 1241:17–32. [DOI] [PubMed] [Google Scholar]
- 4. Sloan B, Scheinfeld N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin Drug Saf 2008; 7:571–7. [DOI] [PubMed] [Google Scholar]
- 5. O’Riordan W, Green S, Overcash JS, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 2019; 380:528–38. [DOI] [PubMed] [Google Scholar]
- 6. O’Riordan W, Cardenas C, Sirbu A, et al. A phase 3 randomized, double-blind, multi-centre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study). In: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain,21–24 April 2018. [Google Scholar]
- 7. Stets R, Popescu M, Gonong JR, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 2019; 380:517–27. [DOI] [PubMed] [Google Scholar]
- 8. Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 9. Doernberg SB, Winston LG, Deck DH, Chambers HF. Does doxycycline protect against development of Clostridium difficile infection? Clin Infect Dis 2012; 55:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 11. Kim O, Leahy RG, Traczewski M, Macone A, Steenbergen J, Tanaka SK. Activity and efficacy of omadacycline against Clostridium difficile [abstract 1325]. In: 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands, 9–12 April 2016. [Google Scholar]
- 12. Moura I, Sherman S, Pilling S, et al. Effects of omadacycline vs moxifloxacin on gut microbiota populations and Clostridium difficile germination, proliferation and toxin production in an in vitro model of the human gut. In: ASM Microbe, Boston, MA, 16–20 June 2016. [Google Scholar]
- 13. Mosley RH. Tetracyclines: hepatotoxicity of antimicrobials and antifungal agents. In: Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Netherlands: Elsevier, 2013. [Google Scholar]
- 14. Bundrant LA, Tzanis E, Garrity-Ryan L, et al. Safety and pharmacokinetics of the aminomethylcycline antibiotic omadacycline administered to healthy subjects in oral multiple-dose regimens. Antimicrob Agents Chemother 2018; 62:e01487–17. doi:10.1128/AAC.01487-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka SK, Villano S. In vitro and in vivo assessments of cardiovascular effects with omadacycline. Antimicrob Agents Chemother 2016; 60:5247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun H, Ting L, Machineni S, et al. Randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother 2016; 60:7431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leggat PA. Safety and efficacy of doxycycline. Clin Med Ther 2009; 1:1069–72. [Google Scholar]
- 18. Carris NW, Pardo J, Montero J, Shaeer KM. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis 2015; 2:ofv178. [DOI] [PMC free article] [PubMed] [Google Scholar]