Abstract
Omadacycline is a novel aminomethylcycline antimicrobial and semisynthetic derivative of tetracycline. In vitro, omadacycline displays potent activity against gram-positive and many gram-negative bacteria, including methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, β-hemolytic streptococci, vancomycin-resistant Enterococcus, and Enterobacteriaceae. Omadacycline is also active against atypical and anaerobic pathogens, including Legionella pneumophila, Mycoplasma spp., Ureaplasma spp., Bacteroides spp., and Clostridioides difficile. This review outlines the microbiology and preclinical studies of omadacycline, including its mechanism of action; spectrum of activity; protein binding; activity in the presence of surfactant, serum, normal, and pH-adjusted urine, or bacterial biofilms; postantibiotic effect; pharmacodynamic properties; and in vitro and in vivo efficacy. The results of in vitro and in vivo animal studies support the observations made in phase III clinical trials and the clinical development of omadacycline.
Keywords: antimicrobial, omadacycline, pharmacodynamics, spectrum of activity, tetracyclines
Omadacycline is an aminomethylcycline antimicrobial of the tetracycline class, approved in the United States for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections (ABSSSI) in adults [1]. Omadacycline has demonstrated in vitro activity against resistant gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), penicillin- and macrolide-resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus (VRE), and Clostridioides difficile, pathogens that have been recognized by the US Centers for Disease Control and Prevention as urgent or serious threats [2]. The efficacy of omadacycline as monotherapy for serious community-acquired bacterial infections, including ABSSSI and community-acquired bacterial pneumonia, has been demonstrated in phase III clinical trials [3–5]. Ongoing clinical trials are investigating omadacycline for the treatment of urinary tract infections.
MECHANISM OF ACTION
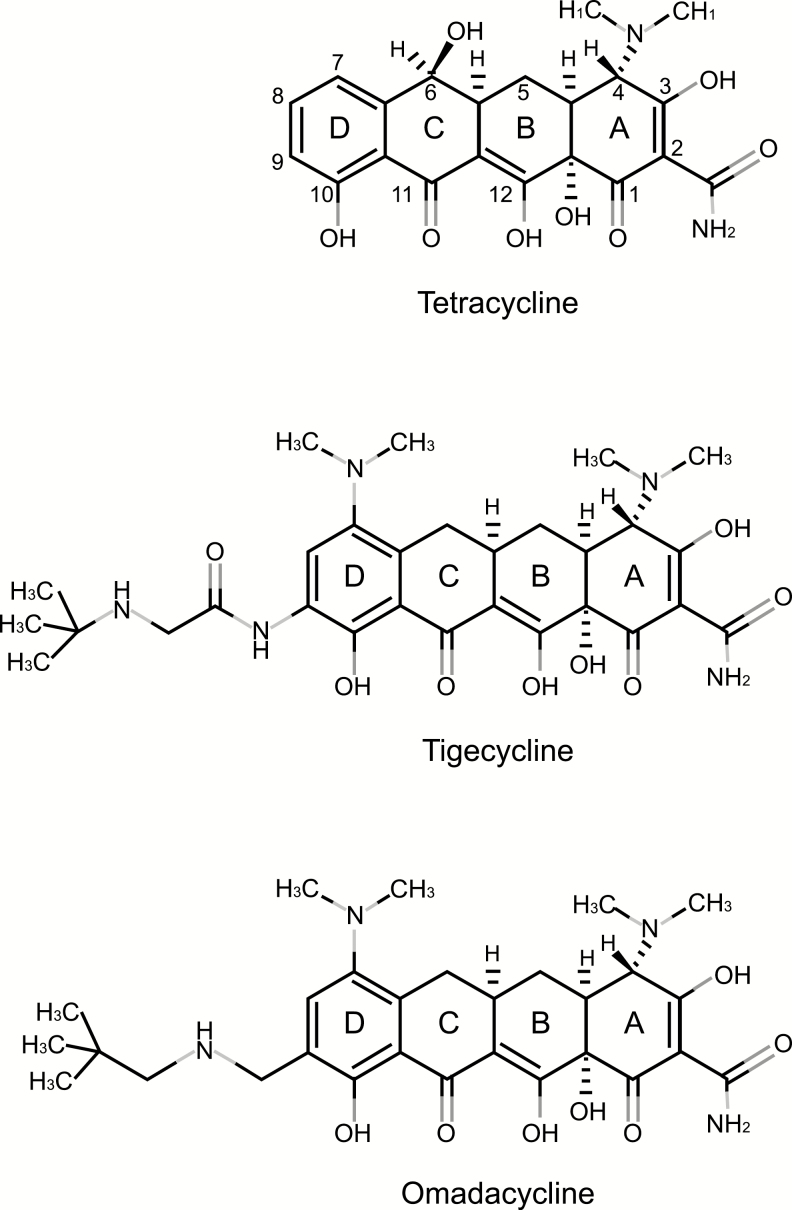
Omadacycline is a semisynthetic tetracycline derivative and displays the same mechanism of action as the tetracycline class. Like tetracyclines, omadacycline inhibits bacterial protein synthesis by binding the primary tetracycline binding site on the 30S subunit of the bacterial ribosome [6]. As demonstrated by whole-cell, macromolecular synthesis, omadacycline directly inhibits bacterial protein synthesis while sparing bacterial DNA, RNA, and peptidoglycan synthesis [7]. Through modifications at the C-7 and C-9 positions of the tetracycline D-ring, omadacycline is able to overcome common tetracycline resistance mechanisms, including tetracycline-specific efflux pumps and ribosomal protection (Figure 1). The C-7 modification circumvents the tetracycline-specific efflux pump resistance mechanism, whereas the C-9 modification overcomes the ribosomal protection resistance mechanism. Although the binding of omadacycline to the primary bacterial ribosome site is similar to that of other tetracyclines, dimethyl sulfate chemical probing and Fenton cleavage studies have revealed that omadacycline, tetracycline, and tigecycline have unique, nonspecific interactions with the 16S rRNA [6]. The unique interaction of omadacycline with the bacterial ribosome may help to explain its ability to overcome the standard tetracycline resistance mechanisms. Omadacycline retains activity against gram-positive pathogens that carry resistance genes for ribosomal protection (ie, tetM, tetO, and tetS) and tetracycline efflux (ie, tetK and tetL) [7, 8]. Thus, omadacycline remains active against tetracycline-resistant bacterial strains. To date, the resistance mechanisms that have been found to inhibit the activity of omadacycline include multidrug efflux pumps (MexXY-OprM and MexAB-OprM) [9] and the tetracycline monooxygenase TetX (Paratek Pharmaceuticals, Inc.; data on file). Although TetX has been shown to inactivate all known tetracyclines, it is not a widespread resistance determinant [10, 11].
Figure 1.
Chemical structures of tetracycline, tigecycline, and omadacycline [6].
SPECTRUM OF ACTIVITY
Omadacycline has been evaluated in a range of studies, including centralized US clinical isolate surveillance studies that began in 2010. Consistency in the spectrum of omadacycline activity over time has been examined by comparing the minimum inhibitory concentrations (MICs) from isolates collected during different surveillance periods. The MIC50/90 values of omadacycline were similar for S. pneumoniae isolates collected in 2010 (0.06/0.12 μg/mL), 2014 (0.06/0.06 μg/mL), and 2016 (0.06/0.12 μg/mL) surveillance programs [12, 13]. Omadacycline has also remained highly active against clinical isolates of S. aureus across studies from 2010 (MIC90 0.25 μg/mL), 2014 (MIC90 0.12 μg/mL), and 2016 (MIC90 0.25 μg/mL) [13, 14]. Similarly, the MIC50/90 values of omadacycline against isolates of Legionella pneumophila remained unchanged from 1995 (0.25/0.25 μg/mL) to 2014 (0.25/0.25 μg/mL) [15]. Omadacycline has been shown to be active against the category A biothreat pathogens Bacillus anthracis (MIC90 0.06 μg/mL) and Yersinia pestis (MIC90 1 μg/mL) [16]. Like other tetracyclines, omadacycline displays no notable activity against Proteus spp. (MIC90 ≥32 μg/mL), Providencia spp. (MIC90 >16 μg/mL), Morganella spp. (MIC90 >16 μg/mL), or Pseudomonas spp. (MIC90 >16 μg/mL) [13, 17]. In general, omadacycline has potent activity against atypical bacterial pathogens and gram-positive aerobes, and a range of activities against gram-negative pathogens.
Gram-positive Aerobes
Omadacycline has potent in vitro activity against gram-positive aerobes, including antimicrobial-resistant pathogens such as MRSA (MIC90 0.25 μg/mL) and penicillin- or macrolide-resistant S. pneumoniae (MIC90 0.12 μg/mL; Table 1; breakpoints available from the US Food and Drug Administration [FDA]) [13, 18]. Omadacycline MIC values have been determined to be comparable for healthcare- and community-associated MRSA [14, 19]. Omadacycline is also active against VRE, with an MIC90 of 0.25 μg/mL for vancomycin-resistant Enterococcus faecalis and of 0.12 μg/mL for vancomycin-resistant Enterococcus faecium. It also retains activity against tetracycline-resistant gram-positive bacteria, including S. aureus (MIC90 0.5 μg/mL), E. faecalis (MIC90 0.25 μg/mL), E. faecium (MIC90 0.12 μg/mL), S. pneumoniae (MIC90 0.12 μg/mL), and β-hemolytic streptococci (MIC90 0.25 μg/mL; Table 1) [13].
Table 1.
In Vitro Activity of Omadacycline and Comparators Against Select Gram-positive Aerobes
| Omadacycline | Tetracycline | Tigecycline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria (No. of Isolates) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSIe | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSI | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSI |
| S. aureus (4215) | 0.12 | 0.25 | ≤0.015 to 8 | 98.6 | ≤0.5 | ≤0.5 | ≤0.5 to 8 | 94.2 | 0.06 | 0.12 | ≤0.015 to 1 | >99.9 |
| S. aureus MSSA (2777) | 0.12 | 0.25 | ≤0.015 to 1 | 99.9 | ≤0.5 | ≤0.5 | ≤0.5 to 8 | 96.3 | 0.06 | 0.12 | ≤0.015 to 0.25 | 100.0 |
| S. aureus MRSA (1438) | 0.12 | 0.25 | 0.03 to 8 | 96.1 | ≤0.5 | 4 | ≤0.5 to 8 | 90.3 | 0.06 | 0.12 | ≤0.015 to 1 | 99.9 |
| S. aureus TR (221)a | 0.12 | 0.5 | 0.03 to 2 | 95.5 | − | − | − | − | 0.12 | 0.25 | 0.03 to 1 | 99.5 |
| E. faecalis (677) | 0.12 | 0.25 | ≤0.015 to 1 | 97.2 | >16 | >16 | ≤0.12 to 16 | 21.4 | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 |
| E. faecalis VS (663) | 0.12 | 0.25 | ≤0.015 to 1 | 97.1 | >16 | >16 | ≤0.12 to 16 | 21.7 | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 |
| E. faecalis VNS (14) | 0.12 | 0.25 | ≤0.015 to 0.25 | 100.0 | >16 | >16 | 0.25 to 16 | 7.7 | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 |
| E. faecalis TR (524)a | 0.12 | 0.25 | ≤0.015 to 1 | 96.4 | − | − | − | − | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 |
| E. faecium (390) | 0.06 | 0.12 | ≤0.015 to 8 | NA | 16 | >16 | ≤0.12 to 16 | 42.6 | 0.03 | 0.06 | ≤0.015 to 1 | NA |
| E. faecium VS (234) | 0.06 | 0.12 | ≤0.015 to 1 | NA | 0.5 | >16 | ≤0.12 to 16 | 58.1 | 0.03 | 0.06 | ≤0.015 to 0.25 | NA |
| E. faecium VNS (156) | 0.06 | 0.12 | ≤0.015 to 8 | NA | >16 | >16 | ≤0.12 to 16 | 19.2 | 0.03 | 0.06 | ≤0.015 to 1 | NA |
| E. faecium TR (217)a | 0.12 | 0.12 | ≤0.015 to 8 | NA | − | − | − | − | 0.06 | 0.06 | ≤0.015 to 1 | NA |
| S. pneumoniae (1314) | 0.06 | 0.12 | ≤0.015 to 1 | 99.7 | ≤0.25 | >8 | ≤0.25 to 8 | 79.5 | 0.03 | 0.06 | 0.015 to 0.25 | 99.4 |
| S. pneumoniae PS (899) | 0.06 | 0.06 | ≤0.015 to 0.5 | 99.9 | ≤0.25 | 0.5 | ≤0.25 to 8 | 92.2 | 0.03 | 0.06 | 0.015 to 0.12 | 99.4 |
| S. pneumoniae PI (263) | 0.06 | 0.12 | ≤0.015 to 1 | 98.9 | 0.5 | >8 | ≤0.25 to 8 | 59.7 | 0.03 | 0.06 | 0.015 to 0.25 | 98.9 |
| S. pneumoniae PR (152) | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 | >8 | >8 | ≤0.25 to 8 | 38.8 | 0.06 | 0.06 | 0.015 to 0.06 | 100.0 |
| S. pneumoniae MRb (413) | 0.06 | 0.12 | ≤0.015 to 1 | 99.5 | >8 | >8 | ≤0.25 to 8 | 44.1 | 0.06 | 0.06 | 0.015 to 0.25 | 99.5 |
| S. pneumoniae TR (263)a | 0.06 | 0.12 | ≤0.015 to 1 | 99.2 | − | − | − | − | 0.06 | 0.06 | 0.015 to 0.25 | 99.2 |
| S. anginosus group (107) | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 | 0.5 | >8 | ≤0.25 to 8 | 67.3 | 0.03 | 0.03 | ≤0.008 to 0.12 | 100.0 |
| S. anginosus group TR (34)a | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 | − | − | − | − | 0.06 | 0.12 | ≤0.015 to 0.12 | 100.0 |
| β-hemolytic streptococcic (966) | 0.06 | 0.12 | 0.03 to 0.5 | NA | 0.5 | >8 | ≤0.25 to 8 | 54.7 | 0.06 | 0.06 | 0.015 to 0.25 | 100.0 |
| β-hemolytic streptococci TR (421)a | 0.12 | 0.25 | 0.03 to 0.5 | NA | − | − | − | − | 0.06 | 0.06 | 0.015 to 0.25 | 100.0 |
| β-hemolytic streptococci MRd (266) | 0.12 | 0.25 | 0.03 to 0.5 | NA | >8 | >8 | ≤0.25 to 8 | 25.6 | 0.06 | 0.06 | 0.015 to 0.25 | 100.0 |
Adapted from Pfaller et al [13]; isolates were collected in the United States and Europe for the 2016 SENTRY Antimicrobial Surveillance Program.
Abbreviations: % S, percentage susceptible; CLSI, Clinical and Laboratory Standards Institute; E, Enterococcus; MIC, minimum inhibitory concentration; MR, macrolide-resistant; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; NA, data not available; PI, penicillin-intermediate; PR, penicillin-resistant; PS, penicillin-susceptible; S., Staphylococcus; TR, tetracycline-resistant; VNS, vancomycin-nonsusceptible; VS, vancomycin-susceptible.
aIsolates were defined as tetracycline-resistant by CLSI (M100-S27, 2017) breakpoint interpretive criteria; MIC50 and MIC90 data for tetracycline vs these isolates were not calculated.
bIncludes erythromycin- and azithromycin-resistant Streptococcus pneumoniae.
cIncludes Streptococcus agalactiae, Streptococcus canis, Streptococcus dysgalactiae, and Streptococcus pyogenes.
dErythromycin-resistant β-hemolytic streptococci.
eBased on Food and Drug Administration breakpoints (see [18]); the ABSSSI breakpoint was used for Staphylococcus aureus.
Gram-negative Aerobes
Omadacycline has in vitro activity against species of Enterobacteriaceae, including Escherichia coli (MIC90 2 μg/mL), Klebsiella pneumoniae (MIC90 8 μg/mL), Klebsiella oxytoca (MIC90 2 μg/mL), Citrobacter spp. (MIC90 4 μg/mL), and Enterobacter cloacae (MIC90 4 μg/mL; Table 2; breakpoints available from the FDA) [13, 18]. Omadacycline is also active against Haemophilus influenzae (MIC90 1 μg/mL; breakpoints available from the FDA) and Moraxella catarrhalis (MIC90 0.25 μg/mL; Table 2) [13, 18]. Omadacycline is not a substrate for extended-spectrum β-lactamases (ESBLs). It displays the same MIC90 values against E. coli (2 μg/mL) and K. pneumoniae (8 μg/mL), whether ESBL negative or positive [20].
Table 2.
In Vitro Activity of Omadacycline and Comparators Against Select Gram-negative Aerobes
| Omadacycline | Tetracycline | Tigecycline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria (No. of Isolates) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSIb | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSI | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | % S by CLSI |
| Enterobacteriaceae (8345) | 1 | 8 | 0.12 to 32 | NA | 2 | >16 | ≤0.25 to 16 | 64.2 | 0.25 | 1 | ≤0.06 to 8 | 97.8 |
| Enterobacteriaceae CNS (1439) | 2 | 8 | 0.12 to 32 | NA | 16 | >16 | 0.5 to 16 | 44.5 | 0.25 | 1 | ≤0.06 to 8 | 98.2 |
| Enterobacteriaceae TR (2737)a | 2 | 16 | 0.12 to 32 | NA | − | − | − | − | 0.5 | 2 | ≤0.06 to 8 | 93.4 |
| E. coli (3541) | 0.5 | 2 | 0.12 to 32 | NA | 2 | >16 | ≤0.25 to 16 | 63.7 | 0.12 | 0.25 | ≤0.06 to 4 | 99.9 |
| E. coli CS (3030) | 0.5 | 2 | 0.12 to 32 | NA | 2 | >16 | ≤0.25 to 16 | 69.1 | 0.12 | 0.25 | ≤0.06 to 2 | 100.0 |
| E. coli CNS (511) | 1 | 2 | 0.12 to 32 | NA | >16 | >16 | 0.5 to 16 | 31.7 | 0.12 | 0.25 | ≤0.06 to 4 | 99.6 |
| E. coli TR (1272)a | 1 | 4 | 0.12 to 32 | NA | − | − | − | − | 0.12 | 0.25 | ≤0.06 to 4 | 99.8 |
| K. pneumoniae (1771) | 2 | 8 | 0.25 to 32 | 89.7 | 2 | >16 | ≤0.25 to 16 | 71.6 | 0.25 | 1 | ≤0.06 to 4 | 98.8 |
| K. pneumoniae CS (1264) | 1 | 4 | 0.25 to 32 | 94.7 | 1 | >16 | ≤0.25 to 16 | 84.1 | 0.25 | 0.5 | ≤0.06 to 4 | 99.3 |
| K. pneumoniae CNS (507) | 2 | 8 | 0.25 to 32 | 77.1 | 16 | >16 | 0.5 to 16 | 40.4 | 0.5 | 2 | ≤0.06 to 4 | 97.4 |
| K. pneumoniae TR (430)a | 4 | 16 | 0.5 to 32 | 67.7 | − | − | − | − | 0.5 | 2 | ≤0.06 to 4 | 94.9 |
| K. oxytoca (423) | 1 | 2 | 0.25 to 32 | NA | 1 | 8 | ≤0.25 to 16 | 89.8 | 0.25 | 0.5 | ≤0.06 to 2 | 100.0 |
| K. oxytoca TR (30)a | 2 | 16 | 0.25 to 32 | NA | − | − | − | − | 0.5 | 1 | ≤0.06 to 2 | 100.0 |
| E. cloacae spp. complex (752) | 2 | 4 | 0.25 to 32 | 93.6 | 2 | 16 | 0.5 to 16 | 85.6 | 0.25 | 0.5 | 0.12 to 4 | 99.2 |
| E. cloacae spp. complex CS (542) | 2 | 4 | 0.5 to 32 | 95.0 | 2 | 4 | 0.5 to 16 | 92.4 | 0.25 | 0.5 | 0.12 to 4 | 99.3 |
| E. cloacae spp. complex CNS (210) | 2 | 4 | 0.25 to 32 | 90.0 | 2 | >16 | 1 to 16 | 68.1 | 0.5 | 1 | 0.12 to 4 | 99.0 |
| E. cloacae spp. complex TR (87)a | 4 | 16 | 1 to 32 | 69.0 | − | − | − | − | 0.5 | 2 | 0.12 to 4 | 95.4 |
| Other Enterobacter spp. (250) | 1 | 4 | 0.5 to 16 | NA | 1 | 4 | 0.5 to 16 | 91.2 | 0.25 | 0.5 | 0.12 to 4 | 99.6 |
| Other Enterobacter spp. TR (17)a | 16 | 16 | 2 to 16 | NA | − | − | − | − | 2 | 2 | 0.5 to 4 | 94.1 |
| Citrobacter spp. (354) | 1 | 4 | 0.25 to 16 | NA | 1 | 4 | 0.5 to 16 | 91.0 | 0.25 | 0.5 | 0.12 to 2 | 100.0 |
| Citrobacter spp. TR (23)a | 4 | 8 | 0.5 to 8 | NA | − | − | − | − | 0.5 | 1 | 0.12 to 2 | 100.0 |
| P. mirabilis (463) | 16 | >32 | 2 to 32 | NA | >16 | >16 | 1 to 16 | 1.1 | 2 | 4 | 0.25 to 8 | 69.8 |
| P. mirabilis TR (458)a | 16 | >32 | 2 to 32 | NA | − | − | − | − | 2 | 4 | 0.25 to 8 | 69.4 |
| Proteus spp. IP (317) | 8 | 32 | 0.5 to 32 | NA | 16 | >16 | 0.5 to 16 | 38.2 | 1 | 2 | 0.12 to 8 | 97.2 |
| Proteus spp. IP, TR (169)a | 8 | 32 | 0.5 to 32 | NA | − | − | − | − | 1 | 2 | 0.12 to 8 | 95.3 |
| H. influenzae (803) | 1 | 1 | 0.12 to 16 | 99.4 | 0.5 | 1 | ≤0.06 to 8 | 99.8 | 0.12 | 0.25 | 0.06 to 1 | 96.1 |
| H. influenzae BLP (201) | 1 | 1 | 0.25 to 4 | 99.0 | 0.5 | 0.5 | 0.25 to 1 | 100.0 | 0.12 | 0.25 | 0.06 to 1 | 93.5 |
| H. influenzae BLN (602) | 1 | 1 | 0.12 to 16 | 99.5 | 0.5 | 1 | ≤0.06 to 8 | 99.7 | 0.12 | 0.25 | 0.06 to 1 | 97.0 |
| M. catarrhalis (408) | 0.25 | 0.25 | 0.06 to 0.5 | NA | 0.25 | 0.5 | 0.12 to 0.5 | 100.0 | 0.06 | 0.06 | ≤0.015 to 0.12 | NA |
Adapted from Pfaller et al [13]; isolates were collected in the United States and Europe for the 2016 SENTRY Antimicrobial Surveillance Program.
Abbreviations: % S, percentage susceptible; BLN, β-lactamase-negative; BLP, β-lactamase-positive; CLSI, Clinical and Laboratory Standards Institute; CNS, ceftazidime-nonsusceptible; CS, ceftazidime-susceptible; E. cloacae, Enterobacter cloacae; E. coli, Escherichia coli; H., Haemophilus; IP, indole-positive; K., Klebsiella; M., Moraxella; MIC, minimum inhibitory concentration; NA, data not available; P., Proteus; TR, tetracycline-resistant.
aIsolates were defined as tetracycline-resistant by CLSI (M100-S27, 2017) breakpoint interpretive criteria; MIC50 and MIC90 data for tetracycline vs these isolates were not calculated.
bBased on Food and Drug Administration breakpoints (see [18]).
Atypical Bacteria and Anaerobes
Omadacycline displays potent in vitro activity against atypical bacteria, with an MIC90 of 2 μg/mL for Mycobacterium abscessus; 0.5 μg/mL for Mycobacterium fortuitum; 0.25 μg/mL for Mycobacterium chelonae, Mycoplasma pneumoniae, Chlamydia pneumoniae, and L. pneumophila; and 0.06 μg/mL for Mycoplasma hominis (Table 3) [17, 21–23]. In addition, omadacycline has in vitro activity against anaerobic pathogens, including Bacteroides fragilis (MIC90 4 μg/mL), Bacteroides thetaiotaomicron (MIC90 4 μg/mL), Bacteroides vulgatus (MIC90 1 μg/mL), Bacteroides ovatus (MIC90 8 μg/mL), C. difficile (MIC90 0.5 μg/mL), Clostridium perfringens (MIC90 16 μg/mL), and anaerobic gram-positive cocci (MIC90 1 μg/mL; Table 4) [24].
Table 3.
In Vitro Activity of Omadacycline and Doxycycline Against Atypical Bacteria
| Omadacycline | Doxycycline | |||||
|---|---|---|---|---|---|---|
| Bacteria (No. of Isolates) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) |
| Mycoplasma hominis (20) | 0.03 | 0.06 | 0.016 to 0.12 | 0.06 | 2 | 0.016 to 2 |
| Mycoplasma pneumoniae (20) | 0.12 | 0.25 | 0.12 to 0.25 | 0.25 | 0.5 | 0.12 to 0.5 |
| Ureaplasma spp.a (20) | 1 | 2 | 0.25 to 2 | 0.25 | 4 | 0.06 to 4 |
| Legionella pneumophila (100) | 0.25 | 0.25 | 0.06 to 1 | 1 | 1 | 0.5 to 1 |
| Chlamydia pneumoniae (15) | 0.06 | 0.25 | 0.03 to 0.5 | 0.125 | 0.125 | 0.06 to 0.25 |
| Mycobacterium abscessus (24) | 1 | 2 | 0.06 to 8 | >64 | >64 | 0.25 to 64 |
| Mycobacterium chelonae (22) | 0.125 | 0.25 | 0.015 to 0.25 | 32 | 64 | 16 to 64 |
| Mycobacterium fortuitum (20) | 0.125 | 0.50 | 0.03 to 1 | 8 | 64 | <0.06 to 64 |
Table 4.
In Vitro Activity of Omadacycline and Comparators Against Anaerobes
| Omadacycline | Tigecycline | |||||
|---|---|---|---|---|---|---|
| Bacteria (No. of Isolates) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | MIC Range (μg/mL) |
| Bacteroides fragilis (21) | 0.5 | 4 | 0.25 to 16 | 0.5 | 2 | 0.5 to 8 |
| Bacteroides thetaiotaomicron (21) | 1 | 4 | 0.12 to 16 | 1 | 8 | 0.25 to 16 |
| Bacteroides vulgatus (21) | 0.12 | 1 | 0.06 to 2 | 0.25 | 1 | 0.12 to 2 |
| Bacteroides ovatus (15) | 0.5 | 8 | 0.06 to 16 | 0.5 | 8 | 0.03 to 16 |
| Clostridioides difficile (21) | 0.25 | 0.5 | 0.25 to 8 | 0.25 | 0.25 | 0.25 to 4 |
| Clostridium perfringens (22) | 4 | 16 | 0.12 to 16 | 8 | >16 | 0.25 to 16 |
| Peptostreptococcus spp.a (22) | 0.12 | 1 | 0.06 to 2 | 0.12 | 2 | 0.06 to 4 |
FACTORS AFFECTING IN VITRO ACTIVITY
The in vitro activity of omadacycline against gram-positive and gram-negative clinical isolates was examined in the presence of either bovine lung surfactant or human or mouse serum [25]. As expected, MIC values of omadacycline against tetracycline-susceptible and tetracycline-resistant strains of S. aureus, S. pneumoniae, H. influenzae, and E. coli did not increase in the presence of surfactant or serum. In these assays, the addition of serum increased the MIC values of doxycycline, and the addition of surfactant increased the MICs of daptomycin. Studies examining the in vitro protein binding of omadacycline have shown that, over a concentration range of 10–10 000 ng/mL, omadacycline displays low binding to human (21%), monkey (21%), rat (26%), and mouse (15%) plasma proteins [26].
The pH is known to affect the activity of a range of antibiotics, because of its effect on the charge of the molecules [27]. Compared with standard pH 7.4 medium, omadacycline MIC values were unaffected by pH 8.0 medium, whereas MIC values were several-fold higher when tested at pH 5.0–6.0 [28]. In addition, in unpublished observations, omadacycline retained activity in pooled human urine (pH 6.6) and in pooled human urine adjusted to pH 7.1 (Paratek Pharmaceuticals, Inc.; data on file). In urine, omadacycline MIC values against E. coli and Staphylococcus saprophyticus were either unaffected or up to 2-fold higher than MICs observed in standard pH 7.3 broth medium. Overall, these assays demonstrate that omadacycline retains activity in human urine.
Intracellular activity was evaluated for omadacycline against both S. aureus and L. pneumophila. In assays of intracellular activity, S. aureus–infected THP-1 human monocytes were treated with 1, 2, 8, or 16 times the MIC of either omadacycline or comparators (tigecycline, linezolid, ceftaroline, levofloxacin, moxifloxacin, or azithromycin) [29]. At 24 hours, omadacycline exhibited bactericidal activity against intracellular S. aureus (methicillin-susceptible and methicillin-resistant strains) with ≥99% growth reduction at 2–16 times the MIC, which was similar to that of levofloxacin and moxifloxacin and was more active than that of tigecycline (≥99% vs <99 to ≥90%), linezolid (≥99% vs <99 to ≥90%), ceftaroline (≥99% vs <90%), and azithromycin (≥99% vs <90%). Similar studies carried out with U937 human monocytes infected with erythromycin-susceptible or erythromycin-resistant strains of L. pneumophila serogroup 1 also demonstrated the robust intracellular activity of omadacycline [30].
These studies demonstrate that omadacycline possesses low binding to plasma proteins and that its activity is not significantly affected by surfactant, serum, high pH, or urine. The human monocyte models demonstrate the intracellular penetration of active omadacycline, which has clinical implications for the treatment of pneumonia.
IN VITRO PHARMACODYNAMIC PROPERTIES
The in vitro pharmacodynamic properties of omadacycline have been studied in a variety of ways. Omadacycline displays bactericidal or bacteriostatic activity that is organism-dependent. In a study examining 85 bacterial isolates, minimum bactericidal concentrations indicated that omadacycline has bactericidal activity (≥3 log reduction of initial inoculum) against S. pneumoniae, H. influenzae, and M. catarrhalis, and that it displays bacteriostatic activity against enterococci, S. aureus, and most isolates of E. coli [31].
In order to determine the residual activity of omadacycline after antibiotic removal, the postantibiotic effect (PAE) was evaluated. The growth inhibition of omadacycline following drug removal has been examined for clinical isolates of S. aureus (including 1 MRSA isolate), S. pneumoniae (including 1 penicillin-resistant isolate), enterococci (including 1 vancomycin-resistant isolate), and E. coli [32]. For these gram-positive and gram-negative aerobes, omadacycline had a PAE ranging from 1.4 to 3.3 hours (1 hour initial exposure at 5 times the MIC), which was similar to that of tigecycline; with the exception of enterococci, for which tigecycline displayed longer PAEs [32]. The activity of omadacycline against E. coli biofilms was determined with the minimum biofilm eradication concentration assay (Innovotech, Edmonton, Alberta, Canada) [33]. Omadacycline displayed dose-dependent activity against established biofilms, with a reduction of ~2 log10 units in biofilm-associated bacteria. At concentrations near the MIC (1.13 μg/mL), omadacycline significantly reduced the total E. coli bioburden. In addition, E. coli biofilms were not propagated in the presence of sub-MIC concentrations of omadacycline. This finding may have clinical implications, as biofilm colonies are known to be resistant to sub-MIC doses of most antibiotics [34].
The effect of omadacycline on the gut microflora and the potential for omadacycline to induce C. difficile infections (CDI) have been investigated in an in vitro human gut model [35]. In this model, the bacterial compositions of the proximal, medial, and distal colon are simulated with a 3-stage continuous system of temperature- and pH-controlled vessels [36]. Antimicrobials that are considered high risk for CDI have been shown to induce simulated CDI in this in vitro human gut model, whereas antimicrobials that are considered low risk for CDI have not induced simulated CDI in this model [36–39]. The 3-vessel system was equilibrated following inoculation with pooled feces from healthy volunteers, and then infected with C. difficile spores, followed by omadacycline treatment [35]. Although omadacycline disrupted the gut microflora (declines in Bifidobacteria, B. fragilis group spp., Lactobacillus spp., Enterococcus spp., Clostridium spp., and Enterobacteriaceae populations), there was no evidence of simulated CDI (ie, C. difficile germination, vegetative cell proliferation, or toxin production). In contrast, moxifloxacin disrupted the gut microflora and induced simulated CDI in this study [35], which supports the clinical finding that fluoroquinolones have one of the highest incidences of CDI [40].
IN VIVO PHARMACODYNAMIC PROPERTIES
The in vivo pharmacodynamics of omadacycline have been examined in 2 animal models: a neutropenic mouse model of pneumonia and a neutropenic mouse thigh infection model [41, 42] (Table 5). The primary pharmacokinetic/pharmacodynamic target predictive of efficacy for omadacycline was noted to be the ratio of the area under the plasma concentration–time curve over 24 hours to the MIC (AUC/MIC). Omadacycline AUC/MIC correlated with microbiological efficacy (r2 = 0.74; mean plasma 24-hour static dose AUC/MIC = 16–20) in mice infected in the lungs with S. pneumoniae, including those with strains with varying susceptibility to tetracyclines, β-lactams, and macrolides [41]. Based on omadacycline concentration measurements in plasma and epithelial lining fluid, omadacycline penetrated well into epithelial lining fluid [41]. In the mouse thigh infection model (which included 10 isolates of S. aureus, including MRSA), the efficacy of omadacycline was defined by the AUC/MIC (r2 = 0.92; mean plasma 24-hour static dose AUC/MIC = 24) [42]. These findings are in agreement with those for tetracyclines, as the AUC/MIC ratio is the pharmacodynamic parameter that best correlates with treatment efficacy for this class [43].
Table 5.
Efficacy of Omadacycline in Animal Models
| Study | Animal Model | Strain | Antimicrobial | Result | |
|---|---|---|---|---|---|
| Lepak et al [41] | Neutropenic mouse pneumonia model (female ICR/Swiss mice, 3 mice/group) |
Streptococcus pneumoniae
a
1293 ATCC 10813 |
SC administrationb | Plasma AUC/MIC | |
| Omadacycline | Emax, 4.85 ED50, 15.60 r2, 0.74 |
||||
| 140 ATCC 49619 |
Omadacycline | Epithelial lining fluid AUC/MIC Emax, 4.91 ED50, 15.11 r2, 0.75 |
|||
| Lepak et al [42] | Mouse neutropenic thigh infection model (4 thigh infections/group) |
SC administrationc Omadacycline |
|||
| Staphylococcus aureus MSSA | Thigh bacterial burden | ||||
| Reduction of 4–5 log10 CFU/thigh compared with untreated controls | |||||
| S. aureus MRSA | Omadacycline | Plasma AUC/MIC | |||
| Emax, 4.62 | |||||
| ED50, 21.7 | |||||
| r 2, 0.92 | |||||
| Macone et al [8] | Mouse IP infection model (male CD-1 mice, 5 mice/group) |
IV administrationd | MIC (μg/mL) | ED50e (mg/kg [95% CI]) |
|
| S. pneumoniae PBS1339 | Omadacycline Tigecycline |
0.125 0.125 |
3.34 ± 1.56 4.13 (2.46 to 5.79) |
||
| S. pneumoniae 700905 | Omadacycline Tigecycline |
≤0.06 0.125 |
0.45 (0.32 to 0.58) 1.72 (0.6 to 2.82) |
||
| S. aureus 29213 | Omadacycline Tigecycline |
0.25 0.125 |
1.74 (0.91 to 2.58) 0.73 (0.69 to 0.76) |
||
| S. aureus USA300 | Omadacycline Tigecycline |
0.25 0.125 |
0.90 (0.33 to 1.46) 0.58 (0.40 to 0.75) |
||
| S. aureus MRSA5 | Omadacycline Tigecycline |
0.25 ≤0.06 |
0.30 (0.295 to 0.305) 1.74 (0.91 to 2.57) |
||
| Escherichia coli PBS1478 | Omadacycline Tigecycline |
1 ≤0.06 |
2.02 (1.09 to 2.96) 1.75 (1.12 to 2.38) |
||
| Endermann et al [44] | Mouse postoperative polymicrobial peritonitis model (female CFW-1 mice, 10 mice/group) | Enterococcus faecalis TR | IV administrationf | 10-day survival | |
| Omadacycline | 80% | ||||
| Enterococcus faecium VR | Imipenem | 70% | |||
| Linezolid | 30% | ||||
| McKenney et al [45] | Mouse urinary tract infection model (male CD-1 mice) | E. coli C189P4 | IV administrationg | MIC (μg/mL) | Kidney bacterial burden |
| (ED50, mg/kg) | |||||
| Omadacycline | 0.5 | 4.3 | |||
| Minocycline | 0.5 | 4.5 <1.0 |
|||
| Ciprofloxacin | ≤0.06 | ||||
| Steenbergen et al [16] | Mouse whole-body aerosol infection model, delayed treatment exposure (female BALB/c mice, 9 or 10 mice/group) | Bacillus anthracis Ames | IP administrationh | 40-day survival | |
| Omadacycline | 60% | ||||
| Doxycycline | 70% | ||||
| Ciprofloxacin | 80% | ||||
| Vehicle | 0% | ||||
| Mouse whole-body aerosol infection model, postexposure prophylaxis (female BALB/c mice, 10 mice/group) | Yersinia pestis CO92 | IP administrationi | 40-day survival | ||
| Omadacycline | 90% (40 mg/kg dose group) | ||||
| Doxycycline | 90% (40 mg/kg dose group) | ||||
| Ciprofloxacin | 100% | ||||
| Vehicle | 0% | ||||
| Kim et al [46] | Hamster C. difficile infection model (male LGV Golden Syrian hamsters, 10 hamsters/group) | Clostridioides difficile ATCC 43596 | Oral administrationj | Median survival (days) | |
| Omadacycline | 12 (P = .0004) | ||||
| Vancomycin | 2 (P = .0293) |
Abbreviations: AUC, area under the plasma concentration–time curve; CFU, colony-forming units; CI, confidence interval; ED50, 50% effective dose; Emax, maximum effect; IP, intraperitoneal; IV, intravenous; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; SC, subcutaneous; TR, tetracycline-resistant; VR, vancomycin-resistant.
aThere were 4 strains examined, with varying susceptibility to tetracyclines, β-lactams, and macrolides.
bThere were 4-fold increasing doses examined, from 0.1 to 25.6 mg/kg.
cDoses increased 4-fold every 12 hours, from 0.25 to 64 mg/kg.
dAt least 4 dose levels per experiment, with doses typically ranging from 0.11 to 18 mg/kg (dose minimum–maximum, 0.08–54 mg/kg); only tigecycline comparator is shown.
eData are represented as means ± standard deviations from 7 independent experiments.
fTwo doses of 10 mg/kg administered at 4 hours and 18 hours postsurgery.
gIncreasing single doses on day 4 postinfection.
hOmadacycline: 15 mg/kg; doxycycline: 15 mg/kg; ciprofloxacin: 30 mg/kg; vehicle: 0.2 mL saline. All treatments began 48 ± 1 hours postinfection, and were given twice daily for 14 days.
iOmadacycline: 5, 10, 20, or 40 mg/kg; doxycycline: 5, 10, 20, or 40 mg/kg; ciprofloxacin: 15 mg/kg; vehicle: 0.2 mL saline. All treatments began 24 ± 1 hours postinfection, and were given twice daily for 7 days.
jGiven for 5 days at 50 mg/kg/day.
IN VIVO EFFICACY
The in vivo efficacy of omadacycline has been demonstrated in a number of animal models (Table 5). Omadacycline, administered as a single intravenous dose, displayed potent efficacy in a mouse model of intraperitoneal infection with E. coli or tetracycline-susceptible and tetracycline-resistant strains of S. aureus and S. pneumoniae [8] (Table 5). Overall in this model, the efficacy of omadacycline was similar to, or greater than, that of comparators. In a mouse intra-abdominal infection model of postoperative polymicrobial peritonitis, 2 intravenous doses of omadacycline showed increased 10-day survival over comparators [44] (Table 5). In a urinary tract infection model, mice were infected with E. coli directly into the bladder and then given increasing single doses of omadacycline on day 4 postinfection. Omadacycline performed as well as minocycline (50% effective dose, 4.3 vs 4.5 mg/kg) in this model [45] (Table 5). Against the biothreat pathogens—B. anthracis and Y. pestis—omadacycline displayed in vivo efficacy in murine whole-body aerosol infection models [16] (Table 5).
The in vivo efficacy of omadacycline has also been examined in a hamster model of CDI [46] (Table 5). In this model, CDI was induced in hamsters by the subcutaneous administration of clindamycin 24 hours before infection with C. difficile by oral gavage. At 24 hours postinfection, hamsters were treated with oral omadacycline for 5 days. Omadacycline demonstrated efficacy in this model, with overall median survival of 12 days in omadacycline-treated hamsters compared with 2 days in vancomycin-treated hamsters.
In these in vivo models, omadacycline demonstrated efficacy greater than, or similar to, comparator antimicrobials.
CONCLUSIONS
Unlike older tetracyclines, omadacycline is active against bacterial isolates that express tetracycline-specific efflux pumps and/or ribosomal protection resistance mechanisms. The in vitro antimicrobial activity of omadacycline covers a wide range of gram-positive and many gram-negative pathogens, including MRSA, penicillin- and macrolide-resistant S. pneumoniae, β-hemolytic streptococci, VRE, and Enterobacteriaceae, such as E. coli and Klebsiella spp. In addition, omadacycline displays in vitro antimicrobial activity against atypical and anaerobic organisms, including Mycoplasma spp., L. pneumophila, Ureaplasma spp., and C. difficile.
Omadacycline remains active at pH 8.0 and in the presence of serum, lung surfactant, or urine. In addition, omadacycline is active against bacterial biofilms and does not propagate biofilm formation. It has low binding to plasma proteins, displays a PAE of 1.4–3.3 hours (at 5 times the MIC, 1-hour exposure), and demonstrates intracellular activity against S. aureus and L. pneumophila.
In vivo pharmacodynamic studies have shown that, similar to other tetracyclines, omadacycline AUC/MIC has the strongest correlation with bacteriological outcome. The efficacy of omadacycline has been validated in a human gut model of C. difficile infection (in vitro) and in animal models (in vivo), including models of pneumonia, thigh infection, systemic infection, intra-abdominal infection, urinary tract infection, and CDI. Taken together, these studies demonstrate the activity of omadacycline against bacterial pathogens commonly associated with serious, community-acquired bacterial infections, including infections of the skin, lungs, and urinary tract.
Notes
Author contributions. All authors provided critical revisions to the manuscript for intellectual content and approved the final version for publication.
Acknowledgments. Editorial support was provided by Theresa E. Singleton, PhD, and Samantha Scott, PhD, of Innovative Strategic Communications, LLC, Milford, Pennsylvania.
Financial support. This work was supported by Paratek Pharmaceuticals, Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Supplement sponsorship. This article appears as part of the supplement “Omadacycline: A New Option in an Era of Increasing Antibiotic Resistance,” sponsored by Paratek Pharmaceuticals, Inc., King of Prussia, Pennsylvania.
Potential conflicts of interest. G. G. Z. received a research grant from Paratek Pharmaceuticals, outside the submitted work. J. S. is an employee and stockholder of Paratek Pharmaceuticals. J. A. K. reports no potential conflicts of interest. The authors received no financial compensation for the preparation of this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. NUZYRA (omadacycline) prescribing information Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209816_209817lbl.pdf. Accessed 3 May 2019.
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 3 May 2019.
- 3. O’Riordan W, Green S, Overcash JS, et al. . Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 2019; 380:528–38. [DOI] [PubMed] [Google Scholar]
- 4. O’Riordan W, Cardenas C, Sirbu A, et al. . A phase 3 randomized, double-blind, multi-centre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study). Abstract O0425 at 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Madrid, Spain). 2018. [Google Scholar]
- 5. Stets R, Popescu M, Gonong JR, et al. . Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 2019; 380:517–27. [DOI] [PubMed] [Google Scholar]
- 6. Heidrich CG, Mitova S, Schedlbauer A, et al. . The novel aminomethylcycline omadacycline has high specificity for the primary tetracycline-binding site on the bacterial ribosome. Antibiotics 2016; 5: E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Draper MP, Weir S, Macone A, et al. . Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 2014; 58:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macone AB, Caruso BK, Leahy RG, et al. . In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother 2014; 58:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruzin A, Dzink-Fox J, Jones AK, Dean CR, Bradford PA. Studies on the mechanism of resistance to PTK796 in Pseudomonas aeruginosa and Klebsiella pneumoniae. Abstract CI-1413 at 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (Boston, MA). 2010. [Google Scholar]
- 10. Leski TA, Bangura U, Jimmy DH, et al. . Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int J Antimicrob Agents 2013; 42:83–6. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh S, LaPara TM, Sadowsky MJ. Transformation of tetracycline by TetX and its subsequent degradation in a heterologous host. FEMS Microbiol Ecol 2015; 91. doi: 10.1093/femsec/fiv059. [DOI] [PubMed] [Google Scholar]
- 12. Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program (2014). Diagn Microbiol Infect Dis 2018; 90:143–7. [DOI] [PubMed] [Google Scholar]
- 13. Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: report from the SENTRY Antimicrobial Surveillance Program, 2016. Antimicrob Agents Chemother 2018;62: e02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother 2017; 61:e02411–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubois J, Dubois M, Martel J, Tanaka S. In vitro activity of omadacycline against Legionella pneumophila. Abstract F770 at 55th Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC) (San Diego, CA). 2015. [Google Scholar]
- 16. Steenbergen J, Tanaka SK, Miller LL, Halasohoris SA, Hershfield JR. In vitro and in vivo activity of omadacycline against two biothreat pathogens, Bacillus anthracis and Yersinia pestis. Antimicrob Agents Chemother 2017; 61:e02434-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villano S, Steenbergen J, Loh E. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol 2016; 11:1421–34. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. Omadacycline injection and oral products Available at: https://www.fda.gov/drugs/development-resources/omadacycline-injection-and-oral-products. Accessed 3 May 2019.
- 19. Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 2016; 24:6409–19. [DOI] [PubMed] [Google Scholar]
- 20. Huband MD, Rhomberg PR, Sader HS, Schuchert JE, Flamm RK. In vitro activity of omadacycline and comparators against gram-negative bacterial isolates collected from patients in European medical centres (2016): Results from the SENTRY antimicrobial surveillance program. Abstract P1253 at 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Vienna, Austria). 2017. [Google Scholar]
- 21. Waites KB, Crabb DM, Liu Y, Duffy LB. In vitro activities of omadacycline (PTK 0796) and other antimicrobial agents against human Mycoplasmas and Ureaplasmas. Antimicrob Agents Chemother 2016; 60:7502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohlhoff SA, Huerta N, Hammerschlag MR. In vitro activity of omadacycline against Chlamydia pneumoniae. Antimicrob Agents Chemother 2019; 63:e01907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shoen C, Benaroch D, Sklaney M, Cynamon M. In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob Agents Chemother 2019; 63:e02522-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stapert L, Wolfe C, Shinabarger D, Marra A, Pillar C. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicrob Agents Chemother 2018; 62:e00047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macone AB, Donatelli J, Draper MP, Tanaka SK. In vitro activity of omadacycline (PTK796) in broth, broth plus lung surfactant or human serum. Abstract P1141 at 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Milan, Italy). 2011. [Google Scholar]
- 26. Villano S, Tzanis E, Tanaka SK. In vitro protein binding with omadacycline, a first in class aminomethylcycline antibiotic. Abstract 518 at American Society of Microbiology (ASM)Microbe; (Boston, MA: ). 2016. [Google Scholar]
- 27. Loftin KA, Adams CD, Meyer MT, Surampalli R. Effects of ionic strength, temperature, and pH on degradation of selected antibiotics. J Environ Qual 2008; 37:378–86. [DOI] [PubMed] [Google Scholar]
- 28. Thwaites M, Shinabarger D, Pillar C. The impact of non-standard test conditions on the in vitro activity of omadacycline by broth microdilution. Abstract P1263 at 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Vienna, Austria). 2017. [Google Scholar]
- 29. Dubois J, Dubois M, Martel J, Steenbergen J. In vitro extracellular and intracellular activity of omadacycline against Staphylococcus aureus. Abstract P1254 at 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Vienna, Austria). 2017. [Google Scholar]
- 30. Dubois J, Dubois M, Martel J, Tanaka S. In vitro intracellular activity of omadacycline against Legionella pneumophila. Abstract 551 at American Society of Microbiology (ASM) Microbe (Boston, MA). 2016. [Google Scholar]
- 31. Hawser S, Siegmund C, Jeandey P, et al. . Bactericidal activity of omadacycline, a novel aminomethylcycline. Abstract 1322 at 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Amsterdam, The Netherlands). 2016. [Google Scholar]
- 32. Hinshaw R, Stapert L, Shinabarger D, Pillar C. Post-antibiotic effect of omadacycline against target pathogens. Abstract 512 at American Society of Microbiology (ASM) Microbe (Boston, MA). 2016. [Google Scholar]
- 33. Diehl D, Bionda N, Cady N, Strickland A, Tanaka S. In vitro activity of omadacycline against E. coli biofilms. Abstract 552 at American Society of Microbiology (ASM) Microbe (Boston, MA). 2016. [Google Scholar]
- 34. Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs 2011; 34:737–51. [DOI] [PubMed] [Google Scholar]
- 35. Moura IB, Buckley AM, Ewin D, et al. . Omadacycline gut microbiome exposure does not induce Clostridium difficile proliferation or toxin production in a model that simulates the proximal, medial and distal human colon. Antimicrob Agents Chemother 2019;63(2): pii e01581-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freeman J, O’Neill FJ, Wilcox MH. Effects of cefotaxime and desacetylcefotaxime upon Clostridium difficile proliferation and toxin production in a triple-stage chemostat model of the human gut. J Antimicrob Chemother 2003; 52:96–102. [DOI] [PubMed] [Google Scholar]
- 37. Baines SD, Freeman J, Wilcox MH. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J Antimicrob Chemother 2005; 55:974–82. [DOI] [PubMed] [Google Scholar]
- 38. Baines SD, Saxton K, Freeman J, Wilcox MH. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother 2006; 58:1062–5. [DOI] [PubMed] [Google Scholar]
- 39. Freeman J, Baines SD, Jabes D, Wilcox MH. Comparison of the efficacy of ramoplanin and vancomycin in both in vitro and in vivo models of clindamycin-induced Clostridium difficile infection. J Antimicrob Chemother 2005; 56:717–25. [DOI] [PubMed] [Google Scholar]
- 40. Tariq R, Cho J, Kapoor S, et al. . Low risk of primary Clostridium difficile infection with tetracyclines: a systematic review and metaanalysis. Clin Infect Dis 2018; 66:514–22. [DOI] [PubMed] [Google Scholar]
- 41. Lepak AJ, Zhao M, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Streptococcus pneumoniae in the murine pneumonia model. Antimicrob Agents Chemother 2017; 61:e02368-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lepak AJ, Zhao M, Marchillo K, VanHecker J, D A. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Staphylococcus aureus (SA) in the murine thigh infection model. Abstract 1531 at IDWeek (San Diego, CA). 2017. [Google Scholar]
- 43. Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006; 58:256–65. [DOI] [PubMed] [Google Scholar]
- 44. Endermann R, Ladel C, Broetz-Oesterhelt H, Labischinski H. BAY 73-7388 is highly efficacious in animal models of intraabdominal infections caused by a range of aerobic and anaerobic organisms, including VRE. Abstract P928 at 14th European Congress of Clinical Microbiology and Infectious Disease (ECCMID) (Prague, Czech Republic). 2004. [Google Scholar]
- 45. McKenney D, Quinn J, Jackson C, et al. . Evaluation of PTK 0796 in experimental models of infections caused by gram-positive and gram-negative pathogens. Abstract 2627 at 43rd Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC) (Chicago, IL). 2003. [Google Scholar]
- 46. Kim O, Leahy RG, Traczewski M, Macone A, Steenbergen J, Tanaka SK. Activity and efficacy of omadacycline against Clostridium difficile. Abstract 1325 at 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Amsterdam, The Netherlands). 2016. [Google Scholar]