Abstract
Background
Early clinical response (ECR) is a new endpoint to determine whether a drug should be approved for community-acquired bacterial pneumonia in the United States. The Omadacycline for Pneumonia Treatment In the Community (OPTIC) phase III study demonstrated noninferiority of omadacycline to moxifloxacin using this endpoint. This study describes the performance of the ECR endpoint and clinical stability relative to a posttreatment evaluation (PTE) of clinical success.
Methods
ECR was defined as symptom improvement 72–120 hours after the first dose of study drug (ECR window), no use of rescue antibiotics, and patient survival. Clinical success at PTE was an investigator assessment of success. Clinical stability was defined based on vital sign stabilization, described in the American Thoracic Society and Infectious Diseases Society of America community-acquired pneumonia treatment guidelines.
Results
During the ECR window, ECR was achieved in 81.1% and 82.7% of omadacycline and moxifloxacin patients, respectively. Similar numbers of patients achieved clinical stability in each treatment group (omadacycline 74.6%, moxifloxacin 77.6%). The proportion of patients with improved symptoms who were considered clinically stable increased across the ECR window (69.2–77.6% for omadacycline; 68.0–79.7% for moxifloxacin). There was high concordance (>70%) and high positive predictive value (>90%) of ECR and clinical stability with overall clinical success at PTE.
Conclusions
Omadacycline was noninferior to moxifloxacin, based on a new ECR endpoint. Clinical stability was similarly high when measured in the same time frame as ECR. Both ECR and clinical stability showed high concordance and high positive predictive value with clinical success at PTE.
Clinical Trials Registration
Keywords: community-acquired bacterial pneumonia, omadacycline, early clinical response, clinical stability
Pneumonia, along with influenza, remains a leading cause of death in the United States [1]. The annual incidence of community-acquired pneumonia (CAP) is high, especially in older adults, and is associated with high rates of hospitalization and mortality as well as with economic burden [2, 3]. While CAP also encompasses pneumonia caused by viral pathogens, community-acquired bacterial pneumonia (CABP) specifically refers to pneumonia caused by bacterial pathogens, with Streptococcus pneumoniae the most commonly identified causative pathogen globally [4]. New therapies to treat CABP are required, due to resistance to, and safety concerns with, existing antibiotics.
The Omadacycline for Pneumonia Treatment In the Community (OPTIC) phase III clinical trial demonstrated the noninferiority of omadacycline to moxifloxacin for the treatment of adults with CABP, and was the basis for the US Food and Drug Administration (FDA) approval in October 2018 of omadacycline for the treatment of CABP [5]. The trial was designed in accordance with FDA guidance on developing antibiotic drugs for the treatment of CABP [6]. The FDA guidelines require an early clinical response (ECR) primary efficacy endpoint based on improvement in pneumonia symptoms (cough, sputum production, pleuritic chest pain, and dyspnea). The FDA, with the help of the Biomarkers Consortium of the Foundation for the National Institutes of Health, developed the ECR endpoint based on a review of historical and modern symptom response data that suggest antibiotic treatment effects are most apparent during the first few days of therapy [6]. Omadacycline is the first antibiotic for CABP to be approved using the ECR endpoint.
National guidelines for the management of CAP (which includes CABP) from the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) suggest criteria to define when a hospitalized patient with CAP has reached clinical stability [7, 8]. Clinical stability is a marker of a clinical response to therapy and is considered an early clinical outcome, while the time to complete symptom resolution and radiographic improvement are considered as late outcomes. The ATS and IDSA guidelines, which were published in 2001 and 2007, suggested 2 sets of criteria to define clinical stability in hospitalized patients with CAP [7, 8]. The definition of clinical stability in the 2001 guidelines was based on patient symptoms, such as cough and shortness of breath, along with signs of systemic response, such as fever and elevated white blood cell count [8]. These criteria were initially reported by Ramirez et al in 1995 [9]. The definition of clinical stability in the 2007 guidelines was based on a decrease under fixed thresholds of vital parameters, including temperature, heart rate, respiratory rate, blood pressure, mental status, and oxygenation [7]. These criteria were first reported by Halm et al in 1998 [10]. A study comparing the 2 sets of criteria concluded that both are equivalent and can be used to identify early clinical stability in hospitalized patients with CAP [11]. The achievement of clinical stability is, then, an opportunity to consider a switch to oral therapy and hospital discharge in patients initially hospitalized and treated with intravenous (IV) antibiotics.
To date, there has been no evaluation of how ECR performs relative to clinical stability and later assessments of clinical success in patients with CABP. We used the omadacycline OPTIC study to describe the performance of the ECR endpoint and clinical stability criteria relative to a posttreatment evaluation (PTE) of clinical success.
METHODS
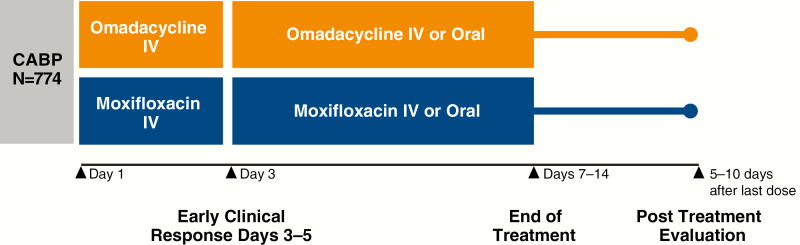
OPTIC was a global, phase III, double-blind, double-dummy, randomized, noninferiority CABP study (Figure 1). It compared 7–14 days of omadacycline at 100 mg IV every 24 hours (q24h; initial 2 doses were every 12 hours [q12h]), including an option to transition to 300 mg orally q24h after 3 days of treatment, or moxifloxacin at 400 mg IV q24h, including an option to transition to 400 mg orally q24h after 3 days of treatment. Adults aged ≥18 years of age with ≥3 protocol-specified CABP symptoms (cough, production of purulent sputum, dyspnea, pleuritic chest pain); abnormal vital signs; laboratory abnormalities associated with CABP; disease categorized as being Pneumonia Outcomes Research Team (PORT) risk class II, III, or IV at screening; and radiographically confirmed pneumonia were enrolled. Full entry criteria for this study and an overview of causative pathogens have been published previously [5].
Figure 1.
OPTIC study design. Abbreviations: CABP, community-acquired bacterial pneumonia; IV, intravenous; OPTIC, Omadacycline for Pneumonia Treatment In the Community.
The ECR primary efficacy endpoint was programmatically defined as symptom improvement at 72–120 hours after the first dose of study drug, no use of rescue antibiotics, and patient survival. CABP symptoms were characterized using a 4-point scale (absent, mild, moderate, or severe) by the investigator. Symptom improvement was defined as ≥1 level improvement (eg, severe to moderate) in ≥2 CABP symptoms, with no worsening by ≥1 level in other CABP symptoms.
Investigator assessment of clinical response was determined at the PTE (5–10 days after the last dose of study drug). Clinical success at PTE was defined as survival with resolution or improvement in signs and symptoms of infection and with no need for further antibacterial therapy.
Patients were considered clinically stable if they achieved all of the following criteria: (1) temperature ≤37.8°C (100°F); (2) heart rate ≤100 beats/minute; (3) respiratory rate ≤24 breaths/minute; (4) systolic blood pressure ≥90 mmHg; and (5) arterial oxygen saturation ≥90% or partial pressure of oxygen ≥60 mmHg on room air [10]. For each clinical stability criterion, the last observation carried forward (LOCF) was utilized to impute missing values.
ECR and clinical success at PTE were determined in the intent-to-treat (ITT) population (defined as all patients randomized to a study treatment), according to the original prespecified statistical analysis plan. Clinical stability in the ITT population during the ECR measurement window (72–120 hours) after the first dose of study drug was assessed to allow comparison with ECR. The ECR and clinical stability were provided by overall study and by PORT risk class. Dichotomized ECR responses (symptoms improved, symptoms not improved) and clinical stability (stable, not stable) during the ECR measurement window were combined to demonstrate the clinical trajectory of patients who did or did not meet symptom improvement and clinical stability criteria at 72–120 hours. Concordance of ECR or clinical stability with clinical success at PTE was determined, as well as the test characteristics (sensitivity, specificity, positive predictive value, negative predictive value) of ECR or clinical stability with clinical success at PTE. All analyses are presented by treatment group.
RESULTS
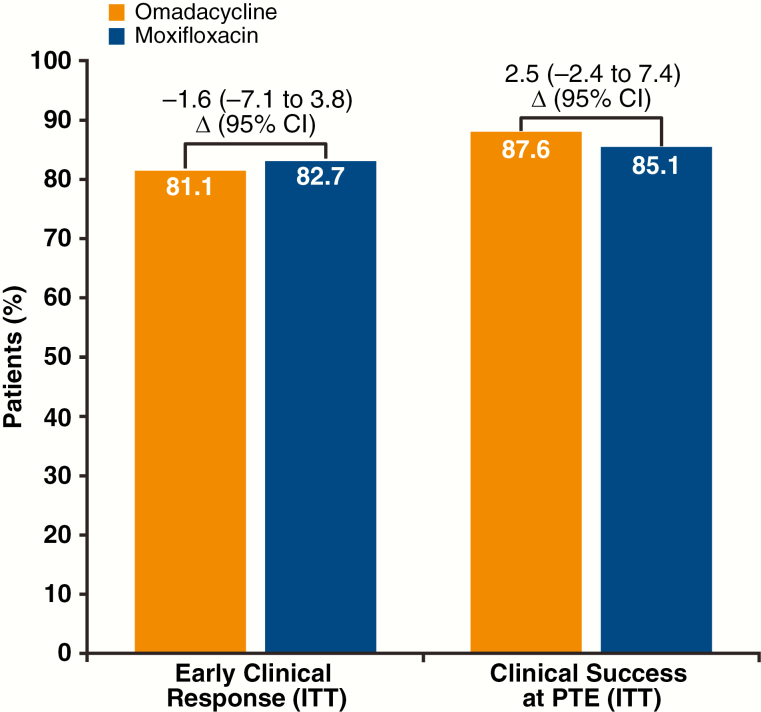
ECR and clinical success at PTE for omadacycline and moxifloxacin are presented for the overall study in Figure 2. Omadacycline was noninferior to moxifloxacin for ECR; the lower bound of the 95% confidence interval for the treatment difference was within the prespecified 10% noninferiority margin. Clinical success at PTE was high and similar between omadacycline and moxifloxacin. A baseline pathogen was identified in 49.9% of the patients in the ITT population. Clinical success rates at PTE were similar for omadacycline and moxifloxacin in patients with an identified bacterial pathogen (89% vs 87%, respectively) and by individual pathogens: 85% vs 88% for S. pneumoniae, 73% vs 82% for Staphylococcus aureus, 81% vs 100% for Haemophilus influenzae, 94% vs 88% for Mycoplasma pneumoniae, 95% vs 97% for Legionella pneumophila, and 89% vs 89% for Chlamydia pneumoniae, for patients treated with omadacycline vs moxifloxacin, respectively [5].
Figure 2.
Early clinical response and clinical success at PTE. Abbreviations: CI, confidence interval; ITT, intent-to-treat; PTE, posttreatment evaluation.
At baseline, 16.4% of patients in the ITT population were clinically stable, including 22.9% of patients with a PORT risk class of II, 13.5% of patients with a PORT risk class of III, and 18.9% of patients with a PORT risk class of IV. During the ECR assessment window (72–120 hours), the majority of patients in the study had reached clinical stability (288/386 [74.6%] for omadacycline and 301/388 [77.6%] for moxifloxacin; difference –3.0, 95% confidence interval –9.2 to 3.0). A vital sign measurement was missing for both treatment groups in ~6% of patients; however, an oxygen saturation measurement was missing in ~15%. If patients with missing values are removed, rather than utilizing LOCF, 288/324 (88.9%) omadacycline and 301/337 (89.3%) moxifloxacin patients showed clinical stability (Table 1).
Table 1.
Stabilization of Vital Signs Associated With Community-acquired Bacterial Pneumonia at 72–120 Hours After First Dose of Study Drug
| Vital Sign Finding | Omadacycline (n = 386), n/N1a (%) | Moxifloxacin (n = 388), n/N1a (%) |
|---|---|---|
| Stabilization of all vital signs | 288/324 (88.9) | 301/337 (89.3) |
| Heart rate ≤100 beats/minute | 357/363 (98.3) | 357/365 (97.8) |
| Temperature ≤37.8°C | 350/363 (96.4) | 348/365 (95.3) |
| Systolic blood pressure ≥90 mmHg | 363/363 (100.0) | 365/365 (100.0) |
| Respiratory rate ≤24 breaths/minute | 340/362 (93.9) | 344/365 (94.2) |
| PaO2 ≥60 mmHg by arterial blood gas or SaO2 ≥90% by pulse oximetry | 318/325 (97.8) | 325/357 (96.4) |
Data are from the intent-to-treat population.
Abbreviations: PaO2, partial pressure of oxygen; SaO2, oxygen saturation.
aFor each categorical parameter, the denominator for the percentage was the number of patients who had that parameter assessed (ie, only patients with available data were included in the denominators).
ECR, clinical success at PTE, and clinical stability for omadacycline and moxifloxacin are also presented by PORT risk class, using LOCF (Table 2). The results demonstrate that ECR, clinical success at PTE, and clinical stability rates by PORT risk class were similar between omadacycline and moxifloxacin patients. Clinical success rates at PTE were higher than ECR rates in the ITT population for both treatment groups and by PORT risk class. The clinical stability rate was higher in PORT risk classes III and IV patients, compared with PORT risk class II patients. The clinical stability rate was similar to the ECR rate in all PORT risk classes, in both treatment groups.
Table 2.
Early Clinical Response, Clinical Success at Posttreatment Evaluation, and Clinical Stability by PORT Risk Class
| PORT Risk Class | Endpoint | Omadacycline, n/N (%) | Moxifloxacin, n/N (%) | Difference (95% CI) |
|---|---|---|---|---|
| II | ECR | 43/57 (75.4) | 41/56 (73.2) | 2.2 (–14.0 to 18.4) |
| PTE | 47/57 (82.5) | 47/56 (83.9) | –1.5 (–15.7 to 12.8) | |
| Clinical stability | 41/57 (71.9) | 43/56 (76.8) | –4.9 (–22.7 to 12.9) | |
| III | ECR | 191/227 (84.1) | 187/216 (86.6) | –2.4 (–9.1 to 4.2) |
| PTE | 206/227 (90.7) | 190/216 (88.0) | 2.8 (–3.0 to 8.7) | |
| Clinical stability | 182/227 (80.2) | 175/216 (81.0) | –0.8 (–8.6 to 7.0) | |
| IV | ECR | 79/102 (77.5) | 93/116 (80.2) | –2.7 (–13.8 to 8.1) |
| PTE | 85/102 (83.3) | 93/116 (80.2) | 3.2 (–7.4 to 13.4) | |
| Clinical stability | 78/102 (76.5) | 92/116 (79.3) | –2.8 (–14.8 to 9.2) |
Clinical stability was determined using LOCF. Data are from the intent-to-treat population.
Abbreviations: CI, confidence interval; ECR, early clinical response; LOCF, last observation carried forward; PORT, Pneumonia Outcomes Research Team; PTE, posttreatment evaluation.
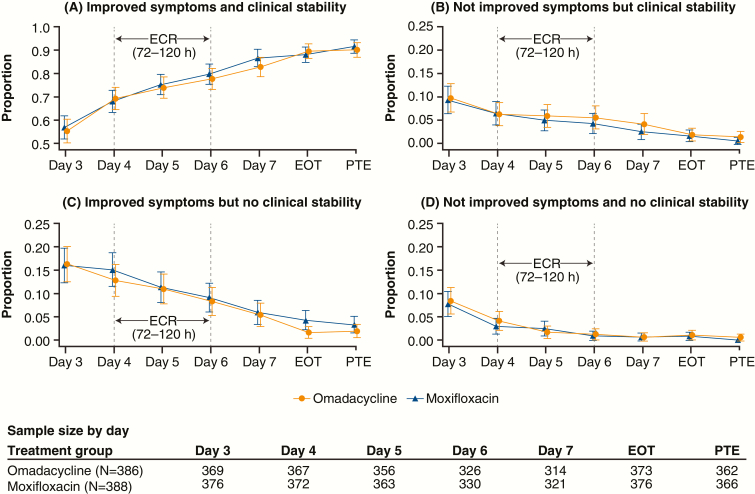
The 4 possible trajectories of CABP symptoms and clinical stability for each visit during the OPTIC study (day 3 through PTE) are shown in Figure 3A–D. The proportion of patients with improved symptoms who were considered clinically stable (Figure 3A) increased from the beginning (day 4) to the end (day 6) of the ECR window (69.2–77.6% for omadacycline; 68.0–79.7% for moxifloxacin). By the end of treatment (EOT), 89.5% of omadacycline patients and 88.0% of moxifloxacin patients had symptom improvement and were considered clinically stable. The proportions of patients without symptom improvement and with continued instability (Figure 3D) by the end of the ECR treatment window were 1.2% and 0.9% of the omadacycline and moxifloxacin treatment groups, respectively. At the end of the ECR window, more patients had symptom improvement and no clinical stability (8.3% omadacycline, 9.1% moxifloxacin; Figure 3C) than patients without symptom improvement but with clinical stability (5.5% omadacycline, 4.2% moxifloxacin; Figure 3B). By EOT, the proportions of omadacycline (1.9%) and moxifloxacin (4.3%) patients with symptom improvement but no clinical stability (Figure 3C) continued to decline. Similarly, by EOT, the proportions of omadacycline (1.9%) and moxifloxacin (1.6%) patients without symptom improvement but with clinical stability (Figure 3B) continued to decline.
Figure 3.
Symptom improvement and clinical stability (using last observation carried forward) during the OPTIC study. Abbreviations: ECR, early clinical response window; EOT, end of treatment; OPTIC, Omadacycline for Pneumonia Treatment In the Community; PTE, posttreatment evaluation.
Table 3 shows high concordance (>75%) of ECR with clinical success at PTE. Fewer omadacycline patients than moxifloxacin patients with ECR subsequently had clinical failure at PTE (2.3% vs 4.4%, respectively). More omadacycline patients than moxifloxacin patients who did not have an ECR were subsequently considered as having achieved clinical success at PTE (9.1% vs 7.7%, respectively). Slightly lower concordance (>70%) was seen between clinical stability and clinical success at PTE (Table 4). Both ECR and clinical stability demonstrated high sensitivity and positive predictive value for clinical success at PTE (Table 5). Negative predictive value was poor for both ECR and clinical stability (<50%).
Table 3.
Concordance of Early Clinical Response With Clinical Success at Posttreatment Evaluation
| Clinical Success at Posttreatment Evaluation | ||||
|---|---|---|---|---|
| Treatment Group | Early Clinical Response (72–120 hours) | Clinical Success | Clinical Failure | Indeterminatea |
| Omadacycline (n = 386) | Clinical success | 298 (77.2) | 9 (2.3) | 6 (1.6) |
| Clinical failure | 35 (9.1) | 13 (3.4) | 1 (0.3) | |
| Indeterminatea | 5 (1.3) | 10 (2.6) | 9 (2.3) | |
| Moxifloxacin (n = 388) | Clinical success | 295 (76.0) | 17 (4.4) | 9 (2.3) |
| Clinical failure | 30 (7.7) | 15 (3.9) | 2 (0.5) | |
| Indeterminatea | 5 (1.3) | 10 (2.6) | 5 (1.3) | |
Data are presented as No. (%) and are from the intent-to-treat population.
aDue to missing data.
Table 4.
Concordance of Clinical Stability (72–120 Hours) With Clinical Success at Posttreatment Evaluation
| Clinical Success at Posttreatment Evaluation | ||||
|---|---|---|---|---|
| Treatment Group | Clinical Stability (72–120 hours) | Clinical Success | Clinical Failure | Indeterminatea |
| Omadacycline (n = 386) | Stable | 271 (70.2) | 13 (3.4) | 4 (1.0) |
| Not stable | 62 (16.1) | 10 (2.6) | 3 (0.8) | |
| Indeterminatea | 5 (1.3) | 9 (2.3) | 9 (2.3) | |
| Moxifloxacin (n = 388) | Stable | 275 (70.9) | 17 (4.4) | 9 (2.3) |
| Not stable | 49 (12.6) | 13 (3.4) | 2 (0.5) | |
| Indeterminatea | 6 (1.6) | 12 (3.1) | 5 (1.3) | |
Data are presented as No. (%) and are from the intent-to-treat population.
aDue to missing data.
Table 5.
Validity of Predictive Value of Early Clinical Response or Clinical Stability With Clinical Success at Posttreatment Evaluation
| Treatment Group | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| ECR and clinical success at PTE | ||||
| Omadacycline (n = 386) | 88.2 | 68.8 | 95.2 | 45.2 |
| Moxifloxacin (n = 388) | 89.4 | 55.2 | 91.9 | 47.8 |
| Clinical stability (72–120 hours) and clinical success at PTE | ||||
| Omadacycline (n = 386) | 80.2 | 64.6 | 94.1 | 31.6 |
| Moxifloxacin (n = 388) | 83.3 | 55.2 | 91.4 | 36.8 |
Data are presented as % and are from the intent-to-treat population.
Abbreviations: ECR, early clinical response; NPV, negative predictive value; PPV, positive predictive value; PTE, posttreatment evaluation.
DISCUSSION
ECR is a new endpoint for regulators to determine whether a drug should be approved for CABP in the United States, but it is an endpoint that is unfamiliar to practicing clinicians. From a regulatory perspective, ECR is similar to a patient-reported outcome tool and is consistent with a clinically meaningful outcome measure that (1) incorporates how the patient feels, functions, or survives; and (2) can provide strong evidence for a treatment effect, and therefore drug approval [12]. From the clinician’s perspective, the ECR endpoint contains elements of the symptom improvement assessments routinely performed at the bedside to document clinical improvement of CABP.
Omadacycline is the first antibiotic approved for CABP using the ECR endpoint. In the ITT population, omadacycline (n = 386) was noninferior (10% noninferiority margin) to moxifloxacin (n = 388) for ECR (81.1% vs 82.7%, respectively; Figure 2). The secondary endpoint of clinical success at PTE, also recommended by the FDA CABP guidance, confirmed that ECR resulted in a durable clinical success following treatment completion. During the ECR window (72–120 hours), similar rates of clinical stability were seen for omadacycline and moxifloxacin. The achievement of clinical stability provides complementary information to ECR and supports the clinical improvement as measured by the ECR endpoint. Consistent results were also observed for ECR and clinical stability when analyzed by PORT risk class. Additionally, clinical responses against individual bacterial pathogens were similar between omadacycline and moxifloxacin at the ECR and PTE time points [5].
Studies have indicated that the time to clinical stability after the initiation of antibiotic therapy for CABP is approximately 3–4 days, though, in more severely ill patients, other data suggest that this may take longer [10, 11, 13, 14]. Once clinical stability is achieved (regardless of definition), the risk of serious deterioration requiring treatment in the intensive care unit was ≤1% [10]. Conversely, hospital discharges with ≥1 instability criteria resulted in higher readmission or death rates [15]. The OPTIC data suggest that components of the ECR and clinical stability are improving in parallel, and that patients are achieving both ECR and clinical stability at high rates in the ECR window of 72–120 hours; this is consistent with prior observations [10, 11, 13, 14].
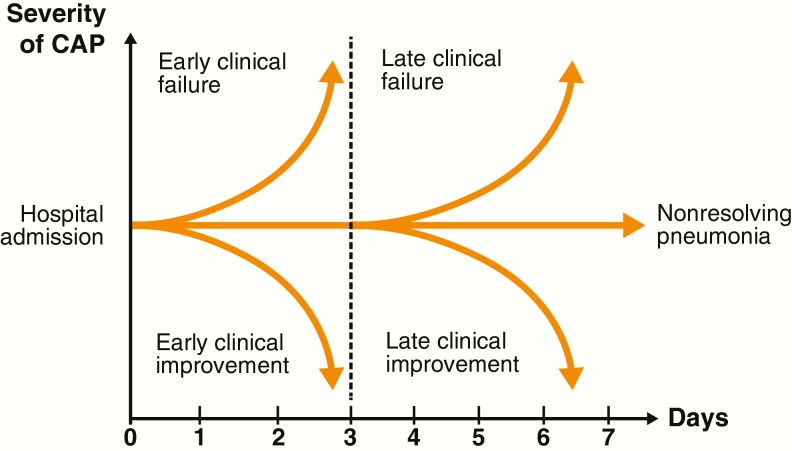
A suggested framework for future research on ECR and clinical stability in hospitalized patients with CAP is depicted in Figure 4. The clinical course during the first 7 days of hospitalization can be characterized as clinical improvement, clinical failure, or nonresolving CAP (ie, progression of pneumonia, or slow or incomplete resolution of pneumonia despite appropriate therapy).
Figure 4.
Clinical course of hospitalized patients with CAP could be categorized into 5 potential outcomes. Reproduced with permission of the ERS 2019 [14]. Abbreviation: CAP, community-acquired pneumonia; ERS, European Respiratory Society.
The FDA ECR endpoint is currently evaluated 72–120 hours after initiating treatment: during this window, a clinical response is defined as “early.” In clinical practice, clinical stability is documented in some patients after only 24 or 48 hours of antibiotic therapy. In future trials, the criteria for ECR (symptom improvement or vital sign stabilization) should be evaluated daily. Some patients may have an ECR and some may have a late clinical response, but a daily determination may allow for a better evaluation of different antimicrobial therapies. For each antibiotic, a time to clinical response can be defined.
In the OPTIC trial, ECR and attainment of clinical stability both predicted clinical success at PTE. This indicates that clinical stability, and possibly ECR, identify a time in the clinical course of CABP when a transition from IV to oral therapy and a hospital discharge may be considered without serious adverse consequences. The clinical course of a patient with a lack of ECR and/or lack of clinical stability results may represent a patient who is taking extra time to improve or a patient who may have failed. According to Figure 4, a patient who did not reach clinical response or clinical stability by day 7 can be classified as a nonresolving CAP. In the OPTIC trial, approximately half of the patients with a lack of ECR or lack of clinical stability continued to improve through the EOT and PTE periods, and a small number of patients in both treatment groups had symptom improvement and/or clinical stability after day 7.
At this point, a complete reevaluation of the patient may be necessary, to define the reasons for the lack of response. Some of these patients may have a delayed response to therapy due to the presence of loculated infections, such as empyema. In other patients, noninfectious etiologies, or mimics of CAP, may explain the lack of improvement. In clinical practice, the CABP pathogen is commonly not identified and susceptibility data are not available to guide therapy. Some patients may be infected with pathogens that produce subacute or chronic CAP, such as Nocardia, fungi, or mycobacteria. Lack of reaching ECR or clinical stability after 6–7 days should trigger a work-up to define an etiology, but should not be associated automatically with a change or escalation of antibiotic therapy. Altering or escalating antibiotic therapy based solely on the lack of ECR or failure to achieve clinical stability within 72–120 hours could expose patients to unnecessary additional antibiotics.
ECR was a programmatic determination of an investigator assessment. Validation of the ECR and development of a validated patient-reported outcome tool should be investigated [16]. A combination of ECR symptoms and clinical stability vital signs may provide better criteria for the detection of CABP improvement. The first 2–3 days following hospitalization and initiation of IV antibiotic therapy is a critical period during which healthcare providers want to assess ECR and clinical stability. Data for ECR and clinical stability were collected according to protocol-defined visits; therefore, the data from the OPTIC trial cannot identify earlier clinical responses or earlier clinical stability, or provide a time to ECR or a time to clinical stability. Time to ECR and time to clinical stability analyses could improve our understanding of the most appropriate window for ECR and clinical stability measurement, and its optimal concordance with clinical success at a PTE. Additional work to improve the negative predictive value of ECR and clinical stability endpoints, and to improve the identification of treatment failures, may also be helpful.
In conclusion, omadacycline was noninferior to moxifloxacin based on a new ECR endpoint. Clinical stability was similarly high when measured in the same time frame as ECR. Both ECR and clinical stability showed high concordance and high positive predictive value with clinical success at PTE.
Notes
Author contributions. All authors provided critical revisions to the manuscript for intellectual content and approved the final version for publication.
Acknowledgments. The authors thank the investigators who participated in the OPTIC study. Editorial support was provided by Theresa E. Singleton, PhD, and Samantha Scott, PhD, of Innovative Strategic Communications, LLC, Milford, Pennsylvania.
Financial support. This work was supported by Paratek Pharmaceuticals, Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Supplement sponsorship. This article appears as part of the supplement “Omadacycline: A New Option in an Era of Increasing Antibiotic Resistance,” sponsored by Paratek Pharmaceuticals, Inc., King of Prussia, Pennsylvania.
Potential conflicts of interest. J. A. R. reports grants from Pfizer and personal fees from The Medicines Company, Nabriva Therapeutics, Paratek Pharmaceuticals, and Achaogen, outside the submitted work. S. C. is a consultant to Paratek Pharmaceuticals. C. K. is an employee of Paratek Pharmaceuticals Inc. E. T., M. C., R. N., A. M., and P. C. M. are employees and shareholders of Paratek Pharmaceuticals. The authors received no financial compensation for the preparation of this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the article have been disclosed.
References
- 1. Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep 2016; 65:1–96. [PubMed] [Google Scholar]
- 2. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65:1806–12. [DOI] [PubMed] [Google Scholar]
- 3. Hayes BH, Haberling DL, Kennedy JL, Varma JK, Fry AM, Vora NM. Burden of pneumonia-associated hospitalizations: United States, 2001–2014. Chest 2018; 153:427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci 2016; 17: pii: E2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stets R, Popescu M, Gonong JR, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 2019; 380:517–27. [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration. Guidance for industry community-acquired bacterial pneumonia: developing drugs for treatment. 2014. Available at: https://www.fda.gov/media/75149/download. Accessed 7 June 2019. [Google Scholar]
- 7. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niederman MS, Mandell LA, Anzueto A, et al. ; American Thoracic Society Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163:1730–54. [DOI] [PubMed] [Google Scholar]
- 9. Ramirez JA, Srinath L, Ahkee S, Huang A, Raff MJ. Early switch from intravenous to oral cephalosporins in the treatment of hospitalized patients with community-acquired pneumonia. Arch Intern Med 1995; 155:1273–6. [PubMed] [Google Scholar]
- 10. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–7. [DOI] [PubMed] [Google Scholar]
- 11. Aliberti S, Zanaboni AM, Wiemken T, et al. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur Respir J 2013; 42:742–9. [DOI] [PubMed] [Google Scholar]
- 12. Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H; Community-Acquired Bacterial Pneumonia–Acute Bacterial Skin and Skin Structure Infections Project Team. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis 2012; 55:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menéndez R, Torres A, Rodríguez de Castro F, et al. ; Neumofail Group Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis 2004; 39: 1783–90. [DOI] [PubMed] [Google Scholar]
- 14. Aliberti S, Blasi F. Early outcomes in CAP: clinical stability, clinical failure and nonresolving pneumonia. Eur Respir Monogr 2014; 63:205–18. [Google Scholar]
- 15. Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med 2002; 162:1278–84. [DOI] [PubMed] [Google Scholar]
- 16. Talbot GH, Powers JH, Hoffmann SC; Biomarkers Consortium of the Foundation for the National Institutes of Health Community-Acquired Bacterial Pneumonia–Acute Bacterial Skin and Skin Structure Infections and HABP-VABP Project Teams Developing outcomes assessments as endpoints for registrational clinical trials of antibacterial drugs: 2015 update from the biomarkers consortium of the foundation for the National Institutes of Health. Clin Infect Dis 2016; 62:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]