Abstract
Background
Human papillomavirus (HPV)-related biomarkers have shown good cross-sectional performance for anal precancer detection in human immunodeficiency virus–positive (HIV+) men who have sex with men (MSM). However, the long-term performance and risk stratification of these biomarkers are unknown. Here, we prospectively evaluated high-risk (HR) HPV DNA, HPV16/18 genotyping, HPV E6/E7 messenger RNA (mRNA), and p16/Ki-67 dual stain in a population of HIV+ MSM.
Methods
We enrolled 363 HIV+ MSM between 2009–2010, with passive follow-up through 2015. All had anal cytology and a high-resolution anoscopy at baseline. For each biomarker, we calculated the baseline sensitivity and specificity for a combined endpoint of high-grade squamous intraepithelial lesion (HSIL) and anal intraepithelial neoplasia grade 2 or more severe diagnoses (HSIL/AIN2+), and we estimated the 2- and 5-year cumulative risks of HSIL/AIN2+ using logistic and Cox regression models.
Results
There were 129 men diagnosed with HSIL/AIN2+ during the study. HR-HPV testing had the highest positivity and sensitivity of all assays, but the lowest specificity. HPV16/18 and HPV E6/E7 mRNA had high specificity, but lower sensitivity. The 2- and 5-year risks of HSIL/AIN2+ were highest for those testing HPV16/18- or HPV E6/E7 mRNA–positive, followed by those testing dual stain–positive. Those testing HR-HPV– or dual stain–negative had the lowest 2- and 5-year risks of HSIL/AIN2+.
Conclusions
HPV-related biomarkers provide long-term risk stratification for anal precancers. HR-HPV– and dual stain–negativity indicate a low risk of HSIL/AIN2+ for at least 2 years, compared with negative anal cytology; however, the high positivity of HR-HPV in HIV+ MSM may limit its utility for surveillance and management in this population.
Keywords: anal cancer, biomarkers, screening, prospective, HPV
The human papillomavirus (HPV)-related biomarkers of HPV- and dual stain–negativity indicate a low risk of anal precancer for at least 2 years in human immunodeficiency virus–positive men who have sex with men, compared with negative anal cytology.
Anal cancer incidence and mortality rates have been increasing over the past decade [1]. While the incidence of anal cancer is low in the general population (1.9 per 100 000), it is much higher in men who have sex with men (MSM) and in MSM living with human immunodeficiency virus (HIV) infections (HIV+ MSM; ranging from 77 to 137 per 100 000) [2, 3]. Most anal cancers are caused by high-risk human papillomavirus (HR-HPV), with a majority attributed to HPV16 [4]. Like cervical cancer, anal cancer is thought to develop through squamous epithelial precursor lesions that can be detected by exfoliative cellular sampling and diagnosed by high-resolution anoscopy (HRA) with directed biopsy [5]. Although there are no formal guidelines for anal cancer screening, some clinics use anal cytology for screening HIV+ MSM [6]. Anal cytology is highly subjective, lacks sensitivity, and needs to be repeated at frequent intervals (eg, every 1–2 years) [7]. Although HRA and anal biopsy are considered the gold standards for diagnosing anal lesions, these procedures require training and specialized expertise [8, 9].
These challenges underscore the need for more objective, molecular markers for anal precancer detection. Cervical cancer screening is transitioning to primary HPV testing, based on its superior sensitivity and negative predictive value when compared with cytology [10, 11]. However, given the high prevalence of anal HPV infections in HIV+ MSM, the clinical utility of HPV testing in this population is unclear [12]. Other biomarkers of HPV-related carcinogenesis have shown good performance for the detection of prevalent anal precancers [12–15]; however, prospective studies are needed to determine the duration of reassurance provided by a negative test result. In a previous study of HIV+ MSM, we demonstrated that HR-HPV testing, HPV16/18 genotyping, HPV E6/E7 messenger RNA (mRNA), or p16/Ki-67 dual staining may improve the detection of prevalent anal precancers [13]. The current study adds 5 years of follow-up data to evaluate the longitudinal performance of these biomarkers.
METHODS
Study Population
This study was conducted at the Anal Cancer Screening Clinic at Kaiser Permanente Northern California (KPNC, San Francisco, California). HIV+ men aged 18 years or older who were identified through the Kaiser HIV registry and were not previously diagnosed with anal precancer were eligible for this study. Information about HIV status and medication, sexually transmitted infections, and histopathology results were obtained from the KPNC clinical database, and risk factor information was obtained from a self-administered questionnaire. A total of 363 men were enrolled between August 2009 and June 2010, with passive follow-up of electronic medical records for cytology and histology endpoints through October 2015. The study was reviewed and approved by the institutional review boards at KPNC and the National Cancer Institute.
Cytology, High-resolution Anoscopy, and Histology
Per KPNC clinical protocols, all HIV+ patients are screened with anal cytology and HRA, with directed biopsies of suspicious lesions taken at the discretion of the anoscopist. In this study, 2 cytology specimens were collected during the clinical examination, as previously described [16]. Both specimens were transferred to PreservCyt medium (Hologic, Bedford, Massachusetts). All biomarkers were measured at the first visit (baseline) out of these specimens. A ThinPrep slide was prepared from the first specimen for routine Pap staining. Anal cytology results were reported as either negative for intraepithelial lesion or malignancy (NILM); atypical cells of undetermined significance or more severe cytology (ASC-US); atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; a low-grade squamous intraepithelial lesion (LSIL); or a high-grade squamous intraepithelial lesion (HSIL) [17]. HSIL cytology was further distinguished as HSIL with more severe abnormalities (HSIL-AIN3 or severe dysplasia) or HSIL with less severe abnormalities (HSIL-AIN2 or moderate dysplasia). All participants received a digital anorectal examination followed by HRA, with directed biopsies of suspicious lesions. Histology results were reported as benign, condyloma acuminatum, and anal intraepithelial neoplasia (AIN) grades 1–3. Men with normal HRAs were referred for a repeat cytology and HRA in 1 year, and those with low-grade histology results were referred for a repeat cytology and HRA within 6 months to 1 year. Men with high-grade cytology results but normal HRAs were referred to a repeat cytology and HRA within 6 months.
Human Papillomavirus DNA Testing
HPV testing was carried out using cobas 4800 on the second cytology specimen by Roche (Pleasanton, California), as previously described [18]. The cobas assay provides 3 HPV positive/negative results: HPV 16, HPV 18, and 12 other HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, pooled). We evaluated the performance of HPV DNA testing for all high-risk types (HR-HPV), and the performance of HPV16/18 genotyping.
Human Papillomavirus E6/E7 Messenger RNA Testing
HPV mRNA testing was conducted from the second cytology specimen by PreTect HPV-Proofer assay (NorChip AS, Klokkarstua, Norway), as previously described [13, 19]. The detection of HPV E6 and E7 mRNA of HPV types 16, 18, 31, 33, and 45 was carried out by real-time multiplex Nucleic Acid Sequence Based Amplification.
Dual Staining: p16/Ki-67
Dual staining with p16/Ki-67 was performed on the residual cytologic specimen by Roche MTM Laboratories (Heidelberg, Germany), using the CINtec PLUS Kit according to the manufacturer’s instructions. Samples with insufficient cellularity were excluded from evaluation. A trained cytotechnologist reviewed all cases for the presence of cells staining positively with both markers. A slide was considered positive if 1 or more squamous epithelial cell(s) stained positive for both p16 and Ki-67 and dual stain–positive cells were semi-quantitatively assessed (0, 1, 2–5, 6–50, >50).
Statistical Analysis
We created combined outcomes, based on cytology and histology results, to reduce the misclassification of anal precancer endpoints, as previously described [13]. We defined a composite HSIL/AIN2+ endpoint that included men with AIN2 or AIN3 histology and/or HSIL cytology. Men with benign, condyloma, or AIN1 histology or those without biopsy but with <HSIL cytology were categorized as <HSIL/AIN2. We calculated the sensitivity and specificity of each biomarker separately and in select combinations for the detection of HSIL/AIN2+ at baseline, both overall and stratified by age (<50 years vs ≥50 years). We used logistic regression to estimate the probabilities and 95% confidence intervals (CI) of the prevalent diseases at baseline and Cox proportional hazards to estimate the incident disease risks in men with follow-up who did not have prevalent disease. The cumulative risk was calculated using the following equation:
Where p(x) is the probability of prevalent disease for those with test values x, estimated from the logistic regression, and S(t; x) is the survival probability by time t for those without prevalent disease and with test values x, estimated from the Cox proportional hazards model.
To compare the biomarker performance to current clinical practice, we also evaluated the prevalent and cumulative risks of HSIL/AIN2+ by cytology (ASC-US or more severe cytology [ASC-US+] versus NILM). We used the baseline probability of HSIL/AIN2+ in men testing NILM as a threshold for a 1-year return interval. We compared these thresholds against the cumulative risk curves for each biomarker. For each cumulative risk estimate, we generated 100 bootstrap samples to calculate 95% CIs.
RESULTS
Study Population
The median age of the 363 HIV+ MSM was 53 years (range 26–79 years). At enrollment, over 90% were using highly active antiretroviral therapy, had an HIV viral load <75 copies/ml, and had a CD4 cell count >200. Approximately 73% reported having 10 or more lifetime anal intercourse partners, and 45% reported having at least 1 anal intercourse partner in the past 6 months. At enrollment, 23 men had inadequate cytology (6%), 112 had NILM (31%), 73 had ASC-US (20%), 27 had ASC-H (7%), 67 had LSIL (18%), and 60 had HSIL (17%). All men underwent HRAs and 265 received a biopsy (73%). Of these, 84 with normal histology or condyloma (32%), 113 had AIN1 (43%), 46 had AIN2 (17%), and 22 (8%) had AIN3 at baseline (Table 1). A total of 104 men (29%) had HSIL/AIN2+ at baseline. The prevalence of HSIL/AIN2+ did not significantly differ by age (29.5% in men <50 years vs 28.6%, in men 50+ years). Of the 259 men with <HSIL/AIN2 at baseline, a total of 135 (52%) had follow-up information (median 2.6 years, interquartile range [IQR] 2.3 years) and 25 developed incident HSIL/AIN2+ (10 AIN2 and 15 AIN3; median time to diagnosis 2.1 years, IQR 1.0 year). Of the 135 men with follow-up, 77 had at least 2 or more follow-up visits (57%). Men diagnosed with incident HSIL/AIN2+ had an average of 4.6 follow-up visits (3.3 cytology and 4.1 HRA visits) and men with <HSIL/AIN2 during follow-up had an average of 1.75 follow-up visits (1.6 cytology and 1.1 HRA visits). Men with missing follow-up data were similar in terms of baseline age, viral load, and biomarker and cytology positivity to those with follow-up (P > .05; data not shown). Men without follow-up were more likely to have a CD4 cell count of 350+ (86% versus 78%; P = .04) and 10 or more lifetime sex partners (79% versus 65%; P = .02) compared to men with follow-up.
Table 1.
Baseline Anal Cytology and Histology in 363 Human Immunodeficiency Virus–positive Men Who Have Sex With Men at Kaiser Permanente Northern California
| Anal Histology | ||||||||
|---|---|---|---|---|---|---|---|---|
| Anal Cytology | Missing | No Biopsy | Benign | Condyloma | AIN1 | AIN2 | AIN3 | Total |
| Inadequate/Missing | 0 | 7 | 6 | 1 | 8 | 1 | 1 | 24 (6.6%) |
| NILM | 1 | 48 | 30 | 3 | 21 | 7 | 2 | 112 (30.9%) |
| ASC-US | 0 | 17 | 19 | 0 | 27 | 6 | 4 | 73 (20.1%) |
| ASC-H | 0 | 7 | 7 | 1 | 4 | 8 | 0 | 27 (7.4%) |
| LSIL | 0 | 10 | 6 | 4 | 36 | 12 | 3 | 67 (18.5%) |
| HSIL-AIN2 | 1 | 1 | 3 | 2 | 6 | 1 | 2 | 16 (4.4%) |
| HSIL-AIN3 | 0 | 6 | 1 | 1 | 15 | 11 | 10 | 44 (12.1%) |
| Total | 2 (0.6%) | 96 (26.4%) | 72 (19.8%) | 12 (3.3) | 113 (31.1%) | 46 (12.7%) | 22 (6.1%) | 363 (100.0%) |
Abbreviations: AIN1–3, anal intraepithelial neoplasia grades 1 through 3; ASC-H, atypical squamous cells that cannot exclude high-grade intraepithelial lesions; ASC-US, atypical cells of undetermined significance or more severe cytology; HIV+, human immunodeficiency virus–positive; HSIL-AIN2, high-grade squamous intraepithelial lesion with less severe abnormalities; HSIL-AIN3, high-grade squamous intraepithelial lesion with more severe abnormalities; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
PERFORMANCE CHARACTERISTICS OF CYTOLOGY AND BIOMARKERS
HR-HPV testing had the highest positivity (79%), followed by p16/Ki-67 dual stain (69%), cytology (67.0%), HPV E6/E7 mRNA (48%), and HPV16/18 (38%; Table 2). For all biomarkers, men <50 years of age were significantly more likely to test positive, compared with men aged 50+ years (chi-square P value <.05, respectively). We observed higher sensitivity, but lower specificity, for all biomarkers and cytology in men <50 years of age compared to those aged 50+ years; these differences were particularly pronounced for HR-HPV, dual stain, and HPV E6/E7 mRNA testing.
Table 2.
Biomarker Performance Characteristics for Baseline Detection of High-grade Squamous Intraepithelial Lesion/Anal Intraepithelial Neoplasia Grades 2 and 3, Overall and by Age Group
| Biomarker (% positive) | <HSIL/AIN2 (n) | HSIL/AIN2+ (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| HR-HPV (79.1) | 255 | 104 | 100.0 | 29.4 |
| <50 years (86.9) | 102 | 43 | 100.0 | 18.6 |
| ≥50 years (73.8) | 149 | 61 | 100.0 | 36.9 |
| HPV16/18 (38.4) | 255 | 104 | 64.4 | 72.2 |
| <50 years (44.1) | 102 | 43 | 67.4 | 65.7 |
| ≥50 years (34.8) | 149 | 61 | 62.3 | 76.5 |
| Dual staina (69.0) | 229 | 103 | 93.2 | 41.9 |
| <50 years (77.1) | 97 | 43 | 97.7 | 32.0 |
| ≥50 years (63.0) | 129 | 60 | 90.0 | 49.6 |
| HPV E6/E7 mRNA (47.5) | 255 | 103 | 79.6 | 65.5 |
| <50 years (55.6) | 101 | 43 | 83.7 | 56.4 |
| ≥50 years (41.9) | 150 | 60 | 76.7 | 72.0 |
| Cytologyc (67.0) | 237 | 102 | 91.2 | 43.5 |
| <50 years (70.1) | 95 | 42 | 95.2 | 41.1 |
| ≥50 years (64.7) | 138 | 60 | 88.3 | 45.7 |
| Biomarker combinations b | ||||
| Dual stain or HPV16/18 (75.5) | 239 | 104 | 96.2 | 33.5 |
| <50 years (83.0) | 98 | 43 | 100.0 | 24.5 |
| ≥50 years (70.2) | 137 | 61 | 93.4 | 40.2 |
| Dual stain and HPV16/18 (31.0) | 245 | 103 | 60.0 | 83.8 |
| <50 years (38.2) | 101 | 43 | 65.1 | 73.3 |
| ≥50 years (26.4) | 141 | 60 | 58.3 | 87.2 |
| Dual stain or E6/E7 mRNA (74.8) | 233 | 104 | 96.2 | 34.8 |
| <50 years (81.3) | 96 | 43 | 100.0 | 27.1 |
| ≥50 years (70.1) | 133 | 61 | 93.4 | 40.6 |
| Dual stain and E6/E7 mRNA (41.6) | 251 | 102 | 76.5 | 72.5 |
| <50 years (51.7) | 102 | 43 | 81.4 | 60.8 |
| ≥50 years (34.6) | 146 | 59 | 72.9 | 80.8 |
| HPV16/18 or E6/67 mRNA (55.2) | 253 | 104 | 85.6 | 57.3 |
| <50 years (62.5) | 101 | 43 | 88.4 | 48.5 |
| ≥50 years (50.2) | 148 | 61 | 83.6 | 63.5 |
Counts may not sum to 363 due to missing biomarker data.
Abbreviations: AIN2, anal intraepithelial neoplasia grade 2; AIN2+, anal intraepithelial neoplasia grades 2 and 3; ASC-US+, atypical cells of undetermined significance or more severe cytology; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous intraepithelial lesion; mRNA, messenger RNA.
The threshold for a p16/Ki-67 dual stain was 1 positive cell.
Biomarkers can either be combined as positive for at least 1 (ie, “or”) or positive for both (ie, “and”).
The threshold for cytology was ASC-US+.
Cytology
The sensitivity of ASC-US+ cytology for prevalent HSIL/AIN2+ was 91.2%, and the specificity was 43.5% (Table 2). In men with ASC-US+, the probability of detecting prevalent HSIL/AIN2+ was 41.0% (95% CI 34.8–47.5%) and the 2- and 5-year cumulative risks of HSIL/AIN2+ were 51.6% (95% CI 39.9–59.4%) and 61.4% (95% CI 40.0–69.3%), respectively. Among men with NILM cytology, the probability of prevalent HSIL/AIN2+ was 8.0% (95% CI 4.2–14.7%) and the 2- and 5-year cumulative risks of HSIL/AIN2+ were 11.9% (95% CI 6.5–18.5%) and 16.9% (95% CI 7.8–26.7%%), respectively (Table 3).
Table 3.
Baseline and Cumulative Risk of High-grade Squamous Intraepithelial Lesion/Anal Intraepithelial Neoplasia Grades 2 and 3 by Candidate Biomarkers and Cytology
| Prevalence, % (95% CI) | 2-Year CumulativeRisk, % (95% CI) | 5-Year CumulativeRisk, % (95% CI) | |
|---|---|---|---|
| HR-HPV | |||
| Positive | 36.6 (31.2–42.4) | 46.5 (32.9–54.8) | 56.7 (32.9–64.2) |
| Negative | -- | 3.3 (0.0–9.4) | 7.3 (0.0–17.4) |
| HPV16/18 | |||
| Positive | 48.6 (40.3–56.9) | 59.6 (51.0–67.6) | 71.6 (61.6–80.9) |
| Negative | 16.7 (12.4–22.3) | 22.6 (14.8–29.6) | 29.3 (19.4–37.4) |
| Dual staina | |||
| Positive | 41.9 (35.7–48.4) | 52.0 (38.3–59.0) | 63.8 (38.3–70.6) |
| Negative | 6.8 (3.6–13.6) | 7.6 (2.9–13.6) | 9.4 (2.9–16.9) |
| HPV E6/E7 mRNA | |||
| Positive | 48.2 (40.8–55.7) | 60.3 (51.6–68.2) | 72.7 (60.3–83.1) |
| Negative | 11.2 (7.4–16.5) | 14.3 (9.0–20.7) | 20.0 (11.8–28.8) |
| Cytologyb | |||
| Positive | 41.0 (34.8–47.5) | 51.6 (39.9–59.4) | 61.4 (40.0–69.3) |
| Negative | 8.0 (4.2–14.7) | 11.9 (6.5–18.5) | 16.9 (7.8–26.7) |
| Biomarker combinations c | |||
| Dual stain orHPV16/18 | |||
| Positive | 38.6 (32.9–44.7) | 48.9 (41.3–55.0) | 59.9 (51.5–68.6) |
| Negative | 4.8 (1.8–12.0) | 4.8 (1.8–12.0) | 4.8 (1.8–12.0) |
| Dual stain andHPV16/18 | |||
| Positive | 58.3 (48.8–67.2) | 69.2 (59.3–77.3) | 81.5 (71.0–89.5) |
| Negative | 16.7 (12.5–21.9) | 21.2 (15.1–28.1) | 29.5 (20.9–36.9) |
| Dual stain orHPV E6/E7 mRNA | |||
| Positive | 39.7 (33.8–45.9) | 50.8 (36.1–58.0) | 62.0 (36.1–69.8) |
| Negative | 4.7 (1.8–11.9) | 5.8 (1.1–26.8) | 7.7 (1.1–30.8) |
| Dual stain andHPV E6/E7 mRNA | |||
| Positive | 53.1 (45.0–61.0) | 63.7 (55.9–71.6) | 76.4 (65.5–84.3) |
| Negative | 11.7 (7.9–16.8) | 14.2 (8.9–20.1) | 20.0 (11.9–28.2) |
| HPV16/18 orHPV E6/E7 mRNA | |||
| Positive | 45.2 (38.4–52.2) | 54.9 (48.9–64.7) | 67.6 (56.9–76.8) |
| Negative | 9.4 (5.7–15.0) | 12.8 (7.3–20.2) | 18.3 (9.5–28.7) |
The baseline prevalence was estimated by calculating the probability of high-grade squamous intraepithelial lesion/anal intraepithelial neoplasia grades 2 and 3 (HSIL/AIN2+) at enrollment from a logistic regression model. The cumulative risk was calculated by adding the probability of baseline HSIL/AIN2+ and the risk of incident disease, estimated from a Cox Proportional Hazards model.
Abbreviations: ASC-US+, atypical cells of undetermined significance or more severe cytology; CI, confidence interval; HPV, human papillomavirus; HR-HPV, high-risk HPV; mRNA, messenger RNA.
The threshold for a p16/Ki-67 dual stain was 1 positive cell.
The threshold for cytology was ASC-US+.
Biomarkers can either be combined as positive for at least 1 (ie, “or”) or positive for both (ie, “and”).
High-risk Human Papillomavirus Testing
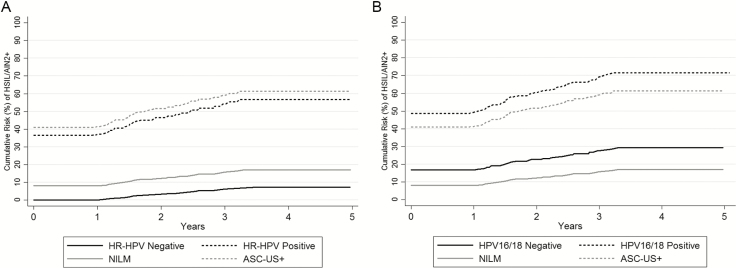
HR-HPV testing had the highest baseline sensitivity (100%), but the lowest specificity (29.4%), as compared to other assays (Table 2). At baseline, the probability of detecting HSIL/AIN2+ in HR-HPV–positive men was 36.6% (95% CI 31.2–42.4%). The 2- and 5-year risks of HSIL/AIN2+ in HR-HPV–positive men were 46.5% (95% CI 32.9–54.8%) and 56.7% (95% CI 32.9–64.2%), respectively, and in HR-HPV–negative men were 3.3% (95% CI 0.0–9.4%) and 7.3% (95% CI 0.0–17.4%), respectively (Table 3 and Figure 1A).
Figure 1.
Cumulative risk of HSIL/AIN2+ by baseline HR-HPV and HPV16/18 genotyping results. The cumulative 5-year risk curves for HSIL/AIN2+ are shown according to (A) baseline HR-HPV testing results (the solid black line indicates HR-HPV negativity and the dashed black line indicates HR-HPV positivity) and (B) baseline HPV16/18 genotyping results (the solid black line indicates HPV16/18 negativity and the dashed black line indicates HPV16/18 positivity). The corresponding risk estimates for the cytology categories of NILM (solid gray line) and ASC-US+ (dashed gray line) are also shown for reference in each plot. Abbreviations: AIN2+, anal intraepithelial neoplasia grades 2 and 3; ASC-US+, atypical cells of undetermined significance or more severe cytology; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
Human Papillomavirus 16/18 Genotyping
HPV16/18 genotyping had the highest baseline specificity for HSIL/AIN2+ detection (72.2%), but the lowest sensitivity (64.4%), compared with the other assays (Table 2). The probability of detecting HSIL/AIN2+ at baseline in HPV16/18-positive men was 48.6% (95% CI 40.3–56.9%), and the cumulative risks of HSIL/AIN2+ were 59.6 (95% CI 51.0–67.6%) at 2 years and 71.6% (95% CI 61.6–80.9%) at 5 years. In HPV16/18-negative men, the probability of prevalent HSIL/AIN2+ was 16.7% (95% CI 12.4–22.3) and the cumulative risks of HSIL/AIN2+ were 22.6% (95% CI 14.8–29.6%) and 29.3% (95% CI 19.4–37.4%) at 2 and 5 years, respectively (Table 3 and Figure 1B).
As an ancillary analysis, we evaluated the performance of HPV16/18 genotyping in men with NILM cytology. Among men with NILM, 21% were positive for HPV16/18 and 46% were positive for other HR-HPV types. In men who were NILM or HPV16/18-positive, the baseline risk of HSIL/AIN2+ was 21.7% (95% CI 9.3–42.8%) and the 2- and 5-year cumulative risks were 36.2% (95% CI 9.2–61.0%) and 44.4% (95% CI 9.2–71.4%), respectively. In men who were NILM or other HR-HPV–positive, the baseline risk of HSIL/AIN2+ was 7.8% (95% CI 3.0–19.1%) and the 2- and 5-year cumulative risks were 9.2% (95% CI 2.1–24.4%) and 12.9% (95% CI 2.1–29.3%), respectively.
Dual Stain: p16-Ki-67
The p16/Ki-67 dual stain showed similar performance characteristics as cytology, with dual staining having a slightly higher baseline sensitivity for HSIL/AIN2+ (93.2%), but a slightly lower specificity (41.9%; Table 2). At baseline, the probability of detecting HSIL/AIN2+ among men testing dual stain–positive was 41.9% (95% CI 35.7–48.4%) and the cumulative 2-year and 5-year risks of HSIL/AIN2+ were 52.0% (95% CI 38.3–59.0%) and 63.8% (95% CI 38.3–70.6%), respectively. In men who tested dual stain–negative, the probability of detecting prevalent HSIL/AIN2+ was 6.8% (95% CI 3.6–13.6%) and the 2-year and 5-year cumulative risks of HSIL/AIN2+ were 7.6 (95% CI 2.9–13.6%) and 9.4% (95% CI 2.9–16.9%), respectively (Table 3 and Figure 2).
Figure 2.
Cumulative risk of HSIL/AIN2+ by baseline p16/Ki-67 dual stain results. The cumulative 5-year risk curves for HSIL/AIN2+ are shown according to baseline p16/Ki-67 dual stain results (the solid black line indicates dual stain–negative results and the dashed black line indicates dual stain–positive results). The corresponding risk estimates for cytology categories NILM (solid gray line) and ASC-US+ (dashed gray line) are also shown for reference. Abbreviations: AIN2+, anal intraepithelial neoplasia grades 2 and 3; ASC-US+, atypical cells of undetermined significance or more severe cytology; DS, dual stain; HSIL, high-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
Additionally, we evaluated the performance of a higher positivity threshold of 2 or more dual stain–positive cells. At this cutoff, the overall positivity decreased from 69% to 58.9% and the specificity improved from 41.9% to 54.8% at baseline, with somewhat reduced sensitivity (from 93.2% to 89.3%). The baseline risk of HSIL/AIN2+ in men with 2 or more dual stain–positive cells was 47.2% (95% CI 40.3–54.2%), compared to 8.1% (95% CI 4.5%-14.0%) in men with 0–1 dual stain–positive cells. The cumulative 2- and 5-year risks of HSIL/AIN2+ with 2 or more dual stain–positive cells were 57.6% (95% CI 50.0–65.5%) and 69.7% (95% CI 60.4–81.1%), respectively, and with 0–1 dual stain–positive cells were 11.1% (95% CI 6.8–16.9%) and 17.3% (95% CI 10.9–24.5%), respectively.
Human Papillomavirus E6/E7 Messenger RNA
HPV E6/E7 mRNA had similar performance characteristics as HPV16/18 genotyping, showing high specificity for HSIL/AIN2+ (65.5%), but much lower sensitivity compared with most other tests (79.6%). The probability of detecting HSIL/AIN2+ at baseline in men testing HPV E6/E7 mRNA–positive was 48.2% (95% CI 40.8 -55.7%) and the cumulative 2- and 5-year risks of HSIL/AIN2+ were 60.3% (95% CI 51.6–68.2%) and 72.7% (95% CI 60.3–83.1%), respectively. Among men testing HPV E6/E7–negative, the probability of detecting HSIL/AIN2+ at baseline was 11.2% (95% CI 7.4–16.5%) and the cumulative 2- and 5-year risks of HSIL/AIN2+ were 14.3% (95% CI 9.0–20.7%) and 20.0% (95% CI 11.8–28.8%), respectively (Table 3 and Figure 3).
Figure 3.
Cumulative risk of HSIL/AIN2+ by baseline HPV E6/E7 mRNA results. The cumulative 5-year risk curves for HSIL/AIN2+ are shown according to baseline HPV E6/E7 mRNA results (the solid black line indicates HPV E6/E7 mRNA negativity and the dashed black line indicates HPV E6/E7 mRNA positivity). The corresponding risk estimates for cytology categories NILM (solid gray line) and ASC-US+ (dashed gray line) are also shown for reference. Abbreviations: AIN2+, anal intraepithelial neoplasia grades 2 and 3; ASC-US+, atypical cells of undetermined significance or more severe cytology; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; mRNA, messenger RNA; NILM, negative for intraepithelial lesion or malignancy.
Biomarker Combinations
Overall and by age group, combinations of biomarkers did not substantially reduce positivity or improve sensitivity and specificity, as compared to individual tests (Table 2). The lowest 2- and 5-year risk estimates for HSIL/AIN2+ were observed in men testing negative for both dual stain and HPV16/18 (4.8% [95% CI 1.8–12.0%] for both timepoints; Table 3).
DISCUSSION
The high rates of anal cancer in HIV+ MSM underscore the need for prevention approaches that effectively reduce the burden of disease in this population. Anal cytology has poor reproducibility and needs to be repeated frequently to account for its low sensitivity [7]. Biomarkers of HPV-related carcinogenesis have shown promising clinical performance for the detection of anal precancers [12–15]; however, their long-term risk stratifications have not been evaluated. In this 5-year prospective study of HIV+ MSM, we assessed HR-HPV, HPV16/18 genotyping, p16/Ki-67 dual staining, and HPV E6/E7 mRNA for the detection of HSIL/AIN2+. We used the baseline probability of HSIL/AIN2+ in men testing cytology-negative as a benchmark for a 1-year return, to compare risk estimates for the various biomarkers [7].
Our data demonstrate that, while an HR-HPV–negative test result provides excellent long-term reassurance against HSIL/AIN2+, nearly 80% of HIV+ MSM tested HR-HPV positive, limiting the efficiency of HR-HPV testing. Type-restricted assays, such as HPV16/18 genotyping and HPV E6/E7 mRNA testing, had lower positivity compared to HR-HPV testing; however, negative test results for these assays did not provide adequate long-term nor immediate reassurance against HSIL/AIN2+. In contrast, p16/Ki-67 dual staining had higher sensitivity, resulting in baseline and 2-year cumulative risks of HSIL/AIN2+ among dual stain–negative men that were below the baseline risk of men with NILM cytology. Increasing the dual stain–positivity threshold to 2 or more positive cells resulted in a substantial improvement of specificity and a 10% reduction of positivity compared to cytology, while baseline and 2- and 5-year HSIL/AIN2+ cumulative risk estimates among dual stain–negative men were equivalent to those of cytology.
In contrast to cervical cancer screening, anal cancer screening focuses on high-risk groups, such as HIV+ MSM, who have a high HPV prevalence and disease burden. While cervical HPV infection peaks around age 20, anal HPV prevalence in HIV+ MSM is high across most age groups [12]. Among men aged <50 years in our study, all markers had higher sensitivity but lower specificity compared to men aged 50 years or older, despite a similar disease prevalence. This shows that age needs to be considered when evaluating the performance of biomarkers for anal cancer screening. Importantly, no management thresholds currently exist for anal cancer screening; in some settings, cytology is either performed concurrent with an HRA, or patients with ASC-US+ cytology are referred to an HRA and those with NILM undergo repeat testing. Our data show that the 2-year risks of HSIL/AIN2+ in men testing HR-HPV– or dual stain–negative were lower than the baseline risks in men with NILM cytology, suggesting that test intervals could be extended in HR-HPV– and/or dual stain–negative men; however, more precise risk estimates are needed to establish clinical action thresholds and optimal screening intervals.
The strengths of our study include a large, representative clinical population of HIV+ MSM, with disease ascertainment based on highly-standardized anal cytology and HRAs, performed on all men [7, 13, 19]. However, our findings, similar to previous studies, show that both anal cytology and HRA have limited sensitivity for detecting high-grade lesions [20–23]. To address this limitation, we used combined anal cytology and HRA endpoints to account for potential misclassification by HRA and biopsy placement [23]. Because HSIL cytology was included in the combined endpoint, the evaluation of cytology as a screening test may have been partially biased. However, this bias would tend to favor the performance of cytology. A limitation of this study is the ascertainment of disease endpoints through passive follow-up. Although clinical guidelines at KPNC recommend frequent repeat screening in men with normal HRA results or low-grade biopsies, loss to follow-up was common in our study and, thus, we were not able to observe endpoints in all men. In addition, although our study population was homogenous, it is not clear whether our findings are generalizable to other high-risk populations, such as HIV-negative MSM and women at high risk of anal cancer.
In summary, our study demonstrates that biomarkers evaluated for cervical cancer screening show long-term risk stratification for HSIL/AIN2+ in HIV+ MSM. In comparison to cervical cancer screening, HR-HPV testing in HIV+ MSM provides long-term reassurance against future disease risk only for a small group of patients, given the high burden of HPV in this population. Screening with p16/Ki-67 dual staining may provide greater reassurance against anal precancer, as compared to cytology, or, at a higher threshold of positivity, may reduce the number of HIV-MSM who need further evaluation.
Notes
Financial support. This work was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (grant number Z01 CP010124-21).
Potential conflicts of interest. P. E. C. has received human papillomavirus tests and assays for research from Roche, Cepheid, Becton Dickinson, and Arbor Vita Corporation at no or reduced cost. T. M. D. has received non financial support from Hologic and personal fees from Becton Dickinson, Roche, TheVax, and Antiva outside of this work. J. C. G. and N. W. are employed by the National Cancer Institute and have received cervical cancer screening assays in-kind or at reduced cost from Becton Dickinson and Roche, to their institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. D’Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2008; 48:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverberg MJ, Lau B, Justice AC, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis 2002; 35:1127–34. [DOI] [PubMed] [Google Scholar]
- 6. Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg 2016; 8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darragh TM, Tokugawa D, Castle PE, et al. Interrater agreement of anal cytology. Cancer Cytopathol 2013; 121:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hillman RJ, Cuming T, Darragh T, et al. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis 2016; 20:283–91. [DOI] [PubMed] [Google Scholar]
- 9. Wentzensen N, Massad LS, Mayeaux EJ Jr, et al. Evidence-based consensus recommendations for colposcopy practice for cervical cancer prevention in the United States. J Low Genit Tract Dis 2017; 21:216–22. [DOI] [PubMed] [Google Scholar]
- 10. Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst 2014; 106. pii: dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis 2015; 19:91–6. [DOI] [PubMed] [Google Scholar]
- 12. Clarke MA, Wentzensen N. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol 2018; 126: 447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wentzensen N, Follansbee S, Borgonovo S, et al. Human papillomavirus genotyping, human papillomavirus mRNA expression, and p16/Ki-67 cytology to detect anal cancer precursors in HIV-infected MSM. AIDS 2012; 26:2185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin F, Roberts JM, Grulich AE, et al. ; SPANC Research Team The performance of human papillomavirus biomarkers in predicting anal high-grade squamous intraepithelial lesions in gay and bisexual men. AIDS 2017; 31:1303–11. [DOI] [PubMed] [Google Scholar]
- 15. Phanuphak N, Teeratakulpisarn N, Keelawat S, et al. Use of human papillomavirus DNA, E6/E7 mRNA, and p16 immunocytochemistry to detect and predict anal high-grade squamous intraepithelial lesions in HIV-positive and HIV-negative men who have sex with men. PLOS One 2013; 8:e78291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gage JC, Ghosh A, Borgonovo S, et al. A comparison of dacron versus Flocked nylon swabs for anal cytology specimen collection. Acta Cytol 2011; 55:364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–9. [DOI] [PubMed] [Google Scholar]
- 18. Wentzensen N, Follansbee S, Borgonovo S, et al. Analytic and clinical performance of cobas HPV testing in anal specimens from HIV-positive men who have sex with men. J Clin Microbiol 2014; 52:2892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castle PE, Follansbee S, Borgonovo S, et al. A comparison of human papillomavirus genotype-specific DNA and E6/E7 mRNA detection to identify anal precancer among HIV-infected men who have sex with men. Cancer Epidemiol Biomarkers Prev 2013; 22:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nahas CS, da Silva Filho EV, Segurado AA, et al. Screening anal dysplasia in HIV-infected patients: is there an agreement between anal pap smear and high-resolution anoscopy-guided biopsy? Dis Colon Rectum 2009; 52:1854–60. [DOI] [PubMed] [Google Scholar]
- 21. Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum 2009; 52:239–47. [DOI] [PubMed] [Google Scholar]
- 22. Mathews WC, Cachay ER, Caperna J, Sitapati A, Cosman B, Abramson I. Estimating the accuracy of anal cytology in the presence of an imperfect reference standard. PLOS One 2010; 5:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machalek DA, Poynten IM, Jin F, et al. ; SPANC study team A composite cytology-histology endpoint allows a more accurate estimate of anal high-grade squamous intraepithelial lesion prevalence. Cancer Epidemiol Biomarkers Prev 2016; 25:1134–43. [DOI] [PubMed] [Google Scholar]