Abstract
Background
Within the last decade, methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a frequent cause of purulent skin and soft tissue infections. New therapeutic options are being investigated for these infections.
Methods
We report an integrated analysis of 2 randomized, controlled studies involving omadacycline, a novel aminomethylcycline, and linezolid for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Omadacycline in Acute Skin and Skin Structure Infections Study 1 (OASIS-1) initiated patients on intravenous omadacycline or linezolid, with the option to transition to an oral formulation after day 3. OASIS-2 was an oral-only study of omadacycline versus linezolid.
Results
In total, 691 patients received omadacycline and 689 patients received linezolid. Infection types included wound infection in 46.8% of patients, cellulitis/erysipelas in 30.5%, and major abscess in 22.7%. Pathogens were identified in 73.2% of patients. S. aureus was detected in 74.7% and MRSA in 32.4% of patients in whom a pathogen was identified. Omadacycline was noninferior to linezolid using the Food and Drug Administration primary endpoint of early clinical response (86.2% vs 83.9%; difference 2.3, 95% confidence interval –1.5 to 6.2) and using the European Medicines Agency primary endpoint of investigator-assessed clinical response at the posttreatment evaluation. Clinical responses were similar across different infection types and infections caused by different pathogens. Treatment-emergent adverse events, mostly described as mild or moderate, were reported by 51.1% of patients receiving omadacycline and 41.2% of those receiving linezolid.
Conclusions
Omadacycline was effective and safe in ABSSSI.
Clinical Trials Registration
NCT02378480 and NCT02877927.
Keywords: omadacycline, skin infection, acute bacterial skin and skin structure infections, tetracyclines, MRSA
Skin and skin structure infections are among the most common infectious diseases, with an estimated incidence of almost 500 cases per 10 000 person-years [1]. Skin and skin structure infections vary in severity and depth, from mild infections that can be treated with topical antibiotics to life-threatening necrotizing fasciitis requiring surgical intervention. In an immunocompetent host, the vast majority of these infections are due to Staphylococcus aureus and β-hemolytic streptococci, primarily Streptococcus pyogenes [1–3]. In patients with compromised immune systems, other, less-virulent β-hemolytic streptococci (eg, Streptococcus agalactiae in patients with diabetes mellitus), and even gram-negative pathogens, may cause skin and skin structure infections [4, 5].
S. aureus is the predominant pathogen identified in skin infection due to its greater proclivity, compared with streptococci, to form abscesses, which also increases the probability of obtaining a positive culture [1, 6]. Methicillin-resistant S. aureus (MRSA) strains are resistant to all clinically available β-lactam antibiotics, except ceftaroline, through their expression of penicillin-binding protein 2A, encoded by the mecA gene [7, 8]. From the 1990s until about 2013, significant increases in the number of MRSA skin infections were noted in the United States, coinciding with a steady increase in hospitalizations [9]. The incidence of MRSA in the United States is slowly decreasing, but remains sufficiently high that empiric coverage of MRSA is often initiated in treating purulent skin and soft tissue infections [10, 11].
For clinical trial assessments of antimicrobial therapeutics in skin and soft tissue infections, the US Food and Drug Administration (FDA) defines an acute bacterial skin and skin structure infection (ABSSSI) as cellulitis/erysipelas, a wound infection, or a major cutaneous abscess that has an area of erythema, induration, or fluctuance of ≥75 cm2 [12]. This definition guides how antibiotics are developed for ABSSSI, which can differ from clinical practice by excluding common diseases such as diabetic foot infections, animal bite wounds, and catheter-associated skin infections [13].
Omadacycline is an aminomethylcycline, which is a semisynthetic tetracycline antibiotic, approved in the United States for the treatment of ABSSSI and community-acquired bacterial pneumonia [14]. Omadacycline demonstrated potent in vitro activity against common gram-positive aerobes, many gram-negative aerobes, anaerobes, and atypical bacterial pathogens [15, 16]. Omadacycline is active against strains that exhibit common mechanisms of resistance specific to tetracyclines (efflux pumps and ribosomal protection), as well as strains that are resistant to currently available antibiotics for ABSSSI, including β-lactams, glycopeptides, macrolides, and fluoroquinolones [15]. This article reports the integrated analysis of 2 phase III studies of omadacycline in the treatment of ABSSSI.
METHODS
The Omadacycline in Acute Skin and Skin Structure Infections Study (OASIS) program was composed of 2 phase III, multicenter, randomized, double-blind, double-dummy, noninferiority studies: the global OASIS-1 (NCT02378480) and the US-only OASIS-2 (NCT02877927) [17, 18]. Both studies enrolled adults with ABSSSI meeting the FDA-established criteria, and compared outcomes of treatment with omadacycline or linezolid. OASIS-1 initiated patients on intravenous (IV) omadacycline or IV linezolid, with the option to transition to oral formulations after day 3 if there was evidence of clinical improvement. OASIS-2 investigated oral-only formulations of omadacycline versus linezolid (Table 1).
Table 1.
Study Design Characteristics
| Characteristic | OASIS-1 | OASIS-2 |
|---|---|---|
| Treatment duration | 7–14 days | 7–14 days |
| Omadacycline dosing | 100 mg IV q12h for 2 doses, then 100 mg IV q24h for 2 days Optional at >3 days: transition to 300 mg PO q24ha |
450 mg PO q24h for 2 doses, then 300 mg PO q24h |
| Linezolid dosing | 600 mg IV q12h Optional at >3 days: transition to 600 mg PO q12h |
600 mg PO q12h |
| FDA primary endpointb | ECR at 48–72 h | ECR at 48–72 h |
| EMA primary endpointc | Investigator-assessed clinical response at PTE | Investigator-assessed clinical response at PTE |
| Prior antibiotics prohibited | Within 72 h of randomization, any other systemic or topical antibiotic agent potentially effective for ABSSSI | Within 72 h of randomization, any other systemic or topical antibiotic agent potentially effective for ABSSSId |
| Concomitant antibiotics prohibited | Any other systemic antibiotic against known/suspected ABSSSI pathogens, except in cases of clinical failure Any topical antibacterial agent active against known/suspected ABSSSI pathogen on study infection |
Any other systemic antibiotic agent potentially effective for ABSSSI, except in cases of clinical failure Any topical antibacterial agent active against known/ suspected ABSSSI pathogen on study infection |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infections; ECR, early clinical response; EMA, European Medicines Agency; FDA, Food and Drug Administration; IV, intravenous; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; PO, oral; PTE, posttreatment evaluation; q12h, every 12 hours; q24h, every 24 hours.
aA transition from the IV to PO study drug was an option if there was evidence of local and systemic improvement (eg, temperature ≤100°F, return of white blood cell count and differential toward normal range, no increase in lesion area compared with baseline, and decrease in extent and intensity of ≥1 inflammatory finding).
bECR was defined as: patient alive, with a reduction in lesion area of ≥20% vs baseline and no receipt of rescue antibacterial therapy.
cPTE occurred at 7–14 days after treatment initiation.
dA single dose of short-acting non-oxazolidinone antibacterial administered within 72 h prior to randomization was allowed for ≤25% of patients.
The primary efficacy population for the FDA was the modified intent-to-treat (mITT) population, which included all randomized patients without a baseline sole gram-negative pathogen, as the comparator linezolid does not provide gram-negative pathogen coverage. The coprimary efficacy populations for the European Medicines Agency (EMA) were the mITT population and the clinically evaluable (CE) population, which consisted of all patients in the mITT population who received a study drug, had a qualifying ABSSSI, had an assessment outcome, and met all other criteria for evaluation. The microbiological mITT (micro-mITT) population consisted of all patients in the mITT population who had ≥1 gram-positive causative pathogen. The microbiologically evaluable population consisted of all patients included in both the micro-mITT and CE populations, and the safety population included all intent-to-treat patients who received ≥1 dose of a study drug.
Endpoints were investigated to meet requirements for the 2 regulatory agencies. The FDA primary endpoint for both studies was early clinical response (ECR) at 48–72 hours after treatment initiation, defined as: patient alive, with a reduction in lesion area of ≥20% vs baseline and no receipt of rescue antibacterial therapy. The EMA coprimary endpoint for both studies was the investigator-assessed clinical response (IACR) at the posttreatment evaluation (PTE), which occurred 7–14 days after last dose for both the mITT and CE populations. A successful IACR was defined as the patient being alive, with resolution of the signs and symptoms of infection such that further antibacterial treatment was not needed. The EMA coprimary efficacy endpoints were also secondary efficacy endpoints for the FDA.
Both studies also assessed the microbiological response at the end of treatment (EOT) and at PTE. Infection-site samples and blood were obtained for culture and microbiological testing, including appropriate protocol-defined samples (eg, punch biopsy, leading edge aspirate) from those with cellulitis/erysipelas. The numbers of patients with a favorable (eradication and presumed eradication) and an unfavorable (persistence, presumed persistence, and indeterminate) microbiological response were calculated. A superinfection was defined as a nonbaseline pathogen isolated from the primary ABSSSI site in a patient assessed as exhibiting clinical failure while the patient was still on the study drug, and a new infection was defined similarly except for its occurrence while the patient was no longer on the study drug. Safety was assessed by measuring vital signs, electrocardiograms, standard laboratory parameters (ie, chemistry, hematology, and coagulation), and adverse events.
Data presented here are integrated analyses from OASIS-1 and OASIS-2. A noninferiority margin of 10% was used for ECR, based on historical data comparing antibacterial drugs vs nonantibacterial treatments and current guidance from the FDA [12]. A noninferiority margin of 10% for IACR was based on guidance from the EMA [19]. The 2-sided 95% confidence intervals (CI) for the differences in ECR and IACR clinical success rates were calculated using the Miettinen and Nurminen method, with stratification [20]. Noninferiority of omadacycline to linezolid was concluded if the lower limit of the 95% CI for the treatment difference was greater than –10%. No control for multiplicity was employed for the analysis of IACR, since this was the primary endpoint for the EMA. No inferential analyses were conducted for other endpoints or for analysis populations.
RESULTS
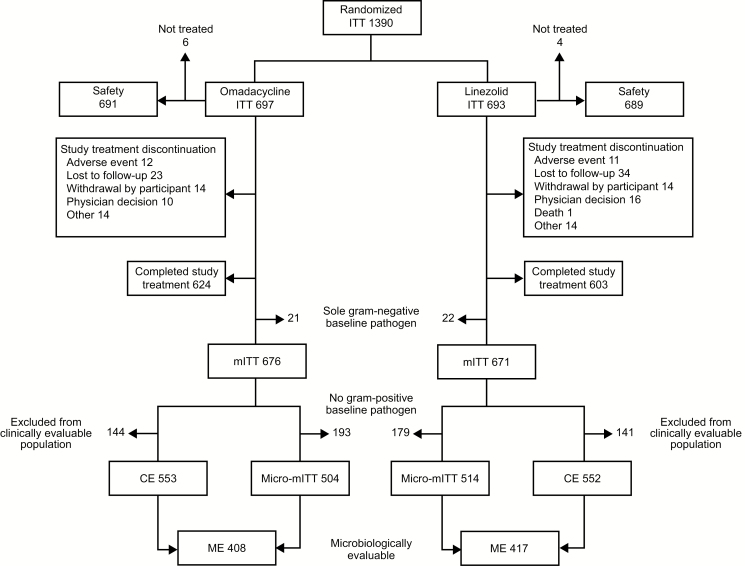
Patient demographic and baseline characteristics were similar in the omadacycline and linezolid groups (Table 2). The mean age was 45.1 years (standard deviation [SD] 14.2), and 92.6% of patients were ≤65 years of age. Overall, 63.6% of patients were male. A total of 82.6% of patients were enrolled from a US site, 12.5% from a European Union site, and 4.9% from a site located outside the United States or European Union. In total, 89.5% (624/697) of patients receiving omadacycline and 87.0% (603/693) receiving linezolid completed study treatment (Figure 1). Reasons for study treatment discontinuation for patients receiving omadacycline or linezolid included being lost to follow-up (3.3% and 4.9%, respectively); withdrawal of consent (both 2.0%); physician decision (1.4% and 2.3%); death (0.0% and 0.1%); and having an adverse event (1.7% and 1.6%). IV drug use was reported in 59.6% of patients, a history of ABSSSI was reported in 51.4%, and diabetes was present in 5.6% of patients receiving omadacycline and 9.7% of patients receiving linezolid.
Table 2.
Demographic and Baseline Characteristics for Patients in the Phase III ABSSSI Studies
| OASIS-1 and OASIS-2 | |||
|---|---|---|---|
| Characteristic | Omadacycline (n = 691) | Linezolid (n = 689) | All Patients (n = 1380) |
| Age, years | |||
| Mean (SD) | 44.7 (14.2) | 45.5 (14.2) | 45.1 (14.2) |
| Min, max | 18, 88 | 18, 90 | 18, 90 |
| Sex | |||
| Male | 445 (64.4) | 433 (62.8) | 878 (63.6) |
| Race | |||
| White | 621 (89.9) | 641 (93.0) | 1262 (91.4) |
| Ethnicity | |||
| Hispanic or Latino | 238 (34.4) | 247 (35.8) | 485 (35.1) |
| Not Hispanic or Latino | 449 (65.0) | 440 (63.9) | 889 (64.4) |
| Not reported/unknown | 4 (0.6) | 2 (0.3) | 6 (0.4) |
| Region | |||
| United States | 570 (82.5) | 570 (82.7) | 1140 (82.6) |
| Non–United States | 121 (17.5) | 119 (17.3) | 240 (17.4) |
| European Uniona | 85 (12.3) | 88 (12.8) | 173 (12.5) |
| BMI (kg/m2)b | |||
| <25 | 260 (37.6) | 245 (35.6) | 505 (36.6) |
| 25–30 | 221 (32.0) | 243 (35.3) | 464 (33.6) |
| >30 | 210 (30.4) | 200 (29.1) | 410 (29.7) |
| Creatinine clearance | |||
| >89 mL/min | 603 (87.6) | 612 (89.5) | 1215 (88.6) |
| 60–89 mL/min | 64 (9.3) | 51 (7.5) | 115 (8.4) |
| <60 mL/min | 21 (3.1) | 21 (3.1) | 42 (3.1) |
| Type of primary infection | |||
| N (mITT population) | 676 | 671 | 1347 |
| Wound infection | 312 (46.2) | 318 (47.4) | 630 (46.8) |
| Cellulitis/erysipelas | 209 (30.9) | 202 (30.1) | 411 (30.5) |
| Major abscess | 155 (22.9) | 151 (22.5) | 306 (22.7) |
| Pathogenc | |||
| N (micro-mITT population) | 504 | 514 | 1018 |
| Gram-positive aerobes | 490 (97.2) | 497 (96.7) | 987 (97.0) |
| Staphylococcus aureus | 376 (74.6) | 384 (74.7) | 760 (74.7) |
| MRSA | 173 (34.3) | 157 (30.5) | 330 (32.4) |
| MSSA | 208 (41.3) | 232 (45.1) | 440 (43.2) |
| Streptococcus pyogenes | 40 (7.9) | 34 (6.6) | 74 (7.3) |
| Streptococcus anginosus groupd | 104 (20.6) | 82 (16.0) | 186 (18.3) |
| Gram-positive anaerobes | 33 (6.5) | 32 (6.2) | 65 (6.4) |
| Gram-negative aerobes | 52 (10.3) | 53 (10.3) | 105 (10.3) |
| Gram-negative anaerobes | 28 (5.6) | 25 (4.9) | 53 (5.2) |
Data are presented as No. (%) and are from the safety population, unless otherwise noted. The denominator for the percentage was the number of patients who had that parameter assessed.
Abbreviations: ABSSSI, acute bacterial skin and skin structure infections; BMI, body mass index; max, maximum; min, minimum; mITT, modified intent-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; SD, standard deviation.
aThe European Union data were a subset of non–United States data, not a mutually exclusive subgroup.
bThe highest BMI measured in the study was 71.3 kg/m2.
cIn each infection, >1 pathogen may have been present; therefore, numbers sum to >100%. Pathogens present in >5% of patients in the micro-mITT population (all mITT patients who had ≥1 causative pathogen) are shown.
d Streptococcus anginosus group consists of S. anginosus, S. intermedius, and S. constellatus.
Figure 1.
Disposition of patients enrolled in OASIS-1 and OASIS-2. Abbreviations: CE, clinically evaluable; ITT, intent-to-treat; ME, microbiologically evaluable; micro-mITT, all mITT patients with ≥1 causative pathogen; mITT, modified ITT; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study.
The most common lesion locations were the lower extremity (38.3% for omadacycline vs 36.1% for linezolid) and the upper extremity (29.9% for omadacycline vs 31.6% for linezolid). Baseline infection types were similar in both groups: wound infection was seen in 46.8% of all patients, cellulitis/erysipelas in 30.5%, and major abscess in 22.7% (Table 2). In patients with major abscesses, 72.9% of omadacycline and 67.5% of linezolid patients had an allowed drainage procedure prior to (or within 48 hours following) the first dose. The mean baseline lesion area was 437 cm2 (SD 403) for patients randomized to omadacycline and 444 cm2 (SD 491) for those randomized to linezolid. Mean lesion surface areas across both treatment groups were >400 cm2 for wound infections and cellulitis, and >300 cm2 for major abscesses.
The mean treatment duration was 8.7 days (SD 2.8) for the omadacycline group and 8.5 days (SD 3.0) for the linezolid group. In the omadacycline group, 37.9% (262/691) had a treatment duration of 4–8 days, and 54.1% (374/691) had a treatment duration of 9–14 days. In the linezolid group, 40.3% (278/689) had a treatment duration of 4–8 days and 49.5% (341/689) had a treatment duration of 9–14 days.
Clinical Efficacy
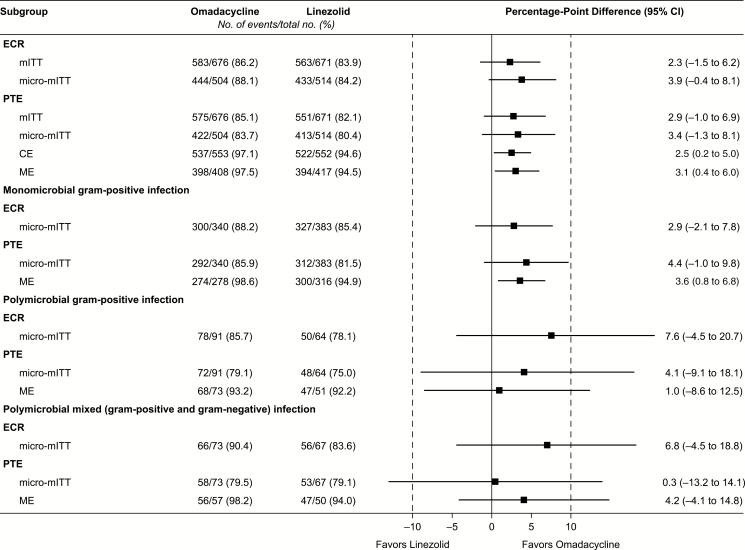
Omadacycline was noninferior to linezolid for the FDA primary endpoint of ECR in the mITT population (86.2% vs 83.9%; difference 2.3, 95% CI –1.5 to 6.2). The most common reason for not achieving ECR was that the lesion area was not reduced by ≥20% (5.5% omadacycline, 6.0% linezolid); notably, most of these patients went on to have IACR at EOT and PTE. Similarly, omadacycline was noninferior to linezolid for the EMA coprimary endpoint of IACR at PTE in the mITT population (85.1% vs 82.1%; difference 2.9, 95% CI –1.0 to 6.9) and CE population (97.1% vs 94.6%; difference 2.5, 95% CI 0.2–5.0). The most common reason for failure at PTE was that the patient required additional antibiotic therapy for the infection under study (omadacycline 1.6%, linezolid 1.6%). Efficacy results for both ECR and IACR at PTE were similar in additional analysis populations, including the micro-mITT and microbiologically evaluable populations (Figure 2).
Figure 2.
Forest plots for US Food and Drug Administration and European Medicines Agency endpoints in different analysis populations show that omadacycline had statistically similar outcomes to linezolid. Abbreviations: CE, clinically evaluable; CI, confidence interval; ECR, early clinical response; ME, microbiologically evaluable; micro-mITT, all mITT patients with ≥1 causative pathogen; mITT, modified intent-to-treat; PTE, posttreatment evaluation.
No differences in efficacy were observed by infection type or by lesion area (Table 3). ECR and IACR at PTE were >80% for each infection type, except for cellulitis (ECR of 78.9% for omadacycline, vs 81.2% for linezolid) and wound infections (IACR at PTE of 82.1% for omadacycline, vs 78.0% for linezolid). The vast majority of patients had baseline lesion areas <600 cm2. ECR and IACR at PTE were >80% in both treatment groups for baseline lesion areas <1000 cm2. For baseline lesion areas >1000 cm2, omadacycline had lower ECR rates than linezolid (73.3% vs 80.0%, respectively), but had higher IACR at PTE rates (82.2% vs 74.0%). Altogether, 82 patients in each treatment group met ≥2 criteria for systemic inflammatory response syndrome (SIRS) at baseline [21]. In omadacycline- and linezolid-treated patients who met SIRS criteria at baseline, ECR (82.9% and 80.5%, respectively) and IACR (80.5% and 79.3%) were similar at PTE.
Table 3.
Clinical Response by Infection Type and Size
| Parameter | Omadacycline (n = 676) | Linezolid (n = 671) | Difference (95% CI) |
|---|---|---|---|
| Wound infection | 312 | 318 | |
| ECR | 278 (89.1) | 269 (84.6) | 4.5 (–0.8 to 9.9) |
| IACR-PTE | 256 (82.1) | 248 (78.0) | 4.1 (–2.2 to 10.3) |
| Cellulitis/erysipelas | 209 | 202 | |
| ECR | 165 (78.9) | 164 (81.2) | –2.2 (–10.0 to 5.5) |
| IACR-PTE | 188 (90.0) | 178 (88.1) | 1.8 (–4.3 to 8.1) |
| Major abscess | 155 | 151 | |
| ECR | 140 (90.3) | 130 (86.1) | 4.2 (–3.1 to 11.8) |
| IACR-PTE | 131 (84.5) | 125 (82.8) | 1.7 (–6.6 to 10.2) |
| Lesion area ≤300 cm2 | 322 | 332 | |
| ECR | 286 (88.8) | 276 (83.1) | 5.7 (0.4 to 11.1) |
| IACR-PTE | 290 (90.1) | 271 (81.6) | 8.4 (3.1 to 13.8) |
| Lesion area >300–600 cm2 | 222 | 219 | |
| ECR | 192 (86.5) | 188 (85.8) | 0.6 (–5.9 to 7.2) |
| IACR-PTE | 178 (80.2) | 186 (84.9) | –4.8 (–11.9 to 2.4) |
| Lesion area >600–1000 cm2 | 87 | 70 | |
| ECR | 72 (82.8) | 59 (84.3) | –1.5 (–13.2 to 10.8) |
| IACR-PTE | 70 (80.5) | 57 (81.4) | –1.0 (–13.2 to 11.9) |
| Lesion area >1000 cm2 | 45 | 50 | |
| ECR | 33 (73.3) | 40 (80.0) | –6.7 (–24.0 to 10.5) |
| IACR-PTE | 37 (82.2) | 37 (74.0) | 8.2 (–8.9 to 24.8) |
Data are presented as No. (%) and are from the mITT population.
Abbreviations: CI, confidence interval; ECR, early clinical response; IACR, investigator-assessed clinical response; mITT, modified intent-to-treat; PTE, posttreatment evaluation.
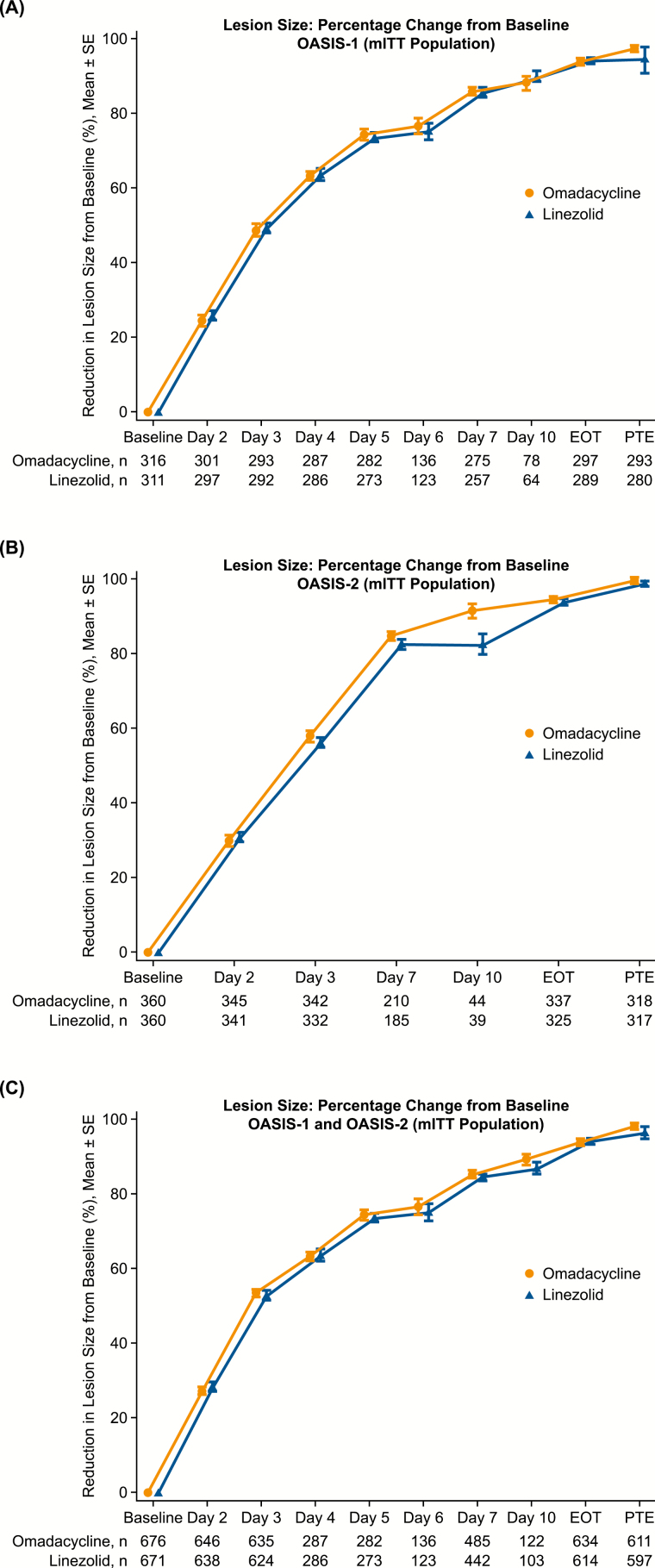
Lesion areas were reduced early and substantially from baseline in both groups (Figure 3). In each trial and in the integrated analysis, a ≥20% reduction in lesion area was observed by day 2. At day 3, the mean reduction from baseline in lesion area was 53.4% (SD 25.8%) for omadacycline-treated patients and 53.0% (SD 24.2%) for linezolid-treated patients. By EOT, the mean reduction in lesion area was 93.9% (SD 14.7%) and 93.7% (SD 13.5%) for omadacycline- and linezolid-treated patients, respectively.
Figure 3.
Reduction in lesion size from baseline to posttreatment evaluation in mITT population: A, OASIS-1 intravenous to oral study; B, OASIS-2 oral-only study; and C, combined data from OASIS-1 and OASIS-2. In all graphs, omadacycline shows a similar trend to linezolid in lesion size over the study duration. Error bars represent the standard error. Lines are offset horizontally to better visualize the data points. Abbreviations: EOT, end of treatment; mITT, modified intent-to-treat; OASIS, Omadacycline in Acute Skin and Skin Structure Infections Study; PTE, posttreatment evaluation; SE, standard error.
Clinical Response by Baseline Pathogen
At least 1 gram-positive ABSSSI pathogen was identified in 1018/1390 (73.2%) intent-to-treat patients in the phase III ABSSSI studies (Table 2). S. aureus was the most common pathogen, detected in 74.6% of patients in the omadacycline group and 74.7% of patients in the linezolid group in whom a pathogen was identified (micro-mITT; Table 2). MRSA was isolated in 34.3% and 30.5% of patients in whom a pathogen was identified in the omadacycline and linezolid groups, respectively.
Omadacycline demonstrated similar efficacy to linezolid in treating infections caused by S. aureus (including MRSA), S. pyogenes, and Streptococcus anginosus (Table 4). Overall clinical success rates at PTE against gram-positive aerobes were 83.3% (408/490) for omadacycline and 80.3% (399/497) for linezolid. Similar rates of clinical success were observed against gram-positive anaerobes (omadacycline: 90.9%, 30/33; linezolid: 81.3%, 26/32), gram-negative aerobes (omadacycline: 76.9%, 40/52; linezolid: 75.5%, 40/53), and gram-negative anaerobes (omadacycline: 78.6%, 22/28; linezolid: 80.0%, 20/25). Responses were also similar across treatments for polymicrobial gram-positive infections and for mixed gram-positive and gram-negative infections (Figure 2).
Table 4.
Clinical Response by Baseline Pathogen
| Pathogen | Omadacycline (n = 504) | Linezolid (n = 514) |
|---|---|---|
| Staphylococcus aureus, n | 376 | 384 |
| ECR | 332 (88.3) | 325 (84.6) |
| IACR-PTE | 312 (83.0) | 312 (81.3) |
| MRSA, n | 173 | 157 |
| ECR | 159 (91.9) | 139 (88.5) |
| IACR-PTE | 146 (84.4) | 128 (81.5) |
| MSSA, n | 208 | 232 |
| ECR | 178 (85.6) | 190 (81.9) |
| IACR-PTE | 171 (82.2) | 187 (80.6) |
| Streptococcus pyogenes, n | 40 | 34 |
| ECR | 32 (80.0) | 30 (88.2) |
| IACR-PTE | 28 (70.0) | 25 (73.5) |
| Staphylococcus lugdunensis, n | 11 | 3 |
| ECR | 10 (90.9) | 3 (100.0) |
| IACR-PTE | 10 (90.9) | 2 (66.7) |
| Enterococcus faecalis, n | 18 | 25 |
| ECR | 16 (88.9) | 20 (80.0) |
| IACR-PTE | 17 (94.4) | 21 (84.0) |
| Enterobacter cloacae, n | 8 | 7 |
| ECR | 8 (100.0) | 6 (85.7) |
| IACR-PTE | 7 (87.5) | 7 (100.0) |
| Klebsiella pneumoniae, n | 11 | 11 |
| ECR | 10 (90.9) | 9 (81.8) |
| IACR-PTE | 8 (72.7) | 6 (54.5) |
| Streptococcus anginosus group, na | 104 | 82 |
| ECR | 93 (89.4) | 63 (76.8) |
| IACR-PTE | 84 (80.8) | 59 (72.0) |
Data are presented as No. (%) and are from the micro-mITT population. Baseline pathogens were identified by culture of blood or ABSSSI site specimens. An acceptable ABSSSI site specimen was defined as a specimen obtained from a biopsy of involved cutaneous or subcutaneous tissue, preferably from the advancing margin of the lesion; debrided tissue; tissue scraping (using curette or scalpel); needle aspirate of involved, nonpurulent cutaneous or subcutaneous tissue; pus or infected tissue collected during an incision and drainage procedure; or pus aspirated into a syringe or a deep swab of purulent material (only if collected from infected tissue that had been incised or was draining). Surface swabs of wounds, inflamed skin, or drainage (including purulent material) were not considered valid sampling techniques.
Abbreviations: ABSSSI, acute bacterial skin and skin structure infections; ECR, early clinical response; IACR, investigator-assessed clinical response; micro-mITT, all modified intent-to-treat patients who had ≥1 causative pathogen; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; PTE, posttreatment evaluation.
aThe Streptococcus anginosus group consists of S. anginosus, S. intermedius, and S. constellatus.
Baseline bacteremia was identified in 13 omadacycline-treated patients and 17 linezolid-treated patients. Most patients with bacteremia at baseline (30/1347) had S. aureus identified as a causative pathogen. For patients with bacteremia at baseline, ECR was achieved in 8/13 (61.5%) omadacycline-treated and 14/17 (82.4%) linezolid-treated patients. There were 3 omadacycline-treated patients (2 with methicillin-susceptible S. aureus and 1 with S. pyogenes) and 1 linezolid-treated patient (with methicillin-susceptible S. aureus) who had bacteremia and were considered failures at ECR. At PTE, clinical success by IACR was achieved in 10/13 (76.9%) omadacycline-treated patients and 14/17 (82.4%) linezolid-treated patients.
Microbiological responses at PTE were favorable in 423/504 (83.9%) and 413/514 (80.4%) omadacycline-treated and linezolid-treated patients, respectively, and were consistent with clinical responses. Superinfection and new infection each occurred in 2 (0.4%) omadacycline-treated patients and in 1 (0.2%) and 5 (1.0%) linezolid-treated patients, respectively. No pathogen developed resistance to a study drug during therapy.
Safety
Treatment-emergent adverse events (TEAEs) were reported by 353/691 (51.1%) patients receiving omadacycline and 284/689 (41.2%) patients receiving linezolid (Table 5). Most of these patients reported TEAEs that were mild (omadacycline, 32.3%; linezolid, 26.0%) or moderate (omadacycline, 17.1%; linezolid, 12.8%) in severity. Severe TEAEs were reported in 1.7% of patients in the omadacycline group and 2.5% of those in the linezolid group. Serious TEAEs and discontinuations due to TEAEs and serious TEAEs were infrequent (Table 5). There was 1 death (0.1%) in the omadacycline group (opiate overdose) and 3 deaths (0.4%) in the linezolid group (1 cardiac arrest, 1 cardiac failure, and 1 illicit drug overdose). No death was considered related to the study treatment.
Table 5.
Overview of Treatment-emergent Adverse Events, by Treatment Group
| Parameter | Omadacycline (n = 691) | Linezolid (n = 689) |
|---|---|---|
| Patients with any TEAE | 353 (51.1) | 284 (41.2) |
| Number of patients (%) with: | ||
| Drug-related TEAE | 197 (28.5) | 111 (16.1) |
| Serious TEAE | 16 (2.3) | 13 (1.9) |
| Drug-related serious TEAE | 0 | 1 (0.1) |
| TEAE leading to deatha | 1 (0.1%) | 3 (0.4) |
| TEAE leading to early discontinuation of study drug | 12 (1.7) | 10 (1.5) |
| TEAE leading to dose interruption of study drug | 2 (0.3) | 0 |
| Serious TEAEs leading to early discontinuation of study drug | 6 (0.9) | 5 (0.7) |
Data are presented as No. (%) and are from the safety population. Percentages were based on the number of patients. If a patient had >1 TEAE with the same preferred term, the patient was counted only once for that preferred term.
Abbreviation: TEAE, treatment-emergent adverse event.
aCauses of death: 1 opiate overdose in the omadacycline group; 1 cardiac arrest, 1 cardiac failure, and 1 illicit drug overdose in the linezolid group.
The most frequently reported TEAEs were nausea (omadacycline, 21.9%; linezolid, 8.7%) and vomiting (omadacycline, 11.4%; linezolid, 3.9%; Table 6). All gastrointestinal (GI) TEAEs were reported as mild or moderate in patients receiving omadacycline. GI TEAEs led to study drug discontinuation in 2 (0.3%) patients receiving omadacycline and in 1 (0.1%) patient receiving linezolid (see Opal et al, in this supplement, for further details).
Table 6.
Most Frequent Treatment-emergent Adverse Events (≥3% Incidence for Any Drug), by Treatment Group and Preferred Term
| Parameter | Omadacycline (n = 691), n (%) | Linezolid (n = 689), n (%) |
|---|---|---|
| Patients with ≥1 TEAE | 353 (51.1) | 284 (41.2) |
| Nausea | 151 (21.9) | 60 (8.7) |
| Vomiting | 79 (11.4) | 27 (3.9) |
| Wound infection | 30 (4.3) | 22 (3.2) |
| ALT increased | 28 (4.1) | 25 (3.6) |
| Infusion-site extravasation | 28 (4.1) | 19 (2.8) |
| Cellulitis/erysipelas | 27 (3.9) | 24 (3.5) |
| AST increased | 25 (3.6) | 24 (3.5) |
| Headache | 23 (3.3) | 21 (3.0) |
| Subcutaneous abscess | 23 (3.3) | 27 (3.9) |
| Diarrheaa | 22 (3.2) | 20 (2.9) |
Data are from the safety population. Percentages were based on the number of patients in each treatment group. Patients may have been counted in >1 row. Coding of preferred terms was based on the Medical Dictionary for Regulatory Activities, Version 17.1.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAE, treatment-emergent adverse event.
aThere were no cases of infection with Clostridioides difficile in either study.
Infusion-site extravasations were reported in 8.7% of patients receiving omadacycline and 5.9% of those receiving linezolid in OASIS-1, were considered unrelated to the study drug, and were related to difficulty obtaining IV access in patients with a history of IV drug use. Skin and skin structure infection TEAEs included wound infection (omadacycline, 4.3%; linezolid, 3.2%), cellulitis/erysipelas (omadacycline, 3.9%; linezolid, 3.5%), and subcutaneous abscess (omadacycline, 3.3%; linezolid, 3.9%). These events represented worsening of the study infection, a recurrent infection at the index ABSSSI site, or a new ABSSSI distinct from the study infection, and were considered unrelated to the study treatment.
No clinically significant trends in standard laboratory parameters (ie, chemistry, hematology, and coagulation), vital signs, or electrocardiogram measurements were observed in either treatment group. TEAEs of increased liver transaminases were similar in both treatment groups (Table 6). Postbaseline increases in alanine aminotransferase >3 × upper limit of normal occurred in 4.7% and 4.1% of omadacycline-treated and linezolid-treated patients, respectively. Postbaseline changes in total bilirubin >2 × upper limit of normal occurred in 0.7% and 0.2% of omadacycline-treated and linezolid-treated patients, respectively. No patients met the criteria for Hy’s Law.
DISCUSSION
In this integrated analysis of OASIS-1 and OASIS-2, omadacycline was noninferior to linezolid in the treatment of ABSSSI for the primary endpoints. The efficacy results were consistent between the FDA early (ECR) and EMA late (PTE) assessments; ECR and IACR at PTE results within subpopulations were also consistent with the primary efficacy results. Omadacycline demonstrated a similar rate of clinical response to linezolid in the integrated analysis, with a reduction of ≥20% in the lesion areas observed by day 2. Overall, the integrated analysis of almost 1400 patients demonstrated that omadacycline is as effective as linezolid, a commonly used ABSSSI therapy.
Omadacycline was effective in ABSSSI caused by S. aureus, including MRSA (Table 2). Due to the rise in MRSA in the early 2000s, MRSA remains the most prevalent and resistant ABSSSI pathogen requiring effective treatment in clinical practice. It should be noted that the mean lesion area in these trials was almost 6 times larger than the minimum lesion area for trial enrollment of 75 cm2; omadacycline was effective against large ABSSSI lesions. In light of these efficacy results, healthcare providers may consider omadacycline an appropriate empiric treatment option prior to culture availability in patients with a large area of skin involvement, in particular when MRSA coverage is required.
Omadacycline was safe and well tolerated, with safety results similar to linezolid. Consistent with studies of tetracyclines, GI adverse events were the most common TEAEs reported [22]. Oral administration of omadacycline was associated with higher rates of GI TEAEs, compared with IV omadacycline or linezolid; however, there were few study drug discontinuations related to GI TEAEs. These higher rates of nausea and vomiting were associated with the 450 mg loading dose during the first 2 days of the oral-only OASIS-2 study; rates of nausea and vomiting decreased thereafter (see Opal et al, in this supplement).
While clinicians have many antimicrobial options in the treatment of ABSSSI, the current treatment paradigms in ABSSSI call for increased efficiency and streamlining in the overall approach to care. Omadacycline’s spectrum of activity includes: (1) typical gram-positive ABSSSI pathogens (including antibiotic-resistant strains), which make it useful for the empiric treatment of cellulitis and major abscess; and (2) many gram-negative and anaerobic pathogens (as reported by Pfaller et al [23] and Stapert et al [24]), which may contribute to wound infections, especially in patients with comorbidities that compromise innate immunity (eg, diabetes mellitus). With both IV and oral formulations, omadacycline may allow for the improved utilization of hospitalization resources, particularly for the many patients hospitalized for ABSSSI who show no systemic symptoms and have limited comorbid conditions [25]. Many patients with ABSSSI are admitted to the hospital solely for administration of IV antibiotics [26]. Once hospitalized, the average length of hospital stay for ABSSSI treatment is ~4–7 days [3, 27, 28]. Treatment with omadacycline and its availability in both IV and oral formulations may facilitate transitions from inpatient to outpatient therapy, thereby reducing lengths of hospitalization or even possibly avoiding admissions altogether [29].
The limitations of the integrated analysis are similar to the limitations of the individual studies. The studies enrolled few elderly patients or patients with diabetes, which reflects the epidemiology of participants enrolled in the FDA ABSSSI registration trials [1, 30, 31]. In alignment with regulatory guidance, patients with some types of common community-acquired skin infections, including bite wounds and chronic skin infections (eg, diabetic foot infections), were excluded. Additionally, because the comparator, linezolid, does not provide coverage against gram-negative bacteria, the OASIS studies were not able to analyze the efficacy of omadacycline against these pathogens. Future research, including postmarketing real-world evidence data, should expand upon omadacycline’s utility in treating these important subgroups and infection types.
CONCLUSIONS
Omadacycline was noninferior to linezolid for ECR and late clinical responses in ABSSSI. Omadacycline had high efficacy, similar to that of linezolid, in the treatment of ABSSSI caused by gram-positive pathogens, including MRSA. Omadacycline showed an acceptable safety profile and is another therapeutic option for ABSSSI.
Notes
Author contributions. All authors provided critical revisions to the manuscript for intellectual content and approved the final version for publication.
Acknowledgments. The authors thank the investigators who participated in OASIS-1 and -2. Editorial support was provided by Agnella Matic, PhD, and Samantha Scott, PhD, of Innovative Strategic Communications, LLC, Milford, Pennsylvania.
Financial support. This work was supported by Paratek Pharmaceuticals, Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Supplement sponsorship. This article appears as part of the supplement “Omadacycline: A New Option in an Era of Increasing Antibiotic Resistance,” sponsored by Paratek Pharmaceuticals, Inc., King of Prussia, Pennsylvania.
Potential conflicts of interest. F. M. A. has received speaking honoraria from Allergan, Merck, Melinta Therapeutics, Grifols, The Medicines Company, and Seqirus; consulting fees from Paratek Pharmaceuticals, Melinta Therapeutics, Nabriva Therapeutics, Grifols, Seqirus, Cempra Pharmaceuticals, Summit Therapeutics, Tetraphase Pharmaceuticals, and Janssen Research & Development; research grants from Cempra Pharmaceuticals, Optimer Pharmaceuticals, and Merck; and manuscript review fees from Elsevier, all outside the submitted work. G. S. has received speaking honoraria from Allergan Plc, Sunovion Pharmaceuticals, Melinta, and The Medicines Company; has received consulting fees from Allergan and Paratek Pharmaceuticals; and is on the scientific advisory boards of Cidara Therapeutics and Arsanis Pharmaceuticals. A. F. D. is a consultant for Paratek Pharmaceuticals and has received personal fees from Achaogen, AntibioTx, Cempra, Contrafect, Iterum Tx, Melinta, Nabriva, Tetraphase, Wochardt, Theravance, and Zavante, all outside the submitted work. P. B. E. has received personal fees from Spero Therapeutics, Zavante Therapeutics, Utility Therapeutics, and InClin outside the submitted work; and is a consultant for Paratek Pharmaceuticals. E. T., A. M., J. S., and P. C. M. are employees and shareholders of Paratek Pharmaceuticals. The authors received no financial compensation for the preparation of this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the article have been disclosed.
References
- 1. Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a US population: a retrospective population-based study. BMC Infect Dis 2013; 13:252. PMID: 23721377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pulido-Cejudo A, Guzmán-Gutierrez M, Jalife-Montaño A, et al. . Management of acute bacterial skin and skin structure infections with a focus on patients at high risk of treatment failure. Ther Adv Infect Dis 2017; 4:143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zervos MJ, Freeman K, Vo L, et al. . Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol 2012; 50:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carey CF, Dall L. Diagnosis of cellulitis in the immunocompromised host. Can J Infect Dis 1990; 1:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanai H, Hamasaki H, Tsuda N, et al. . Group B streptococcus infection and diabetes: a review. J Microbiol Antimicrob 2012; 4:1–5. [Google Scholar]
- 6. Stevens DL, Bisno AL, Chambers HF, et al. . Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:147–59. [DOI] [PubMed] [Google Scholar]
- 7. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother 2009; 53:4961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maranan MC, Moreira B, Boyle-Vavra S, Daum RS. Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect Dis Clin North Am 1997; 11:813–49. [DOI] [PubMed] [Google Scholar]
- 9. Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PLOS One 2013; 8:e52722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landrum ML, Neumann C, Cook C, et al. . Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA 2012; 308:50–9. [DOI] [PubMed] [Google Scholar]
- 11. Hultén KG, Mason EO, Lamberth LB, Forbes AR, Revell PA, Kaplan SL. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining community acquired methicillin-resistant Staphylococcus aureus infections at a large children’s hospital. Pediatr Infect Dis J 2018; 37:235–41. [DOI] [PubMed] [Google Scholar]
- 12. Center for Drug Evaluation and Research. Guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. Silver Spring, Maryland: US Department of Health and Human Services, Food and Drug Administration, 2013. [Google Scholar]
- 13. Falcone M, Concia E, Giusti M, et al. . Acute bacterial skin and skin structure infections in internal medicine wards: old and new drugs. Intern Emerg Med 2016; 11:637–48. [DOI] [PubMed] [Google Scholar]
- 14. Stets R, Popescu M, Gonong JR, et al. . Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 2019; 380:517–27. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 2016; 24:6409–19. [DOI] [PubMed] [Google Scholar]
- 16. Macone AB, Caruso BK, Leahy RG, et al. . In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother 2014; 58:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Riordan W, Green S, Overcash JS, et al. . Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 2019; 380:528–38. [DOI] [PubMed] [Google Scholar]
- 18. O’Riordan W, Cardenas C, Sirbu A, et al. . A Phase 3 randomized, double-blind, multi-centre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study). In: Program and abstracts of the 28th European Congress of Clinical Microbiology and Infectious Diseases (Madrid, Spain). 2018. Presentation O0425. [Google Scholar]
- 19. European Medicines Agency. Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections [document EMA/CHMP/351889/2013]. 2013. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/addendum-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections_en.pdf. Accessed 7 June 2019. [Google Scholar]
- 20. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 21. Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence 2014; 5:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heta S, Robo I. The side effects of the most commonly used group of antibiotics in periodontal treatments. Med Sci 2018; 6:6. PMID: 30720776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2018; 62:pii: e02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stapert L, Wolfe C, Shinabarger D, Marra A, Pillar C. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicrob Agents Chemother 2018; 62:pii: e00047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lodise TP, Fan W, Sulham KA. Hospital admission patterns in adult patients with skin and soft tissue infections: identification of potentially avoidable hospital admissions through a retrospective database analysis. Hosp Pract (1995) 2015; 43:137–43. [DOI] [PubMed] [Google Scholar]
- 26. Verastegui JE, Hamada Y, Nicolau DP. Transitions of care in the management of acute bacterial skin and skin structure infections: a paradigm shift. Expert Rev Clin Pharmacol 2016; 9:1039–45. [DOI] [PubMed] [Google Scholar]
- 27. Pollack CV Jr, Amin A, Ford WT Jr, et al. . Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med 2015; 48:508–19. [DOI] [PubMed] [Google Scholar]
- 28. Suaya JA, Mera RM, Cassidy A, et al. . Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 2014; 14:296. PMID: 24889406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LaPensee K, Lodise T. Potential cost-savings with once-daily aminomethylcycline antibiotic versus vancomycin in hospitalized patients with acute bacterial skin and skin structure infections. Am Health Drug Benefits 2018; 11:449–59. [PMC free article] [PubMed] [Google Scholar]
- 30. Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLOS One 2015; 10:e0143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edelsberg J, Taneja C, Zervos M, et al. . Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]