Summary
Background
Girls and women need effective, safe, and affordable menstrual products. Single-use products are regularly selected by agencies for resource-poor settings; the menstrual cup is a less known alternative. We reviewed international studies on menstrual cup leakage, acceptability, and safety and explored menstrual cup availability to inform programmes.
Methods
In this systematic review and meta-analysis, we searched PubMed, Cochrane Library, Web of Science, Popline, Cinahl, Global Health database, Emerald, Google Scholar, Science.gov, and WorldWideScience from database inception to May 14, 2019, for quantitative or qualitative studies published in English on experiences and leakage associated with menstrual cups, and adverse event reports. We also screened the Manufacturer and User Facility Device Experience database from the US Food and Drug Administration for events related to menstrual cups. To be eligible for inclusion, the material needed to have information on leakage, acceptability, or safety of menstrual cups. The main outcome of interest was menstrual blood leakage when using a menstrual cup. Safety outcomes of interest included serious adverse events; vaginal abrasions and effects on vaginal microflora; effects on the reproductive, digestive, or urinary tract; and safety in poor sanitary conditions. Findings were tabulated or combined by use of forest plots (random-effects meta-analysis). We also did preliminary estimates on costs and environmental savings potentially associated with cups. This systematic review is registered on PROSPERO, number CRD42016047845.
Findings
Of 436 records identified, 43 studies were eligible for analysis (3319 participants). Most studies reported on vaginal cups (27 [63%] vaginal cups, five [12%] cervical cups, and 11 [25%] mixed types of cups or unknown) and 15 were from low-income and middle-income countries. 22 studies were included in qualitative or quantitative syntheses, of which only three were of moderate-to-high quality. Four studies made a direct comparison between menstrual cups and usual products for the main outcome of leakage and reported leakage was similar or lower for menstrual cups than for disposable pads or tampons (n=293). In all qualitative studies, the adoption of the menstrual cup required a familiarisation phase over several menstrual cycles and peer support improved uptake (two studies in developing countries). In 13 studies, 73% (pooled estimate: n=1144; 95% CI 59–84, I2=96%) of participants wished to continue use of the menstrual cup at study completion. Use of the menstrual cup showed no adverse effects on the vaginal flora (four studies, 507 women). We identified five women who reported severe pain or vaginal wounds, six reports of allergies or rashes, nine of urinary tract complaints (three with hydronephrosis), and five of toxic shock syndrome after use of the menstrual cup. Dislodgement of an intrauterine device was reported in 13 women who used the menstrual cup (eight in case reports, and five in one study) between 1 week and 13 months of insertion of the intrauterine device. Professional assistance to aid removal of menstrual cup was reported among 47 cervical cup users and two vaginal cup users. We identified 199 brands of menstrual cup, and availability in 99 countries with prices ranging US$0·72–46·72 (median $23·3, 145 brands).
Interpretation
Our review indicates that menstrual cups are a safe option for menstruation management and are being used internationally. Good quality studies in this field are needed. Further studies are needed on cost-effectiveness and environmental effect comparing different menstrual products.
Funding
UK Medical Research Council, Department for International Development, and Wellcome Trust.
Introduction
Girls and women need effective, safe, and affordable menstrual products. Globally, an estimated 1·9 billion women—around 26% of the population—were of menstruating age in 2017, spending on average 65 days in the year dealing with menstrual blood flow.1 Menstruation is a normal body function and a sign of reproductive health. Few solutions are available to manage menstruation; additionally, ignorance, prejudice, costs, and safety fears can impede girls and women from testing the full range of products available. A lack of affordable and effective menstrual products can result in leakage and chaffing in menstruating girls and women and can affect their health.2, 3 Use of poor-quality materials has been shown to predispose women to an increased risk of urogenital infections including bacterial vaginosis.4, 5, 6 In some situations, mostly researched in low-income and middle-income countries, menstruation can affect girls' schooling,7 make women and girls a target of sexual violence or coercion,8, 9 and affect employment and work experiences of women.10, 11 In low-income and middle-income countries, a lack of water, sanitation, and hygiene, inadequate education, and poor disposal facilities, raise public health concerns, particularly among schoolgirls.7, 12 In several countries, the number of policy-led initiatives and donations to provide menstrual products have increased—eg, to keep girls in school. To allow such organisations to make informed decisions, information is needed on the full range of menstrual products.
Research in context.
Evidence before this study
A lack of affordable and effective menstrual products can result in leakage and chaffing in menstruating girls and women and can affect their health and education. The number of programmes that provide menstrual products to assist women and girls has increased. The menstrual cup, a receptacle used to collect menstrual blood flow, has received little attention, which in part might reflect concerns about insertable products as either culturally unacceptable or because of previous public health alerts associated with highly absorbent tampons (eg, toxic-shock syndrome). Information about leakage, acceptability, and safety of menstrual cups is needed to support organisations to make informed decisions and provide more comprehensive menstrual health education for girls and women. We searched PubMed, Cochrane Library, Web of Science, Popline, Cinahl, Global Health database, Emerald, Google Scholar, Science.gov, and WorldWideScience from database inception on Nov 24, 2018, for publications in English using the keywords (“Menstrual Cup”) AND “Review” to determine if a review of menstrual cups was available with information on leakage, acceptability, and safety. No review was identified, but a literature review on menstrual management in emergency contexts noted a lack of empirical evidence examining the introduction and testing of menstrual cups in humanitarian settings.
Added value of this study
To our knowledge, this is the first systematic review and meta-analysis examining girls' and women's experiences of menstrual cups, aggregating outcomes from 43 studies and 3319 participants who were asked about their use or willingness to use menstrual cups. We provide information on leakage compared with other products, a listing of known adverse events, and quantitative and qualitative information on acceptability in both high-income countries and low-income and middle-income countries. We also assessed availability and prices of menstrual cups. Serious adverse events were not common, with five reported cases of toxic-shock syndrome. However, the number of menstrual cup users is unknown, so comparisons of risk of toxic-shock syndrome between menstrual cups, tampons, or the intravaginal diaphragm cannot be made. Although menstrual cups are manufactured and available globally, they are not commonly mentioned on websites offering educational materials on puberty for girls.
Implications of all the available evidence
Menstrual cups seem to be an effective and safe alternative to other menstrual products. Information on menstrual cups should be provided in puberty education materials. Policy makers and programmes can consider this product as an option in menstrual health programmes. Further research globally can provide more information on acceptability and is needed to monitor adverse events and assess best practice to shorten the familiarisation phase required for safe and effective use, and on cost-effectiveness and environmental effects.
The menstrual cup is not commonly known, despite its long history (appendix p 2).13 Like tampons, menstrual cups are inserted into the vagina, but the blood is collected in the receptacle, which can hold 10–38 mL of blood. The menstrual cup should be emptied every 4–12 h, depending on menstrual flow and type of cup. Two types of cup are available, a vaginal cup, which is generally bell-shaped and placed in the vagina, and a cervical cup, which, like a diaphragm for contraception, is placed around the cervix high in the vagina. Menstrual cups are made of medical-grade silicone, rubber, latex, or elastomer and can last up to 10 years; disposable single-use menstrual cups also exist.
We aimed to summarise current knowledge about leakage, safety, and acceptability of menstrual cups and compared, when available, with other menstrual products. We compiled information on global availability and costs of menstrual cups, did preliminary estimates on costs and waste savings, and examined online guidance materials on menarche in selected regions of the world for reference to menstrual cup as a product option.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched PubMed, Cochrane Library, Web of Science, Popline, Cinahl, Global Health database, Emerald, Google Scholar, Science.gov, and WorldWideScience for material in English from the inception of the database until May 14, 2019, using the keywords (“menstrual” AND “cup”) OR (“menses” AND “cup”) OR (“menstruation” AND “cup”) OR (“vaginal” AND “cup”). We also screened the Manufacturer and User Facility Device Experience (MAUDE) database from the US Food and Drug Administration (FDA) for events related to menstrual cups (10-year limit, last search done on May 28, 2019).14 For information on costs and availability, we screened websites of menstrual cup manufacturers using different web listings and web searches and consulted experts (full lists are in the appendix [pp 35–39]).
To ensure we covered a broad range of the available literature, we searched the reference lists of relevant studies, websites of pertinent professional bodies (eg, FDA), non-governmental organisations, and grey literature (eg, reports or conference abstracts), and we contacted experts in the field to recommend relevant reports. For information on costs and availability, our search included individually going through every list of menstrual cup brands we could find and searching where they were being sold (via web lists, Google searches, Pinterest boards, Facebook pages, and experts working in countries where cups appeared to be unavailable to confirm).
Study eligibility, data extraction, and risk-of-bias assessment were done independently by two reviewers (AMvE and ML for quantitative and LM and GZ for qualitative studies), and conflicts were resolved via discussion until an agreement was reached. To be eligible for inclusion, the material needed to have information on leakage, acceptability, or safety of menstrual cups. Quantitative, qualitative, or mixed design studies were included. Animal studies, and studies using menstrual cups to collect vaginal fluids without participants' reported experiences during menstruation were ineligible.
The main outcome of interest was menstrual blood leakage when using the menstrual cup. Additional outcomes of interest were acceptability of use of menstrual cups, difficulty with insertion or removal, comfort of wearing, and intention to use in future. Safety outcomes of interest included serious adverse events, such as toxic shock syndrome; vaginal abrasions and effects on vaginal microflora (eg, vaginal discharge, infections); effects on the reproductive, digestive, or urinary tract; and safety in poor sanitary conditions. Other safety issues we identified only during our review were documented, and all material was re-reviewed to ensure completeness of the safety assessment.
Data analysis
Data were manually extracted from studies using spreadsheets. If the same results from the same study were presented in several reports, we used data from the report with the largest sample size. For quality and bias assessments, we used the Cochrane tool for trials, an adaptation of the Newcastle-Ottawa tool (appendix p 5) for observational studies,15, 16 and the Critical Appraisal Skills Programme tool17 for qualitative studies.
We tabulated our findings as a narrative synthesis. If trials or studies presented sufficiently homogeneous data in terms of design, we pooled results as proportions using meta-analyses and a random-effects model with heterogeneity quantified using the I2 statistic (appendix p 3). We examined the following sources of heterogeneity if sufficient data were available using subgroup analysis: setting of the study (high-income vs low-income and middle-income countries), study population (adult women vs adolescents), year of study (study conducted before or after 2000), type of menstrual cup used (cervical vs vaginal cup), and duration of menstrual cup use. We assessed publication and small-study bias by visual inspection of funnel plots and Egger's test. We integrated the quantitative and qualitative analyses for the acceptability of use of menstrual cups.
For estimations on costs of disposable pads and tampons, we explored prices for commonly used products in six countries (the USA, the UK, India, Spain, China, and Canada) and calculated average costs per product. Extrapolating information on content and weight of menstrual products,18 we estimated waste and costs for a range of 9–25 units per product per month and compared these with consistent use of one menstrual cup for 10 years. Additional information on methods used to assess menstrual cup information, availability and prices, qualitative studies, and costs and waste, and additional information on data extraction are in the appendix (pp 3–5).
We did a sensitivity analysis of low versus moderate-to-good quality studies, as determined by the quality assessment and assessed small-study effect using funnel plots and the Egger's test. We used two-tailed p values of less than 0·05 to indicate statistical significance. We did statistical analyses using Metaprop, Stata version 14.2.2. This systematic review is registered on PROSPERO, number CRD42016047845.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility to submit for publication.
Results
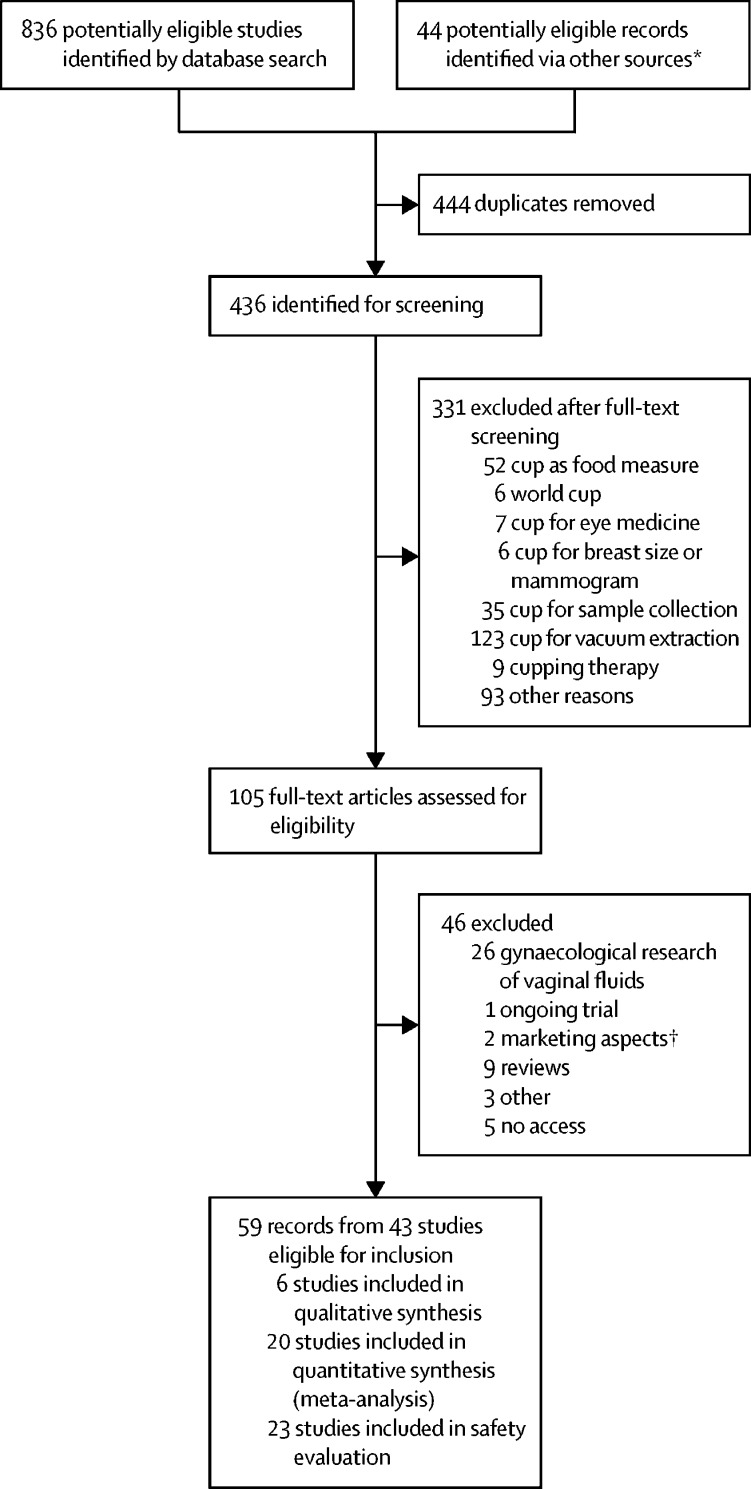
Of 436 unique records identified (appendix p 6), 59 were identified as relevant (figure 1), and 43 studies were included in our analysis (table 1). In these 43 studies, 3319 participants used or were asked about the menstrual cup.5, 13, 14, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 Seven studies were completed among schoolgirls (ie, aged 12–19 years) in low-income and middle-income countries (647 [19·5%] participants).5, 27, 33, 43, 58, 59 Three studies were done in the early 1960s, six in the late 1980s, and 26 in 2009–18. 15 studies were from low-income and middle-income countries. Most studies reported on vaginal cups (27 [63%] vaginal cups, five [12%] cervical cups, and 11 [25%] mixed types of cups or unknown) and 35 (81%) were journal articles. Although some studies did not report the type or brand of menstrual cup used, at least seven described menstrual cups that are no longer available (Tassette, Tassaway, and Gynaeseal). The quality of quantitative studies was low, with only two that were of moderate-to-high quality (table 1; appendix pp 7–8). Many studies did not clearly identify where their participants were from, or participants were not representative of the community. Only six studies, all from low-income and middle-income countries, provided qualitative information (appendix p 10).
Figure 1.
Study selection
*Reference lists of relevant studies, websites of pertinent professional bodies (eg, US Food and Drug Administration), non-governmental organisations, grey literature (eg, reports or conference abstracts), and records recommended by experts. †For example, advertising approaches.
Table 1.
Characteristics of studies contributing to menstrual cup review
| Source and study design | Location and date | Sample size and population | Age and heavy menstrual flow as defined by source | Menstrual cup brand*(type) | Comparison | Follow-up | Outcomes | Loss to follow-up (%) | Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Trials | ||||||||||
| Beksinska et al (2015)19, 20, 21 | Journal article; individually randomised crossover | Durban, South Africa; 2013 | 110 women | 29 years (SD 6; range 18–45); heavy flow 46·2% | Mpower Mcup; (vaginal) | Usual product (ie, disposable pads, tampons, cloths) | 6 cycles (3 cycles each product) | Acceptability and performance | 4·5% | 5 |
| Hoffmann et al (2014)22 | Journal article; cluster randomised | Jehanabad district, Bihar, India; 2012 | 960 women; 174 randomly assigned to cup group and 46 chose to use cup | 29·9 years (SD 6·7); NR | NR | Usual product (ie, cloth and disposable pads) | 8 months | Acceptability, demand for high-barrier menstrual cup and low-barrier sanitary pads | 15·8% (6 months) | 4 |
| Howard et al (2011)23 | Journal article; individually randomised | Vancouver, Canada; 2006–07 | 110 women; 56 in cup group | Range 19–40 years; heavy flow 11·1% | Divacup (vaginal) | Tampons | 4 cycles | Use, use in future, costs, and waste | 11·8% | 3 |
| Oster et al (2011),24 Oster et al (2012),25 Oster et al (2009)26, 27 | Journal article; individually randomised | Bharatpur, Chitwan district, Nepal; 2006–08 | 199 schoolgirls; 98 in cup group | 14·2 years (SD 1·2); NR | Mooncup (vaginal) | Usual product (ie, cloths and disposable pads) | 15 months | School attendance, peer effect | 0·5% | 3 |
| Phillips-Howard et al (2016),5 Nyothach et al (2015),28 Juma et al (2017),29 Mason et al (2015),30 Oduor et al (2015),31 van Eijk et al (2018)32 | Journal article; cluster randomised | Gem district, Siaya Province, Kenya; 2012–13 | 766 schoolgirls; 229 in cup group | 14·6 years (SD 0·7); heavy flow 20·8% | Mooncup (vaginal) | Disposable pads and usual practice (ie, cloths and pads) | Median 10·9 months | School drop-out, STIs, reproductive tract infections | 15·7% | 6 |
| Observational studies | ||||||||||
| APHRC (2010)33, 34, 35 | Report; cohort study | Nairobi, Kenya; 2008 | 36 women and 60 schoolgirls | NR; NR | Mooncup (vaginal) | Disposable pads, cloths, cotton wool, tampons | 3 cycles | Feasibility | 6·3% | 2 |
| Averbach et al (2009)36 | Journal article; survey and focus group discussions | Epworth, Zimbabwe; 2007–08 | 43 women | Range 18–45 years | Duet (cervical, re-usable) | Cotton wool, cloths, disposable pads, tissue | NA | Consideration of menstrual cup use | NA | ND |
| Borowski et al (2011)37 | Master's thesis; survey | USA; 2011 | 155 women | Age ≥18 years; NR | No particular brand | NR | NA | Consideration of eco-friendly menstrual products | NA | ND |
| Care International in Uganda (2018)38 | Report; cohort | Refugee settlement, Uganda; 2018 | 80 girls and women and 20 female trainers | n=25 15–18 years, n=41 19–25 years, n=34 26–30 years; NR | Ruby cup | Disposable and re-usable pads, cloths | 3 months | Menstrual cup use | 53·8% | 2 |
| Cattanach et al (1991),39Cattanach et al (1990)40† | Journal article; cohort | Hawthorn, Australia; NR | 80 women | Range 17–42 years; NR | Gynaeseal (cervical) | NR | 18 months | Acceptability | 69·1% | 2 |
| Cheng et al (1995)41 | Journal article; cohort | NR, Canada; 1991–92 | 51 women | 46 (90%) of 51 <40 years; moderate to heavy flow: 42 (82%) of 51 | Menses cup‡ (vaginal) | Tampons and disposable pads | 2–13 cycles | Acceptability of menstrual cup for measuring flow | NR | 2 |
| Chintan et al (2017)42 | Journal article; cohort | India (several sites); NR | 100 women | Range 14–55 years; NR | Flow care (vaginal) | Disposable pads and tampons | 8 weeks | Menstrual cup use | NR | 2 |
| Femme International (2017)43§ | Report; cohort | Kilamanjaro region, Tanzania; 2016–17 | 184 adolescents and 38 women | Range 12–54 years; NR | Ruby cup (vaginal) | NR | 6–12 months | Menstrual cup use | 37–88% | 2 |
| Ganyaglo et al (2018),44 Ryan et al (2018)45 | Journal article and abstract; repeated measures design | Ghana; 2016 | 11 women | 43·6 years (SD 12·3); NR | Diva cup | Pads | 4 h | Menstrual cup use for vesicovaginal fistula | 0 | 5 |
| Gleeson et al (1993)46 | Journal article; cohort | Dublin, Ireland; NR | 22 women | NR; 12 (55%) women had menorrhagia | Gynaeseal (cervical) | Tampons | 1 cycle | Leakage, ease, use for measuring flow | 0 | 3 |
| Grose et al (2014)47 | Journal article; survey | California, USA; NR | 151 undergraduates | Range 18–23 years; NR | Brand not reported | NR | NA | Consideration of menstrual cup | NA | ND |
| Kakani et al (2017)48 | Journal article; cohort | Dharpur, Gujarat, India; NR | 158 women | 31 years (SD 6·1; range 21–50); heavy flow: 20 (13%) of 150 | NR: 44 mm diameter, thin walled silicon¶ | Cloths, disposable pads, tampons | 3 cycles | Acceptability and efficacy | 5·1% | 3 |
| Madziyire et al (2018)49, 50§ | Journal article; cohort | Epworth, Zimbabwe; 2016–17 | 54 women | Range 18–45 years; no information on heavy flow | Butterfly (vaginal) | NR | 3 cycles; 1 year | Acceptability, leakage | 3·7% | 3 |
| North et al (2011)13 | Journal article; cohort | USA (7 centres); NR | 406 women | Range 18–55 years; no information on heavy flow | Soft cup‖ (disposable cervical) | Disposable pads or tampons, or both | 3 cycles | Safety, effectiveness, and acceptability | 24·1% | 3 |
| Parker et al (1964)51 | Journal article; cohort | Ann Arbor, USA; NR | 65 women | NR; 46 women with menorrhagia, 19 with normal flow | Tassette (vaginal) | Tampons and disposable pads | 2–6 months | Acceptability | 15·2% | 3 |
| Pena et al (1962)52 | Journal article; cohort | Florida, USA; NR | 125 women (100 with normal flow and 25 with vaginal infections) | Range 20–45 years; all participants had normal flow | Tassette (vaginal) | Tampons and disposable pads | 3 cycles | Not clear | NR | 2 |
| Shihata et al (2014)53 | Journal article; cohort | Sweden, USA, Mexico, Brazil, Colombia; 2013 | 146 women | Range 18–40 years; NR | FemmyCycle (one size, vaginal)** | Disposable pads, tampons | 3 cycles | Leakage, acceptability | 28·1% | 2 |
| Stewart et al (2010)54 | Journal article; cohort | Nottingham, UK; 2008–09 | 54 women | Mean 22·5 (SD NR); NR | Mooncup (vaginal) | Tampons and disposable pads | 6 cycles (3 with cup) | Leakage, acceptability | 61·1% | 2 |
| Stewart et al (2009)55 | Journal article; survey | Nottingham, UK; NR | 69 clinic patients | n=18 <30 years, n=21 30–40 years, n=30 >40 years; NR | Mooncup (vaginal) | Tampons and disposable pads | NA | Consideration of menstrual cup | NA | ND |
| Tellier et al (2012)56 | Report; cohort study | Kitgum, Uganda; NR | 31 women | 24 years (SD NR); NR | Ruby cup (vaginal) | Cloths, gauze, disposable pads | 3–5 cycles | Acceptability, safety | 51·6% | 3 |
| Wiebe et al (2012)57 | Journal article; retrospective chart survey | Vancouver, Canada; 2009 | 930 women; 96 used menstrual cups | 75 (59%) of 96 <30 years; NR | No particular brand or type | NA | 6 weeks | IUD expulsion within 6 weeks after placement, by menstrual product used | NA | ND |
| Studies with only qualitative information | ||||||||||
| Hyttel et al (2017)58†† | Journal article; two focus group discussions and six semi-structured interviews | Bungatira, Gulu, Uganda; 2013 | 36 schoolgirls (purposely selected) | 14·6 years (SD 0·7; range 13–17); NA | Ruby cup (vaginal) | NA | 4 months after study start | Willingness and ability to use | NA | Medium |
| Sundqvist et al (2015)59 | Thesis; in-depth interviews | Msiriwa, Tanzania; 2014 | 15 schoolgirls | Range 14–15 years; NA | Lady cup (vaginal) | NA | NR | Effect of menstrual cup use on education and social interactions | NA | Strong |
| Case reports | ||||||||||
| Adedokun et al (2017)60 | Abstract; case report | Brno, Czech Republic; NR | 1 woman | 30 years; NA | NR | NA | NR | Hydronephrosis | NA | ND |
| Nunes-Carneiro et al (2018)61 | Journal article; case report | Porto, Portugal; NR | 1 woman | 26 years; NA | NR | NA | 5 days | Uretero-hydronephrosis | NA | ND |
| Stolz et al (2019)62 | Journal article; case report | NR; NR | 1 woman | 47 years; NA | NR | NA | A “couple of weeks” | Hydronephrosis | NA | ND |
| Day et al (2012)63 | Journal article; case report | London, UK; NR | 1 woman | 20 years; NA | Mooncup (vaginal) | NA | NR | Menstrual cup retention | NA | ND |
| FDA MAUDE database14 | Results database search; case reports | USA; 1950–June, 2018 | 12 women | NR; NA | Mooncup, Diva cup, Femmy cycle, Softcup (vaginal and cervical) | NA | Variable | Adverse events (in table 2) | NA | ND |
| Seale et al (2019)64 | Journal article; case series | Denver, CO, USA; NR | 7 women | n=1 16 years: n=6 22–25 years; NA | NR | NA | 2–12 months | IUD expulsion | NA | ND |
| Goldberg et al (2016)65 | Journal article; case report | New Brunswick, Canada; 2013 | 1 woman | 39 years; NA | NR (vaginal) | NA | NR | Use as diagnostic aid of vesicouterine fistula | NA | ND |
| Mitchell et al (2015)66 | Journal article; case report | Ontario, Canada; NR | 1 woman | 37 years; NA | DivaCup (vaginal) | NA | 2 weeks post-admission | Possible TSS | NA | ND |
| Russell et al (2016)67 | Journal article; case reports | Utah, USA; NR | 3 women | 54 years, 60 years, and 68 years; NA | NR (vaginal) | NA | NR | Use as enterovaginal or vesicovaginal fistula control | NA | ND |
| Spechler et al (2003)68 | Journal article; case report | Bethesda, MD, USA; NR | 1 woman | 41 years; NA | Keeper (vaginal) | NA | 2 years post-surgery | Adenomyosis and endometriosis | NA | ND |
| Other types of study with relevant information | ||||||||||
| Cattanach et al (1989)69† | Journal article; vaginal samples | Hawthorn, Australia; 1986–88 | 5 women | Range 19–32 years; NA | Gynaeseal* (cervical) | NA | 3–22 months | Menstrual cup safety, effect on vaginal flora | NA | ND |
| Karnaky et al (1962)70 | Journal article; vaginal observations and samples | Houston, TX, USA; NR | Two cohorts of 50 and 97 women; and a survey of 20 women | NR; NA | Tassette (vaginal) | NA | Unclear for cohort studies | Menstrual cup safety, effects on vagina | NA | ND |
| Tierno et al (1989)71 | Journal article; in-vitro study | New York, NY, USA; NR | NA | NA; NA | 16 menstrual cups, brands not reported | NA | NA | Ability to induce TSST-1 production by TSS-associated strains of Staphylococcus | NA | ND |
| Tierno et al (1994)72 | Journal article; in-vitro study | New York, NY, USA; NR | NA | NA; NA | Six Tassaway cups (vaginal) | NA | NA | Ability to induce TSST-1 by a TSS strain of Staphylococcus aureus MN8 | NA | ND |
| Nonfoux et al (2018)73 | Journal article; in-vitro study | France; NR | NA | NA; NA | 2 be'Cup and 2 MeLuna (vaginal) | NA | NA | Effect on S aureus growth and TSST-1 production using the modified sac method | NA | ND |
Where data are missing, it was not provided in the source material. Cycles refer to menstrual cycles. A quality score of 5–6 indicates a moderate-to-high quality study, and a score of less than 5 indicates a medium-to-low quality study. For qualitative studies, levels of study quality were strong, medium, and weak. The quality score components of individual studies are in the appendix (pp 7, 8, 10). Cloths=pieces of material (clothing, blankets, socks) that are used for menstruation and can be reused after washing or disposed of after use. NR=not reported. STI=sexually transmitted infection. APHRC=African Population and Health Research Center. NA=not applicable. ND=not done (these studies were not assessed for quality). IUD=intrauterine device. FDA=US Food and Drug Administration. MAUDE=Manufacturer and User Facility Device Experience. TSS=toxic shock syndrome. TSST-1=toxic shock syndrome toxin 1.
Manufacturing company, city, country, and website where available, are listed in the appendix (p 8).
The study author was the developer of Gynaeseal (a disposable cup covering the cervix. that can also be worn during intercourse); we assumed the articles from 1990 and 1991 described the same study and used the publication with the larger sample size (1991).
This type of cup has a drainage tube that can be opened to let menstrual fluids pass.
Additional information obtained from internal report or author.
Description in article is like a cervix-covering cup (“The device- the menstrual cup we utilized for the study is an internally worn device with a pliable rim 44mm in diameter and a thin-walled reservoir to collect and hold the menstrual fluid. It was designed to minimize bulk in order to facilitate insertion and removal. Once inserted; it opens to an oval shape, positioned between the posterior fornix and the notch behind the pubic bone, covering the cervix. Removal is accomplished by hooking a finger over the rim behind the pubic bone. It is made up of health grade non-toxic non- allergic silicon”), but image is of a low vaginal cup.
Instead Softcup (a rebrand of Softcup): disposable cup covering the cervix. This type of cup can also be worn during intercourse.
Author has patent on this menstrual cup.
Part of a larger study (Gulu Schoolgirl Menstrual Cup Study, n=194), for which no other publication could be retrieved.
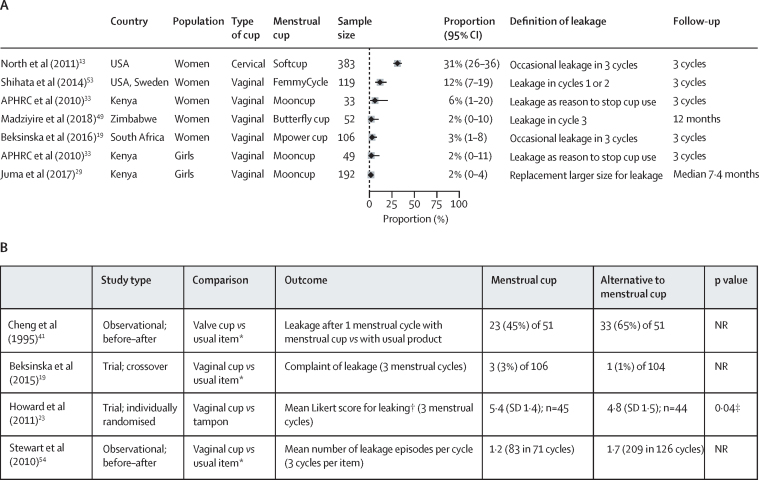
With regard to leakage, only four studies (n=293) made direct comparisons between menstrual cups and usual products. The outcomes in each of these studies were different, but leakage between products was similar in three studies and significantly less among menstrual cups for one study (figure 2).23 In studies that assessed menstrual cups that are still available, the proportion of leakage among the participants who reported use of the menstrual cup was 2–31% for a wide range of definitions, as shown in figure 2. Some factors mentioned in association with leakage by study authors included menorrhagia,48 unusual anatomy of the uterus,53 need for a larger size of menstrual cup,5 and incorrect placement of the menstrual cup, or that it had filled to capacity.39, 53
Figure 2.
Menstrual cup and leakage
(A) Proportion of participants who had menstrual leakage in seven studies using different types of menstrual cups and definitions. (B) Reports of leakage among menstrual cup users versus users of other menstrual products. APHRC=African Population and Health Research Center. NR=not reported. *Disposable pad or tampon. †Likert scale: 7-point score, in which 1=terrible and 7=great. ‡p value reported in article for Mann-Whitney test.
When looking at safety, use of the menstrual cup was not associated with abnormalities in the vagina or cervix in three studies with vaginal examinations (n=370; table 2).13, 52, 70 Three users reported vaginal wounds in case reports, which could not be confirmed with medical records. In one case report, severe pain on removal was self-reported and in another case report severe pain was self-reported when wearing the menstrual cup,14 and two participants in two different cohort studies reported vaginal or cervical irritation without clinical consequences.39, 41 Three adverse events that were reported in one cohort study and three case reports were possibly related to an allergy; one case of silicone allergy necessitated reconstructive vaginal surgery.13, 14, 48 Difficulty with removal that required professional assistance—an adverse event we did not anticipate—was reported 47 times for cervical cups (one participant from a cohort study, and 46 case reports) and twice for vaginal cups (both case reports).13, 14, 46, 63
Table 2.
Safety and side-effects of the menstrual cup
| n (%) or description | Notes | Data source | ||
|---|---|---|---|---|
| Handling and positioning of menstrual cup | ||||
| Vaginal wound | ||||
| Cup not clear (Divacup or softcup) | Event April, 2012; vaginal wound due to use of menstrual cup, needing treatment from physician for vaginal bleeding | Complete medical records were not available for evaluation | FDA database14 | |
| Softcup (cervical) | Reported April, 2012; long-term customer of softcup product claimed vaginal scarring due to use | Medical director did not find anything in medical records provided by customer related to vaginal health | FDA database14 | |
| Softcup (cervical) | FDA database case report: “…cup wore through the vaginal wall, damaging an artery that required surgical repair” | Event could not be confirmed; no medical records were available | North et al (2011)13 | |
| Vaginal pain on removal | ||||
| Divacup (vaginal) | Event March, 2017; extreme pain on removal (first use), individual stopped using the cup | Self-report; no medical report available | FDA database14 | |
| Pelvic pain | ||||
| Softcup (cervical) | Event February, 2017; pain in lower pelvis and rectum and nausea about 1 h after insertion, no longer present approximately 30 min after removal | Self-report; no medical evaluation available; individual stopped use after trying twice (possibly vascular compression) | FDA database14 | |
| Vaginal irritation | ||||
| Gynaeseal (cervical) | One (1%) of 73 | Self-report by participant | Cattanach et al (1991)39 | |
| Cervix irritation | ||||
| Menses cup (vaginal) | One (2%) of 51 | Cervical smear was normal | Cheng et al (1995)41 | |
| Allergy and rash | ||||
| NR, vaginal cup | Allergy: one (1%) of 150; and rash: two (1%) of 150 | · · | Kakani et al (2017)48 | |
| Softcup (cervical) | FDA database: two case reports | NR | North et al (2011)13 | |
| Mooncup (vaginal) | Event 2010: silicone allergy in one individual | Surgery was needed for vaginal repair; manufacturer noted that silicone allergy is very rare | FDA database14 | |
| Difficulty with removal requiring professional assistance | ||||
| Gynaeseal (cervical) | One (5%) of 22 | · · | Gleeson et al (1993)46 | |
| Softcup (cervical) | FDA database: three case reports reported by North 2011; one event in 2018 | · · | North et al (2011),13 FDA database14 | |
| Softcup (cervical) | Reported complaints to company 2003–08: 42 individuals underwent physician-assisted removal | Other complaints reported to company included poor fit (n=102), leakage (n=168), messy (n=98) | North et al (2011)13 | |
| Mooncup (vaginal) | Case report: menstrual cup lodged on cervix, difficult to remove, requiring assistance | Moderate cervical inflammation after retrieval | Day et al (2012)63 | |
| Divacup (vaginal) | Event April, 2015: one case report required an emergency room visit for removal | · · | FDA database14 | |
| Reproductive tract observations with use of menstrual cup | ||||
| Vulva abnormalities | ||||
| Softcup (cervical) | Baseline: four (1%) of 393; cycle 1: eight (2%) of 365; cycle 2: six (2%) of 326; cycle 3: five (2%) of 305 | Vulva-vaginal inspection at baseline and monthly for 3 months; no p values reported | North et al (2011)13 | |
| Abnormalities of vaginal wall | ||||
| Softcup (cervical) | Zero of 44 | Vulva-vaginal inspection at baseline and monthly for 3 months | North et al (2011)13 | |
| Tassette (vaginal) | Zero of 12 | Vaginal inspection after 3 months | Pena et al (1962)52 | |
| Tassette (vaginal) | Zero of 50 | Vaginal inspection done; timing of inspections not clear | Karnaky et al (1962)70 | |
| Abnormalities of cervix | ||||
| Softcup (cervical) | Baseline: 23 (6%) of 390; cycle 1: ten (3%) of 345; cycle 2: six (2%) of 326; cycle 3: four (1%) of 300 | Inspection of cervix; no p values reported for differences | North et al (2011)13 | |
| Softcup (cervical) | Abnormal cervical smear test: baseline: one (<1%) of 406; cycle 1: one (<1%) of 368; cycle 2: two (1%) of 329; cycle 3: zero of 308 | Abnormal cervical smear test results were exclusion criteria at admission, and a reason for discontinuation of the study; no p values reported for differences | North et al (2011)13 | |
| Condition of vaginal and cervical epithelium | ||||
| Softcup (cervical) | 44 women examined at baseline, 37 at 2–3 months, and 25 at 5–6 months | “The Softcup caused no alteration or disruption in vaginal or cervical epithelium, as assessed by colposcopy and cervical cytology” | North et al (2011)13 | |
| Vaginal flora and infections with use of menstrual cup | ||||
| pH changes of vagina | ||||
| Tassette (vaginal) | Zero of 50 | No abnormalities, vaginal areas where menstrual cup was placed were more acid | Karnaky et al (1962)70 | |
| Softcup (cervical) | Mean pH at baseline: 4·6 (n=400); cycle 1: 4·6 (n=368); cycle 2: 4·6 (n=329); cycle 3: 4·5 (n=308) | No p values reported | North et al (2011)13 | |
| Clue cells (vaginal smear) Lactobaccilus | ||||
| Softcup (cervical) | Number with clue cells: baseline n=6; cycle 1 n=6; cycle 2 n=2; cycle 3 n=4 | Sample sizes and p values were not reported | North et al (2011)13 | |
| Lactobaccilus | ||||
| Softcup (cervical) | “…before, during, and after use of the cup, vaginal Lactobacillus (normal vaginal flora) was maintained at normal levels.” | Data in figure 3 in publication cannot be extracted; no significant changes according to authors | North et al (2011)13 | |
| Gardnerella vaginalis | ||||
| Softcup (cervical) | No significant changes from baseline-cycle 3 according to authors | Data in figure 3 in publication cannot be extracted | North et al (2011)13 | |
| Bacterial vaginosis | ||||
| Softcup (cervical) | No significant changes from baseline to cycle 3 according to authors | Data in figure 3 in publication cannot be extracted | North et al (2011)13 | |
| Mooncup (vaginal) | Study end survey: cup 21 (15%) of 144; pads 40 (20%) of 202, and usual practice (control) 32 (21%) of 156; cup vs control p=0·11 and cup vs pads p=0·13; among girls enrolled for ≥9 months: cup 13 (13%) of 101, pads 29 (20%) of 143, usual practice 20 (19%) of 104; cup vs control p=0·07, and cup vs pads p=0·018 | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Candidiasis | ||||
| Softcup (cervical) | Number with candidiasis: baseline n=6; cycle 1 n=6; cycle 2 n=3; cycle 3 n=6 | Sample sizes not reported; according to authors, yeast decreased significantly from month 1 to 2 | North et al (2011)13 | |
| Ruby cup (vaginal) | Zero of 18 participants had vaginal candidiasis at follow-up (3–5 months) | NA | Tellier et al (2012)56 | |
| Tassette (vaginal) | Candida albicans decreased with the use of the cup | NR | Karnaky et al (1962)70 | |
| Mooncup (vaginal) | Study end survey: cup 11 (8%) of 143, pads 19 (10%) of 200, usual practice (control) 13 (9%) of 156; cup vs control p=0·87, and cup vs pads p=0·68 | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Group B Streptococcus | ||||
| Softcup (cervical) | No differences between baseline and cycle 1 to cycle 3 | Data in figure 3 in publication cannot be extracted; no significant changes according to authors | North et al (2011)13 | |
| Enterococcus | ||||
| Softcup (cervical) | Increase in Enterococcus from cycle 2 to cycle 3 (p=0·03) | “… this increased frequency persisted for 3 months after discontinuing use of the cup, suggesting that factors or behavior other than cup use may have influenced colonization”; data in figure 3 in publication cannot be extracted | North et al (2011)13 | |
| E coli | ||||
| Softcup (cervical) | No significant changes from baseline to cycle 3 according to authors | Data in figure 3 in publication cannot be extracted | North et al (2011)13 | |
| Escherichia coli on menstrual cup | ||||
| Mooncup (vaginal) | Nine (53%) of 17 if used cup for <6 months; four (22%) of 18 if used for ≥6 months (p=0·12); association between E coli with heavy periods: 61·5% of girls reporting heavy periods had E coli on cups, compared with 22·7% of those stating they did not have heavy periods (p=0·022, no numbers presented) | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Juma et al (2017)29 | |
| Chlamydia trachomatis | ||||
| Mooncup (vaginal) | Study end survey: cup three (2%) of 144, pads three (2%) of 201, usual practice (control) seven (5%) of 154; cup vs control p=0·20, and cup vs pads p=0·63 | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Trichomonas vaginalis | ||||
| Softcup (cervical) | Zero cases at baseline, and cycles 1 to 3 | Sample sizes not reported | North et al (2011)13 | |
| Mooncup (vaginal) | Study end survey: cup two (1%) of 143, pads five (3%) of 200, usual practice (control) seven (5%) of 154; cup vs control p=0·12, and cup vs pads p=0·36 | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Ruby cup (vaginal) | Zero of 18 at baseline, and at 3–5 months of follow-up | NA | Tellier et al (2012)56 | |
| Neisseria gonorrhoea | ||||
| Mooncup (vaginal) | Study end survey: cup one (1%) of 144, pads one (1%) of 201, usual practice (control) one (1%) of 154; cup vs control p=0·96, and cup vs pads p=0·81 | Cluster randomised trial of schools; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Ruby cup (vaginal) | Zero of 18 at baseline, and at 3–5 months of follow-up | NA | Tellier et al (2012)56 | |
| Staphylococcus aureus | ||||
| Softcup (cervical) | No significant changes in cycles 1–3 compared with baseline | Data in figure 3 of publication cannot be extracted | North et al (2011)13 | |
| Mooncup (vaginal) | Among menstrual cup users: four (11%) of 38 in first month of intervention, 13 (9%) of 139 after first month; p=0·83 (median follow-up 4 months, range 2–11 for this substudy); prevalence was 21 (11%) of 197 in sanitary pads group, and 16 (11%) of 153 in usual practice group | Cluster randomised trial in schools; median follow-up 11 months (range 3–15); samples from vaginal swab (self-swabbing) | Juma et al (2017)29 | |
| be'Cup (vaginal) | Silicone cup: potentially more S aureus after incubation for 8 h with shaking in a plastic bag with S aureus in one of two cups used, but not when no shaking | In-vitro study | Nonfoux et al (2018)73 | |
| Me Luna (vaginal) | Thermoplastic isomer cup: no more S aureus after incubation for 8 h with shaking in plastic sac, and not when no shaking | In-vitro study | Nonfoux et al (2018)73 | |
| TSST-1 | ||||
| Mooncup (vaginal) | 49 schoolgirls with vaginal S aureus had second swab: ten yielded S aureus, two had TSST-1, both in sanitary pad group; the cases were asymptomatic | Cluster randomised trial in schools; median follow-up 11 months (range 3–15); sample from vaginal swab (self-swabbing) | Juma et al (2017)29 | |
| NR | No TSST-1 in supernatant of S aureus cultivated for 24 h (incubated aerobically in a still growth environment) in the presence of elastic polymer menstrual cup (n=16 menstrual cups) | In-vitro study | Tierno et al (1989)71 | |
| Tassaway (vaginal) | S aureus MN8 produced no TSST-1 when grown in the presence of Tassaway (elastomeric polymer, n=6), washed or unwashed, no shaking, incubation overnight | In-vitro study | Tierno at al (1994)72 | |
| be'Cup (vaginal) | Silicone cup: potentially more TSST-1 production after incubation for 8 h with shaking in plastic bag with S aureus compared with control, but not when not shaken or with pieces of cup | In-vitro study | Nonfoux et al (2018)73 | |
| Me Luna (vaginal) | Thermoplastic isomer cup: potentially more TSST-1 production after incubation for 8 h with shaking in plastic bag with S aureus compared with control, but not when not shaken or with pieces of cup | In-vitro study | Nonfoux et al (2018)73 | |
| TSS | ||||
| Mooncup (vaginal) | Zero of 192 in trial in Kenya | “Safety monitoring components comprised routine nurse-based screening, population-based monitoring (school and community) and clinical evaluation of infection with laboratory confirmation” | Juma et al (2017)29 | |
| Softcup (cervical) | Two case reports in the FDA database | Both unconfirmed cases of TSS | North et al (2011)13 | |
| Divacup (vaginal) | One case report: blood cultures and urine culture negative, no culture of the menstrual cup was done | Woman had history of Hashimoto's thyroiditis and chronic menorrhagia | Mitchell et al (2015)66 | |
| Mooncup (vaginal) | Event February, 2012: TSS 2 days after using of first and new Mooncup resulting in 9 days of inpatient hospital stay; vaginal swab positive for S aureus | Had an IUD, Mooncup was not sent for bacteriological testing | FDA database14 | |
| Divacup (vaginal) | Event February, 2015: TSS from Streptococcus resulting in 5 days of i-patient hospital stay; culture of cup isolated group A and B streptococcus | Woman had used Divacup for menstrual period, which started 3 days before illness; menstrual cup was in for 18 h on admission to hospital | FDA database14 | |
| UTI | ||||
| Ruby cup (vaginal) | Baseline: four (13%) of 31; at follow-up (after 3–5 months) three (17%) of 18; p=0·65, McNemar test | One participant with a UTI at enrolment and follow-up had her cup stolen and used toilet paper in vagina as a tampon | Tellier et al (2012)56 | |
| Gynaeseal (cervical) | One (1%) of 73 had transient dysuria | “The woman who developed dysuria did not seek treatment and the problem subsided within 24–48 hours” | Cattanach et al (1991)39 | |
| Softcup (cervical) | Urine analysis done; detailed results not reported | “Monthly monitoring of gynecological health via urinalysis, pelvic examination with visual evaluation of tissues, vaginal pH, and microscopic wet mount showed no adverse effects of cup use” | North et al (2011)13 | |
| Softcup (cervical) | Event August, 2014: UTI confirmed by urine cultures twice after use of softcup | Medical records were not available for evaluation | FDA database14 | |
| Infections overall | ||||
| Tassette (vaginal) | “The amount of bacterial contamination was greatest with the pad, next with the tampon and least with the rubber cup” | No data provided; study reported to make cultures from vaginal wall samples and to examine fresh and stained smears for C albicans, Trichomonas vaginalis, Haemophilus vaginalis, and for predominance of Gram-positive or Gram-negative cocci, small rods or long-rod bacilli (Doederlein bacilli) | Karnaky et al (1962)70 | |
| Softcup (cervical) | FDA database: one case report | Vaginal infection not further specified; could not be confirmed at follow-up | North et al (2011)13 | |
| Butterfly cup (vaginal) | “…none of the women sought treatment for a pelvic infection. There was no onset or worsening of dysmenorrhoea in 83%, dyspaurenia in 94%, pelvic pain in 92% and vaginal discharge in 92% of the participants during the 12 months of cup use”; n=52 | NA | Madziyire et al (2018)49, 50 | |
| Gynaeseal | “There was no increased pathogenicity detected in vaginal flora. There was a trend towards smaller numbers of potentially pathogenic bacteria for 4 of the women, and the remaining woman showed no change. None of the women developed any significant medical problems” | Vaginal swabs before and after use, five women, median follow-up 14 months (range 3–22) | Cattanach et al (1989)69 | |
| STIs | ||||
| Mooncup (vaginal) | Study end survey: menstrual cup six (4%) of 144, pads nine (5%) of 202, and usual practices (control) 12 (8%) of 156; cup vs control p=0·11, and cup vs pads p=0·87; when follow-up was ≥9 months: cup four (40%) of 101, pads seven (5%) of 143, and usual practice 11 (11%) of 104; cup vs control p=0·004, and cup vs pads p=0·60 | Presence of either C trachomatis, T vaginalis or N gonorrhoea; cluster randomised trial of schools in Kenya; median follow-up 11 months (range 3–15)* | Phillips-Howard et al (2016)5 | |
| Reproductive tract infections | ||||
| Mooncup (vaginal) | Study end survey: cup 31 (22%) of 144, pads 58 (29%) of 202, and usual practice (control) 42 (27%) of 156; cup vs control p=0·36, and cup vs pads p=0·19 | Presence of either B vaginosis or C albicans; cluster randomised trial of schools in Kenya; median follow-up 11 months (range 3–15) | Phillips-Howard et al (2016)5 | |
| Other adverse events | ||||
| Urinary incontinence | ||||
| Femcap (first model of femmycycle, vaginal) | FDA database: one case report; event July, 2014; pelvic pain and urinary incontinence when wearing and removing menstrual cup; urine sample negative for infection | Self-report; stopped using menstrual cup | FDA database14 | |
| Displacement of IUD when using menstrual cup | ||||
| NR | IUD expulsion 6–8 weeks after insertion: menstrual cup five (4%) of 135, tampon 11 (2%) of 469, pads: seven (4%) of 169; cup vs tampon p=0·57, and cup vs pads: p=0·92 | Retrospective cohort; expulsion of an IUD occurs in approximately one in 20 women and is most common in the first 3 months after insertion; expulsion commonly occurs during menstruation; some recommend not to use internal sanitary protection for 3–6 weeks after insertion because of an increased infection risk | Wiebe et al (2012)57 | |
| Mooncup (vaginal) | FDA database: one case report; event July, 2012; potential IUD dislodgment after Mooncup removal; patient had an ectopic pregnancy and needed surgery | Patient felt pain after removal of Mooncup and had the position of the IUD checked at a health centre where it was declared in position; 2 months later she was found to be pregnant | FDA database14 | |
| NR | Case series of seven women with IUD expulsion when removing menstrual cup; expulsion occurred 1 week to 13 months after insertion of IUD and was recurrent in two women; of seven women, two choose to use different contraception; the five others had their IUD re-inserted | Two women opted for cutting the wires of the IUD close to the cervix to avoid the problem; authors also stress importance of releasing vacuum of menstrual cup before removal | Seale et al (2019)64 | |
| Endometriosis because of menstrual backflow via use of menstrual cup | ||||
| Tassette (vaginal) | Position of cup confirmed with X-ray imaging | “Hence the free space available in the upper vagina plus the capacity of the cup itself are ample to accommodate several times the amount of blood passed in a complete menstrual cycle” | Pena et al (1962)52 | |
| Tassette (vaginal) | No evidence for backflow | “Thin watery solutions could not be introduced under high pressures during the menstrual flow in 6 multiparous women” | Karnaky et al (1962)70 | |
| Keeper (vaginal) | Case report: dysmenorrhoea 2 years after start of menstrual cup use (10 years ago tubal ligation); laparoscopy showed adenomyosis and endometriosis, treated with laser; patient stopped use of menstrual cup; pain decreased after surgery; 2 years of follow-up | “The observations in our patient suggest that it may be useful to inquire about use of these devices in women with pelvic pain or endometriosis”; petition for revoking of market approval to US FDA rejected because of lack of evidence74 | Spechler et al (2003)68 | |
| Hydronephrosis (ie, renal colic) | ||||
| NR | Case report: severe colicky flank pain; CT scan showed menstrual cup was slightly dislocated, pressing into left ureter | “The extraction of the menstrual cup resulted in resolution of hydronephrosis and associated symptoms” | Adedokun et al (2017)60 | |
| NR | Case report: 3 h of back pain on the right side; low-dose unenhanced CT scan showed entrapment of left vaginal wall and part of interolateral bladder wall; improperly positioned menstrual cup | Symptoms and swelling disappeared after removal of menstrual cup, confirmed by another CT scan; patient had used a menstrual cup for a long time with no previous problems, and continued use of cup; no problems at follow-up after several weeks | Stolz et al (2019)62 | |
| NR | Case report: 3 h of pain in the right flank, and nausea during menstruation; X-ray imaging showed menstrual cup orientated to the right | “The symptoms and the ureterohydronephrosis relieved completely after the removal of the device”; patient had used a menstrual cup for 2 years | Nunes-Carneiro et al (2018)61 | |
Entries in FDA database14 for softcup not entered if before 2011, to avoid double reporting with North et al (2011).13 NR=not reported. NA=not applicable. TSST-1=toxic shock syndrome toxin-1. TSS=toxic shock syndrome. FDA=US Food and Drug Administration. IUD=intrauterine device. UTI=urinary tract infection. STI=sexually transmitted infection.
The decrease in STIs in the trial in Kenya in the groups in which either menstrual cups or sanitary pads were provided is thought to be an indirect effect because of the decrease in risky sexual behaviour to obtain money to buy pads.
We found no increased infection risk (reproductive tract or systemic infection) associated with use of a menstrual cup among European,54, 55 North American, and African women and girls,5, 19 compared with other menstrual products (table 2). A decrease in candidiasis was reported with use of the menstrual cup in two of four studies that investigated this infection; one study found no candidiasis infections at follow-up in 18 participants, and the other, a randomised feasibility pilot among schoolgirls (aged 14–16 years) in Kenya comparing menstrual cups, sanitary pads, and usual practice (cloths, pads, tissue, or other makeshift materials), showed no difference in the prevalence of candidiasis by study group (menstrual cup 11 [8%] of 143, pads 19 [10%] of 200, and usual practice 13 [9%] of 156; menstrual cup vs pads p=0·68 and menstrual cup vs usual practice p=0·87; table 2).5, 13, 56, 70 One study70 reported lower prevalence of bacterial infections among users of the menstrual cup than among users of tampons or pads (not further specified), and a randomised pilot study5 in Kenya reported lower prevalence of bacterial vaginosis among users of the menstrual cup than users of pads and usual practice enrolled for 9 months or longer (menstrual cup 13 [13%] of 101, pads 29 [20%] of 143, usual practice 20 [19%] of 104; menstrual cup vs pads p=0·018 and menstrual cup vs usual practice p=0·074; table 2).5, 70 Toxic shock syndrome was identified in five case reports;13, 14, 66 microbiological confirmation was available with cultures from menstrual cup and blood showing streptococcus for one case.14 In two participants from two case reports, concomitant conditions were present (intrauterine device [IUD] in situ; an immunodeficiency disease).14, 66 A potential additional case of toxic shock syndrome was identified in a web blog (appendix pp 43–44): we could not determine whether this case has separately been reported in the MAUDE system or in a case report and thus it has been left out of our analysis. The prevalence of vaginal Staphylococcus aureus was examined among Kenyan schoolgirls participating in a randomised pilot study;5, 29 no difference was seen between menstrual cup, pads, and usual practice groups.29 No expression of toxic shock syndrome toxin 1 (TSST-1) was found in S aureus positive samples from menstrual cup users in this study.29 In-vitro studies of production of TSST-1 in the presence of menstrual cup material showed conflicting results (table 2).72, 73
An initial case report of a menstrual cup user about dislodgement of her IUD during use of a menstrual cup was followed by a case series of seven women who reported dislodgement of an IUD during removal of the menstrual cup between 1 week and 13 months of IUD insertion.14, 64 A retrospective chart survey did not find an increased risk for IUD expulsion within 6–8 weeks after insertion among menstrual cup users (five [4%] of 135), compared with tampons users (11 [2%] of 469) or pad users (seven [4%] of 169).57
One case-report68 suggested use of a menstrual cup might have been associated with the development of endometriosis;68 however, this hypothesis was not considered plausible by the regulatory authority and we did not identify any further reports on this possible association. We found three case reports60, 61, 62 of hydronephrosis and one14 of incontinence when using the menstrual cup; however, symptoms disappeared after menstrual cup removal (table 2).14, 60, 61 Other uses of menstrual cups—eg, as a contraceptive or temporary fistula control—are in the appendix (p 9)
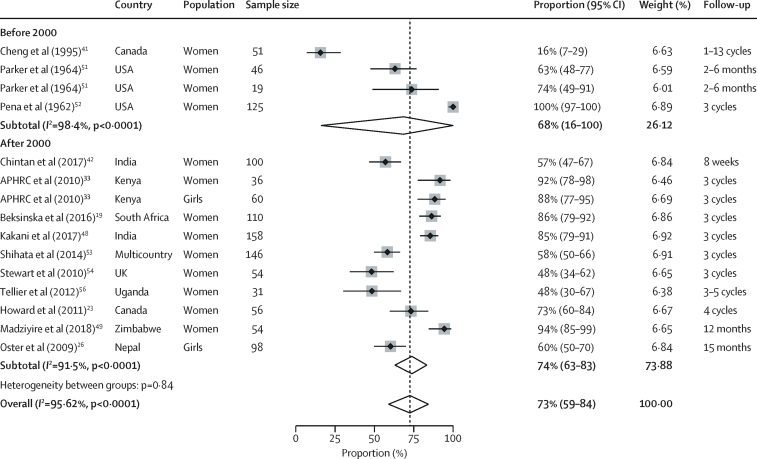
When assessing uptake and acceptability, all six relevant qualitative studies were from low-income and middle-income countries (appendix pp 10–13),30, 33, 34, 38, 56, 58, 59 whereas 20 studies with quantitative information on uptake and acceptability were from low-income and middle-income countries and high-income countries (appendix pp 14–22).13, 19, 22, 23, 24, 32, 33, 38, 39, 41, 42, 43, 46, 48, 49, 51, 52, 53, 54, 56 In low-income and middle-income countries, usual products for menstruation included cloths, disposable pads, cotton wool, tissue paper, or other items, and leakage and chaffing is a common concern.30, 33, 34, 58, 75 All studies that assessed use of menstrual cups used some form of education and training on the menstrual cup. Girls and women expressed initial concerns in qualitative studies, noting the size of the menstrual cup.30 Many were concerned it could cause pain (and noted it often did so at first) or worried about reproductive harms (eg, infertility). In quantitative studies, 3% (pooled estimate: n=1251, 95% CI 1–6%, 11 studies; I2=79·3%) of participants reported they could not insert the menstrual cup and 11% (n=1190, 95% CI 3–23%, ten studies; I2=96·4%) reported discontinuation related to the menstrual cup (table 3). Pain on insertion was reported for ten (9%) of 106 menstrual cup users versus none of 104 using their usual method at 3 months of follow-up in a South African crossover trial (p value not reported).19 Initial discomfort on insertion was reported by 20% of participants (pooled estimate: n=1061, 95% CI 12–30%, 17 studies; I2=92·3%). All qualitative studies described user familiarisation with the menstrual cup over time, with practice, peer support, and training being key to success.30, 33, 34, 38, 58, 59 Longitudinal quantitative studies in low-income and middle-income countries showed a learning curve of 2–5 months (appendix p 22); colour change of the menstrual cup as an objective measure suggested use increased throughout the first year among Kenyan schoolgirls.32 A Nepalese study25 noted that self-reported increased use 2 months after distribution was associated with the presence of friends who successfully used the menstrual cup. In India22 and Tanzania,43 the uptake of menstrual cups was significantly slower than uptake of pads (appendix p 24).22, 43 In 15 studies with relevant information, 73% (pooled estimate: n=1144, 95% CI 59–84; I2=96%) of participants reported willingness to continue use of the menstrual cup after the study (figure 3). All qualitative and some quantitative studies reported a positive effect of use of the menstrual cup on participants' lives, decreased stress concerning staining and leakage, and improvements in mobility.13, 51, 53 Challenges described included difficulties with cleaning and storage of the menstrual cup in low-income and middle-income countries.34, 58 Other challenges were associated with emptying the menstrual cup in school or public toilets,28, 34, 58 which was also reported by participants in high-income countries.37 Menstrual cups were associated with a decrease in the average number of changes per cycle in a UK study compared with tampons or sanitary pads.54 Three qualitative studies implied that school attendance, concentration, and performance improved after participants were given a menstrual cup.30, 34, 58 No measured difference in school absence or test results between products were reported (appendix p 24).5, 27, 34 A study in Nepal noted a significant decrease in time spent doing laundry for menstrual cup users compared with those using usual practice.27
Table 3.
Pooled estimates of meta-analyses for different outcomes of acceptability
| Pooled prevalence | Number of studies (or subgroups) | Total participants | I2 | pheterogeneity | p value for Z test* | ||
|---|---|---|---|---|---|---|---|
| Could not insert cup | 2·8% (0·8–5·6) | 11 | 1251 | 79·3% | <0·0001 | 0·0002 | |
| Used menstrual cup at least once (verbal report) | 79·3% (68·5–88·4) | 25 | 2367 | 97·1% | <0·0001 | <0·0001 | |
| Menstrual cup-related discontinuation | 10·7% (2·7–22·6) | 10 | 1190 | 96·4% | <0·0001 | 0·0004 | |
| Discontinuations for other reasons | 9·0% (3·8–15·9) | 15 | 1783 | 94·9% | <0·0001 | <0·0001 | |
| Difficult to insert (among users) | 20·3% (11·7–30·4) | 17 | 1061 | 92·3% | <0·0001 | <0·0001 | |
| First cycle | 35·3% (15·4–58·1) | 5 | 272 | 92·7% | <0·0001 | <0·0001 | |
| Later cycles† | 13·0% (8·1–18·7) | 12 | 789 | 74·3% | <0·0001 | <0·0001 | |
| Uncomfortable to wear | 12·6% (5·9–21·3) | 12 | 958 | 91·9% | <0·0001 | <0·0001 | |
| First cycle | 32·9% (2·2–76·2) | 3 | 221 | 97·5% | <0·0001 | 0·0148 | |
| Later cycles‡ | 7·9% (4·0–12·9) | 9 | 737 | 77·1% | <0·0001 | <0·0001 | |
| Difficulty removing | 9·3% (2·9–18·3) | 7 | 461 | 84·7% | <0·0001 | 0·0001 | |
| Continue using the cup | 72·5% (59·0–84·3) | 15 | 1144 | 95·6% | <0·0001 | <0·0001 | |
| Study before 2000 | 68·5% (16·1–100) | 4 | 241 | 98·4% | <0·0001 | 0·0014 | |
| Study after 2000§ | 73·8% (63·0–83·3) | 11 | 903 | 91·5% | <0·0001 | 0·0001 | |
Data in parentheses are 95% CIs. No significant difference was found for any of the subgroup analyses (high-income vs low-income and middle-income countries, adult women vs adolescents, type of cup, duration of follow-up, or high vs low or moderate study quality).
A significant Z test indicates that the pooled prevalence is different from zero.
Difficult to insert: first vs later menstrual cycles: p=0·15.
Uncomfortable to wear: first vs later menstrual cycles: p=0·13.
Continue to use cup: before vs after 2000: p=0·29.
Figure 3.
Proportion of women who wanted to continue menstrual cup use after the study
All studies herein used vaginal cups. In Cheng et al (1995),41 a cup with a valve in the stem was used. In Parker at al (1964),51 one study population had menorrhagia (n=46), and the other population had normal flow (n=19). APHRC=African Population and Health Research Center.
An economic advantage of a menstrual cup emerged in qualitative studies, with participants (and families) citing monthly cost savings from not needing to purchase pads or soap for laundry. Two qualitative studies included quotes from participants showing that menstrual cups might decrease the need for transactional sex to purchase pads.34, 38 This finding might be corroborated by results from a randomised controlled study among schoolgirls (aged 14–16 years) in rural western Kenya that noted a significantly lower prevalence of sexually transmitted infections among participants who were provided by the study with either menstrual cups or disposable pads versus controls (ie, using usual practice), citing lower exposure to transactional sex as a probable reason (table 2; appendix p 24).5
A study in schoolgirls in Kenya (aged 14–16 years) in an area with poor water and sanitation76 reported dropping of menstrual products during changing of cloths or disposable pads, or emptying of the cup.31 The frequency was similar for menstrual cups and sanitary pads. Factors involved included young age, and lack of time and privacy. Dropping of the menstrual cup decreased with increasing experience (approximately 23% in the first 3 months and 10% at or after 12 months). This dropping was associated with Escherichia coli isolated in cultures from swabs of menstrual cups, which was higher in new users than in experienced users (table 2).29 The proportion of girls who washed their hands before changing of the menstrual cup, by verbal self-report, was 95% (204 of 215) in a Kenyan report,28 70% (16 of 23) in a Ugandan report,56 and 94% (15 of 16) in a study in a refugee camp.38 When toilets have a lack of water, some participants reported carrying a bottle of water for when they emptied their menstrual cup.28 Others said they had to empty the menstrual cup only twice a day, so they could avoid emptying in public places.38 In two studies, women reported that use of menstrual cups saved water because of less leaking and washing of cloths.33, 38 Privacy was mainly mentioned as a problem when boiling (ie, cleaning) or storing menstrual cups.38, 56
We identified considerable heterogeneity in outcomes of acceptability of menstrual cups in the pooled meta-analyses (table 3). Subgroup analyses by study quality (low vs moderate-to-good) for outcomes examined did not show significant differences for the outcomes examined, but the sample size for moderate-to-good quality studies was small (appendix p 23). Smaller studies sometimes show different, often larger, treatment effects than large studies (ie, small-study effect); except for the outcome of “could not insert cup” (p=0·041), we did not find evidence for the presence of small-study effects (appendix p 23).
In the assessment of visibility in three studies in high-income countries, only 11–33% of the women interviewed (n=375) were aware of menstrual cups (appendix p 25).37, 47, 55 On 69 websites with educational materials on puberty and menarche from 27 countries, disposable pads were mentioned as an option by 53 (77%), tampons by 45 (65%), menstrual cups by 21 (30%), and reusable pads by 15 (22%; appendix pp 26–28). In the assessment of costs and availability, we identified 199 brands of menstrual cups, and availability in 99 countries with prices ranging US$0·72–46·72 (median 23·3 [IQR 16·54–29·20], from 145 brands with prices available; appendix pp 29–39).
When considering financial and environment costs, using accumulated estimates over 10 years, purchase costs and waste from consistent use of a menstrual cup (vaginal cup) would be a small fraction of the purchase costs and waste of pads or tampons—eg, if compared with using 12 pads per period, use of a menstrual cup would comprise 5% of the purchase costs and 0·4% of the plastic waste, and compared with 12 tampons per period, use of a menstrual cup would comprise 7% of the purchase costs and 6% of the plastic waste (appendix pp 40–42).
Discussion
Women, girls, and transgender people require hygienic menstrual products monthly to live healthy and productive lives. In this systematic review and meta-analysis, we assessed the menstrual cup, combining information from medical and grey literature to inform choice and strengthen the evidence base for programmes supporting menstrual health, such as for schoolgirls in low-income and middle-income countries or among refugees. Leakage was similar or less when using the menstrual cup than when using disposable pads and tampons. The adoption of a menstrual cup required a familiarisation phase and peer support seemed to be important for uptake in low-income and middle-income countries. Challenges in resource constrained settings (eg, lack of sanitation, hygiene, and privacy) did not stop women from using the cup. Around 70% of participants in 13 studies declared wanting to continue use. We identified several incidental case reports of vaginal damage, toxic shock syndrome, or urinary tract complaints after menstrual cup use, and difficulty retrieving the menstrual cup was also reported. Use of menstrual cups has been described as a factor for IUD dislodgement. Menstrual cups were infrequently mentioned in online educational materials on puberty and menstruation for adolescent girls; the lack of information appears to be global. Brands of menstrual cups were identified in 99 countries with a wide range in prices with a median of US$23·30.
In studies that examined the vagina and cervix during follow-up, no mechanical harm was evident from use of a menstrual cup.13, 52, 70 Infection risk did not appear to increase with use of a menstrual cup, and compared with pads and tampons, some studies indicated a decreased infection risk.5, 13, 70 A study in Kenya that detected lower bacterial vaginosis in users of a menstrual cup than in those who used sanitary pads postulated that the inert material of the menstrual cup might assist in maintaining a healthy vaginal pH and microbiome.5 Reported pain might relate to variations in the pelvic anatomy or wrong positioning of the menstrual cup leading to internal pressure. These factors could account for case reports of hydronephrosis or urinary incontinence. Allergies to the materials used in menstrual cups are not common, but women should be aware of the possibility and keep this in mind when starting use. However, for women who start using a menstrual cup, discrimination between discomfort as part of the normal learning curve and pathology might be difficult. Laboratory studies have shown contradicting results on the possibility of development of TSST-1 in the presence of menstrual cups,71, 72, 73 but clinical data in humans using cups have so far not shown reason for concern.29 The reported risk of toxic shock syndrome with use of a menstrual cup seems low, with five cases identified via our literature search. Although aggregated data on the number of menstrual cups sold or used is unavailable, we anticipate the number of girls and women using the 199 different brands globally is likely to be in the thousands. In the USA, the incidence of all types of toxic shock syndrome was around 0·8–3·4 per 100 000 population, whereas menstrual toxic shock syndrome was reported in 6–12 per 100 000 users of high-absorbency tampons in 1980.77 Similarly, among women using female barrier contraceptives, which also use medical-grade silicone or latex products, toxic shock syndrome is low (approximately 2·25 cases per 100 000 users per year).78
The combination of an IUD and use of a menstrual cup might need further study. Women with IUDs might need to consider an alternative option for either family planning or menstrual flow. Given the few reports on menstrual cups thus far, we cannot yet exclude other issues with the use of menstrual cups. Few studies directly compared menstrual cups and other menstrual products or materials; however, data do not suggest the menstrual cup is less effective than other sanitary products. Menstrual cups can collect more blood than tampons or sanitary pads and have been adopted by women with menorrhagia.51 The studies we reviewed report that under challenging conditions (eg, with little water or privacy), menstrual cups can be used. Alternatives to menstrual cups and disposable sanitary pads include reusable pads, so far assessed in few studies.75, 79, 80, 81 In Uganda, privacy to dry these pads was a challenge, suggesting additional packs would be needed to ensure effective laundering.82
Our study had several limitations. We used leakage as a primary outcome; however, the outcomes identified in the reports and studies reviewed varied by different timepoints and designs, prohibiting combination of results when directly comparing menstrual cups with another item. The quality of studies was a limitation, with only three assessed to be of good quality, which will potentially have contributed to bias in the meta-analysis. Some data were from older studies when reporting requirements were less stringent or with menstrual cups that are no longer available, from reports not published in peer-reviewed journals, and from studies using the menstrual cup to assess other topics (eg, understanding how uncertainty barriers can be overcome in economics,22 use of reusable menstrual products because of environmental concerns,37 the association between self-objectification and attitudes toward an alternative menstrual product47). Recruitment in observational studies was not representative or clear. Studies mostly depended on self-reporting, which might have overestimated use of the menstrual cup. One study comparing self-reporting against a conservative but objective measure of the colour change of the menstrual cup found uptake of use of the menstrual cup was slower than self-reported—eg, by 4 months, 75% of recipients stated they had started using the cup, whereas only by 10 months did 75% of menstrual cups show appropriate colour change.32 The number of countries where menstrual cups were available might be underestimated because producers of menstrual cups in low-income and middle-income countries might not always have websites. Our search was in English, and thus lacked information from many countries, such as Russia or China. The heterogeneity for the pooled prevalence was high in the meta-analyses (I2≥74%), indicating inconsistency in outcomes across studies. Given the high variability in study design, study period, study population, and products examined, this heterogeneity might not be unexpected. What proportion of adverse events are under-reported is unknown; we did not identify many adverse events (one case of toxic shock syndrome) when exploring the internet (appendix pp 43–44). The MAUDE database only allows searches for the past 10 years. Our cost and waste estimates are illustrative and do not account for the combined use of menstrual products during a period or inflation and production costs.
This systematic review suggests that menstrual cups can be an acceptable and safe option for menstrual hygiene in high-income, low-income, and middle-income countries but are not well known. Our findings can inform policy makers and programmes that menstrual cups are an alternative to disposable sanitary products, even where water and sanitation facilities are poor. However, provision of information, training, and follow-up on correct use might be needed. Further studies are needed on cost-effectiveness and environmental impact comparing different menstrual products, and to examine facilitators for use of menstrual cups, with monitoring systems in place to document any adverse outcomes.
Acknowledgments
Acknowledgments
This study was funded by the UK Medical Research Council, Department for International Development, and Wellcome Trust (Joint Global Health Trials scheme; award MR/N006046/1). We thank Obsidian for her work and dedication. We also thank Cheryl Giddings for her support. The Kenya Medical Research Insitute Director approved publication of this report.
Contributors
AMvE and PAP-H led the conception and design of the study. AMvE, GZ, ML, and LM led the development of the data collection instrument, data collection, and quality assessment. AMvE, GZ, ML, and LM did the statistical analysis, interpreted the data, and wrote and revised the manuscript. MS, EN, HU, and KL contributed to study design, assisted in data interpretation, and revised the manuscript. All authors contributed to data interpretation and revised the intellectual content of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.United Nations Population division. World population prospects. 2019. https://esa.un.org/unpd/wpp/
- 2.Mason L, Nyothach E, Alexander K. ‘We keep it secret so no one should know’ - a qualitative study to explore young schoolgirls attitudes and experiences with menstruation in rural Western kenya. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebert Kuhlmann A, Peters Bergquist E, Danjoint D, Wall LL. Unmet menstrual hygiene needs among low-income women. Obstet Gynecol. 2019;133:238–244. doi: 10.1097/AOG.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 4.Das P, Baker KK, Dutta A. Menstrual hygiene practices, WASH access and the risk of urogenital infection in women from Odisha, India. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips-Howard PA, Nyothach E, Ter Kuile FO. Menstrual cups and sanitary pads to reduce school attrition, and sexually transmitted and reproductive tract infections: a cluster randomised controlled feasibility study in rural Western Kenya. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torondel B, Sinha S, Mohanty JR. Association between unhygienic menstrual management practices and prevalence of lower reproductive tract infections: a hospital-based cross-sectional study in Odisha, India. BMC Infect Dis. 2018;18:473. doi: 10.1186/s12879-018-3384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer M, Caruso BA, Sahin M. A time for global action: addressing girls' menstrual hygiene management needs in schools. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips-Howard P, Olilo G, Burmen B. Menstrual needs and associations with sexual and reproductive risks in rural Kenyan females: a cross-sectional behavioural survey linked with HIV prevalence. J Womens Health (Larchmt) 2015;24:801–811. doi: 10.1089/jwh.2014.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer M. Where the education system and women's bodies collide: the social and health impact of girls' experiences of menstruation and schooling in Tanzania. J Adolesc. 2010;33:521–529. doi: 10.1016/j.adolescence.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Sommer M, Chandraratna S, Cavill S, Mahon T, Phillips-Howard P. Managing menstruation in the workplace: an overlooked issue in low- and middle-income countries. Int J Equity Health. 2016;15:86. doi: 10.1186/s12939-016-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Côté I, Jacobs P, Cumminx D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol. 2002;100:683–687. doi: 10.1016/s0029-7844(02)02094-x. [DOI] [PubMed] [Google Scholar]
- 12.van Eijk AM, Sivakami M, Thakkar MB. Menstrual hygiene management among adolescent girls in India: a systematic review and meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North B, Oldham M. Preclinical, clinical, and over-the-counter postmarketing experience with a new vaginal cup: menstrual collection. J Womens Health (Larchmt) 2011;20:303–311. doi: 10.1089/jwh.2009.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration Manufacturer and user facility device experience database (MAUDE) Oct 6, 2019. https://www.fda.gov/medicaldevices/deviceregulationandguidance/postmarketrequirements/reportingadverseevents/ucm127891.htm
- 15.Higgins JP, Altman DG, Gøtzsche PC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 17.Critical Appraisal Skills Programme; Oxford: 2017. CASP checklists.http://www.casp-uk.net/casp-tools-checklists [Google Scholar]
- 18.Mazgaj M, Yaramenka K, Malovana O. Royal Institute of Technology Stockholm; 2006. Comparative life cycle assessment of sanitary pads and tampons.http://docplayer.net/39797321-Comparative-life-cycle-assessment-of-sanitary-pads-and-tampons.html Master's thesis. [Google Scholar]
- 19.Beksinska ME, Smit J, Greener R. Acceptability and performance of the menstrual cup in South Africa: a randomized crossover trial comparing the menstrual cup to tampons or sanitary pads. J Womens Health (Larchmt) 2015;24:151–158. doi: 10.1089/jwh.2014.5021. [DOI] [PubMed] [Google Scholar]
- 20.Beksinska M, Smit J, Greener R. ‘It gets easier with practice’. A randomised cross-over trial comparing the menstrual cup to tampons or sanitary pads in a low resource setting. Eur J Contracept Reprod Health Care. 2016;21(suppl 1):138. [Google Scholar]
- 21.Beksinska M, Smit J, Greener R, Maphumulo V, Mabude Z. Better menstrual management options for adolescents needed in South Africa: what about the menstrual cup? S Afr Med J. 2015;105:331. doi: 10.7196/samj.9205. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann V, Adelman S, Sebastian A. Learning by doing something else: Experience with alternatives and adoption of a high-barrier menstrual hygiene technology. Feb 10, 2014. https://docplayer.net/41533102-Learning-by-doing-something-else-experience-with-alternatives-and-adoption-of-a-high-barrier-menstrual-hygiene-technology.html
- 23.Howard C, Rose CL, Trouton K. FLOW (finding lasting options for women): multicentre randomized controlled trial comparing tampons with menstrual cups. Can Fam Physician. 2011;57:e208–e215. [PMC free article] [PubMed] [Google Scholar]
- 24.Oster E, Thornton R. Menstruation, sanitary products, and school attendance: evidence from a randomized evaluation. Am Econ J Appl Econ. 2011;3:91–100. [Google Scholar]
- 25.Oster E, Thornton R. Determinants of technology adoption: peer effects in menstrual cup up-take. J Eur Econ Assoc. 2012;10:1263–1293. [Google Scholar]
- 26.Oster E, Thornton R. The National Bureau of Economic Research; Cambridge, MA: March, 2009. Determinants of technology adoption: private value and peer effects in menstrual cup take-up.http://www.nber.org/papers/w14828 [Google Scholar]
- 27.Oster E, Thornton R. The National Bureau of Economic Research; Cambridge, MA: April, 2009. Menstruation and education in Nepal.http://www.nber.org/papers/w14853 [Google Scholar]
- 28.Nyothach E, Alexander KT, Oduor C. Handwashing for menstrual hygiene management among primary schoolgirls in rural Western Kenya. Waterlines. 2015;34:279–295. [Google Scholar]
- 29.Juma J, Nyothach E, Laserson KF. Examining the safety of menstrual cups among rural primary school girls in western Kenya: observational studies nested in a randomised controlled feasibility study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason L, Laserson K, Oruko K. Adolescent schoolgirls' experiences of menstrual cups and pads in rural western Kenya: a qualitative study. Waterlines. 2015;34:15–30. [Google Scholar]
- 31.Oduor C, Alexander KT, Oruko K. Schoolgirls' experiences of changing and disposal of menstrual hygiene items and inferences for WASH in schools. Waterlines. 2015;34:397–411. [Google Scholar]
- 32.van Eijk AM, Laserson KF, Nyothach E. Use of menstrual cups among school girls: longitudinal observations nested in a randomised controlled feasibility study in rural Western Kenya. Reprod Health. 2018;15:139. doi: 10.1186/s12978-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.APHRC . African Population and Health Research Center; Nairobi: 2010. Attitudes towards, and acceptability of, menstrual cups as a method for managing menstruation: experiences of women and school girls in Nairobi, Kenya - policy brief No. 21.http://www.susana.org/_resources/documents/default/2-984-policy-brief-no-21-2010-attitudes-and-acceptability.pdf [Google Scholar]
- 34.APHRC . The African Population and Health Research Center; Nairobi: 2010. Use of menstrual cup by adolescent girls and women: potential benefits and key challenges – policy brief 22.https://www.susana.org/en/knowledge-hub/resources-and-publications/library/details/985 [Google Scholar]
- 35.APHRC . The African Population and Health Research Center; Nairobi: 2010. Experiences and problems with menstruation among poor women and schoolgirls in Nairobi, Kenya – policy brief 20.http://www.communityledtotalsanitation.org/sites/communityledtotalsanitation.org/files/PolicyBrief_Mooncups_Kenya.pdf [Google Scholar]
- 36.Averbach S, Sahin-Hodoglugil N, Musara P, Chipato T, van der Straten A. Duet for menstrual protection: a feasibility study in Zimbabwe. Contraception. 2009;79:463–468. doi: 10.1016/j.contraception.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Borowski A. Rochester Insitute of Technology; 2011. Are American women turning to reusable and greener menstrual products due to health and environmental pollution concerns?http://scholarworks.rit.edu/theses/544/ Master's thesis. [Google Scholar]
- 38.Care International in Uganda . CARE International in Uganda; Kampala: 2018. Ruby cups: girls in Imvepi refugee settlement taking control.http://womena.dk/wp-content/uploads/2018/12/Ruby-Cups-Girls-in-Imvepi-Refugee-Settlement-Taking-Control-03.12.18-Final-report.pdf [Google Scholar]
- 39.Cattanach JF. A diaphragm tampon applied to an ovulation method in a birth control system. Contraception. 1991;44:607–621. doi: 10.1016/0010-7824(91)90081-p. [DOI] [PubMed] [Google Scholar]
- 40.Cattanach JF. The Gynaeseal diaphragm tampon. Med J Aust. 1990;152:52–53. doi: 10.5694/j.1326-5377.1990.tb124442.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheng M, Kung R, Hannah M, Wilansky D, Shime J. Menses cup evaluation study. Fertil Steril. 1995;64:661–663. [PubMed] [Google Scholar]
- 42.Chintan S, Dipesh P, Maitri P. Use of Flow Care Menstrual Cups over conventional menstrual products in India. Int J Adv Res Dev. 2017;2:78–82. [Google Scholar]
- 43.Runli F. Femme International; 2017. Monitoring & evaluation report. Kilimanjaro Region, 2017. Successes and lessons learned from the Thaweza program.https://www.femmeinternational.org/wp-content/uploads/2018/09/Femme-International-ME-Report-2017.pdf [Google Scholar]
- 44.Ganyaglo GYK, Ryan N, Park J, Lassey AT. Feasibility and acceptability of the menstrual cup for non-surgical management of vesicovaginal fistula among women at a health facility in Ghana. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan NE. Feasibility, acceptability, and appropriateness of the vaginal menstrual cup for short term non-surgical management of vesicovaginal fistula (VVF) among potential users and stakeholders. Implement Sci. 2018;13(suppl 4):S80. [Google Scholar]
- 46.Gleeson N, Devitt M, Buggy F, Bonnar J. Menstrual blood loss measurement with gynaeseal. Aust N Z J Obstet Gynaecol. 1993;33:79–80. doi: 10.1111/j.1479-828x.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 47.Grose RG, Grabe S. Sociocultural attitudes surrounding menstruation and alternative menstrual products: the explanatory role of self-objectification. Health Care Women Int. 2014;35:677–694. doi: 10.1080/07399332.2014.888721. [DOI] [PubMed] [Google Scholar]
- 48.Kakani CR, Bhatt JK. Study of adaptability and efficacy of menstrual cup in managing menstrual health and hygiene. Int J Reprod Contracept Obstet Gynecol. 2017;6:3045–3053. [Google Scholar]
- 49.Madziyire MG, Magure TM, Madziwa CF. Menstrual cups as a menstrual management method for low socioeconomic status women and girls in Zimbabwe: a pilot study. Womens Reprod Health. 2018;5:59–65. [Google Scholar]
- 50.Madziyire MG, Magure TM, Madziwa CF. The safety of menstrual cups in women of low socio-economic status in Zimbabwe: pilot study. Cent Afr J Med. 2018;64:59–65. [Google Scholar]
- 51.Parker J, Bushell RW, Behrman SJ. Hygienic control of menorrhagia: use of rubber menstrual cup. Int J Fertil. 1964;9:619–621. [PubMed] [Google Scholar]
- 52.Pena EF. Menstrual protection. Advantages of the menstrual cup. Obstet Gynecol. 1962;19:684–687. [PubMed] [Google Scholar]
- 53.Shihata A, Brody S. An innovative, reusable menstrual cup that enhances the quality of women's lives during menstruation. Br J Med Med Res. 2014;4:3581–3590. [Google Scholar]
- 54.Stewart K, Greer R, Powell M. Women's experience of using the Mooncup. J Obstet Gynaecol. 2010;30:285–287. doi: 10.3109/01443610903572117. [DOI] [PubMed] [Google Scholar]
- 55.Stewart K, Powell M, Greer R. An alternative to conventional sanitary protection: would women use a menstrual cup? J Obstet Gynaecol. 2009;29:49–52. doi: 10.1080/01443610802628841. [DOI] [PubMed] [Google Scholar]
- 58.Hyttel M, Thomsen CF, Luff B, Storrusten H, Nyakato VN, Tellier M. Drivers and challenges to use of menstrual cups among schoolgirls in rural Uganda: a qualitative study. Waterlines. 2017;36:109–124. [Google Scholar]
- 56.Tellier M, Hyttel M, Gad M. WoMena, Uganda Red Cross Society; Kampala: 2012. Assessing acceptability and hygienic safety of menstrual cups as a menstrual management method for vulnerable young women in Uganda Red Cross Society's Life Planning Skills Project.http://womena.dk/wp-content/uploads/2015/11/Menstrual-Cups-_-WoMena-_-Uganda-Pilot-Study-Report-Dec-2012-new-version.pdf [Google Scholar]
- 57.Wiebe ER, Trouton KJ. Does using tampons or menstrual cups increase early IUD expulsion rates? Contraception. 2012;86:119–121. doi: 10.1016/j.contraception.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Sundqvist J. Linnaeus University; 2015. A cup of freedom?: A study of menstrual cup's impact on girls' capabilities. Independent thesis Basic Level.http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A783661&dswid=-5356 [Google Scholar]
- 60.Adedokun BO. 43 - Hydronephrosis associated with the use of menstrual cup. Eur Urol Suppl. 2017;16 [Google Scholar]
- 61.Nunes-Carneiro D, Couto T, Cavadas V. Is the menstrual cup harmless? A case report of an unusual cause of renal colic. Int J Surg Case Rep. 2018;46:28–30. doi: 10.1016/j.ijscr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stolz A, Meuwly J-Y, Roussel A, Nicodème Paulin E. An improperly positioned menstrual cup complicated by hydronephrosis: a case report. Case Rep Womens Health. 2019;22 doi: 10.1016/j.crwh.2019.e00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Day S. A retained menstrual cup. Int J STD AIDS. 2012;23:367–368. doi: 10.1258/ijsa.2011.011277. [DOI] [PubMed] [Google Scholar]
- 64.Seale R, Powers L, Guiahi M, Coleman-Minahan K. Unintentional IUD expulsion with concomitant menstrual cup use: a case series. Contraception. 2019 doi: 10.1016/j.contraception.2019.03.047. published online April 11. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg L, Elsamra S, Hutchinson-Colas J, Segal S. Delayed diagnosis of vesicouterine fistula after treatment for mixed urinary incontinence: menstrual cup management and diagnosis. Female Pelvic Med Reconstr Surg. 2016;22:e29–e31. doi: 10.1097/SPV.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell MA, Bisch S, Arntfield S, Hosseini-Moghaddam SM. A confirmed case of toxic shock syndrome associated with the use of a menstrual cup. Can J Infect Dis Med Microbiol. 2015;26:218–220. doi: 10.1155/2015/560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell KW, Robinson RE, Mone MC, Scaife CL. Enterovaginal or vesicovaginal fistula control using a silicone cup. Obstet Gynecol. 2016;128:1365–1368. doi: 10.1097/AOG.0000000000001745. [DOI] [PubMed] [Google Scholar]
- 68.Spechler S, Nieman LK, Premkumar A, Stratton P. The Keeper, a menstrual collection device, as a potential cause of endometriosis and adenomyosis. Gynecol Obstet Invest. 2003;56:35–37. doi: 10.1159/000072329. [DOI] [PubMed] [Google Scholar]
- 69.Cattanach J. A new type of sanitary tampon, (Gynaeseal) that can also be used as an alternative diaphragm has been developed. Aust N Z J Obstet Gynaecol. 1989;29:275. [PubMed] [Google Scholar]
- 70.Karnaky KJ. Internal menstrual protection with the rubber menstrual cup. Obstet Gynecol. 1962;19:688–691. [PubMed] [Google Scholar]
- 71.Tierno PM, Jr, Hanna BA. Ecology of toxic shock syndrome: amplification of toxic shock syndrome toxin 1 by materials of medical interest. Rev Infect Dis. 1989;11(suppl 1):S182–S186. doi: 10.1093/clinids/11.supplement_1.s182. [DOI] [PubMed] [Google Scholar]
- 72.Tierno PM, Hanna BA. Propensity of tampons and barrier contraceptives to amplify Staphylococcus aureus Toxic shock syndrome toxin-I. Infect Dis Obstet Gynecol. 1994;2:140–145. doi: 10.1155/S1064744994000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nonfoux L, Chiaruzzi M, Badiou C. Impact of currently marketed tampons and menstrual cups on Staphylococcus aureus growth and TSST-1 production in vitro. Appl Environ Microbiol. 2018;84:e0035–e0118. doi: 10.1128/AEM.00351-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Museum of Menstruation and Women's Health Do menstrual cups cause endometriosis? 2003. http://www.mum.org/fdacups2.htm
- 75.Budhathoki SS, Bhattachan M, Pokharel PK, Bhadra M, van Teijlingen E. Reusable sanitary towels: promoting menstrual hygiene in post-earthquake Nepal. J Fam Plann Reprod Health Care. 2017;43:157–159. doi: 10.1136/jfprhc-2016-101481. [DOI] [PubMed] [Google Scholar]
- 76.Alexander KT, Oduor C, Nyothach E. Water, sanitation and hygiene conditions in Kenyan rural schools: are schools meeting the needs of menstruating girls? Water. 2014;6:1453–1466. [Google Scholar]
- 77.Hajjeh RA, Reingold A, Weil A, Shutt K, Schuchat A, Perkins BA. Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis. 1999;5:807–810. doi: 10.3201/eid0506.990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwartz B, Gaventa S, Broome CV. Nonmenstrual toxic shock syndrome associated with barrier contraceptives: report of a case-control study. Rev Infect Dis. 1989;11(suppl 1):S43–S48. doi: 10.1093/clinids/11.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 79.Montgomery P, Ryus CR, Dolan CS, Dopson S, Scott LM. Sanitary pad interventions for girls' education in Ghana: a pilot study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montgomery P, Hennegan J, Dolan C, Wu M, Steinfield L, Scott L. Menstruation and the cycle of poverty: a cluster quasi-randomised control trial of sanitary pad and puberty education provision in Uganda. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crofts T, Fisher J. Menstrual hygiene in Ugandan schools: an investigation of low-cost sanitary pads. J Water Sanit Hyg Dev. 2012;2:50–58. [Google Scholar]
- 82.Hennegan J, Dolan C, Wu M, Scott L, Montgomery P. Measuring the prevalence and impact of poor menstrual hygiene management: a quantitative survey of schoolgirls in rural Uganda. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.