Thyroid hormones play important roles in metabolic and reproductive function; however, they have not been described in detail for any river dolphin species. We characterize these hormones across different age, sex and pregnancy status in the Amazon river dolphin in order to provide baseline normal values for this increasingly threatened species.
Keywords: Boto, fetal demise, neonatal loss, river dolphins, thyroxine, triiodothyronine
Abstract
This study was conducted to characterize immunoreactive thyroid hormone concentrations in wild Amazon river dolphins, also called boto (Inia geoffrensis) by age group, sex, pregnancy and lactation status, and to determine if thyroid hormone concentration differences could be detected between pregnant females with and without successful parturition outcomes. Radioimmunoassays were used to analyse total T3 and total T4 in 182 serum samples collected from 172 botos living in the Mamirauá Sustainable Development Reserve, in the Brazilian Amazon from 2003 through 2015. Age significantly affected tT3 and tT4 concentrations in males, with values in immature males and females being significantly lower than those in adult males, whereas no age effects were noted between immature females and adult non-pregnant, non-lactating females. Significant sex differences were noted in tT3 concentrations between immature males and females and in tT4 concentrations between adult males and females. These resulted in significant differences in the tT3:tT4 ratio between males and females within the immature and adult groups. Lactating and non-pregnant adult females had significantly higher tT3 concentrations than pregnant females, and this difference was primarily driven by a 12% drop in tT3 concentrations during the last two-thirds of pregnancy. No differences in thyroid hormone concentrations were detected between females diagnosed as pregnant and later found to have or not have a live calf. These results are the first to define thyroid hormone reference intervals and normal physiological variations in a wild population of river dolphins.
Introduction
Global freshwater habitats are often adjacent to or within dense and expanding human populations and are increasingly being degraded due to anthropogenic activity. As a result, freshwater megafauna species, including all species of river dolphins, are under direct pressures, and if they continue unchecked, it may result in their extinction (He et al., 2017; Huang et al., 2012; Williams et al., 2016). Currently, of an estimated 10 species of fresh water dolphins, six are listed as endangered, critically endangered or functionally extinct, two are vulnerable and two populations are considered “status unknown” (Braulik and Smith, 2017; He et al., 2017; Reeves et al., 2011). While it is clear that freshwater dolphins are at extreme risk, what is less understood is how anthropogenic pressures are affecting their survival. In addition, as apex predators, these species often directly compete with man for food, and many of the environmental factors that may be detrimental towards their overall health and well-being could also affect human populations. Thus, these species, and cetaceans in general, may represent one of the best environmental sentinels for human populations living nearby (Bossart, 2006; Chen et al., 2017; Lailson-Brito et al., 2008).
Despite the importance of river dolphins for habitat health (Turvey et al., 2012), and as markers of adverse environmental threats, little is known about their physiology, or how various species may be affected by deteriorating conditions. The Amazon river dolphin, or boto (Inia geoffrensis), exists in habitats that are currently relatively free from industrial development and high population density (da Silva et al., 2018). Despite this, recent evidence suggests this species is in decline due to anthropogenic pressures created from direct harvesting of the animals for use as bait to catch commercially valuable catfish, the piracatinga (Calophysus macropterus), by native fisherman (Mintzer et al., 2013; da Silva et al., 2018). In addition, multiple proposals exist for hydroelectric projects throughout the Amazon river basin that may threaten the population through habitat fragmentation (Finer and Jenkins, 2012). These current and future pressures may threaten boto sustainability, and therefore, there may be a limited time period during which physiologic health markers and physiological reference ranges can be defined and used as “normal” controls for future population health assessments.
Several health assessments of wild cetacean populations have been published over the last 20 years; the value of which has been demonstrated by recent research on wild bottlenose dolphin populations adversely impacted by a regional oil spill (Schwanke et al., 2014). Data from both captive and wild healthy populations have enabled researchers to develop models identifying the adverse short- and long-term impacts from this oil spill (Hall et al., 2018; Lane et al., 2015; Smith et al., 2017). Recently, health assessments of wild cetaceans have been applied to an increasing number of marine and freshwater cetacean species (Flower et al., 2015; Nabi et al., 2018; Smith et al., 2017; Van Bressem et al., 2009). Blood and other biological samples collected from animals during these assessments provided the opportunity to examine multiple organ and endocrine systems. For cetaceans, overfishing and climate change have elevated the importance of monitoring thyroid function via thyroid hormones to detect critical homeostatic changes in response to these external stressors (Fair et al., 2011; Flower et al., 2015; St. Aubin et al., 1996; Wasser et al., 2017). However, before detecting abnormal function, normal concentrations during varying life history events must be defined.
Thyroid hormones have been documented to vary by age and different physiological conditions, e.g. pregnancy and lactation, in multiple mammalian species, including cetaceans (Fair et al., 2011; Flower et al., 2015; St. Aubin, 2001; St. Aubin et al., 1996; West et al., 2014). Comparatively high hormone concentrations and proportionally large thyroid glands of marine cetaceans (Fair et al., 2011; Ridgway and Patton, 1971) have led researchers to speculate that cold environmental temperatures combined with high heat conductivity of water habitats requires an increased metabolic response to maintain core body temperature, which is driven by enhanced thyroid activity. In addition, changes in thyroid hormone concentrations that parallel seasonal environment changes and prey availability have been described for belugas (Delphinapterus leucas) and killer whales (Orcinus orca; Flower et al., 2015; Wasser et al., 2017).
Multiple thyroid pathologies have been identified in marine cetaceans, including colloid depletion, fibrosis, thyroiditis and squamous cysts (Cowan and Tajima, 2006; Harrison, 1969; Schumacher et al., 1993). Abnormal thyroid gland function during pregnancy either in response to iodine deficiency or due to primary thyroid pathology has been known to be associated with both fetal and placental growth abnormalities that can translate into a number of pathologies including abortion, premature birth, low birth weight and neonatal failure to thrive syndrome (Glinoer, 2007). While no reports of thyroid pathology exist for the boto, or other river dolphin species, the frequency of its occurrence in marine cetaceans warrants such an investigation.
In addition to direct effects of thyroid pathology, malnutrition has been indirectly linked to thyroid dysfunction, and if this deficiency occurs during pregnancy, fetal growth restriction and other developmental abnormalities may result. Some recent evidence in beef cows demonstrated that changes in the percentage of protein while maintaining normal energy content can affect fetal thyroid development and programming without any changes in circulating maternal thyroid hormone concentrations (Micke et al., 2015). In humans, maternal malnutrition has resulted in normal T4, and significantly low T3 concentrations compared with controls (Mahajan, et al., 2005). Recent attempts to use fecal thyroid hormone concentrations as an indicator of nutritional stress and subsequent fetal loss in the killer whale have provided some support for this premise (Wasser et al., 2017). While a direct link between malnutrition, thyroid dysfunction and fetal or neonatal abnormalities remains elusive, evaluation of normal thyroid function in the boto would provide baseline data from which future effects of nutritional stress may be evaluated.
Currently no significant ex situ populations of this species exist whereby thyroid hormone concentrations could be determined to establish baseline, and to our knowledge, only one report of thyroid hormone concentrations in wild botos exists. In that report, which analysed serum from eight wild animals, mean total thyroxine (tT4) concentration was determined without any information on sex, age or reproduction status of the animals that had been sampled (Ridgway et al., 1970). Therefore, the goal of this research was to describe both tT4 and total triiodothyronine (tT3) concentrations in wild botos. Specific objectives were to (i) determine if concentrations of thyroid hormones are significantly affected by sex, age and female reproductive status; (ii) develop thyroid reference ranges for wild botos relative to sex, maturity and female reproductive status (non-pregnant nonlactating, pregnant and lactating); (iii) determine if thyroid hormone concentrations vary during trimesters of gestation; and (iv) determine if thyroid hormone concentrations in females diagnosed as pregnant differ between females who are subsequently observed to have had a live calf or are observed without a calf.
Methods
Animals and sample collections
A total of 182 samples was collected from 172 animals who were temporarily restrained during an annual capture–recapture campaign of Projeto Boto at the Mamirauá Sustainable Development Reserve, Brazilian Amazon, between 2003 and 2015. Once restrained, total length, sex, and reproductive status of adult females (non-pregnant, pregnant or lactating) was determined by a combination of visual inspection and transabdominal ultrasonography (Figure 1). Blood samples were collected from the ventral tail fluke using a 19-gauge winged blood collection set (Figure 1). Whole blood was collected into BD Vacutainers (Becton Dickenson, Franklin Lakes, NJ, USA) containing activated thrombin, allowed to clot for 20 min, and then centrifuged at 1000 g for 15 to 30 min. Serum was collected and stored at −20°C until analysis. Evidence indicates that minimal change in thyroid hormone concentrations occurs when stored at −25°C for up to 23 years (Männistö et al., 2007). For pregnancy determination, all examinations were performed using a Sonosite 180 Plus ultrasound unit (Pyramid Medical Systems, São Paulo, São Paulo, 04181-110, Brazil) in conjunction with a 2- to 5-MHz multi-frequency transducer (Convex Array 180 Plus/Elite-C60, Pyramid Medical Systems). Animals were determined to be pregnant by visualization of a conceptus (placental membranes, fetus) and uterine fluid (amniotic, chorionic or allantoic fluid). Fetal age was assigned to the first (0 to 112 days), second (113 to 224 days) or third trimester (225 to 333 days) of pregnancy using total length, biparital or transthoracic measurements, by reference to published measurements for bottlenose dolphins (Tursiops truncatus; Lacave et al., 2004). Bottlenose dolphin fetal growth rates were chosen to estimate fetal age in boto due to the similarity in gestation length between the two species (Martin & da Silva, 2018). Based on serum testosterone in males (Amaral et al., manuscript in prep), males were classified as immature (calf or juvenile males < 188 cm total body length) or adult (males ≥188 cm total body length). Females were classified based on shortest size at first pregnancy (Martin & da Silva, 2018); therefore, immature females were <180 cm total body length and adult females were ≥180 cm total body length. Adult females were further categorized based on physiological state—non-pregnant, pregnant, or lactating.
Figure 1.
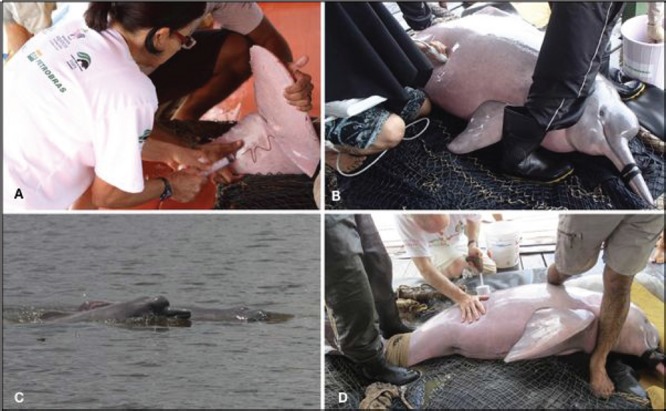
Health assessment on wild Amazon river dolphins (boto, Inia geoffrensis). Images depict blood sampling (a), ultrasound body examination (b), wild boto with calf (c) and freeze branding of animal prior to release (d). Images credited to Projeto Boto/INPA
Thyroid hormone radioimmunoassays
Thyroid hormones (total T4 and T3) were measured in serum using commercial solid phase radioimmunoassay (RIA) kits [Siemens Medical Solutions Diagnostics, Los Angeles, CA: total T4 (tT4) catalog number TKT45; total T3 (tT3) catalog number TKT35]. All RIAs were conducted in accordance with the manufacturer's instructions and were validated for use in dolphins based on observed parallelism between serial dilutions of pooled serum samples and the respective standard curves and >90% recovery of respective hormone standards from pooled serum samples. Assay sensitivities were 2.5 ng/ml for tT4, 0.1 pg/ml for fT4, 0.07 ng/ml for tT3 and 0.02 pg/ml for fT3. For all assays, intra- and inter-assay coefficients of variation were <15%. The relative percentage of total immunoreactive T3 and T4 were determined by dividing the mean value across all groups for each hormone, respectively, by the sum of both mean concentrations (μtT3 + μtT4 = total immunoreactive thyroid hormone concentrations).
Statistical analysis
Data were analysed using Stata® (version 14; StataCorp LP, College Station, TX, USA). For females, to control for repeated samples from the same animal, and an unequal number of sampling among animals, we analysed data using a linear mixed effect restricted maximum likelihood regression model (West et al., 2015) to quantify the relationship between the dependent variable (hormone concentration) and fixed effect variables, with animal ID as the random intercept variable with an unstructured covariance. The fixed effect categorical variables included age group (immature male and female, adult male and adult non-lactating, non-pregnant female), physiological state (pregnant, non-pregnant, non-pregnant lactating) and trimester (i.e. first trimester, second trimester, third trimester). Separate analyses were performed for each fixed effect variables and repeated for each dependent variable (i.e. hormone concentrations (tT3, tT4 and T3:T4 ratio).
All final mixed models were checked for normality using quantile plots of the standard residuals. If quantile–quantile (qnorm) plots of standardized residuals exhibited non-normal distribution, then data were transformed as indicated by the Shapiro–Wilk test (Ladder command, STATA) of raw data until model residuals were normalized. Pairwise comparisons of estimated marginal means were conducted using the margins command with Sidak correction or as paired contrasts without correction. Unless specified, data are expressed as back transformed marginal means and 95% confidence intervals. For all analyses, P < 0.05 was considered significant.
Comparisons of thyroid hormone concentration across gestational stages in pregnant females (as determined by ultrasonography during gestation) that had live calves versus pregnant females that did not have a live calf were determined by observations during the next annual field assessment. Since not all females were observed each year, only females which could be confirmed as having a calf within a 2-year period after they were diagnosed as pregnant (this length of time was used to accommodate for the various stages of gestation when they were initially assessed, and this the time interval to when a calf would have been born) were compared using unpaired two-tailed t test with unequal variances using the Welch (1947) approximation for degrees of freedom. Due to the length of time between examinations or observations of females we could not determine at which point the fetus or calf was lost. Therefore, the calf loss classification would include females that experienced an abortion, or stillbirth and calves that died during the perinatal period or that failed to thrive.
Results
Overall results
Across all sex and age groups, mean (±SD, 95% CI) tT3 and tT4 were 0.52 ± 0.15 ng/ml (0.49–0.55 ng/ml) and 38.6 ± 0.84 ng/ml (37.0–40.3 ng/ml), respectively. Based on mean concentrations, the relative percentage contribution towards total immunoreactive thyroid concentrations for tT3 and tT4 were 1.3% and 98.7%, respectively.
Thyroid hormone reference ranges for the boto
Percentiles (2.5, 25, 50, 75, 97.5) for tT3, tT4 and the tT3:tT4 by age class and for adult female physiological status are shown in Table 1. Generally, lactating females had tT3 values that tended to be higher at all percentage cut-offs than the other female groups and adult males, while pregnant females consistently had the lowest concentrations. For tT4, juveniles of both sexes tended to have the greatest concentrations. Finally, and similarly for tT3, the tT3:tT4 ratio was greatest for lactating females and adult males.
Table 1.
Serum thyroid hormone (total T3 ng/ml, total T4, T3:T4 ratio ×1000) reference concentrations for the Amazon river dolphin (Inia geoffrensis) in samples collected from 2003 to 2015
| Age class | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TH | Percentile | JF (n = 8) |
JM (n = 23) |
AM (n = 65) |
AF (n = 26) |
PF (n = 40) |
1st (n = 15) |
2nd (n = 21) | LF (n = 14) |
| tT3 | 2.5 | 0.28 | 0.38 | 0.25 | 0.13 | 0.24 | 0.27 | 0.24 | 0.31 |
| 25 | 0.32 | 0.43 | 0.43 | 0.39 | 0.33 | 0.34 | 0.32 | 0.45 | |
| 50 | 0.46 | 0.57 | 0.49 | 0.50 | 0.39 | 0.44 | 0.37 | 0.57 | |
| 75 | 0.52 | 0.90 | 0.59 | 0.75 | 0.46 | 0.46 | 0.42 | 0.67 | |
| 97.5 | 0.71 | 1.53 | 1.05 | 1.1 | 0.76 | 0.76 | 0.53 | 0.94 | |
| tT4 | 2.5 | 31.7 | 23.1 | 20.7 | 16.5 | 15.8 | 15.6 | 20.8 | 14.7 |
| 25 | 39.3 | 34.9 | 29.4 | 32.1 | 33.2 | 31.9 | 35.8 | 25.6 | |
| 50 | 44.1 | 42.0 | 33.1 | 39.7 | 38.2 | 42.7 | 37.7 | 36.0 | |
| 75 | 46.7 | 50.9 | 40.0 | 51.1 | 44.5 | 47 | 42.6 | 44.2 | |
| 97.5 | 51.2 | 68.7 | 51.5 | 96.7 | 61.5 | 61.8 | 50.3 | 67.8 | |
| T 3:T4 | 2.5 | 6.0 | 7.5 | 7.2 | 4.7 | 5.4 | 5.4 | 6.5 | 4.6 |
| 25 | 8.4 | 10.6 | 12.9 | 9.3 | 8.0 | 8.1 | 7.7 | 11.7 | |
| 50 | 10.2 | 13.6 | 15.2 | 11.9 | 10.2 | 11.1 | 10.0 | 15.6 | |
| 75 | 10.7 | 17.6 | 18.2 | 18.6 | 14.2 | 16.8 | 13.5 | 26.2 | |
| 97.5 | 12.3 | 32.9 | 27.7 | 23.0 | 24.7 | 24.7 | 14.4 | 44.2 | |
JF: juvenille female, JM: juvenille male, AM: adult male, AF: adult female (nonpregnant, nonlactating), PF: pregnant female (pregnancy with resulting calf), 1st: first trimester, 2nd: combined second and third trimester, LF: lactating female.
Mean thyroid hormone concentrations between age and sex in boto
For age, the only significant differences in tT3 and tT4 concentrations were found between immature males (tT3: 0.62 ng/ml, tT4: 41.6 ng/ml) and adult males (tT3: 0.51 ng/ml, tT4: 33.6), whereas no differences in thyroid hormone concentrations between immature and adult non-pregnant, non-lactating females were detected (Table 2). Sex differences in tT3 were detected between immature males and immature females (0.66 ng/ml). For tT4, adult males (33.6 ng/ml) were significantly lower than adult non-lactating non-pregnant females (38.9 ng/ml; Table 2). And finally, the tT3:tT4 ratio was significantly higher for immature males compared with immature females and lower in adult females compared with adult males (Table 2).
Table 2.
Comparisons of marginal mean (95% CI) total T3 (ng/ml), total T4 (ng/ml) and T3:T4 ratio (×1000) between sex within age groups (adult female vs adult male), between age groups within the same sex (juvenile vs adult) and in adult females during different physiologic states (pregnant, lactating, or non-lactating non-pregnant adult)
| Group | tT3 | tT4 | tT3:tT4 |
|---|---|---|---|
| Juvenile male (n = 23) |
0.62 (0.54–0.71) |
41.6 (37.7–46.0) |
14.5 (12.4–16.9) |
| Juvenile female (n = 8) |
0.43 (0.34–0.54) |
42.6 (34.6–52.5) |
9.4 (7.9–11.1) |
| Adult male (n = 65) |
0.51 (0.47–0.55) |
33.6 (31.7–35.5) |
15.2 (14.2–16.2) |
| Adult femalea (n = 26) |
0.49 (0.43–0.56) |
38.9 (35.0–43.3) |
12.2 (10.5–14.2) |
| Pregnant femaleb (n = 40) |
0.39 (0.35–0.43) |
37.9 (34.8–41.4) |
10.5 (9.4–11.8) |
| Lactating femalec (n = 14) |
0.56 (0.47–0.67) |
34.7 (28.6–42.1) |
16.3 (12.0–16.9) |
| Pairwise comparisons of meansd | IF < IM; AM < IM; PF < AF and LF | AM < IM AM < AF |
IF < IM; AF < AM AF & PF < LF |
aNon-pregnant, non-lactating adult females.
bOnly females with “successful pregnancies” were included in this category. These were females which were diagnosed as pregnant and then were observed to have a calf the following season.
cNon-pregnant females.
dOnly groups with differences (P < 0.05) were reported.
Mean thyroid hormone concentrations across female boto reproductive states
The concentrations of tT3 and tT4 and were similar during the second and third trimesters and were therefore combined for statistical analysis. Overall, tT3 was significantly lower in pregnant (0.39 ng/ml) compared with adult (non-pregnant, non-lactating) females. During pregnancy, females experienced a mean 12% decrease in tT3 from the first (0.42 ng/ml) to the combined second and third trimesters (0.37 ng/ml, Table 3). Therefore this decrease was primarily responsible for the overall significant reduction in tT3 during pregnancy (Table 3). Overall, pregnancy values were significantly lower than those in lactating females. For tT4, no significant differences were detected among the adult female groups, although lactating females had a significantly increased tT3:tT4 compared with all other adult female groups (Table 3).
Table 3.
Comparisons of marginal mean (95% CI) total T3 (ng/ml), total T4 (ng/ml) and the T3:T4 ratio (×1000) during the first and combined second and third trimesters of pregnancy with non-pregnant, non-lactating, adult females and non-pregnant, lactating females
| Group | tT3 | tT4 | tT3:tT4 | |
|---|---|---|---|---|
| Adult female (n = 26) |
0.49 (0.43–0.56) |
38.9 (35.0–43.3) |
12.2 (10.5–14.2) |
|
| First trimester (n = 17) |
0.42 (0.35–0.49) |
39.2 (34.1–44.9) |
11.6 (9.5–14.0) |
|
| Second and third trimester (n = 23) |
0.37 (0.32–0.43) |
37.3 (32.3–42.2) |
9.7 (8.2–11.5) |
|
| Lactating female (n = 14) |
0.56 (0.47–0.67) |
35.0 (29.5–41.7) |
16.3 (13.1–20.4) |
|
| Sidak Groups | Second and third < AF First, second, and third < LF |
NSD | AF, first, second, and third < LF | |
Females with confirmed calf loss either during gestation or after delivery were not included in this analysis.
NSD: no significant difference.
Thyroid hormone concentrations in boto with successful versus non-successful pregnancies
Throughout gestation, no significant differences were detected in thyroid hormone concentrations between pregnant females that lost calves and those that successfully gave birth. In addition, no significant differences in thyroid hormone concentrations were found when comparing trimesters between normal and abnormal pregnancies.
Discussion
Boto thyroid hormone reference ranges
This study is the first report of thyroid hormone concentration reference ranges in relation to sex, age and reproductive status for any species of river dolphin and provides novel information regarding river dolphin physiology, species that are some of the least understood and most endangered of the cetaceans (He et al., 2017). For example, it has been postulated that relatively high concentrations of T4 in marine cetaceans (beluga, bottlenose dolphins) compared with non-human terrestrial mammals may be an adaptation to the relatively cold climate associated with an aquatic environment (Fair et al., 2011; Flower et al., 2015). The reference range for tT4 in the boto supports this concept, as this cetacean species inhabits tropical waters that are considerably warmer than most encountered by other marine cetaceans (Sioli, 1984), and their concentrations were approximately half those of cold-water cetacean species. Further, tT4 concentrations appeared to be similar to terrestrial mammals (for review, see Fair et al., 2011) and the semi tropical marine mammal manatee (Trichechus manatus, Ortiz et al., 2000).
In addition to adaptive variations, boto reference ranges provide evidence that thyroid hormone concentrations decrease with age in both males and females, similar to bottlenose dolphins (Fair et al., 2011; West et al., 2014) and belugas (Flower et al., 2015). A decrease in thyroid hormones with age has been observed in humans (Kapelari et al., 2008; Lem et al., 2012) and other mammals (Malinowski et al., 1996) and may in part be explained by growth hormone, which stimulates peripheral T3 production in young animals (Lapierre et al., 1990). Defining age specific changes in thyroid hormone concentrations allows for improved sensitivity for the detection of thyroid hormone deficiencies. When these deficiencies occur in juveniles, they can have profound effects on normal development; and therefore, defining the normal changes with age is an important component for the development of population health assessment criteria (Dwyer and Stickland, 1992; Lem et al., 2012).
Normal changes or reference ranges within adult groups can be beneficial for identifying thyroid pathology—thyroiditis, hypo and hyper thyroidism—some of which can have profound effects on fetal and neonatal growth and development. While tT4 was relatively consistent among groups, tT3 and the tT3:tT4 ratio demonstrated large variation in concentration ranges, especially between pregnant and lactating females. In general, T3:T4 changes are used to reflect the efficiency of thyroid production and predict clinical disease, such as thyrotoxicosis, euthyroid sick syndrome, iodine deficiency, follicular cysts, thyroiditis and hyperthyroidism (Mortoglou and Candiloros, 2004). To our knowledge, most animals in this study were healthy at the time of sample collection, and combined with a fairly large sample size, these results should provide a robust reference interval that will be important for continued monitoring of individual animals and potentially provide a useful indicator for assessing potential changes in overall population health.
For the boto, the major thyroid hormone in serum is tT4 (98.7%) with only ~ 1.3% represented as tT3. This proportional distribution is nearly identical to that observed for wild (98 to 99%; Fair et al., 2011) and captive (99.3%; West et al., 2014) bottlenose dolphins. This finding is also in line with the understanding in other mammalian species that T4 functions primarily as a prohormone reserve for intracellular conversion to the biologically active form T3 (for review, see Brent, 2012).
Boto thyroid hormone concentration comparisons by age and sex
There were significant age differences in thyroid hormone levels, but these were not always consistent. For example, T3 and T4 were significantly increased in immature males compared with mature males but not between immature and adult females (non-pregnant, non-lactating females). For sex differences, significant differences in thyroid hormone concentrations were observed between immature male and female (T3, T3:T4) and adult male and female (T4, T3:T4) boto. These age and sex results differ from a study in wild bottlenose dolphins where significant differences in T4 between sex and age groups were largely due to juvenile males (Fair et al., 2011). Similarly, immature females within both studies had the lowest T3:T4 ratio. In addition, our results are comparable with an earlier study in wild bottlenose dolphin whereby adult females had increased tT4 compared with adult males. However, unlike our findings, they also observed an increase in T3 in adult females compared with adult males. This observed difference may have been caused by the absence of controlling for lactating females in their comparisons with adult males, potentially increasing the overall mean T3 concentration in the female group (St. Aubin et al., 1996). Research with belugas demonstrated a significant decrease with age in both tT3 and tT4 and significantly higher mean concentrations for male versus females; however, they did not directly compare sex differences between age classes. For the boto and beluga, the increase in T3 production observed in immature males versus immature females may reflect an increased or prolonged growth rate for dimorphically larger males whom require longer periods of time to reach sexual maturity (Robeck et al., 2005; Martin and da Silva, 2006), and therefore, a prolonged growth hormone mediated increase in thyroid hormone production.
Effect of reproductive state on thyroid hormone in adult female boto
We observed a significant 20% decrease in mean T3 concentration with no change in T4 during pregnancy as compared with non-pregnant non-lactating females. While our results were similar to a small number (n = 5) of captive beluga (Flower et al., 2015) and to a recent report in mares when controlling for season (Fazio et al., 2016a), they contrast with reported results from captive bottlenose dolphins and many other mammalian species whereby thyroid hormone concentrations are significantly increased during pregnancy (Todini, 2007; West et al., 2014). However, even within bottlenose dolphins, conflicting evidence exists, although results in wild animals were similar to ours in that tT3 and tT4 were reduced in pregnant females when compared to non-pregnant females (Fair et al., 2011). For the captive bottlenose dolphin (West et al., 2014), the significant increase detected in thyroid hormone during pregnancy compared with non-pregnant animals was primarily due to differences detected in the first trimester, whereby all thyroid hormones measured were increased. One possible explanation for the conflicting results observed between captive versus wild bottlenose dolphins and boto is that captive animals had multiple serial samples collected throughout pregnancy, while only single point samples were “randomly” collected from wild animals. Therefore, samples may have been clustered towards the end of the first trimester of gestation, a period when thyroid hormones are already decreasing towards the second trimester. Therefore, inaccuracy in trimester determination and/or clustering of samples in the late first trimester could theoretically result in missing peak concentrations of thyroid hormones observed during early pregnancy. For example, when weekly thyroid hormone concentrations were determined in horses, differences between pregnant versus non-pregnant controls were detected for tT3; however, these were primarily due to discrete significant increases during Weeks 7 and 12 post-ovulation (Fazio et al., 2016b). If this pattern of secretion holds true for cetaceans, single point sampling from animals, which is typical for wild animal population studies, is more likely to results in misinterpretation of the thyroid hormone secretion pattern compared with repeated weekly measurements throughout gestation.
Clearly more work is required to understand normal cetacean thyroid function during pregnancy versus non-pregnancy, but what appears consistent between the boto and other cetacean and artiodactyl species (Todini, 2007; Todini et al., 2007) is that thyroid hormone concentrations decrease from levels during early gestation to significantly lower concentrations during mid to late pregnancy. The mechanism for this change is believed to be due to a combination of factors that include increased metabolic need, an increase in production of thyroid binding globulin and direct pituitary stimulation from chorionic gonadotropins (Kennedy et al., 2010). However, while these mechanisms have been advanced as explanations for increases observed during human pregnancy, they do not appear to apply directly to cetaceans. For example, the increase in thyroid binding protein is believed to be stimulated by early increase in estrogens, an event that does not occur in at least two odontocete species, bottlenose dolphins (Steinman et al., 2016) and killer whales (Robeck et al., 2017). In addition, no chorionic gonadotropin activity similar to that which is observed in humans (hCG) and horses (eCG) has been identified in cetaceans (Bergfelt et al., 2012). Finally, the probable inappropriateness of comparing maternal thyroid hormone concentrations during pregnancy between species with different placentation should not be overlooked. For example, evidence suggests that little if any thyroxine crosses the placenta in mammals with epitheliochorial placentation (ruminants, cetaceans, etc.) after complete development of the maternal-placental barrier (mid to late gestation, Comline et al., 1970; Hopkins and Thorburn, 1971), while significant transfer appears to occur for species with hemochorial placentation (primates). Therefore, species with active or passive transfer of thyroid hormones or their trophic precursors across the maternal-fetal barrier will have concentrations that reflect a combined homeostatic equilibrium of maternal and fetal requirements during the various stages and metabolic requirements of gestation, while in the former group, one would expect maternal concentrations to reflect metabolic demands for the dam, largely independent of fetal requirements. As the fetal thyroid develops, competition for circulating iodine, which readily diffuses across all types of placentas, may be the significant factor that results in decreased maternal circulating thyroid hormone hormones (Yarrington and Pearce, 2011) and maybe a partial explanation for the reduced thyroid hormone during late-term pregnancy in boto. Measurement of maternal iodine concentrations during pregnancy may provide evidence to support or refute this hypothesis.
The reduction in T3:T4 during late pregnancy provides some evidence to support the concept that iodine competition across the placenta, along with increased renal clearance of iodine may be, in part, the cause of this decreased ratio (Fisher, 1996). Because the T3:T4 ratio is believed to represent an indirect measurement of peripheral T4 deiodination to active T3 concentration (Todini et al., 2007), a reduced ratio during pregnancy in the boto may reflect a decrease in maternal tT4 which is limited by iodine availability combined with an increased need for tT3. Accordingly, this phenomenon was not observed in captive or wild bottlenose dolphins (Fair et al., 2011; West et al., 2014), whereby their diet which consist of marine fish typically have an overabundance of iodine (Ridgway and Patton, 1971), whereas the fresh water fish food source on which the boto rely for are a magnitude lower in iodine content (Eckhoff and Maage, 1997; Fordyce, 2003). It has been noted that wild pregnant and captive bottlenose dolphins had increased thyroid gland mass compared with non-pregnant females (Cowan and Tajima, 2006; Kot et al., 2012), and the goiterogenic effects of moderate iodine restriction during pregnancy in humans are well documented (Glinoer, 2004). In addition to possible iodine deficiency, the change in T3:T4 could simply indicate the inability of the thyroid to keep up with high metabolic demands of late pregnancy. Without further evidence, especially maternal iodine and TSH concentrations, and normal values for comparisons, the cause remains speculative.
Similar to lactating wild bottlenose dolphins (Fair et al., 2011), we found a significant increase in tT3, and a non-significant decrease in tT4 (9%) compared with adult non-pregnant non-lactating females, and a corresponding significant increase in tT3:tT4 during lactation in the boto. These results are also compatible with an observed increase in thyroid size in wild lactating bottlenose dolphins (Cowan and Tajima, 2006) and an observed increase in thyroid volume during lactation in captive Indo-Pacific bottlenose dolphins (Tursiops aduncus, Kot et al., 2012). Whether the observed thyroid hypertrophy was a residual effect from the known goitrogenic effects of pregnancy, as has been observed in humans (Glinoer, 2004), or a true response to increase demand during lactation for thyroid hormone is unknown.
Thyroid hormones appear to have both species-specific and time-specific alterations during lactation (Polat et al., 2014; Micke et al., 2015; Fazio et al., 2016a). Our results most resemble those of women during the post-partum period when a transient decrease in post-partum tT4 occurs in conjunction with an increased tT3 believed to be caused by the energetic demands of lactation (Weeke et al., 1982). The increase in T3:T4 also supports the concept that tT4 is being metabolized to tT3 at a faster rate than can be compensated for by thyrotrophic hormones or rate limiting effect of iodine availability. In support, a significant increase in the T3:T4 ratio paired with increased TSH concentrations is considered diagnostic for hypothyroidism in humans (Mortoglou and Candiloros, 2004). Without measures of TSH concentration in conjunction with thyroid hormone concentrations, it will be impossible to classify these significant changes as hypo- or hyper-thyroidism, but these changes illustrate and confirm the shifting demand on maternal thyroid function during these various reproductive states in the boto.
Thyroid hormone concentrations in boto with successful versus non-successful pregnancies
Pregnant boto females, as diagnosed by ultrasonography, that were not observed with a calf the following year were assumed to have lost the fetus from an abortion, perinatal loss or had a calf that failed to thrive and died. We did not detect any differences in thyroid hormone concentrations during pregnancy between females that lost calves verse those with confirmed normal calves. These results contrast with those of captive bottlenose dolphins where a significant decrease in thyroid hormone concentrations during the second and thirrd trimesters in females that had perinatal loss was observed (West et al., 2014). Our results may simply indicate that thyroid function was not a factor in the loss of the calves from these females or that the low numbers of females sampled would not allow enough sensitivity to detect significant changes. Given the potentially diverse array of etiologies for perinatal calf loss in wild populations, much larger sample sizes likely are needed to determine exact cause of such losses.
Conclusions
Similar to many other wildlife species (Kersey and Dehnhard, 2014), little is known about thyroid function in any river dolphin species including the boto, and this study represents the first significant report. As the enviable loss or change in prey availability continues, and exposure to endocrine disrupting contaminants increases, both factors which have been implicated as affecting thyroid function (Fair et al., 2011; Hall et al., 2018), the data presented herein will provide a critical base of information from which the effects of these changes on the boto can be monitored. In addition, this information provides for comparisons with other species of river dolphins that are currently experiencing many of these anthropogenic threats to such a degree they are currently threatened with extinction (He et al., 2017).
Acknowledgements
All procedures described herein were reviewed and approved by the National Institute of Amazonian Research (INPA) Animal Care and Use Committee, and this project was approved by the following Brazilian government agencies: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA); and Sistema de Autorizacão em Biodiversidade (SISBio; permit numbers 2344/9611-AC, 3552/9312-AC, 2002001.002344/96-11, 3462-1 to 13462-5, 13157-1 to 13157-4, 50544-1 to 50544-3). Research was conducted under the NMFS permit 16193 and Brazilian CITES export permit number 15BR018682/DF. This study was part of Projeto Boto, a cooperative agreement between the National Amazon Research Institute (INPA/MCTI) and the Mamirauá Sustainable Development Institute (MSDI-OS/MCTI).
We thank Nivia do Carmo of LMA/INPA for help with sample collection and K. Steinman of SeaWorld and Busch Gardens Species Preservation Laboratory for help with sample processing. This is a SeaWorld Technical manuscript contribution number 2018-17.
Funding
Partial funding of this study was provided by Associação Amigos do Peixeboi (AMPA)/Petrobras Socioambiental programme.
References
- Bergfelt DR, Thompson DL Jr, Brown JL, Presley NA, West KL, Campbell M, Adams GP (2012) Investigation of an immunoreactive chorionic gonadotropin-like substance in the placenta, serum, and urine of pregnant bottlenose dolphins (Tursiops truncatus). Aquatic Mammals 38: 354–361. [Google Scholar]
- Bossart GD. (2006) Marine mammals as sentinel species for oceans and human health. Oceanography 19: 134–137. [DOI] [PubMed] [Google Scholar]
- Braulik GT, Smith BD (2017) Platanista gangetica. The IUCN Red List of Threatened Species Version 2017http://www.iucnredlist.org. (last accessed 03 August 2018).
- Brent GA. (2012) Mechanisms of thyroid hormone action. J Clin Invest 122: 2035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhuang M, Chou L, Liu J, Shih C, Chen C (2017) Tissue concentrations of four Taiwanese toothed cetaceans indicating the silver and cadmium pollution in the western Pacific Ocean. Mar Poll Bull 124: 993–1000. [DOI] [PubMed] [Google Scholar]
- Cowan DF, Tajima Y (2006) The thyroid gland in bottlenose dolphins (Tursiops truncatus) from the Texas coast of the Gulf of Mexico: normal structure and pathologic changes. J Comp Path 135: 217–225. [DOI] [PubMed] [Google Scholar]
- Comline RS, Nathanielsz PW, Silver M (1970) Passage of thyroxine across the placenta in the foetal sheep. Proc. 51st Meeting, the Endocrine Society, pp 126. [PubMed]
- Silva VMF, Freitas CEC, Dias RL, Martin AR (2018) Both cetaceans in the Brazilian Amazon show sustained, profound population declines over two decades. PLoS ONE 13, e0191304 doi.org/10.1371/journal.pone.0191304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer CM, Stickland NC (1992) The effects of maternal undernutrition on maternal and fetal serum insulin-like growth factors, thyroid hormones and cortisol in the guinea pig. J Dev Physiol 18: 303–313. [PubMed] [Google Scholar]
- Eckhoff KM, Maage A (1997) Iodone content in fish and other food products from east africa analyzed by ICP-MS. J Food Comp Anal 10: 270–282. [Google Scholar]
- Fair PA, Montie E, Bathis L, Reif JS, Bossart GD (2011) Influences of biological variables and geographic location on circulating concentrations of thyroid hormones in wild bottlenose dolphins (Tursiops truncatus). Gen Comp Endo 174: 184–194. [DOI] [PubMed] [Google Scholar]
- Fazio E, Medica P, Cravana C, Bruschetta G, Ferlazzo A (2016a) Seasonal thyroid and lipid profiles in thoroughbred pregnant and nonpregnant mares (Equus caballus). Theriogenology 85: 1582–1589. [DOI] [PubMed] [Google Scholar]
- Fazio E, Medica P, Trifiletti C, Ferlazzo A (2016b) The outcome of the first stages of pregnancy on mares bloodstream thyroid hormones. Theriogenology 86: 036–1041. [DOI] [PubMed] [Google Scholar]
- Finer M, Jenkins CN (2012) Proliferation of hydroelectric dams in the Andean Amazonand implications for Andes–Amazon connectivity. PLoS ONE 7, e35126. doi: 10.1371/journal.pone.0035126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DA. (1996) Physiological variation in thyroid hormones: physiological and pathophysiological considerations. Clin Chem 42: 135–139. [PubMed] [Google Scholar]
- Flower JE, et al. (2015) Circulating concentrations of thyroid hormone in beluga whales (Delphinapterus leucas): influence of age, sex and season. J Zoo Wild Med 46: 456–467. [DOI] [PubMed] [Google Scholar]
- Fordyce FM. (2003) Database of iodine content of food and diets populated with data from published literature. Brit. Geol. Surv. Comm. Rep. CR/03/84N. pp 50.
- Glinoer D. (2004) The regulation of thyroid function during normal pregnancy: importance of the iodine nutritional status. Best Pract Res Clin Endo Metab 18: 133–152. [DOI] [PubMed] [Google Scholar]
- Glinoer D. (2007) Clinical and biological consequences of iodine deficiency during pregnancy. Endo Dev 10: 62–85. [DOI] [PubMed] [Google Scholar]
- Hall AJ, McConnell BJ, Schwacke LH, Ylitalo GM, Willams R, Rowles TK (2018) Predicting the effects of polychlorinated biphenyls on cetacean populations through impacts on immunity and calf survival. Environ Poll 233: 407–418. [DOI] [PubMed] [Google Scholar]
- Harrison RJ. (1969) Endocrine organs: hypophysis, thyroid and adrenal In Andersen HT, ed, The Biology of Marine Mammals. Academic Press, NewYork, pp. 365–372. [Google Scholar]
- He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jänig SC (2017) Disappearing giants: a review of threats to freshwater megafauna. WIREs Water 2017, 4:e1208. doi: 10.1002/wat2.1208. [DOI] [Google Scholar]
- Huang SL, Hao YJ, Mei ZG, Turvey ST, Wang D (2012) Common pattern of population decline for freshwater cetacean species in deteriorating habitats. Freshw Biol 57: 1266–1276. [Google Scholar]
- Hopkins PS, Thorburn GD (1971) Placental permeability to maternal thryoxine in the sheep. J Endocrinol 49: 549–550. [DOI] [PubMed] [Google Scholar]
- Kapelari K, Kirchlechner C, Högler W, Schweitzer K, Virgolini I, Moncayo R (2008) Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr Disord. 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RL, Malabu UH, Jarrod G, Nigam P, Kannan K, Rane A (2010) Thyroid function and pregnancy: before, during and beyond. J Obstetrics Gynaecol 30: 774–783. [DOI] [PubMed] [Google Scholar]
- Kersey DC, Dehnhard M (2014) The use of noninvasive and minimally invasive methods in endocrinology for threatened mammalian species conservation. Gen Comp endocrinol 203: 296–306. [DOI] [PubMed] [Google Scholar]
- Kot BCW, Ying MTC, Brook FM, Kinoshita RE, Kane D, Chan WK (2012) Sonographic evaluation of thyroid morphology during different reproductive events in female Indo-Pacific bottlenose dolphins, Tursiops aduncatus. Mar Mam Sci 28: 733–750. [Google Scholar]
- Lacave G, Eggermont M, Verslycke T, Martin , Brook F, Salbany A, Azevedo , Torres , Roque L, Kinoshita R (2004) Prediction from ultrasonographic measurements of the expected delivery date in two species of bottlenosed dolphin (Tursiops truncatus and Tursiops aduncus). Vet Rec 154: 228–223. [DOI] [PubMed] [Google Scholar]
- Lailson-Brito J Jr, Dorneles PR, Silva VMF, Martin AR, Batos WR, Azevedo-Silva CE, Azevedo AF, Torres JPM, Malm O (2008) Dolphins as indicators of micropollutant trophic flow in amazon basin. Oecol Bras 12: 532–541. [Google Scholar]
- Lane SM, Smith CR, Mitchell J, Balmer BC, Barry KP, McDonald T, Mori CS, Rosel PE, Rowles TK, Speakman TR, et al. (2015) Reproductive outcome and survival of common bottlenose dolphins sampled in Barataria Bay, Louisiana, USA, following the Deepwater Horizon oil spill. Proc. R. Soc. B. 282, 20151944. doi.org/ 10.1098/rspb.2015.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre H, Petitclerc D, Pelletier G, Delorme L, Dubreuil P, Morisset J, Gaudreau P, Couture Y, Brazeau P (1990) Dose effect of human growth hormone-releasing factor and thyrotropin-releasing factor on hormone concentrations in lactating dairy cows. Dom Anim Endocrinol 7: 485–495. [DOI] [PubMed] [Google Scholar]
- Lem AJ, Rijke YB, Toor H, Ridder MAJ, Visser TJ, Hokken-Koelega ACS (2012) Serum thyroid hormone levels in healthy children from birth to adulthood and in short children born small for gestational age. J Clin Endocrinol Metab 97: 3170–3178. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinked R, Singh S, Shah P, Gupta N, Kochupillai N (2005) Thyroid hormone dysregulation in intrauterine growth retardation associated with maternal malnutrition and/or anemia. Horm Metab Res 37: 633–640. [DOI] [PubMed] [Google Scholar]
- Malinowski K, Christensen RA, Hafs HD, Scanes CG (1996) Age and breed differences in thyroid hormones, insulins-like growth factor (IGF)-I and IGF binding proteins in female horses. J Anim Sci 74: 1936–1942. [DOI] [PubMed] [Google Scholar]
- Männistö T, Surcel H-M, Bloigu A, Ruokonen A, Hartikainen A-L, Järvelin M-R, Pouta A, Vääräsmäki M, Suvanto-Luukkonen E (2007) The effect of freezing, thawing, and short-and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem 53: 1986–1987. [DOI] [PubMed] [Google Scholar]
- Martin AR, Silva VMF (2006) Sexual dimorphism and body scarring in the boto (Amazon river dolphin) Inia Geoffrensis. Mar Mamm Sci 22: 25–33. [Google Scholar]
- Martin AR, Silva VMF (2018) Reproductive parameters of the Amazon river dolphin or boto Inia geoffrensis (Cetacea: Iniidae); an evolutionary outlier bucks no trends. Biol J Linn Soc 123: 666–676. [Google Scholar]
- Micke GC, Sullivan TM, Kennaway DJ, Hernandez-Medrano J (2015) Maternal endocrine adaptation throughout pregnancy to nutrient programming of thyroid hormones and development of their progeny. Theriogenology 83: 604–615. [DOI] [PubMed] [Google Scholar]
- Mintzer VJ, Martin AR, Silva VMF, Barbour AB, Lorenzen K, Frazer TK (2013) Effect of illegal harvest on apparent survival of Amazon river dolphins (Inia geoffrensis). Biol Conserv 158: 280–286. [Google Scholar]
- Mortoglou A, Candiloros H (2004) The serum triiodthyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after levothyroxine replacement therapy. Hormones 3: 120–126. [DOI] [PubMed] [Google Scholar]
- Nabi G, Hao Y, Robeck TR, Zheng J, Wang D (2018) Physiological consequences of biologic state and habitat dynamics on the critically endangered Yangtze finless porpoises (Neophocaena asiaeorientalis ssp. Asiaeorientalis) dwelling in the wild and semi-natural environment. Conserv Physiol 6. doi: 10.1093/conphys/coy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz RM, MacKenzie DS, Worthy GAJ (2000) Thyroid hormone concentrations in captive and free-ranging west indian manatees (Trichechus manatus). J Exp Biol 203: 3631–3627. [DOI] [PubMed] [Google Scholar]
- Polat H, Dellal G, Baritci I, Pehlivan E (2014) Changes of thyroid hormones in different physiologic periods in white goats. J Anim Plant Sci 24: 445–449. [Google Scholar]
- Reeves RR, Jefferson TA, Karczmarski L, Laidre K, O’Corry-Crowe G, Rojas-Bracho L, Secchi ER, Slooten E, Smith BD, Wang JY, Zhou K (2011) Inia geoffrensis. The IUCN Red List of Threatened Species; The IUCN Red List of Threatened Species: Version 2017 http://www.iucnredlist.org. (last accessed 30 May 2018). [Google Scholar]
- Ridgway SH, Patton GS (1971) Dolphin thyroid: some anatomical and physiological findings. Physiologie 71: 129–141. [Google Scholar]
- Ridgway SH, Simpson JG, Patton GS, Gilmartin BS (1970) Hematologic findings in certain small cetaceans. J Amer Vet Med Ass 157: 566–575. [PubMed] [Google Scholar]
- Robeck TR, Monfort SL, Calle PP, Dunn JL, Jensen E, Boehm JR, Young S, Clark ST (2005) Reproduction, growth and development in captive beluga (Delphinapterus leucus). Zoo Biol 24: 29–49. [Google Scholar]
- Robeck TR, Steinman KJ, O'Brien JK (2017) Characterization and longitudinal monitoring of serum androgens and glucocorticoids during normal pregnancy in the killer whale (Orcinus orca). Gen Comp Endocrinol 247: 116–129. [DOI] [PubMed] [Google Scholar]
- Sioli H. (1984) The Amazon and its main affluents: hydrography, morphology of the river courses, and river types In Sioli H, ed, The Amazon. Limnology and landscape ecology of a mighty tropical river and its basin. First Edition. Springer, Netherlands, pp. 127–165. [Google Scholar]
- St. Aubin DJ. (2001) Endocrinology In Dierauf L, Gulland F, eds, CRC handbook of marine mammal medicine, EdSecond CRC Press, Boca Raton, Florida, pp. 165–192. [Google Scholar]
- St. Aubin DJ, Ridgway SH, Wells RS (1996) Dolphin thyroid and adrenal hormones: circulating levels in wild and semidomesticated Turiops truncatus, and influence of sex, age and season. Mar Mamm Sci 12: 1–13. [Google Scholar]
- Schwacke LH, Smith CR, Townsend FI, Wells RS, Hart LB, Balmer BC, Collier TK, De Guise S, Fry MM, Guillette LJ Jr, et al. (2014) Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Environ Sci Technol 48:93–103. [DOI] [PubMed] [Google Scholar]
- Schumacher U, Zahler S, Horny H-P, Heidemann G, Skirnisson K, Welsch U (1993) Histological investigations on the thyroid glands of marine mammals (Phoca vitulina, Phocoena phocoena) and the possible implications of marine pollution. J Wild Dis 29: 103–108. [DOI] [PubMed] [Google Scholar]
- Smith CR, et al. (2017) Slow recovery of Barataria Bay dolphin health following the Deepwater Horizon oil spill (2013–2014), with evidence of persistent lung disease and impaired stress response. Endangered Species Res 33: 127–142. [Google Scholar]
- Steinman KJ, Robeck TR, O’Brien JK (2016) Characterization of estrogens, testosterone, and cortisol in normal bottlenose dolphin (Tursiops truncatus) pregnancy. Gen Comp Endocrinol 226: 102–112. [DOI] [PubMed] [Google Scholar]
- Todini L. (2007) Thyroid hormones in small ruminants: effect of endongenous, environmental and nutritional factors. Animal 1: 997–1008. [DOI] [PubMed] [Google Scholar]
- Todini L, Malfatti A, Valbonesi A, Trabalza-Marinucci M, Debenedetti A (2007) Plasma total T3 and T4 concentrations in goats at different physiologic stages, as affected by energy intake. Small Rumin Res 68: 285–290. [Google Scholar]
- Turvey ST, Risley CL, Barrett LA, Hao YJ, Ding W (2012) River dolphins can act as population trend indicators in degraded freshwater systems. PLoS ONE, 7:e37902. doi: 10.1371/journal.pone.0037902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bressem MF, Santos MCO, Oshima JEF (2009) Skin diseases in Guiana dolphins (Sotalia guianensis) from the Paranaguá estuary, Brazil: a possible indicator of a compromised marine environment. Marine Environ Res 67: 63–68. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Lundin JI, Ayres K, Seely E, Giles D, Balcomb K, Hempelmann J, Parsons K, Booth R (2017) Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca). PLoS One 12: E0179824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J, Dybkjaer L, Granlie K, Eskjaer Jensen S, Kjaerulff E, Laurberg P, Magnusson B (1982) A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinologica 101: 531–537. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT (2015) Linear mixed models: a practical guide using statistical software, EdSecond CRC Press, Boca Raton, pp. 199–248. [Google Scholar]
- Welch BL. (1947) The generalization of student's problem when several different population variances are involved. Biometrika 34:28–35. [DOI] [PubMed] [Google Scholar]
- West KL, Ramer J, Brown JL, Sweeney J, Hanahoe EM, Reidarson T, Proudfoot J, Bergfelt DR (2014) Thyroid hormone concentrations in relation to age, sex, pregnancy, and perinatal loss in bottlenose dolphins (Tursiops truncatus). Gen Comp Endo 197: 73–81. [DOI] [PubMed] [Google Scholar]
- Williams R, Moore JE, Gomez-Salazar C, Trujillo F, Burt L (2016) Searching for trends in river dolphin abundance: designing surveys for looming threats, and evidence for opposing trends of two species in the Colombian amazon. Biol Conserv 195: 136–145. [Google Scholar]
- Yarrington C., Pearce E.N., 2011. Iodine and pregnancy. J Thyroid Res 2011, 934101. doi: 10.4061/2011/934104. [DOI] [PMC free article] [PubMed] [Google Scholar]


