Abstract
Background
Human and animal studies have demonstrated that helminth infections are associated with a decreased prevalence of type 2 diabetes mellitus (T2DM). However, very little is known about their biochemical and immunological interactions.
Methods
To assess the relationship between a soil-transmitted helminth, Strongyloides stercoralis (Ss), and T2DM, we examined analytes associated with glycemic control, metabolic processes, and T-cell–driven inflammation at the time of Ss diagnosis and 6 months after definitive anthelmintic treatment. We measured plasma levels of hemoglobin A1c, glucose, insulin, glucagon, adipocytokines, and T-helper (TH) 1-, 2-, and 17- associated cytokines in patients with T2DM with (INF group) or without (UN group) Ss infection. In INF individuals, we again assessed the levels of these analytes 6 months following anthelmintic treatment.
Results
Compared to UN individuals, INF individuals exhibited significantly diminished levels of insulin and glucagon that increased significantly following therapy. Similarly, INF individuals exhibited significantly diminished levels of adiponectin and adipsin that reversed following therapy. INF individuals also exhibited significantly decreased levels of the TH1- and TH17- associated cytokines in comparison to UN individuals; again, anthelmintic therapy augmented these levels. As expected, INF individuals had elevated levels of TH2-associated and regulatory cytokines that normalized following definitive therapy. Multivariate analysis revealed that these changes were independent of age, sex, body mass index, and liver and renal function.
Conclusions
Strongyloides stercoralis infection is associated with a significant modulation of glycemic, hormonal, and cytokine parameters in T2DM and its reversal following anthelmintic therapy. Hence, Ss infection has a protective effect on diabetes-related parameters.
Keywords: Strongyloides stercoralis infection, type 2 diabetes mellitus, adipocytokines, glycemic hormones, cytokines
Helminth infections are associated with diminished levels of pancreatic hormones, adipocytokines, and T-helper (TH) 1-/TH17-associated cytokines. They are also associated with elevated levels of TH2 and regulatory cytokines. Most of these alterations are reversed following anthelmintic treatment.
The “hygiene hypothesis” initially postulated an inverse relationship between allergic diseases and bacterial infections in early childhood [1]. Consequently, the hygiene hypothesis was expanded to include helminth infections, as the immunomodulation seen in chronic helminth infection was also capable of providing a degree of protection from allergic and autoimmune diseases [2, 3]. The prevalence of helminth infections are low to absent in resource-rich (upper income) countries, which has been postulated to account for the increased incidence of allergic, autoimmune, and metabolic (eg, type 2 diabetes mellitus [T2DM]) diseases [4–6]. Epidemiological and experimental evidence suggests that helminths may play a protective role against the development of T2DM [7–13] as a result of decreases in systemic inflammation driven by the immunomodulation seen in chronic helminth infections or by alteration of the intestinal microbiota [14, 15].
T2DM, a chronic inflammatory disease characterized by persistent elevated glucose levels as a result of insulin resistance, is associated with the infiltration of macrophages and T cells into adipose tissue and increased production of proinflammatory cytokines (T-helper [TH] 1 and TH17) [16, 17]. T2DM can coexist with any helminth infection, although attention has focused on the major soil-transmitted helminths—namely, Ascaris lumbricoides, hookworm species, Trichuris trichiura, and Strongyloides stercoralis (Ss). Recent cross-sectional studies in India, Indonesia, and rural China, and in Aboriginal Australians, revealed that the prevalence of helminth infections in T2DM patients were significantly lower than in nondiabetic controls [9, 10, 13, 18]. These studies suggested that helminth infections may prevent or delay the onset of T2DM. Thus, in addition to the more established risk factors, such as sedentary lifestyle and high-energy foods, current deworming programs in parallel with rapid socioeconomic development could perhaps contribute to the development of T2DM in many low- and middle-income countries [19–22].
Because little is known about the relationship between human helminth infections and metabolic diseases of inflammatory origin, we examined the interaction between Ss infection and T2DM and evaluated the impact of Ss infection on parameters important in glycemic control and those hormonal and cytokine factors previously found to be important in T2DM. To this end, we measured comprehensively the circulating levels of glycated hemoglobin (HbA1c) and blood glucose, the pancreatic hormones insulin and glucagon, the adipocytokines (adiponectin, adipsin, resistin, leptin, and visfatin), and a variety of T-cell–derived cytokines in persons with T2DM with or without coincident Ss infection. We also determined the effect of definitive anthelmintic treatment on the aforementioned parameters in Ss-infected subjects.
MATERIALS AND METHODS
Ethics Statement
All participants were examined as part of a natural history study protocol (12-I-073) approved by the institutional review boards of the US National Institute of Allergy and Infectious Diseases and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
Study Population
We recruited 118 individuals consisting of 60 clinically asymptomatic Ss-infected individuals with T2DM (INF group) and 58 individuals with T2DM and no Ss infection (UN group) in Kanchipuram District, Tamil Nadu, South India (Table 1). These individuals were all recruited from a rural population by screening of individuals for helminth infection by stool microscopy and serology as described previously [23–26]. All the recruited individuals were between 18 and 75 years of age. None had previous anthelmintic treatment, a history of helminth infections, or human immunodeficiency virus. Individuals with other helminth infections were excluded from the study, as were pregnant or lactating women.
Table 1.
Demographic Characteristics and Biochemical Parameters
| Characteristic/Parameter | INF (n = 60) | UN (n = 58) |
|---|---|---|
| Sex | ||
| Male | 30 | 30 |
| Female | 30 | 28 |
| Age, years, median (range) | 46 (24–63) | 45 (22–63) |
| Random blood glucose, mg/dL | 179 (140–438) | 180.5 (140–198) |
| Hemoglobin A1c, % | 8.57 (6.5–12.5) | 8.9 (6.5–11.8) |
| Urea, mg/dL | 19.5 (12.34) | 21.9 (11–42) |
| Creatinine, mg/dL | 0.78 (0.3–1) | 0.85 (0.6–1.0) |
| Alanine aminotransferase, U/L | 17.7 (7–60) | 22.4 (7–92) |
| Aspartate aminotransferase, U/L | 27.8 (16–110) | 24.7 (11–68) |
Data are presented as geometric mean (range) unless otherwise indicated.
Abbreviations: INF, individuals with Strongyloides stercoralis infection with type 2 diabetes mellitus; UN, individuals with type 2 diabetes mellitus and no Strongyloides stercoralis infection.
Measurement of Hematological and Anthropometric Parameters
Hematological parameters were measured from fresh venous ethylenediaminetetraacetic acid blood samples on all individuals using an AcT 5diff hematology analyzer (Beckman Coulter, Brea, California). Anthropometric measurements (including height, weight, and waist circumference) and biochemical parameters (including plasma glucose, lipid profiles, and HbA1c) were obtained using standardized techniques as detailed elsewhere [27]. Serum samples were used for biochemical parameters, and plasma was used for the other measurements.
Parasitological Examination and Anthelmintic Treatment
Strongyloides stercoralis infection was diagnosed by the presence of immunoglobulin G antibodies to the recombinant protein Ss-NIE, a 31-kDa antigen derived from S. stercoralis L3 parasites as described previously [24, 26]. This was further confirmed by specialized stool examination with nutrient agar plate cultures [28]. Stool microscopy was used to exclude the presence of other intestinal helminth infections. Filarial infection was excluded in all study participants by virtue of being negative in tests for circulating filarial antigen. All INF individuals were treated with a single dose of ivermectin (12 mg) and albendazole (400 mg), and follow-up blood draws were obtained 6 months later. Following anthelmintic treatment, parasitological examinations were repeated after 6 months to confirm successful chemotherapy.
Determination of T2DM Status
Diabetes was defined as an HbA1c reading of ≥6.5% and/or a random blood glucose (RBG) of >200 mg/dL, according to the American Diabetes Association criteria. All the biochemical parameters were measured after overnight fasting except RBG. All diabetic individuals were referred to the primary healthcare center for diabetic treatment.
Measurement of Plasma Adipocytokines and Cytokine Levels
Plasma levels of pancreatic hormones (insulin and glucagon), adipocytokines (adiponectin, adipsin, resistin, leptin, visfatin), and the TH1 cytokines interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin (IL) 2; the TH17 cytokines IL-17A and IL-22; the TH2 cytokines IL-4, IL-5, and IL-13; and the regulatory cytokine IL-10 were measured using a Bioplex multiplex assay system (Bio-Rad, Hercules, California). Transforming growth factor beta (TGF-β) and IL-17F were measured by conventional enzyme-linked immunosorbent assay according to the manufacturer’s protocol (R&D Systems, Minneapolis, Minnesota).
Statistical Analysis
Geometric means (GMs) were used for measurements of central tendency. Mann-Whitney U tests were used to compare the INF group to the UN group, and the Wilcoxon signed-rank test was used to compare parameters before and after treatment. Multiple comparisons were corrected using the Holm correction. Multiple logistic regression analysis by backward stepwise methods was used to identify factors that were influenced by Ss infection. Analyses were performed using GraphPad Prism version 7.0 (GraphPad, San Diego, California) and Stata version 15 (StataCorp, College Station, Texas).
RESULTS
Study Population Characteristics
The baseline demographic characteristics and biochemical parameters are shown in Table 1. There were no significant differences in age, sex, body mass index, or other biochemical parameters between the 2 groups.
Diminished Circulating Levels of Pancreatic Hormones, Adiponectin, and Adipsin in INF Individuals
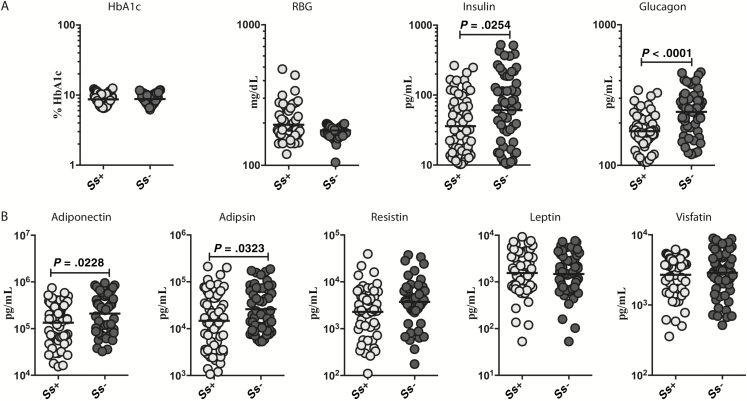
To assess the effect of Ss infection on indices of glucose control in T2DM, we measured the levels of HbA1c and RBG in INF and UN individuals. As shown in Figure 1A, HbA1c and RBG levels were not different between the 2 groups. We next measured insulin and glucagon in INF and UN individuals. As shown in Figure 1A, the levels of insulin (GM, 35.97 vs 61.09 pg/mL; P = .0254) and glucagon (GM, 175.1 vs 240.3 pg/mL; P < .0001) were significantly lower in INF compared to UN individuals. respectively. Next, we assessed the effect of Ss infection on adipocytokines in T2DM. We measured the plasma levels of adiponectin, adipsin, resistin, leptin, and visfatin in INF and UN individuals. As shown in Figure 1B, INF individuals exhibited significantly lower levels of adiponectin than UN individuals (GM, 133808 vs 209547 pg/mL, respectively; P = .0228) and adipsin (GM, 14726 vs 26109 pg/mL, respectively; P = .0323). Thus, Ss infection is associated with lower circulating levels of the pancreatic hormones, adiponectin, and adipsin in Ss-infected subjects with T2DM.
Figure 1.
Diminished circulating levels of pancreatic hormones, adiponectin, and adipsin in individuals with Strongyloides stercoralis infection with type 2 diabetes mellitus (INF). A, Plasma levels of glycated hemoglobin, random blood glucose, insulin, and glucagon in the INF and UN (individuals with type 2 diabetes mellitus and no S. stercoralis infection) groups. B, Plasma levels of adiponectin, adipsin, resistin, leptin, and visfatin in the INF and UN groups. Each dot is an individual subject with the bar representing the geometric mean. Abbreviations: HbA1c, glycated hemoglobin; RBG, random blood glucose; Ss, Strongyloides stercoralis.
Diminished Circulating Levels of TH1 and TH17 Cytokines and Elevated Levels of TH2 Cytokines and TGF-β in INF Individuals
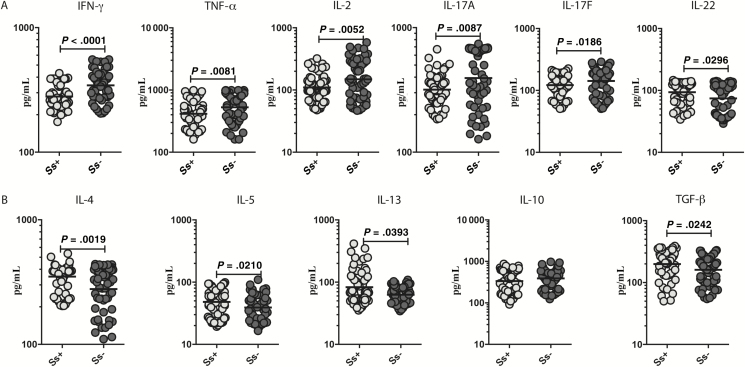
To determine whether helminth infections might improve inflammatory processes by reducing potentially pathological TH1 and TH17 immune responses, we measured the circulating levels of TH1-associated (IFN-γ, TNF-α, and IL-2) and TH17-associated (IL-17A, IL-17F, and IL-22) cytokines in INF and UN individuals. As shown in Figure 2A, INF individuals had significantly lower levels of IFN-γ compared with UN individuals (GM, 280.1 vs 344 pg/mL; P < .0001), TNF-α (GM, 414.4 vs 528.3 pg/mL; P = .0081), IL-2 (GM, 109.8 vs 148.8 pg/mL; P = .0252), IL-17A (GM, 318 vs 394.1 pg/mL; P = .0087), and IL-17F (GM, 121.8 vs 141.9 pg/mL; P = .0186). In contrast, INF individuals had significantly higher levels of IL-22 (GM, 93.36 vs 74.26 pg/mL; P = .0296) in comparison to UN individuals.
Figure 2.
Diminished circulating levels of T-helper (TH) 1 and TH17 cytokines and elevated levels of TH2 cytokines and transforming growth factor beta (TGF-β) in individuals with Strongyloides stercoralis infection with type 2 diabetes mellitus (INF group). A, Plasma levels of TH1 cytokines (IFN-γ, TNF–α, and IL-2) and TH17 cytokines (IL-17A, IL-17F, and IL-22) in the INF and UN (individuals with type 2 diabetes mellitus and no S. stercoralis infection) groups. B, Plasma levels of TH2 (IL-4, IL-5, and IL-13) and regulatory (IL-10 and TGF-β) cytokines in the INF and UN groups were measured. Each dot is an individual subject with the bar representing the geometric mean. Abbreviations: IFN-γ, interferon gamma; IL, interleukin; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; Ss, Strongyloides stercoralis.
Chronic helminth infection has been shown to protect against metabolic disorders by promoting a TH2 (and/or a regulatory) response [29]. To determine the effect of Ss infection on the TH2-associated and regulatory cytokines in T2DM, we measured the plasma levels of TH2-associated (IL-4, IL-5, and IL-13) and regulatory (IL-10 and TGF-β) cytokines in INF and UN individuals. As shown in Figure 2B, IL-4 (GM, 349.7 vs 278 pg/mL; P = .0019), IL-5 (GM, 48.19 pg/mL vs 39.14 pg/mL; P = .0210), IL-13 (GM, 83.11 vs 62.77 pg/mL; P = .0393), and TGF-β (GM, 199.2 vs 160.6 pg/mL; P = .0242) levels were significantly elevated in INF vs UN individuals. Thus, Ss infection with T2DM is associated with lower circulating levels of TH1- and TH17-associated cytokines and higher circulating levels of IL-22, the TH2- associated cytokines, and TGF-β.
Changes in Circulating Levels of Pancreatic Hormones, Adiponectin, and Adipsin Following Anthelmintic Treatment
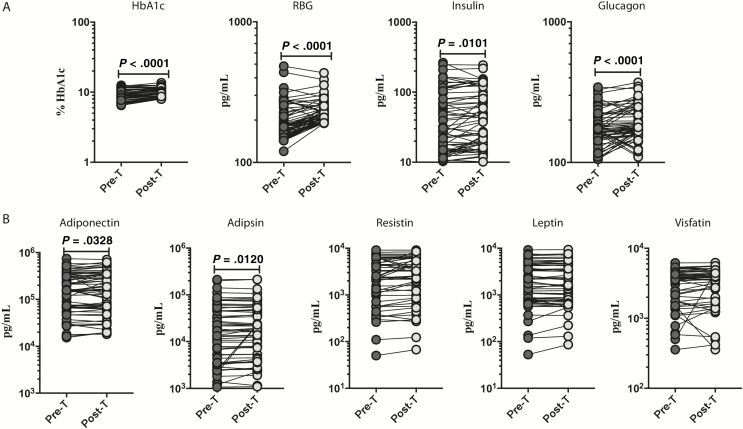
Next, we wanted to determine the effect of anthelmintic treatment on the levels of HbA1c, RBG, and the pancreatic hormones insulin and glucagon. As shown in Figure 3A, the posttreatment levels of HbA1c (percentage increase of 25%; P < .0001), RBG (percentage increase of 18%; P < .0001), insulin (percentage increase of 13%; P = .0101), and glucagon (percentage increase of 18%; P < .0001) were significantly increased compared with pretreatment levels.
Figure 3.
Heightened circulating levels of pancreatic hormones, adiponectin, and adipsin following anthelmintic treatment. A, Plasma levels of HbA1c, RBG, insulin, and glucagon in individuals with Strongyloides stercoralis infection with type 2 diabetes mellitus (INF) Pre-T and 6 months post-T were measured. B, Plasma levels of adiponectin, adipsin, resistin, leptin, and visfatin in INF individuals Pre-T and 6 months Post-T were measured. Abbreviations: HbA1c, glycated hemoglobin; Pre-T, pretreatment; Post-T, posttreatment; RBG, random blood glucose.
To determine the effect of anthelmintic treatment on adipocytokines, we measured the circulating levels of adiponectin, adipsin, resistin, leptin, and visfatin in INF individuals 6 months following anthelmintic treatment. As shown in Figure 3B, the levels of adiponectin (percentage increase of 14%; P = .0328) and adipsin (percentage increase of 12%; P = .0120) levels were significantly elevated in INF individuals posttreatment compared with their pretreatment levels.
Elevated Levels of TH1 and TH17 Cytokines and Diminished Levels of TH2 Cytokines and TGF-β Following Anthelmintic Treatment
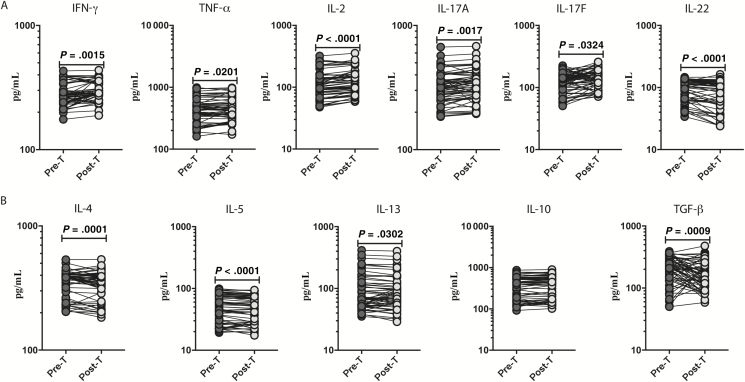
To determine the effect of anthelmintic treatment on circulating levels of TH1-associated (IFN-γ, TNF-α, and IL-2) and TH17-associated (IL-17A, IL-17F, and IL-22) cytokines, we measured the cytokines in INF individuals at 6 months following anthelmintic treatment. At 6 months following anthelmintic treatment, the levels of IFN-γ (percentage increase of 16%; P = .0015), TNF-α (percentage increase of 6%; P < .0001), IL-2 (percentage increase of 18%; P = .0201), IL-17A (percentage increase of 22%; P = .0017), and IL-17F (percentage increase of 21%; P = .0324) all increased following treatment, whereas the IL-22 (percentage decrease of 15%; P < .0001) levels were lower than the pretreatment levels (Figure 4A).
Figure 4.
Heightened circulating levels of pancreatic hormones, adiponectin, and adipsin following anthelmintic treatment. A, Plasma levels of T-helper (TH) 1 cytokines (IFN-γ, TNF–α, and IL-2) and TH17 cytokines (IL-17A, IL-17F, and IL-22) in individuals with Strongyloides stercoralis infection with type 2 diabetes mellitus (INF group) Pre-T and 6 months Post-T were measured. B, Plasma levels of TH2 cytokines (IL-4, IL-5, and IL-13) and regulatory cytokines (IL-10 and TGF–β) in the INF group Pre-T and 6 months Post-T were measured. Abbreviations: IFN-γ, interferon gamma; IL, interleukin; Pre-T, pretreatment; Post-T, posttreatment; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha.
Next, we wanted to determine the effect of anthelmintic treatment on TH2-associated and regulatory cytokines. We measured the TH2 and regulatory cytokines in INF individuals at 6 months following anthelmintic treatment. As shown in Figure 4B, IL-4 (percentage decrease of 9%; P = .0001), IL-5 (percentage decrease of 9%; P < .0001), IL-13 (percentage decrease of 9%; P = .0302), and TGF-β (percentage decrease of 22%; P = .0009) levels were lower in comparison to their respective pretreatment levels.
Multivariate Regression Analysis of Helminth–T2DM Interaction
Multivariate regression analysis was done to assess the influence of Ss infection on the various analytes assessed in this study in T2DM individuals. As shown in Table 2, after adjusting for the effects of age; sex; body mass index; and levels of creatinine, alanine aminotransferase, and aspartate aminotransferase, the levels of RBG, adiponectin, IFN-γ, IL-2, TNF-α, IL-17F, IL-4, IL-13, and TGF-β were all significantly influenced by Ss infection. Thus, our data confirm that Ss infection has a profound influence on various important parameters in individuals with T2DM, including blood glucose levels, and levels of the adipocytokines and the more conventional cytokines.
Table 2.
Multiple Logistic Regression Analysis on Effect of Strongyloides stercoralis Infection on Type 2 Diabetes Mellitus
| Factors | Crude OR (95% CI) |
P Value | Adjusted OR (95% CI) |
P Value |
|---|---|---|---|---|
| Hemoglobin A1c, % | 0.979 (.788–1.216) | .851 | 0.695 (.401–1.206) | .197 |
| Random blood glucose, mg/dL | 1.013 (1.002–1.023) | .015 | 1.013 (1.001–1.024) | .029 |
| Adiponectin, pg/mL | 0.999 (.999–.999) | .012 | 0.999 (.999–.999) | .005 |
| IFN-γ, pg/mL | 0.989 (.984–.994) | .000 | 0.964 (.940–.988) | .004 |
| IL-2, pg/mL | 0.993 (.989–.997) | .001 | 0.979 (.965–.993) | .004 |
| TNF-α, pg/mL | 0.997 (.996–.999) | .003 | 0.994 (.989–.999) | .025 |
| IL-17F, pg/mL | 0.991 (.985–.998) | .011 | 0.976 (.958–.995) | .016 |
| IL-4, pg/mL | 1.006 (1.002–1.011) | .001 | 1.013 (1.006–1.020) | ≤.001 |
| IL-13, pg/mL | 1.016 (1.005–1.028) | .005 | 1.029 (1.010–1.049) | .003 |
| TGF-β, pg/mL | 1.005 (1.000–1.009) | .019 | 1.005 (1.000–1.010) | .043 |
Abbreviations: CI, confidence interval; IFN, interferon; IL, interleukin; OR, odds ratio; TGF, transforming growth factor; TNF, tumor necrosis factor.
DISCUSSION
T2DM is a state of chronic inflammation with alterations in cytokines and chemokines, activation status of different cell types, and metabolic perturbations in various organs [30]. Helminth infections are known modulators of all of the above responses due to their propensity to control detrimental inflammatory responses and support metabolic homeostasis both locally and systemically [31]. Recent studies have shown that helminths may confer protection against the development of T2DM [7, 8], probably by altering host immune responses. Thus, it has been postulated that elimination of helminth infections could contribute to the development of T2DM in many of the economically advanced countries [19]. Previous studies have shown that alterations in the gut microbiota modulate glucose intolerance and adipose tissue inflammation and that helminth infections could alter the gut microbiome during obesity and modulate glucose uptake, inflammation, and insulin sensitivity [32]. Thus, the helminth infection–T2DM interface appears to be counterregulatory, with the effects of helminths on diabetic status still to be fully explored.
We thus first explored the influence of Ss infection on parameters associated with glycemic control in T2DM individuals. Although Ss infection had no significant effect on of HbA1c and RBG in those with coincident T2DM, treatment of Ss infection clearly caused a worsening of both the HbA1c and RBG levels, a finding suggestive of helminth infection–induced modulation of hyperglycemia and perhaps insulin resistance. We speculate that the absence of an effect at baseline could depend on the duration of Ss infection and/or infection load, which is variable in different individuals. In T2DM, insulin deficiency occurs as pancreatic β‐islet cells fail to compensate for insulin resistance by increasing the production of insulin or for a reduction of β‐islet cell mass as a consequence of β‐islet cell apoptosis [33]. Indeed, previous studies have shown that helminths might protect against insulin resistance in T2DM [9]. In line with this, the present study has demonstrated that Ss infection was associated with lower insulin and glucagon levels, levels that reversed following anthelmintic treatment. However, our study did not reveal any direct correlation between infection load and biochemical parameters.
An imbalance between pro- and anti-inflammatory adipokines could also contribute to the development of insulin resistance. Adiponectin is an anti-inflammatory adipokine that is most the abundant adipokine in plasma. Adiponectin, as a modulator of inflammation in a variety of diseases, has recently been highlighted [34]. Circulating adiponectin levels are generally positively correlated with insulin sensitivity [35]. Adipsin was recognized as a proinflammatory product of adipose tissue that is induced in models of diabetes and obesity, providing evidence for a functional link between obesity and inflammation. A recent study shows that measurement of adipsin levels may be used, from a diagnostic standpoint, to identify those patients at high risk of developing β-islet cell failure and accelerated diabetes [36]. In animal models, mice lacking adipsin have worsened glucose homeostasis when placed under the metabolic stress of diet-induced obesity [37]. We have shown that both adiponectin and adipsin are present at diminished levels in the circulation in INF individuals. These levels were significantly increased following anthelmintic treatment, a finding that suggests that Ss infection may modulate adipocytokine levels in INF individuals. Previous studies did not report any difference in adipocytokine levels between individuals with coronary artery disease and coincident helminth infection and those with coronary artery disease alone [38]. In the current study, resistin, leptin, and visfatin were similar between the 2 groups. Thus, modulation of adipocytokines is another mechanism by which helminths could influence glucose homeostasis and insulin resistance in T2DM.
Helminth infections are associated with modulation of the immune responses, including those associated with innate and adaptive responses [39]. We have previously shown that the cytokine profile of Ss-infected asymptomatic individuals had significantly diminished circulating levels of the proinflammatory cytokines and significantly elevated levels of the TH2-associated and regulatory cytokines [40]. The induction of both the TH2- associated and regulatory cytokine responses is postulated to contribute to the modulation of proinflammatory, TH1, and TH17 cytokine responses [31]. A previous study from a high-fat diet–induced mouse model of diabetes showed increased TH2/Treg-associated functional activity and reduction in the levels of IFN-γ and IL-17 compared with helminth-uninfected control mice [11]. In addition, previous reports indicate that CD4+ T cells producing IL-17A and IFN-γ drive inflammation and may lead to insulin resistance [41, 42].
In our study, TH1 (IFN-γ, TNF-α, and IL-2) and TH17 (IL-17A and IL-17F) cytokines were significantly lower in INF individuals than in those without Ss infection. Thus, our data suggest that a mechanistic underpinning of the helminth–diabetes interface involves the modulation of TH1 and TH17 cytokine responses, which has the propensity for improving insulin sensitivity and for promoting barrier function and tissue repair through IL-22 induction [43] (Figure 2). TH2-associated cytokine production is the hallmark of the host response to helminth infection [39]. Helminth infections are also associated with expansion of T cells producing the regulatory cytokines IL-10 and TGF-β [44, 45], which promote insulin sensitivity by inducing the development of alternatively activated macrophages, by promoting eosinophilic infiltration of adipose tissue and by activation of innate lymphoid cells [41, 46].
In summary, our study demonstrates that Ss infection may provide a degree of protection against the severity of T2DM by modulating adipocytokines and the associated cytokine milieu. Our data provide an important link between soil-transmitted helminth infection and the modulation of the severity of T2DM and also opens up novel avenues to reach better glycemic control through a better understanding of helminth-driven immune-mediated and nonimmune-mediated alteration of host metabolism.
Notes
Financial support. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Acknowledgments. The authors thank Dr Satiswaran and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study, N. Pavan Kumar for technical assistance, and the staff of the Department of Epidemiology, National Institute for Research in Tuberculosis, for valuable assistance in recruiting the patients for this study.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Strachan DP, Jarvis MJ, Feyerabend C. Passive smoking, salivary cotinine concentrations, and middle ear effusion in 7 year old children. BMJ 1989; 298:1549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maizels RM, McSorley HJ, Smyth DJ. Helminths in the hygiene hypothesis: sooner or later?Clin Exp Immunol 2014; 177:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yazdanbakhsh M, Matricardi PM. Parasites and the hygiene hypothesis: regulating the immune system?Clin Rev Allergy Immunol 2004; 26:15–24. [DOI] [PubMed] [Google Scholar]
- 4. Guigas B, Molofsky AB. A worm of one’s own: how helminths modulate host adipose tissue function and metabolism. Trends Parasitol 2015; 31:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 2005; 202:1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Q, Sundar K, Mishra PK, et al. . Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun 2009; 77:5347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tracey EF, McDermott RA, McDonald MI. Do worms protect against the metabolic syndrome? A systematic review and meta-analysis. Diabetes Res Clin Pract 2016; 120:209–20. [DOI] [PubMed] [Google Scholar]
- 8. Shen SW, Lu Y, Li F, et al. . The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol 2015; 37:333–9. [DOI] [PubMed] [Google Scholar]
- 9. Wiria AE, Hamid F, Wammes LJ, et al. . Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS One 2015; 10:e0127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hays R, Esterman A, Giacomin P, Loukas A, McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract 2015; 107:355–61. [DOI] [PubMed] [Google Scholar]
- 11. Su CW, Chen CY, Li Y, et al. . Helminth infection protects against high fat diet-induced obesity via induction of alternatively activated macrophages. Sci Rep 2018; 8:4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mendonça SC, Gonçalves-Pires Mdo R, Rodrigues RM, Ferreira A Jr, Costa-Cruz JM. Is there an association between positive Strongyloides stercoralis serology and diabetes mellitus?Acta Trop 2006; 99:102–5. [DOI] [PubMed] [Google Scholar]
- 13. Aravindhan V, Mohan V, Surendar J, et al. . Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83). PLoS Negl Trop Dis 2010; 4:e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol 2006; 28:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kreisinger J, Bastien G, Hauffe HC, Marchesi J, Perkins SE. Interactions between multiple helminths and the gut microbiota in wild rodents. Philos Trans R Soc Lond B Biol Sci 2015; 370. doi: 10.1098/rstb.2014.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol 2011; 23:431–7. [DOI] [PubMed] [Google Scholar]
- 17. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci 2014; 11:1185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Lu J, Huang Y, et al. . Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab 2013; 98:E283–7. [DOI] [PubMed] [Google Scholar]
- 19. Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis 2014; 14:1150–62. [DOI] [PubMed] [Google Scholar]
- 20. de Ruiter K, Tahapary DL, Sartono E, et al. . Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol 2017; 39. doi: 10.1111/pim.12404. [DOI] [PubMed] [Google Scholar]
- 21. Berbudi A, Ajendra J, Wardani AP, Hoerauf A, Hübner MP. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes Metab Res Rev 2016; 32:238–50. [DOI] [PubMed] [Google Scholar]
- 22. Tahapary DL, de Ruiter K, Martin I, et al. . Effect of anthelmintic treatment on insulin resistance: a cluster-randomized, placebo-controlled trial in Indonesia. Clin Infect Dis 2017; 65:764–71. [DOI] [PubMed] [Google Scholar]
- 23. Lipner EM, Gopi PG, Subramani R, et al. . Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am J Trop Med Hyg 2006; 74:841–7. [PubMed] [Google Scholar]
- 24. Bisoffi Z, Buonfrate D, Sequi M, et al. . Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 2014; 8:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buonfrate D, Sequi M, Mejia R, et al. . Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 2015; 9:e0003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect 2015; 21:543–52. [DOI] [PubMed] [Google Scholar]
- 27. Deepa M, Pradeepa R, Rema M, et al. . The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I). J Assoc Physicians India 2003; 51:863–70. [PubMed] [Google Scholar]
- 28. Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg 1995; 53:248–50. [DOI] [PubMed] [Google Scholar]
- 29. Hussaarts L, García-Tardón N, van Beek L, et al. . Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J 2015; 29:3027–39. [DOI] [PubMed] [Google Scholar]
- 30. Donath MY. Inflammation as a sensor of metabolic stress in obesity and type 2 diabetes. Endocrinology 2011; 152:4005–6. [DOI] [PubMed] [Google Scholar]
- 31. Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014; 7:753–62. [DOI] [PubMed] [Google Scholar]
- 32. Cani PD. Gut microbiota and pregnancy, a matter of inner life. Br J Nutr 2009; 101:1579–80. [DOI] [PubMed] [Google Scholar]
- 33. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52:102–10. [DOI] [PubMed] [Google Scholar]
- 34. Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care 2011; 15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116:1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259:87–91. [DOI] [PubMed] [Google Scholar]
- 37. Xu H, Barnes GT, Yang Q, et al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aravindhan V, Mohan V, Surendar J, et al. . Effect of filarial infection on serum inflammatory and atherogenic biomarkers in coronary artery disease (CURES-121). Am J Trop Med Hyg 2012; 86:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 2011; 11:375–88. [DOI] [PubMed] [Google Scholar]
- 40. Anuradha R, Munisankar S, Bhootra Y, et al. . Systemic cytokine profiles in Strongyloides stercoralis infection and alterations following treatment. Infect Immun 2016; 84:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev 2012; 249:116–34. [DOI] [PubMed] [Google Scholar]
- 42. Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 2012; 8:709–16. [DOI] [PubMed] [Google Scholar]
- 43. Broadhurst MJ, Leung JM, Kashyap V, et al. . IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2010; 2:60ra88. [DOI] [PubMed] [Google Scholar]
- 44. Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol 2011; 112:73–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Metenou S, Nutman TB. RegulatoryT cell subsets in filarial infection and their function. Front Immunol 2013; 4:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weng M, Huntley D, Huang IF, et al. . Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol 2007; 179:4721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]