Abstract
The phagocyte NADPH oxidase (the NOX2 complex) generates superoxide, the precursor to reactive oxygen species (ROS). ROS possess both antimicrobial and immunoregulatory function. Inactivating mutations in alleles of the NOX2 complex cause chronic granulomatous disease (CGD), characterized by an enhanced susceptibility to infections and autoimmune diseases such as Systemic lupus erythematosus (SLE). The latter is characterized by insufficient removal of dead cells, resulting in an autoimmune response against components of the cell's nucleus when non-cleared apoptotic cells lose their membrane integrity and present autoantigenic molecules in an inflammatory context. Here we aimed to shed light on the role of the NOX2 complex in handling of secondary necrotic cells (SNECs) and associated consequences for inflammation and autoimmunity during lupus.
We show that individuals with SLE and CGD display accumulation of SNECs in blood monocytes and neutrophils. In a CGD phenotypic mouse strain (Ncf1** mice) build-up of SNECs in Ly6CHI blood monocytes was connected with a delayed degradation of the phagosomal cargo and accompanied by production of inflammatory mediators. Treatment with H2O2 or activators of ROS-formation reconstituted phagosomal abundance of SNECs to normal levels. Induction of experimental lupus further induced increased antibody-dependent uptake of SNECs into neutrophils. Lupus-primed Ncf1** neutrophils took up more SNECs than wild type neutrophils, whereas SNEC-accumulation in regulatory Ly6C−/LO monocytes was lower in Ncf1**mice. We deduce that the inflammatory rerouting of immune-stimulatory necrotic material into inflammatory phagocyte subsets contributes to the connection between low ROS production by the NOX2 complex and SLE.
Abbreviations: CGD, chronic granulomatous disease; NHD, normal healthy donors; NOX2, NADPH oxidase complex 2; Ncf1, neutrophil cytosol factor 1; ROS, reactive oxygen species; SLE, systemic lupus erythematosus; SNEC, secondary necrotic cells; WT, wildtype
Graphical abstract
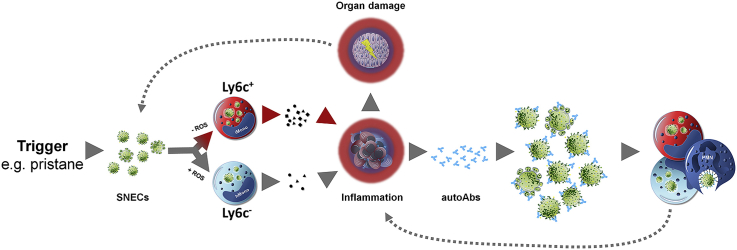
Graphical abstract: Inflammatory rerouting of SNECs occurs in individuals with a dysfunctional NOX2 complex.
1. Introduction
Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease that involves inflammation affecting vital organs such as the kidneys, the lung, and the brain. The immune pathology of SLE is characterized by autoantibodies directed against components of the cell nucleus. Interestingly, a genetic polymorphism of Ncf1, coding for an essential component of the NADPH oxidase complex 2 is the strongest genetic factor for developing SLE and Sjögren's syndrome [1,2], and is also associated with rheumatoid arthritis (RA) [2,3]. Additionally, hypoactive polymorphisms in other subunits of the NOX2 complex have been associated with SLE [[4], [5], [6]]. These connections between the NOX2 complex and SLE are additionally supported by the frequent co-occurrence of cutaneous lupus erythematosus and other autoimmune manifestations with chronic granulomatous disease (CGD), a genetic disorder caused by impaired ROS-production from the NOX2 complex [7,8]. Genome-wide gene expression analysis in human individuals with CGD and a mouse model of CGD also revealed a pronounced type I Interferon (IFN) signature that is typically found in SLE [9]. The genetic association of defects in components of the NOX2 complex with human disease is corroborated in a broad spectrum of animal models of inflammation and autoimmunity [10]. More recently, the NOX2 complex was also confirmed as a critical negative regulator of autoantibody production and autoimmune inflammation in three mouse models of SLE [[11], [12], [13]].
The development of anti-nuclear autoimmunity in SLE is at least partly triggered by impaired or insufficient clearance of apoptotic cells in germinal centers and the circulation. This process may either be due to an increased rate of cell death overwhelming the body's waste disposal machinery, or due to defects in specific clearance mechanisms, such as in the complement system [14]. Apoptotic cells that are not timely removed by phagocytes lose their membrane integrity and, consequently, expose and release cytoplasmic and nuclear autoantigens. This cellular material generated in the absence of proper clearance is designated as secondary necrotic cells (SNECs) [[15], [16], [17]]. The release of nucleic acids and other damage-associated molecular patterns from SNECs triggers an inflammatory response which, in combination with the accessibility of autoantigens, induces a breach of tolerance and precipitates the production of SNEC-specific autoantibodies [15,[18], [19], [20]]. After autoimmunity is established, non-cleared apoptotic material serves as autoantigen repository for the formation of pathogenic immune complexes. These complexes continuously form in the periphery or in situ and deposit in various tissues, especially in the kidney, skin, and joints, where they trigger inflammation and tissue damage [21].
Interestingly, while apoptotic cell clearance is impaired, neutrophils from patients with SLE exhibit enhanced uptake of SNECs [17,22]. In addition, the stimulation of endosomal Toll-like receptors by self nucleic acids and other SNEC-associated molecules has been linked to lupus and other autoimmune conditions [[23], [24], [25], [26]]. Hence, the aim of this study was to investigate the role of the NOX2 complex in the cellular responses of phagocytes to SNECs and its implications for SLE.
We show that low production of ROS is associated with increased accumulation of SNECs in human and mouse blood phagocytes which goes along with enhanced secretion of inflammatory cytokines and chemokines. Treatment with hydrogen peroxide or with chemical inducers of ROS-formation decreased phagocytosis of SNECs into Ly6CHI monocytes from mice with a dysfunctional NOX2 complex to levels seen in mice with normal NOX2-activity. Conversely, removal of ROS with catalase or butyrated hydroxyanisole increased the abundance of SNECs in normal Ly6CHI monocytes. In pristane-induced lupus in mice with a dysfunctional NOX2 complex the same mechanisms cause a bypassing of SNECs from regulatory into inflammatory phagocyte subsets, suggesting that this NOX2-dependent inflammatory rerouting importantly contributes to the development and maintenance of exacerbated SLE in individuals with low activity of the NOX2 complex [1].
2. Materials and methods
2.1. Mice
Mice with a mutated Ncf1 (Ncfm1j/m1j, denoted as Ncf1**) were kept on a BALB/c.Q background. The Ncf1 mutation impairs translocation of the Ncf1 (p47phox) protein to the membrane upon activation, thereby completely blocking function of the NOX2 complex [27,28]. Experiments were performed on female mice frequency-matched for age, and evaluated with blinded identity. All procedures were in accordance with institutional guidelines on animal welfare and were approved by the local ethical committee of the university Erlangen-Nuremberg (permit numbers 54-2532.1-29/12 and TS-12/15 Medizin III Klin Im.).
2.2. Patients
We used blood from 6 normal healthy donors (NHDs), 13 individuals with SLE and 3 patients with CGD during this study. Patients with SLE fulfilled at least 4 of the classification criteria of SLE [29,30]. Diagnosis of CGD was made both clinically and by molecular genetic analysis. Patient 1 showed compound-heterozygous mutations and a typical clinical and granulocyte phenotype, patient 2 had an X-linked CGD with a frameshift mutation in the CYBB gene, and patient 3 carried two mutations in the CYBA gene. Further characteristics of the included subjects are listed in Table S1. All experiments were approved by the ethical committee of the university of Erlangen (permits number Re.-No.3982 and 193_13B). All subjects included in the study gave written informed consent.
2.3. Experimental lupus
Pristane-induced lupus was induced by a single intraperitoneal (i.p.) injection of 500 μl pristane (2,6,10,14-tetramethyl-pentadecane; Sigma-Aldrich) in female mice with an age of 8–12 weeks. Cells were isolated from pristane-injected peritonea 8 weeks after injection of pristane by lavaging the peritonea with cooled PBS.
2.4. Secondary necrotic cells (SNECs)
SNECs were generated by exposing murine thymocytes or human PBMCs (107/ml in PBS) to UVB light for 90 s, and subsequent incubation for 24 h at 37 °C/5% CO2 followed by heat treatment for 30 min at 60 °C for antigen retrieval as previously described [31]. Secondary necrosis was verified by flow cytometry after staining with FITC Annexin V (BioLegend) and propidium iodide (PI) (Sigma-Aldrich). SNECs are Annexin V+PI+ cells. SNECs created by this method have been thoroughly characterized in our lab and have accessible nuclear material [15]. For fluorescent labeling, SNECs were labelled with PI (10 μg/ml) for 30 min at 37 °C or with pHrodo (ThermoFisher) according to the manufacturer's instruction, washed, and stored at 4 °C until further usage. Fluoresbrite YG Carboxylate Microspheres (1 μm) (Polyscience), denoted as “beads” in the manuscript, were sonicated for 5 min and coated with mouse IgG or BSA (2 mg/ml) in 50 mM MES/2 mM EDAC (pH 6.1) for 12 h at room temperature. Beads were washed, resuspended in PBS, and stored at 4 °C.
2.5. Whole blood phagocytosis assay
Lithium-heparinized blood from BALB/c WT and Ncf1** mice or human NHD and SLE/CGD patients was mixed with SNECs in a 1:2 ratio and incubated at 37 °C. In some experiments, the blood was preincubated with varying concentrations of H2O2 (10 min), of the aminoferrocene compound MIS43 (10 min) [32], varying concentrations of catalase (Sigma) or butylated hydroxyanisole (BHA, Sigma) for 15 min before adding SNECs. Cells were stained with anti-mouse CD11b-eFluor450 (eBioscience), anti-mouse Ly6C-APC (BioLegend), Ly6G-PE/Cy7 (BioLegend), CCR2-FITC (BioLegend), I-A/I-E (MHC II)-Alexa Fluor 700 (BioLegend) or anti-human CD14-eFluor450 (eBioscience), CD16-APC/Cy7 and CCR2-PerCP/Cy5.5 (BioLegend), respectively. Samples were subjected to hypotonic water lysis of erythrocytes and analyzed using a Beckman Coulter Gallios™ flow cytometer. The Phagocytosis index (PhIx) was calculated in the following way: PhIx = (%PI/pHrodo-positive phagocytes x MFIPI/pHrodo). For the analysis of cytokine/chemokine production upon phagocytosis, plasma was isolated from blood incubated with SNECs (3 h) and subjected to LegendPlex bead-based assay (BioLegend).
2.6. Plasma swap
Lithium-heparinized blood of BALB/c WT and Ncf1** mice was collected. To determine whether plasma factors affected phagocytosis of SNECs samples were centrifuged at 1000 rpm for 5 min to separate the plasma from the whole blood. Then the plasma was carefully removed and pooled Ncf1** plasma was added to WT cells and vice versa. As a control, cells were also incubated with pooled autologous plasma from the same genotype. Samples were then mixed with pHrodo-labelled SNECs and incubated at 37 °C for up to 4 h. Samples were then stained with anti-mouse CD11b-eFluor450 (eBioscience) and anti-mouse Ly6C-APC (BioLegend) for 20min on ice, followed by hypotonic water lysis of erythrocytes and analysis using a Beckman Coulter Gallios™. The PhIx was then calculated as previously described.
2.7. Depletion of platelets and complement from plasma
Lithium-heparinized blood of BALB/c WT and Ncf1** mice was collected. To evaluate whether platelets or complement affect phagocytosis of SNECs samples were centrifuged at 1000 rpm for 5 min to separate the plasma from the whole blood. The plasma was carefully removed and incubated with 1:1000 Prostaglandin E1 (Sigma) and 1:250 Apyrase (Sigma). Plasma was then used without further treatment or depleted of platelets via centrifugation at 400g and collection of the supernatants. To further deplete complement, platelet free plasma was heat inactivated via incubation in a water bath (57 °C, 30min). Pellets and plasma were checked for platelets via flow cytometry (FSC/SSC and CD61-PE). Prior to adding pHrodo-labelled SNECs samples were reconstituted with either plasma, platelet depleted plasma, or platelet-depleted and heat-inactivated plasma and incubate at 37 °C for 4 h. Samples were then stained with anti-mouse CD11b-eFluor450 (eBioscience) and anti-mouse Ly6C-APC (BioLegend) for 20min on ice, followed by hypotonic water lysis of erythrocytes and determination of phagocytosis indices as described before.
2.8. Western blot analysis of protein degradation
Streptavidin Fluoresbrite YG Carboxylate Microspheres (Polyscience) were coated with biotinylated Protein L (Genescript) in phosphate-buffered saline (PBS) with sodium chloride and bovine serum albumin. Protein L-coated beads were then incubated with blood from WT and Ncf1** mice at 37 °C, 5% CO2 for 1 h for uptake. After removal of erythrocytes by hypotonic lysis, remaining cells were taken up in Opti-MEM I + GlutaMAX-I (Gibco) supplemented with 10% FCS and 1% Penicillin/Streptomycin and incubated at 37 °C, 5% CO2 for another 0–4 h. After washing with PBS, pelleted cells were lysed with Laemmli buffer (Sigma) for 10 min at 95 °C. Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using 8–16% gradient gels (ServaGel TG PRiME, Serva). The protein material was then blotted to polyvinylidene difluoride membranes (Immobilon, Merck). Membranes were blocked for 1 h with Tris-buffered saline (TBS)/5% milk powder and incubated for 1 h with Streptavidin-HRP (Thermo Scientific Cat No. #N100). Finally, bands were visualised by enhanced chemiluminescence (ThermoScientific). Densitometric analysis was performed using ImageJ software (Rasband, WS, ImageJ, NIH, Bethesda, USA). Histone H3 served as a loading control and was visualised using a rabbit anti-histone H3 antibody (ab1791 Abcam). Remaining Protein L content was normalized to histone H3 in every lane.
2.9. Measurement of ROS production in monocytes
For measurement of intracellular ROS in human phagocytes, lithium-heparinized blood was incubated with dihydrorhodamine (DHR) 123 (3 μg/ml, Molecular Probes) for 15 min at 37 °C. Cells were stained with anti–human CD14-eFluor450 (eBioscience, cat.# 48–0149, clone 61D3) and CD16-APC/Cy7 (Biolegend, cat.# 302017, clone 3G8), and anti-human CD192/CCR2 (Biolegend, cat.# 357203, clone K036C2), and ROS production was induced by incubation with PMA (100 ng/ml) for 15 min at 37 °C. Intracellular ROS in mouse CD11b+Ly6CHI iMonos was measured via DHR123 fluorescence after 3 h incubation of mouse blood with various concentrations of MIS43 or RE-02 or 15 min incubation with 100 ng/ml PMA. Before analysis on the Beckman Coulter Gallios™ flow cytometer, human and murine samples were subjected to hypotonic water lysis.
2.10. Measurement of pH in the phagosome
SNECs were labelled with 10 μM SNARF-1 (Sigma) (1 h at room temperature). The average pH in the phagosome was determined 0–4 h after incubation with SNARF-1 labelled SNECs by the ratio of FL6/FL2 on a Gallios™ flow cytometer (Beckman coulter) as described [33].
2.11. Analysis of Fcγ receptor expression
Blood cells of naïve BALB/c WT and Ncf1** mice were analyzed for expression of different Fcγ receptors in steady state or after 2 h of incubation with propidium iodide-labelled SNECs. After hypotonic water lysis cells were stained with anti-CD11b-eFluor450 (eBioscience) and anti-Ly6C-APC (BioLegend), and either anti-CD64-PerCP/Cy5.5, anti-CD16/32-PE/Cy7, or anti-CD16.2-PE (all from BioLegend). Analysis was carried out using a Beckman Coulter Gallios™.
2.12. Statistical analysis
Two group comparisons were performed using 2-tailed Student's t-test or, in the case of non-normally distributed data, by 2-tailed Mann-Whitney U test. Gaussian distribution of samples was checked using D'Agostino-Pearson omnibus normality test. Within each set of experiments shown in one graph multiple comparisons of groups were adjusted using Sidak's multiple comparisons test or Dunnett's test if several values were compared to control values. In case of non-normally distributed data we used Kruskal-Wallis test with Dunn's post-hoc test for multiple comparisons. Outliers within data sets were excluded based on a Grubb's test/extreme studentized deviate (ESD) test for variation from a normal distribution. Adjusted p-values < 0.05 were considered statistically significant. Computations and charts were performed using GraphPad Prism 7 software.
3. Results
3.1. Secondary necrotic cells accumulate in phagocytes from human individuals with low ROS production
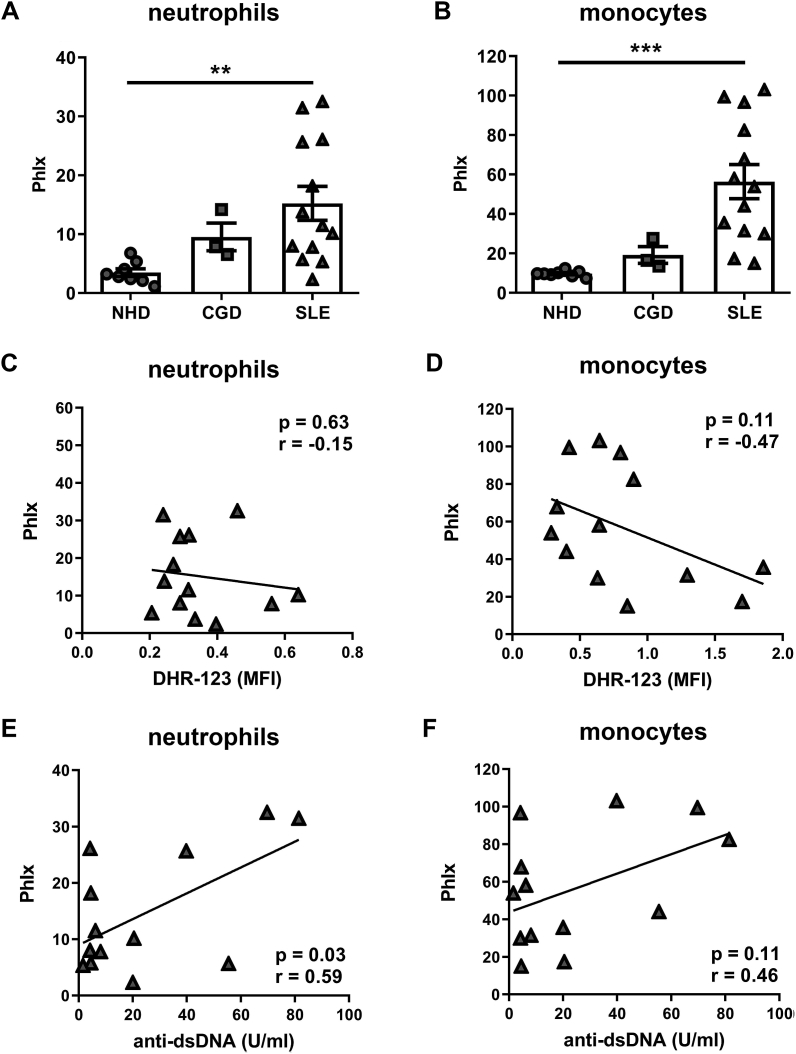
To find out if uptake and clearance of dead cells during SLE is connected to the function of the NOX2 complex in phagocytes, we quantified phagocytosis and intracellular ROS production in a cohort of 13 individuals with SLE, 3 patients with CGD, and 8 normal healthy donors (patient and control characteristics in Supplemental Table S1). Phagocytosis was assessed by a whole blood phagocytosis assay, using SNECs prepared from human PBMCs and labelled with the fluorescent dye propidium iodide (PI). After incubation of fluorescent SNECs with peripheral blood, SNECs taken up by different phagocyte populations were detected by flow cytometry. This technique closely mimics the situation in vivo since it is sensitive to both genetically determined effects and the impact of plasma components. In line with previous results [17], we observed significant accumulation of SNECs in CD14−CD16+CCR2- neutrophils and CD14+ CD16-/LOCCR2+ monocytes derived from the blood of individuals with SLE as compared to healthy controls (Fig. 1A and B). Interestingly, also neutrophils and monocytes from patients with CGD took up more SNECs than phagocytes from healthy controls. Due to the low number of these patients available at our local outpatient clinic (n = 3), the statistical significance of this finding could, however, not be evaluated. We have previously shown that neutrophils from patients with SLE produce normal amounts of ROS spontaneously or upon stimulation with PMA [12]. The same results applied to CD14+CD16-/LOCCR2+ monocytes (Fig. S1). However, spontaneous intracellular production of ROS in monocytes from patients with SLE appeared to show a weak trend to be negatively correlated with phagocytosis of SNECs (Fig. 1C and D). Furthermore, plasma levels of SNEC-binding (anti-dsDNA) antibodies correlated with uptake of SNECs in neutrophils and showed a trend of a correlation in monocytes (Fig. 1E and F).
Fig. 1.
Phagocytosis of SNECs in human blood neutrophils and monocytes is negatively correlated with the oxidative burst. (A, B) Peripheral blood from individuals with systemic lupus erythematosus (SLE; n = 13), chronic granulomatous disease (CGD; n = 3) and from normal healthy donors (NHD; n = 8) was incubated with human PBMC-derived SNECs for 3 h. Phagocytosis indices (PhIx) of CD14−CD16+CCR2- neutrophils (A) and CD14+CD16-/LOCCR2+ monocytes (B) were determined by flow cytometry. Patients with SLE exhibited significant accumulation of SNECs. Graphs show individual values, means ± S.E.M. **p < 0.01, ***p < 0.001, as determined by Student's t-test. (C, D) Correlation analysis between spontaneous intracellular production of ROS (DHR-123 MFI) and phagocytosis indices in blood CD14−CD16+CCR2- neutrophils (C) and CD14+CD16-/LOCCR2+ monocytes (D). (E, F) Correlation analysis between levels of anti-dsDNA autoantibodies and PhIx in blood CD14−CD16+CCR2- neutrophils (E) and CD14+CD16-/LOCCR2+ monocytes (F). Pearson coefficients (r) and p values (determined by 2-tailed Student's t-test) in C-F are depicted within the graphs.
3.2. Dysfunction of the NOX2 complex causes accumulation of SNECS in murine phagocytes accompanied by increased production of inflammatory mediators
To perform functional studies, we used the Ncf1** mouse strain which does not produce ROS via the NOX2 complex due to a mutation in the Ncf1 gene, one of the activating subunits of the NOX2 complex [27,28]. With their increased susceptibility to certain bacteria [34] and their propensity to develop chronic autoimmune and inflammatory diseases [9,10,12] Ncf1** mice are considered a valid model for human CGD.
We again used a whole blood phagocytosis assay employing SNECs labelled with either PI or pHrodo, a pH-sensitive dye that specifically fluoresces in the acidic environment after uptake into phagosomes and lysosomal sequestration (Fig. S2A).
Three main types of phagocytes exist in mouse blood: CD11b+Ly6CINTLy6G+ neutrophils (further demoted as neutrophils), CD11b+Ly6CHICCR2+CD115+ inflammatory monocytes (further denoted as iMonos), which also express high levels of MHC class II, and CD11b+Ly6C−CCR2-/LO (homeostatic) mature monocytes (further denoted as hMonos) (Fig. S2B).
We observed that after 1, 2 and 3 h incubation, iMonos from Ncf1** mice had taken up significantly more SNECs than cells from wildtype (WT) mice (Fig. 2A). A strong trend to increased intracellular accumulation of SNECs was also seen in Ncf1** neutrophils. In contrast, the phagocytic indices (PhIx) of SNECs in hMonos were low in both genotypes. The increased accumulation of SNECs in Ncf1** phagocytes was accompanied by higher supernatant concentrations of inflammation-related cytokines and chemokines, such as TNFα, IL-1β and CXCL1 (Fig. 2B).
Fig. 2.
Accumulation of SNECs in murine phagocytes with a dysfunctional NOX2 complex and associated production of inflammatory mediators. (A) Phagocytosis indices (PhIx) in blood phagocyte subpopulations in naïve BALB/c wild type (WT) and Ncf1** mice after incubation with propidium iodide-labelled SNECs for 0–3 h. Plots show means ± S.E.M, n = 17. *p < 0.05, **p < 0.01, ***p < 0.001 of individual time points or area under the curves, as determined by ANOVA with Sidak's multiple comparisons test. (B) Concentrations of cytokines and chemokines released into the supernatants after 3 h incubation of erylysed whole blood from naïve BALB/c WT and Ncf1** mice with SNECs. Bars show means and S.E.M., n = 5. p < 0.05, **p < 0.01, n.s., not significant, as determined by Student's t-test. (C) Representative Western blot and quantification of remaining Protein L after 0–4 h of intracellular digestion in phagocytes derived from WT and Ncf1** mice. Histone H3 was used as a reference protein. Bars show means and S.E.M, n = 6–7. Amount of protein L normalized to histone H3 at 0 h was set to 100%. *p < 0.05, ***p < 0.001 as determined by ANOVA with Sidak's multiple comparison test. (D) Representative histograms and quantification of the relative expression of I-A/I-E (MHC II) on iMonos, neutrophils and hMonos from naïve mouse blood. Shown are individual values, means ± S.E.M. of the increase in the median fluorescence intensity (ΔMFI) caused by the I-A/I-E-specific antibody. *p < 0.05, n.s., not significant, as determined by ANOVA with Sidak's multiple comparisons test. (E) Absence of phagosomal alkalinization in Ncf1** phagocytes upon phagocytosis. FL6/FL2 ratio in WT and Ncf1** Ly6CHI iMonos and Ly6CINTLy6G+ neutrophils from the blood of naïve mice after 0–4 h incubation with SNARF1-labelled SNECs. **p < 0.01, ***p < 0.001 between individual time points, as determined by ANOVA with Sidak's multiple comparisons test. N = 9.
3.3. Impaired phagosomal alkalinization and delayed protein degradation in Ncf1** phagocytes
Depending on the culture conditions, the phagocytes and the phagosomal cargo the NOX2 complex can either positively or negatively affect the degradation capacity in the phagosome [35,36]. To test if the NOX2 complex affects protein degradation in phagocytes under conditions mimicking the in vivo situation in the blood, we investigated degradation of bead-coupled Protein L. Western blot analysis revealed that protein degradation was delayed in Ncf1** blood phagocytes, resulting in significantly higher amounts of undigested Protein L after 1 and 2 h, while 4 h after bead uptake no significant differences could be found in remaining Protein L content of Ncf1** and WT phagocytes any more (Fig. 2C). These results suggest that a suboptimal degradation of proteins contributes to the accumulation of SNECs in phagocytes from Ncf1** mice. Limited proteolysis of phagosomal cargo correlates with the ability to generate antigenic peptides for presentation by MHC and activation of adaptive immune responses [37]. Supporting this concept, we found increased base-line expression of MHC II on circulating Ncf1** inflammatory monocytes (Fig. 2D). Incubation with SNECs further elevated MHC II expression on all blood phagocyte subsets, with higher upregulation on Ncf1** iMonos and neutrophils and on WT hMonos (Fig. S3).
During the oxidative burst after phagocytosis, the catalytic subunits of the NOX2 complex transport massive amounts of electrons into the phagocytic vacuole which results in a rise in vacuolar pH and a concomitant acidification of the cytosol [38,39]. In the absence of an active NOX2 complex the phagocytic vacuole does not undergo alkalinization and becomes acidic upon fusion with lysosomes. This phenomenon is observed in neutrophils from individuals with CGD [40]. In agreement with these human results, we observed a complete lack of phagosome alkalinization after incubation with SNECs in iMonos and neutrophils from Ncf1** mice (Fig. 2E), detected by SNECs labelled with the ratiometric pH indicator SNARF-1. Since the neutral serine proteases need to be activated by the elevated pH in the phagosome that succeeds the oxidative burst [41], we hypothesize that the lack of phagosome alkalinization is responsible for the delayed degradation of protein cargo in Ncf1** phagosomes.
3.4. Treatment with H2O2 or ROS-inducing compounds reconstitutes physiological phagocytosis of SNECs in inflammatory monocytes
Our finding that in the absence of ROS production from the NOX2 complex SNECs build up in phagocytes, prompted us to investigate if supplementation of ROS levels by incubation with the long-lived ROS H2O2 might reduce SNEC accumulation. Indeed, exposure of Ncf1** whole blood to H2O2 reduced the abundance of SNECs in iMonos to WT levels (Fig. 3A). In contrast, in WT iMonos no reduction in accumulation of SNECs was observed upon incubation with H2O2. The abundance of SNECs in Ncf1** or WT neutrophils or hMonos was not significantly changed after incubation with H2O2. Importantly, H2O2 does not induce cell death under the conditions used in our assay (1 mM, 70 min treatment) [42] (Fig. S4). Thus, the reduced phagocytic activity in H2O2-treated iMonos was not due to cytotoxic effect of H2O2. Similar to H2O2, the aminoferrocene-based compound MIS43 [32] catalyzed ROS production independently of a functional NOX2 complex (Fig. S5A) and dose-dependently decreased accumulation of SNECs in Ncf1** iMonos (Fig. 3B).
Fig. 3.
Treatment of blood with redox-active compounds. (A) 1 h phagocytosis indices (PhIx) of PI-labelled SNECs in phagocytes from blood of naïve mice treated with or without 0,003% (~1 mM) H2O2 before incubation with SNECs. *p < 0.05, n.s., not significant, as determined by ANOVA with Sidak's multiple comparisons test. N = 6. (B) Phagocytotic activity after treatment of iMonos from Ncf1** blood with varying concentrations of the aminoferrocene prodrug MIS43. **p < 0.01, ***p < 0.001, as compared to Ncf1** control, determined by repeated measures ANOVA with Dunnett's post-hoc test. N = 4–8. PhIx in sham-treated Ncf1** iMonos (control) were set to 100%. (C) 3 h PhIx of PI-labelled SNECs in CD11b+Ly6CHI iMonos from blood of WT mice upon treatment with 50 μM of the sulfonamide NOX2 agonist RE-02. n.s., not significant, as determined by paired Student's t-test. (D) 3 h PhIx of pHrodo-labelled SNECs in iMonos upon treatment of blood from WT mice with varying concentrations of catalase and butylated hydroxyanisole (BHA). *p < 0.05, **p < 0.01, ***p < 0.001, as compared to vehicle-treated blood (0), determined by repeated measures ANOVA with Dunnett's post-hoc test. N = 4.
We also treated WT phagocytes with the specific NOX2-activator RE-02, a sulfonamide compound that triggers activation of the NOX2 complex and has been successfully employed for treatment of experimental lupus [12,43]. However, as in the case of H2O2, triggering activation of the NOX2 complex with RE-02 did not induce a further decline of SNEC accumulation in WT iMonos (Fig. 3C, Fig. S5B).
The experiments with H2O2 and the ROS-inducer MIS43 argue for a direct but NOX2-independent dampening effect of ROS on accumulation of SNECs in iMonos. In line with that, in vitro treatment with the enzymatic ROS-scavenger catalase and the antioxidant butylated hydroxyanisole (BHA) increased the accumulation of SNECs in WT iMonos (Fig. 3D).
3.5. Plasma components enhance uptake of SNECs in neutrophils but not in inflammatory monocytes
Aside from a cell-intrinsic influence of the NOX2 complex (per example related to delayed degradation of cargo), extrinsic factors, such as other blood cells or plasma proteins, could additionally contribute to the accumulation of SNECs in Ncf1** blood phagocytes by increasing SNEC-uptake. To assess the impact of Ncf1**-derived plasma components, we conducted phagocytosis assays with WT cells in the presence of pooled Ncf1** plasma and vice versa. Incubation with autologous plasma was used as control. While phagocytosis of SNECs in iMonos was not affected by the plasma swap, incubation with Ncf1**-derived plasma significantly enhanced accumulation in neutrophils. Interestingly, WT serum slightly but significantly increased uptake into hMonos (Fig. 4A).
Fig. 4.
Influence of plasma factors on phagocytosis of SNECs. (A) Phagocytosis indices (PhIx) of pHrodo-labelled SNECs after 2 h incubation with swapped plasma. *p < 0.05, n.s., non significant, as determined by ANOVA with Sidak's multiple comparisons test. N = 4–6. (B) PhIx of pHrodo-labelled SNECs upon depletion of platelets or heat inactivation of complement. *p < 0.05, **p < 0.01 compared to sham-treated sera (control) determined by ANOVA with Dunnett's multiple comparisons test. (C) Fold induction of CD64 (Fcγ receptor I), CD16/32 (Fcγ receptor IIB/III), and CD16.2 (Fcγ receptor IV) in blood phagocytes incubated with SNECs or without stimulation for 3 h. Plots show mean fluorescence intensities (MFI) from individual mice. N = 3–4.
Platelets can affect leukocyte function and the inflammatory response as a whole [44,45]. Especially interaction between platelets and neutrophils has been revealed to initiate or amplify various neutrophil responses including phagocytosis, release of NETs and production of ROS [46]. We, therefore, hypothesized that platelets in plasma also might influence phagocytosis of SNECs. However, depletion of platelets did not have measureable influence on phagocytosis of SNECs in WT or Ncf1** phagocytes (Fig. 4B). In contrast, heat inactivation of plasma reduced uptake into neutrophils and iMonos in both genotypes. This suggests that heat-sensitive plasma components, for example complement proteins, mediate uptake of SNECs independently of the NOX2 complex (Fig. 4B) [47,48].
Fcγ receptors (FcγR) mediate the uptake of nucleic acid-containing immune complexes and SNECs and thereby facilitate their delivery to TLR7-containing endosomes [17,49,50]. We, thus, aimed to determine if differential expression of FcγR in the Ncf1** strain could contribute to the aberrant uptake of SNECs. However, no significant differences in expression of FcγR were found between blood phagocytes derived from WT and Ncf1** mice (Fig. S6). Nevertheless, incubation with SNECs induced the expression of FcγRI (CD64) in iMonos and neutrophils, a high affinity receptor for monomeric IgG2a and low affinity for IgG2b and IgG3. Furthermore, the expression of FcγRIV (CD16.2), a high affinity receptor for IgG2a and 2b, was upregulated in iMonos, neutrophils and hMonos, while the expression of FcγRIIB/III was not influenced by incubation with SNECs (Fig. 4C).
3.6. SNEC-targeting autoantibodies increase uptake into neutrophils
SNECS are preferentially taken up during conditions in which lupus autoimmunity occurs and autoantibodies are produced [15,17,51]. Ncf1** mice on the BALB/c background spontaneously develop elevated levels of SNEC-binding autoantibodies, which is further elevated during experimental lupus [9,12]. To investigate if the NOX2 complex influences phagocytosis of SNECs in the context of lupus, we analyzed phagocytic behavior of cells isolated from WT and Ncf1** mice injected intraperitoneally with the isoprenoid alkane pristane (2,4,6,10-tetramethyl-pentadecane), which induces an inflammatory disease accompanied by production of autoantibodies directed against DNA- and RNA-associated autoantigens and closely resembling human SLE [52]. Uptake of SNECs into blood-derived iMonos was enhanced after pristane-injection in both WT and Ncf1** mice as compared to naïve mice, with no differences between the genotypes (Fig. 5A, left panel). However, engulfment of SNECs by blood neutrophils of pristane-injected Ncf1** mice was significantly increased compared to neutrophils from pristane-primed WT mice (Fig. 5A, middle panel). Interestingly, the opposite was observed for hMonos in which the uptake of SNECs was higher in cells of pristane-injected WT than of Ncf1** mice (Fig. 5A, right panel). Similar results were obtained for uptake of SNECs into iMonos and neutrophils isolated from the pristane-injected peritoneum (Fig. 5B), although the differences did not reach the level of statistical significance. Of note, in agreement with a previous study [53] we could not detect CD11b+Ly6C−/LO hMonos in pristane-injected peritonea. We hypothesize that the enhanced uptake of SNECs into neutrophils derived from the blood and the peritoneum of pristane-injected mice might be caused by the increased local levels of SNEC-binding autoantibodies. To further test this concept, we assessed uptake of IgG- and BSA-coated beads into neutrophils derived from naïve mice. Other than SNECs, latex beads are not degraded by pH-dependent proteinases in the phagosome, so that phagocytosis indices can serve as specific indicator of bead uptake. In contrast to SNECs, the abundance of BSA-coated control beads was not different in neutrophils from naïve Ncf1** and WT mice. Opsonization of beads with IgG increased the uptake into neutrophils of both genotypes (Fig. 5C).
Fig. 5.
Phagocytosis of SNECs and opsonized beads by phagocytes from pristane-injected animals. Phagocytosis indices of propidium iodide-labelled SNECs in phagocyte subpopulations derived from the blood (A) or peritoneum (B) of BALB/c wildtype (WT) and Ncf1** mice 2 months after intraperitoneal injection of pristane. Plots show means and S.E.M. *p < 0.05, **p < 0.01 of individual time points or area under the curves, as determined by ANOVA with Sidak's multiple comparisons test. Dashed lines indicate PhIx in blood phagocytes from naïve animals. N = 16–22 (A) or 8–18 (B), respectively. (C) Phagocytosis indices of neutrophils from naïve wild type and Ncf1** mice after incubation with IgG- or bovine serum albumin (BSA)-precoated beads. Shown are means ± S.E.M. *p < 0.05, **p < 0.01, of individual time points as determined by ANOVA with Sidak's multiple comparisons test.
In summary, our results show aberrant uptake and degradation of necrotic material in the absence of sufficient ROS-production. Thereby, SNECs are rerouted into inflammatory monocytes and neutrophils instead of mature homeostatic monocytes.
4. Discussion
Phagocytosis is defined as the cellular uptake of particles of >0.5 μm diameter within a plasma-membrane-enclosed envelope. The uptake of apoptotic and necrotic cells is termed efferocytosis. The immunologically silent, anti-inflammatory clearing of dead cells can become impaired when apoptosis progresses to necrotic cell death, (with the occurrence of SNECs), resulting in inflammation and possible breach of tolerance. While there is a plethora of ligands described for apoptotic cells (reviewed in Ref. [54]) the uptake of SNECs with compromised membrane integrity is not well characterized.
Although the phagocyte subsets identified in human and mouse blood are not precisely overlapping, their differentiation and contribution to immune defense appear to be quite similar [55]. The major phagocyte populations in mouse blood are defined by the expression of the glycoprotein Ly6C (Gr1) and of chemokine receptors: Ly6CHI CX3CR1+ CCR2+ monocytes (corresponding to CD14+ CD16− or CD16LO in humans), Ly6C−/LO CX3CR1HICCR2– monocytes (CD14INTCD16+ in humans) and Ly6CINTLy6G+ neutrophils (corresponding to human CD14−CD16+ cells). These subsets are characterized by distinct inflammatory properties [56,57].The classical murine Ly6CHI monocytes efficiently infiltrate inflammatory sites, dominating the acute inflammatory response. They are thought to differentiate into classical macrophages in most inflammatory conditions. Ly6CHI cells are therefore often regarded as “inflammatory monocytes”. In pristane-induced lupus inflammatory monocytes are major producers of type I IFNs and thus contribute crucially to pathogenesis [58]. In the absence of inflammation, Ly6CHI monocytes return to the bone marrow, where they are thought to differentiate into Ly6C−/LO monocytes [59]. The non-classical Ly6C−/LO monocytes patrol blood vessels and mostly differentiate into alternatively activated macrophages at inflammatory sites, which secrete immune-regulatory cytokines and contribute to tissue repair [60]. Ly6C−/LO monocytes are regarded as “stationary” or “homeostatic” because they appear to be the only monocyte population that extravasates into tissues under steady-state conditions [57].
Ly6C−/LO monocytes are more efficient than Ly6CHI monocytes in the ingestion of apoptotic cells in the blood in vivo, which promotes tolerance to self-antigens contained in apoptotic cells [61]. In contrast Ly6C+ monocytes were demonstrated to cross-present cell-associated autoantigens upon efferocytosis to CD8+ T cells in lymph nodes and thereby promote cytotoxic T cell development in the presence of inflammatory conditions in lupus [62]. Since preferential uptake of SNECs in Ly6CHI monocytes promoted the production of inflammatory mediators, whereas uptake into Ly6C−/LO monocytes was associated with a milder form of PIL, our data support the pro-inflammatory role of Ly6CHI as opposed to the regulatory role of Ly6C−/LO monocytes.
Phagocytosis triggers the assembly of the phagocyte NADPH oxidase [35,63]. The NOX2 complex is the dominant ROS-generating complex in mammals [64]. ROS production via the catalytic subunits embedded in the phagosomal and plasma membranes is regulated by the cytosolic adaptors p47phox (Ncf1) and p40phox (Ncf4) that bridge interactions of activators of the NOX2 complex with the enzymatic flavocytochrome components embedded in phagosomal and plasma membranes.
The influence of the NOX2 complex on the phagocytic function of murine macrophages has more recently been addressed in two studies [35,36]. Using aged human neutrophils that had undergone spontaneous apoptosis as a cargo and performing phagocytosis assays with peritoneal macrophages, Bagaitkar and colleagues showed delayed proteolytic activity in mice with inactivated X-linked Cybb [35]. These mice serve as model for the most frequent form of human CGD, emerging in males that are hemizygous for mutations causing dysfunction of the gp91phox subunit of the enzymatic center of the NOX2 complex [65]. In contrast, Rybicka et al. had previously shown that degradation of protein coupled to silica beads was increased in bone-marrow derived macrophages from Cybb−/- mice. They had not observed any effects of changes in luminal pH on degradation but had demonstrated that in NOX2-dysfunctional phagocytes cathepsin proteases are protected from redox-mediated deactivation [36]. Both the nature of the ingested particle and the ingesting phagocyte itself thus seem to influence the impact of NOX2 on the degradative capacity in the phagosome. To further characterize the complex role that the NOX2 complex has on degradation of proteins in the phagosome we employed an assay where degradation was assessed after uptake in various phagocyte populations in whole blood. In this assay, digestion of bead-coupled protein L was delayed in the absence of a functional NOX2 complex. Our results suggest that activation of the NOX2 complex is required for the alkalization of the phagosome and in the absence of a functional NOX2 complex further processing of ingested SNECs is therefore inhibited. This effect can be attributed to the fact that the major granule proteases of neutrophils, neutrophil elastase and cathepsin G, work best at a pH higher than 7 [41]. Given the implications of NET-associated neutrophil serine proteases for degradation of inflammatory mediators [66] these results could also offer an additional explanation for the strong exacerbation of pristane-induced lupus observed in mice with a dysfunctional NOX2 complex [11,12].
SLE is characterized by aberrant phagocytosis, with impaired clearance of apoptotic cells but enhanced uptake of autoantigen-loaded SNECs [67]. The phagocytosis of SNECs was elevated in CD11b+Ly6CHICCR2+ monocytes in Ncf1** mice, a phagocyte subset that was shown to crucially contribute to experimental lupus via a TLR7-dependentmechanism [68]. Taking into account their cytokine profiles, these cells instigate a robust inflammatory response after phagocytosis of SNECs. Interestingly, this phenotype was reverted completely by addition of H2O2 or induction of ROS, indicating a role of ROS in the regulation of the inflammatory response induced by SNECs. Interestingly, another study had shown that the uptake of apoptotic neutrophils does not induce the production of pro-inflammatory cytokine production in macrophages, but induced anti-inflammatory mediators that were, however, not different in the absence of a functional NOX2 complex [35]. The enhanced induction of pro-inflammatory cytokines by ROS-deficient phagocytes seems therefore to be specific for secondary necrotic material.
Data from patients with CGD indicated that macrophages with a defective NOX2 complex exhibit impaired phagocytosis of apoptotic cells [69], but increased phagocytosis of bacteria that cannot be killed in the absence of a functional NOX2 complex [70]. Similarly, during SLE, uptake of apoptotic cells is decreased but more SNECs are taken up. These findings argue for a potential discrimination of cargo with a relation to danger. SNECs express various danger signals (e.g., nucleic acids and HMGB-1) that activate pattern recognition receptors and modified autoantigens, and are often opsonized by anti-nuclear antibodies in vivo.
In any case, the final outcome of a dysfunctional NOX2 complex for humans and mice with CGD is enhanced inflammation and autoimmunity. In Ncf1** mice these processes seem to be accelerated by increased accumulation of SNECs in inflammatory monocytes. Furthermore, in Ncf1** mice the induction of lupus shifted the phagocytosis of SNECs towards neutrophils while wild type mice preferentially directed SNECs into Ly6C−/LO regulatory monocytes.
In concordance with previous studies performed in individuals with SLE, the opsonization of SNECs by elevated titers of autoantibodies probably caused this abnormal phagocytic profile [17,19,31,48]. Indeed, IgG coated beads reproduced this effect independently from the ROS-producing capabilities of the phagocytes.
Neutrophils became activated upon SNEC ingestion, with strongly increased expression of CD64 (FcγRI), the activating high affinity FcγR for the potent monomeric subclasses IgG2a and IgG2b. Neutrophils also expressed the other high affinity FcγRIV (CD16.2), which was however even expressed in higher amounts by Ly6C−/LO blood monocytes. This is in line with previous reports [71] and fits to the strong elevation of autoantibody titers of the IgG2a isotype that occurs during PIL in Ncf1** mice [12].
Taken together, the aberrant phagocytosis promoted by an altered autoantibody profile contributed to the detrimental inflammatory milieu and exaggerated disease progression observed in Ncf1** mice. We suggest a role of ROS production in the maintenance of the homeostasis in the phagocytic compartment. We propose a concept in which a trigger or irritant (e.g. pristane or UV exposure) induces a wave of cell death that overburdens the host's clearance machinery. Necrotic cell material is then generated either by direct induction of necrosis by physical or chemical irritants, or alternatively by insufficient clearance of apoptotic cells. In the latter case apoptotic cells lose their membrane integrity and progress to secondary necrosis. SNECs expose autoantigens in an inflammatory context. Deficiency of ROS further inhibits the degradation of dead cell remnants in the efferosome and thereby favors their accumulation in Ly6CHI inflammatory monocytes, which is accompanied by elevated cytokine and chemokine production (inflammatory rerouting of SNECs). The ensuing activation of the immune system increases the production of anti-nuclear autoantibodies that opsonize SNECs and promote enhanced and abnormal uptake into neutrophils, which additionally fuels the inflammatory response. The resulting tissue damage adds additional masses of dead cells that need to be cleared, thus closing a vicious circle bringing about the establishment of chronic inflammation in lupus (see graphical abstract).
RE-02, an activator of the NOX2 complex, has previously been successfully used to treat pristane-induced lupus in mice with a functional NOX2 complex [12]. A novel alternative to RE-02 is the aminoferrocene-based prodrug MIS43, which induces ROS production also in cells with a defective NOX2 complex [32] and prevented inflammatory rerouting of SNECs in Ncf1** phagocytes in our current study. MIS43 is activated by the removal of a triggering moiety by ROS, which leads to the formation of substituted aminoferrocenes. These act as electron donors for endogenous H2O2 and 3O2 leading to formation of ROS (HO• and O−2∙) as well as ferrocenium cations. The latter can be reduced back to the aminoferrocene by endogenous bulk reducing agents, such as GSH and NADPH, thereby closing the catalytic cycle [72]. Aminoferrocenes are powerful ROS amplifiers both in vitro and in vivo. Compounds of this class were originally developed for targeting cancer cells [73] but are promising candidates for the treatment of autoimmune or chronic inflammatory diseases as well.
Author contributions
J.Hahn, M. Euler, E. Kilgus, D. Kienhöfer, J. Knopf and M. Hahn planned and performed experiments, conducted data analysis, helped to write the manuscript and to prepare figures. T. Harrer contributed to experiments using neutrophils from CGD patients and interpretation of the results. M. Hultqvist, P. Olofsson and A. Mokhir developed and provided reagents for induction of ROS and contributed to experimental design. R. Holmdahl, M. Herrmann, G. Schett and L.E. Munoz provided scientific input, strategically planned the experiments, and helped with the manuscript. M.H. Hoffmann supervised the project, planned experiments, conducted data analysis, and wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
This project was supported by the Austrian Science Fund (FWF, Project J3102-B13), the Städtler-Stiftung Nürnberg, the Swedish Governmental Agency for Innovation Systems (project number 2016-01010), the Swedish Science Research Council, the Volkswagen-Stiftung (grant #90361 to MaHe) and the EU H2020-MSCE-RISE-2015 projects Redoxit (Nr. 644035, to MaHo, PO and MaHu) and PANG (Nr. 690836, to LEM), the EU project Neurinox (Health-F2-2011-278611), and the Eurostar project rosaGBS-E!10082. This work received further support from the German Research Foundation (DFG) (project number CRC1181-C03 to JH, EK, MaHo and MaHe) and by the doctoral training program GK1660 of the DFG to JK.
Author disclosure statement
MaHu and PO are co-founders and employees of Redoxis/Pronoxis, which has a commercial interest in the development of NOX2-activating compounds. The other authors declare no competing interests.
Acknowledgements
We thank Hülya Gizem Özkan (Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg) for providing a sample of the amino-ferrocene-based prodrug MIS43.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101279.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Olsson L.M., Johansson A.C., Gullstrand B., Jonsen A., Saevarsdottir S., Ronnblom L. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann. Rheum. Dis. 2017 Sep;76(9):1607–1613. doi: 10.1136/annrheumdis-2017-211287. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J., Ma J., Deng Y., Kelly J.A., Kim K., Bang S.Y. A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat. Genet. 2017 Mar;49(3):433–437. doi: 10.1038/ng.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsson L.M., Nerstedt A., Lindqvist A.K., Johansson S.C., Medstrand P., Olofsson P. Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxidants Redox Signal. 2012 Jan 1;16(1):71–78. doi: 10.1089/ars.2011.4013. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D.L., Eisenstein M., Zidovetzki R., Jacob C.O. Systemic lupus erythematosus-associated neutrophil cytosolic factor 2 mutation affects the structure of NADPH oxidase complex. J. Biol. Chem. 2015 May 15;290(20):12595–12602. doi: 10.1074/jbc.M115.639021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob C.O., Eisenstein M., Dinauer M.C., Ming W., Liu Q., John S. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc. Natl. Acad. Sci. U. S. A. 2012 Jan 10;109(2):E59–E67. doi: 10.1073/pnas.1113251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahelin R.V., Burian A., Bruzik K.S., Murray D., Cho W. Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. J. Biol. Chem. 2003 Apr 18;278(16):14469–14479. doi: 10.1074/jbc.M212579200. [DOI] [PubMed] [Google Scholar]
- 7.Magnani A., Brosselin P., Beaute J., de Vergnes N., Mouy R., Debre M. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2014 Sep;134(3):655–662 e658. doi: 10.1016/j.jaci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 8.De Ravin S.S., Naumann N., Cowen E.W., Friend J., Hilligoss D., Marquesen M. Chronic granulomatous disease as a risk factor for autoimmune disease. J. Allergy Clin. Immunol. 2008 Dec;122(6):1097–1103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelkka T., Kienhofer D., Hoffmann M., Linja M., Wing K., Sareila O. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxidants Redox Signal. 2014 Dec 1;21(16):2231–2245. doi: 10.1089/ars.2013.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M.H., Griffiths H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic. Biol. Med. 2018 Sep;125:62–71. doi: 10.1016/j.freeradbiomed.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Campbell A.M., Kashgarian M., Shlomchik M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012 Oct 24;4(157):157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kienhofer D., Hahn J., Stoof J., Csepregi J.Z., Reinwald C., Urbonaviciute V. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI insight. 2017 May 18;2(10) doi: 10.1172/jci.insight.92920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob C.O., Yu N., Yoo D.G., Perez-Zapata L.J., Barbu E.A., Kaplan M.J. Haploinsufficiency of NADPH oxidase subunit neutrophil cytosolic factor 2 is sufficient to accelerate full-blown lupus in NZM 2328 mice. Arthritis & rheumatology. 2017 Aug;69(8):1647–1660. doi: 10.1002/art.40141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manderson A.P., Botto M., Walport M.J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 15.Biermann M.H.C., Boeltz S., Pieterse E., Knopf J., Rech J., Bilyy R. Autoantibodies recognizing secondary NEcrotic cells promote neutrophilic phagocytosis and identify patients with systemic lupus erythematosus. Front. Immunol. 2018;9:989. doi: 10.3389/fimmu.2018.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janko C., Franz S., Munoz L.E., Siebig S., Winkler S., Schett G. CRP/anti-CRP antibodies assembly on the surfaces of cell remnants switches their phagocytic clearance toward inflammation. Front. Immunol. 2011;2:70. doi: 10.3389/fimmu.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz L.E., Janko C., Grossmayer G.E., Frey B., Voll R.E., Kern P. Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum. 2009 Jun;60(6):1733–1742. doi: 10.1002/art.24535. [DOI] [PubMed] [Google Scholar]
- 18.Urbonaviciute V., Furnrohr B.G., Meister S., Munoz L., Heyder P., De Marchis F. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008 Dec 22;205(13):3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz L.E., Lauber K., Schiller M., Manfredi A.A., Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010 May;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 20.Kruse K., Janko C., Urbonaviciute V., Mierke C.T., Winkler T.H., Voll R.E. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis : an international journal on programmed cell death. 2010 Sep;15(9):1098–1113. doi: 10.1007/s10495-010-0478-8. [DOI] [PubMed] [Google Scholar]
- 21.Mayadas T.N., Tsokos G.C., Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009 Nov 17;120(20):2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janko C., Schorn C., Grossmayer G.E., Frey B., Herrmann M., Gaipl U.S. Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE) Autoimmun. Rev. 2008 Oct;8(1):9–12. doi: 10.1016/j.autrev.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Lau C.M., Broughton C., Tabor A.S., Akira S., Flavell R.A., Mamula M.J. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005 Nov 7;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen S.R., Shupe J., Nickerson K., Kashgarian M., Flavell R.A., Shlomchik M.J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006 Sep;25(3):417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Savarese E., Steinberg C., Pawar R.D., Reindl W., Akira S., Anders H.J. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008 Apr;58(4):1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann M.H., Skriner K., Herman S., Baumann C., Steiner C.W., Ospelt C. Nucleic acid-stimulated antigen-presenting cells trigger T cells to induce disease in a rat transfer model of inflammatory arthritis. J. Autoimmun. 2011 May;36(3–4):288–300. doi: 10.1016/j.jaut.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Huang C.K., Zhan L., Hannigan M.O., Ai Y., Leto T.L. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+ J. Leukoc. Biol. 2000 Feb;67(2):210–215. doi: 10.1002/jlb.67.2.210. [DOI] [PubMed] [Google Scholar]
- 28.Sareila O., Jaakkola N., Olofsson P., Kelkka T., Holmdahl R. Identification of a region in p47phox/NCF1 crucial for phagocytic NADPH oxidase (NOX2) activation. J. Leukoc. Biol. 2013 Mar;93(3):427–435. doi: 10.1189/jlb.1211588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 30.Tan E.M., Cohen A.S., Fries J.F., Masi A.T., McShane D.J., Rothfield N.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 31.Munoz L.E., Janko C., Chaurio R.A., Schett G., Gaipl U.S., Herrmann M. IgG opsonized nuclear remnants from dead cells cause systemic inflammation in SLE. Autoimmunity. 2010 Apr;43(3):232–235. doi: 10.3109/08916930903510930. [DOI] [PubMed] [Google Scholar]
- 32.Daum S., Reshetnikov M.S.V., Sisa M., Dumych T., Lootsik M.D., Bilyy R. Lysosome-targeting amplifiers of reactive oxygen species as anticancer prodrugs. Angew. Chem. 2017 Dec 4;56(49):15545–15549. doi: 10.1002/anie.201706585. [DOI] [PubMed] [Google Scholar]
- 33.Maueroder C., Mahajan A., Paulus S., Gosswein S., Hahn J., Kienhofer D. Menage-a-Trois: the ratio of bicarbonate to CO2 and the pH regulate the capacity of neutrophils to form NETs. Front. Immunol. 2016;7:583. doi: 10.3389/fimmu.2016.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzolla A., Hultqvist M., Nilson B., Grimm M.J., Eneljung T., Jonsson I.M. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J. Immunol. 2012 May 15;188(10):5003–5011. doi: 10.4049/jimmunol.1103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagaitkar J., Huang J., Zeng M.Y., Pech N.K., Monlish D.A., Perez-Zapata L.J. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood. 2018 May 24;131(21):2367–2378. doi: 10.1182/blood-2017-09-809004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rybicka J.M., Balce D.R., Khan M.F., Krohn R.M., Yates R.M. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc. Natl. Acad. Sci. U. S. A. 2010 Jun 8;107(23):10496–10501. doi: 10.1073/pnas.0914867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blander J.M. The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunol. Rev. 2016 Jul;272(1):65–79. doi: 10.1111/imr.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson L.M., Chappell J.B., Jones O.T. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 1988 Apr 15;251(2):563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan D., Capasso M., Musset B., Cherny V.V., Rios E., Dyer M.J. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2009 Oct 20;106(42):18022–18027. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal A.W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 41.Levine A.P., Duchen M.R., de Villiers S., Rich P.R., Segal A.W. Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janko C., Munoz L., Chaurio R., Maueroder C., Berens C., Lauber K. Navigation to the graveyard-induction of various pathways of necrosis and their classification by flow cytometry. Methods Mol. Biol. 2013;1004:3–15. doi: 10.1007/978-1-62703-383-1_1. [DOI] [PubMed] [Google Scholar]
- 43.Hultqvist M., Olofsson P., Wallner F.K., Holmdahl R. Pharmacological potential of NOX2 agonists in inflammatory conditions. Antioxidants Redox Signal. 2015 Aug 10;23(5):446–459. doi: 10.1089/ars.2013.5788. [DOI] [PubMed] [Google Scholar]
- 44.Kral J.B., Schrottmaier W.C., Salzmann M., Assinger A. Platelet interaction with innate immune cells. Transfus. Med. Hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2016 Mar;43(2):78–88. doi: 10.1159/000444807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam F.W., Vijayan K.V., Rumbaut R.E. Platelets and their interactions with other immune cells. Comprehensive Physiology. 2015 Jul 1;5(3):1265–1280. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018 Mar;371(3):567–576. doi: 10.1007/s00441-017-2727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang H., Han S., Li Y., Kienhofer D., Lee P., Shumyak S. A novel mechanism for generating the interferon signature in lupus: opsonization of dead cells by complement and IgM. Arthritis & rheumatology. 2016 Dec;68(12):2917–2928. doi: 10.1002/art.39781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossmayer G.E., Munoz L.E., Weber C.K., Franz S., Voll R.E., Kern P.M. IgG autoantibodies bound to surfaces of necrotic cells and complement C4 comprise the phagocytosis promoting activity for necrotic cells of systemic lupus erythaematosus sera. Ann. Rheum. Dis. 2008 Nov;67(11):1626–1632. doi: 10.1136/ard.2007.081828. [DOI] [PubMed] [Google Scholar]
- 49.Bave U., Magnusson M., Eloranta M.L., Perers A., Alm G.V., Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003 Sep 15;171(6):3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 50.Barrat F.J., Meeker T., Gregorio J., Chan J.H., Uematsu S., Akira S. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005 Oct 17;202(8):1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schett G., Rubin R.L., Steiner G., Hiesberger H., Muller S., Smolen J. The lupus erythematosus cell phenomenon: comparative analysis of antichromatin antibody specificity in lupus erythematosus cell-positive and -negative sera. Arthritis Rheum. 2000 Feb;43(2):420–428. doi: 10.1002/1529-0131(200002)43:2<420::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Reeves W.H., Lee P.Y., Weinstein J.S., Satoh M., Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009 Sep;30(9):455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee P.Y., Li Y., Kumagai Y., Xu Y., Weinstein J.S., Kellner E.S. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 2009 Nov;175(5):2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poon I.K., Lucas C.D., Rossi A.G., Ravichandran K.S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014 Mar;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011 Oct 10;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sprangers S., de Vries T.J., Everts V. Monocyte heterogeneity: consequences for monocyte-derived immune cells. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/1475435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003 Jul;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 58.Lee P.Y., Weinstein J.S., Nacionales D.C., Scumpia P.O., Li Y., Butfiloski E. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 2008 Apr 1;180(7):5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sunderkotter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004 Apr 1;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Zhang L., Yu C., Yang X.F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker research. 2014 Jan 7;2(1):1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng Y., Latchman Y., Elkon K.B. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J. Immunol. 2009 Mar 1;182(5):2777–2785. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larson S.R., Atif S.M., Gibbings S.L., Thomas S.M., Prabagar M.G., Danhorn T. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016 Jun;23(6):997–1003. doi: 10.1038/cdd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016 Mar 15;44(3):463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Nauseef W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004 Oct;122(4):277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 65.Pollock J.D., Williams D.A., Gifford M.A., Li L.L., Du X., Fisherman J. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995 Feb;9(2):202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 66.Hahn J., Schauer C., Czegley C., Kling L., Petru L., Schmid B. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J.: Off. Pub. Feder. Am. Soc. Exp. Biol. 2019 Jan;33(1):1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrmann M., Voll R.E., Zoller O.M., Hagenhofer M., Ponner B.B., Kalden J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998 Jul;41(7):1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 68.Lee P.Y., Kumagai Y., Li Y., Takeuchi O., Yoshida H., Weinstein J. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 2008 Dec 22;205(13):2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez-Boyanapalli R., McPhillips K.A., Frasch S.C., Janssen W.J., Dinauer M.C., Riches D.W. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J. Immunol. 2010 Oct 1;185(7):4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biggar W.D. Phagocytosis in patients and carriers of chronic granulomatous disease. Lancet. 1975 May 3;1(7914):991–995. doi: 10.1016/s0140-6736(75)91943-1. [DOI] [PubMed] [Google Scholar]
- 71.Biburger M., Aschermann S., Schwab I., Lux A., Albert H., Danzer H. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011 Dec 23;35(6):932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Reshetnikov V., Hahn J., Maueroder C., Czegley C., Munoz L.E., Herrmann M. Chemical tools for targeted amplification of reactive oxygen species in neutrophils. Front. Immunol. 2018;9:1827. doi: 10.3389/fimmu.2018.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagen H., Marzenell P., Jentzsch E., Wenz F., Veldwijk M.R., Mokhir A. Aminoferrocene-based prodrugs activated by reactive oxygen species. J. Med. Chem. 2012 Jan 26;55(2):924–934. doi: 10.1021/jm2014937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.