Abstract
With the development of reliable endoscopic closure techniques and tools, endoscopic full-thickness resection (EFTR) is emerging as a therapeutic option for the treatment of subepithelial tumors and epithelial neoplasia with significant fibrosis. EFTR may be categorized as “exposed” and “nonexposed.” In exposed EFTR, the full-thickness resection is undertaken with a tunneled or nontunneled technique, with subsequent closure of the defect. In nonexposed EFTR, a secure serosa-to-serosa apposition is achieved before full-thickness resection of the isolated lesion. This document reviews current techniques and devices used for EFTR and reviews clinical applications and outcomes.
Abbreviations: EFTR, endoscopic full-thickness resection; ESD, endoscopic submucosal dissection; GIST, GI stromal tumor; NOTES, natural orifice transluminal endoscopic surgery; OTSC, over-the-scope clip; POEM, per-oral endoscopic myotomy; PTFE, polytetrafluoroethylene; SET, subepithelial tumor; STER, submucosal tunnel endoscopic resection; TTS, through-the-scope
The American Society for Gastrointestinal Endoscopy (ASGE) Technology Committee provides reviews of existing, new, or emerging endoscopic technologies that have an impact on the practice of GI endoscopy. Evidence-based methodology is used, with a MEDLINE literature search to identify pertinent clinical studies on the topic and a MAUDE (U.S. Food and Drug Administration Center for Devices and Radiological Health) database search to identify the reported adverse events of a given technology. Both are supplemented by accessing the “related articles” feature of PubMed and by scrutinizing pertinent references cited by the identified studies. Controlled clinical trials are emphasized, but in many cases data from randomized, controlled trials are lacking. In such cases, large case series, preliminary clinical studies, and expert opinions are used. Technical data are gathered from traditional and Web-based publications, proprietary publications, and informal communications with pertinent vendors.
Technology Status Evaluation Reports are drafted by 1 or 2 members of the ASGE Technology Committee, reviewed and edited by the committee as a whole, and approved by the Governing Board of the ASGE. When financial guidance is indicated, the most recent coding data and list prices at the time of publication are provided. For this review, the MEDLINE database was searched through May 2017 for articles related to endoscopic full-thickness resection and submucosal tunnel endoscopic resection, using relevant terms including endoscopic full-thickness resection, EFTR, submucosal tunnel endoscopic resection, STER, and submucosal endoscopy, among others. Technology Status Evaluation Reports are scientific reviews provided solely for educational and informational purposes. Technology Status Evaluation Reports are not rules and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment or payment for such treatment.
Introduction
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are established techniques that facilitate the resection of neoplasia involving the mucosal and superficial submucosal layers while leaving the muscularis propria intact to maintain the integrity of the bowel wall. However, some neoplastic lesions, including those involving the muscularis propria, cannot be adequately and/or safely treated with these techniques. With the development of reliable endoscopic closure techniques and tools, endoscopic full-thickness resection (EFTR) is emerging as a therapeutic option for the treatment of these challenging lesions, which include subepithelial tumors (SETs) and epithelial neoplasia extending deeper than the mucosa or associated with significant fibrosis. EFTR may offer a less invasive treatment alternative relative to surgical approaches in selected patients.1 This document reviews current techniques and devices used for EFTR.
Emerging technology under review
EFTR techniques
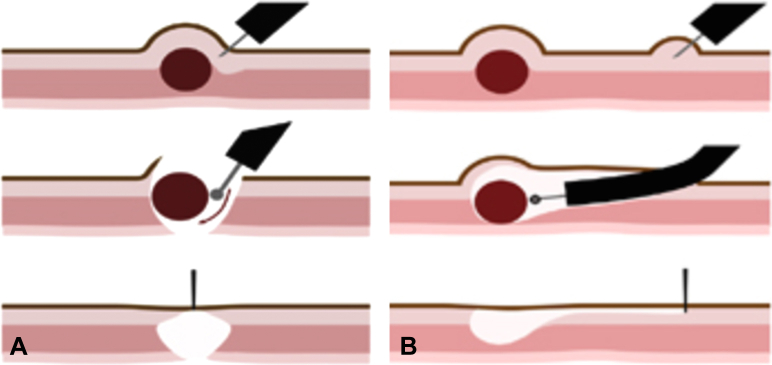
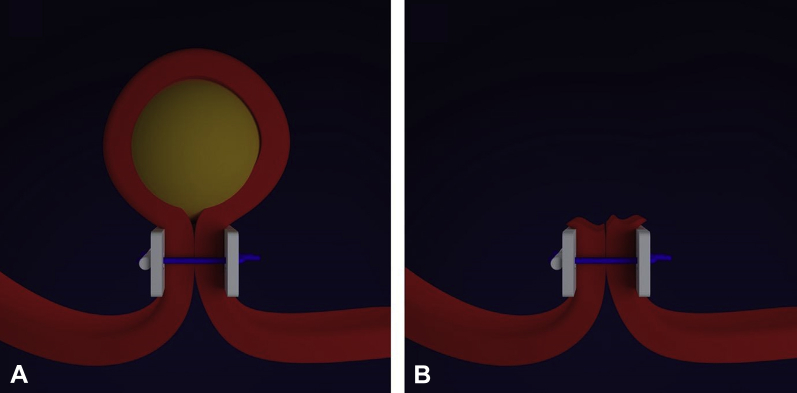
Full-thickness resection in the digestive tract requires robust closure of the resulting defect to avoid leakage of bowel contents and peritonitis. Two general approaches to EFTR have been described: “exposed” and “nonexposed” EFTR.2 In exposed EFTR, the full-thickness resection is undertaken first, with subsequent closure of the defect. The temporary exposure of the peritoneal cavity to the intestinal lumen with this approach is the basis for the term “exposed.” Exposed EFTR can be further classified into tunneled and nontunneled techniques (Figs. 1A and B). In nonexposed EFTR, the bowel wall segment containing the lesion is invaginated toward the lumen to allow a secure serosa-to-serosa apposition before full-thickness resection of the now isolated lesion (Fig. 2). In effect, the closure is achieved before the resection with this approach, and thus the term “nonexposed.”
Figure 1.
A, Nontunneled exposed endoscopic full-thickness resection. Dissection around a subepithelial lesion with disruption of the muscularis propria, followed by defect closure. B, Submucosal tunnel endoscopic resection. Submucosal tunneling is performed to access a submucosal lesion, which is resected and removed through the tunnel, followed by closure of the tunnel entry site.
Figure 2.
Nonexposed endoscopic full-thickness resection. Full-thickness duplication of the intestinal wall (A) is performed before resection of the lesion (B).
Exposed EFTR
The technical basis for exposed EFTR originates with the techniques of ESD; submucosal tunnel endoscopy, as is used in per-oral endoscopic myotomy (POEM); and natural orifice transluminal endoscopic surgery (NOTES). These techniques have been reviewed in detail in prior ASGE Technology reviews.3, 4, 5 In many instances a full-thickness resection may not be initially planned but is eventually undertaken to ensure an en bloc R0 resection.
Nontunneled exposed EFTR
Nontunneled exposed EFTR may be used for either mucosal neoplasia or SETs, typically when lesion involvement of the muscularis propria is extensive. This technique uses a similar approach to ESD, which involves fluid expansion of the submucosal layer and dissection in the submucosal plane to achieve en bloc resection. During nontunneled exposed EFTR, the dissection is then continued through the muscularis propria circumferentially around the lesion. With nontunneled exposed EFTR, the closure must also be full thickness. A variety of techniques for full-thickness closure have been described. Video 1 (available online at www.VideoGIE.org) demonstrates the technique of non-tunneled exposed EFTR.6
A loop-and-clip closure technique involves advancement of a detachable nylon loop through a double-channel endoscope. The loop is opened around the full-thickness defect, and endoscopic clips (advanced through the other channel) are applied over the loop at several locations. The loop is then closed to approximate the edges of the defect.7 A loop-and-clip closure is demonstrated in Video 2 (available online at www.VideoGIE.org). Smaller defects (≤2 cm) may be closed with a cap-mounted clip (over-the-scope clip, OTSC; Ovesco Endoscopy, Tubingen, Germany; Padlock, Aponos Medical Corporation, Kingston, NH, USA). An endoscopic suturing device (Overstitch; Apollo Endosurgery Inc, Austin, Tex, USA), T-tags, and other related devices have also been used for defect closure after EFTR.8, 9, 10
Tunneled exposed EFTR
In submucosal tunnel endoscopy, a mucosal incision is made some distance from the therapeutic target, and a submucosal tunnel toward that site is created by dissecting the submucosal layer. With submucosal tunnel endoscopic resection (STER), the tunnel is approximately 5 cm long and is used to gain access to a subepithelial neoplasm, which is then enucleated and removed via the tunnel.11 Tunneled exposed EFTR refers to STERs that include dissection through the muscularis propria circumferentially around the lesion; however, this distinction is not typically made in the existing literature. As such, this document will refer to tunneled exposed EFTR as STER. STER is typically used for SETs but not for mucosal neoplasia. Inasmuch as the tunnel entry site is distant from the muscular defect, closure of only the mucosal defect is necessary to maintain luminal integrity. A full-thickness closure is not necessary, and the muscular defect is not repaired. Endoscopic clips or endoscopic suturing are typically used to close the mucosal defect after submucosal tunnel endoscopy.8, 12, 13 Given the relatively small size of the tunnel, STER is most feasible for lesions ≤4 cm in diameter. Lesions in the distal esophagus and gastric cardia are most readily accessible via a tunneled approach. A STER procedure is shown in Video 3 (available online at www.VideoGIE.org).
Nonexposed EFTR
Nonexposed EFTR techniques are conceptually related to surgical wedge resections of gastric tumors. During surgical wedge resections, the gastric wall is retracted from the serosal side, resulting in the apposition of 2 intraluminal mucosal walls. A staple line is then fired to achieve closure and isolate the lesion-containing wedge above the staple line, facilitating subsequent resection.
During endoscopic nonexposed EFTR, the bowel segment containing the target lesion is retracted into the lumen, allowing approximation and subsequent secure fixation of 2 serosal surfaces by the use of various devices. This creates an intestinal wall duplication that isolates the target lesion, allowing full-thickness resection “above” the serosal closure. Resection of the isolated tumor above the fixated serosal tissue can be performed with a snare or other ligation device. Video 4 (available online at www.VideoGIE.org) demonstrates a nonexposed EFTR procedure.14 Devices that have been used to perform nonexposed EFTR include endoscopic suturing platforms and over-the-scope devices.15 Use of a cap-mounted clip may aid in hemostasis due to mechanical tamponade. Combined endoscopic and laparoscopic surgical techniques have also been reported.16, 17, 18, 19
Devices for EFTR
Mucosal incision and submucosal dissection
Devices used for mucosal incision and submucosal dissection during EFTR include needle-knife catheters and an array of ESD knives, which have been previously reviewed.5 Knives that perform dual functions of injection and cutting are often used for the performance of submucosal tunneling.
Devices for closure and/or nonexposed EFTR
Through-the-scope clips
Standard through-the-scope (TTS) endoscopic clips marketed for hemostasis have been used both for closure of mucosal defects associated with tunnel endoscopy and for closure of smaller full-thickness defects after nontunneled exposed EFTR. As previously mentioned, clips may be combined with a detachable nylon loop or an omental patch for closure of larger defects.20, 21
Cap-mounted clips
Cap-mounted clips such as OTSC and Padlock have been used for closure of nontunneled exposed EFTR defects,22 mucosal defects in STER, and also in nonexposed EFTR.15 For nonexposed EFTR, suction or retraction of the lesion into the cap and subsequent clip firing creates wall duplication and isolation of the lesion, permitting subsequent snare resection above the clip. Available cap and clip sizes restrict the use of this technique to smaller lesions that can be completely retracted into the cap. Series describing the resection of SETs and of recurrent or incompletely resected mucosal neoplasia have been reported.23, 24
Combined full-thickness resection and closure device
The full-thickness resection device (FTRD; Ovesco Endoscopy) has been cleared by the U.S. Food and Drug Administration (FDA) and is designed as an integrated closure and resection device for use in the lower intestinal tract (Fig. 3).25 The FTRD consists of a transparent applicator cap carrying a modified 14-mm OTSC that can be mounted over a standard colonoscope, similar to the OTSC system. Compared with the conventional OTSC system, the cap has greater depth (23 mm vs 6 mm) to accommodate more tissue. The outer diameter of the device is 20 mm, which hinders use in the upper-GI tract. Additionally, a 13-mm monofilament snare is preloaded in the tip of the cap. The snare catheter runs along the outer surface of the colonoscope, constrained by a plastic sheath. The target lesion is pulled into the cap with a grasping or anchoring device before clip deployment. Immediately after clip deployment, the tissue above the clip is resected with the snare (Video 4).14
Figure 3.

Full-thickness resection device (Ovesco Endoscopy Tubingen, Germany).
Endoscopic plicating and suturing devices
The Plicator (NDO Surgical, Inc, Mansfield, Mass, USA), which was originally designed for antireflux therapy, creates apposition between 2 serosal surfaces by the use of polytetrafluoroethylene (PTFE)-pledgeted sutures.26 The Plicator is no longer produced or sold; however, a similar device, the GERDX (G-Surg, Seeon, Germany), is currently Conformite Europeenne (CE) marked for use as an antireflux device in Europe, and its use for endoscopic resection of gastric SETs has also been described.15 Although the suture is the same as that for the Plicator, the handle has a hydraulic closure mechanism (Fig. 4). The device’s large size and limited manipulation restrict its use to gastric lesions.
Figure 4.

GERDX Device (G-Surg, Seeon, Germany).
The Overstitch device has been used to close full-thickness defects after nontunneled exposed EFTR and mucosotomy defects after STER.2, 8, 27 The lack of availability in many markets outside the United States and the limited scope compatibility of this device restrict its widespread use.2
Endoscopic stapling devices
Two endoscopic stapling systems have been developed and evaluated for nonexposed EFTR.28, 29 However, neither of these devices is currently marketed or available in the United States.
The SurgASSIST System (Power Medical Interventions Deutschland GmbH, Hamburg, Germany) comprises a linear stapling device with a 20-cm flexible shaft that may be advanced coaxially with an endoscope. SurgASSIST has been used for resection of a gastric SET and a gastric T1 carcinoma in 2 human cases.29 The large size and limited maneuverability of this device may pose an increased risk of perforation with passage.
An over-the-scope, 1-step endoluminal full-thickness resection device that combined tissue graspers, a semicircular stapler for serosal closure, and a scalpel for resection was introduced in 2001.28, 30 This device effectively served as a predecessor to the FTRD; however, its large size and its use confined to the left colon segment limited adoption, and it is no longer manufactured.
Applications and outcomes of EFTR
Nontunneled exposed EFTR
In a retrospective case series of exposed EFTR for 23 GI stromal tumors (GISTs) under 2 cm, the authors used OTSC clips for defect closure, facilitated by twin-grasper forceps to retract both sides of the defect into the cap.22 All lesions were successfully resected and closure was achieved, although self-limited, localized peritonitis developed in 2 (9%) patients. A single-center retrospective series of 62 patients with gastric SETs in difficult locations (fundus or lesser curve) compared resection without lesion retraction to the use of a loop or clip plus thread to assist with retraction during exposed EFTR.31 Retraction methods were associated with a reduction in procedure time (40–45 minutes vs 85 minutes, P < .05) and in the need for abdominal decompression (18%–23% vs 63%, P < .05).
A large retrospective study from a Chinese tertiary referral center described endoscopic resection of 733 upper intestinal SETs arising from the muscularis propria (461 leiomyoma, 250 GIST, 22 other) in 726 patients with the use of traditional ESD techniques (n = 536) or STER (n = 197).32 Although all lesions involved the muscularis propria, the therapeutic intent was complete resection without full-thickness resection when possible. The mean tumor size was 1.7 cm (range, 1–4 cm). Complete resection was achieved in 97.1% of cases, with a mean procedure time of 49.2 ± 14.3 minutes. In this series there were 88 immediate perforations (12.1%), effectively indicating full-thickness resection. The majority (88.3%) of adverse events were managed endoscopically, with 11 patients requiring laparoscopic surgery. Five patients in this series developed localized peritonitis.
STER
A systematic review and meta-analysis summarized 28 studies of STER for upper-GI SETs, mostly leiomyomas and GISTs.33 Twenty retrospective and 8 prospective series comprising 1041 patients and 1085 lesions were included, and all but 1 of the studies were conducted in China. The pooled estimate for complete resection was 97.5% (95% CI, 96.0%–98.5%), and the pooled en bloc resection rate was 94.6% (95% CI, 91.5%–96.7%). The pooled prevalence of perforation was 5.6% (95% CI, 3.7%–8.2%), and subcutaneous emphysema and/or pneumomediastinum was reported in 14.8% (95% CI, 10.5%–20.5%), suggesting that the majority of these STERs did not require full-thickness resection.
A retrospective series from a Chinese tertiary referral center described long-term outcomes with STER for 180 upper intestinal SETs (69% esophageal, 31% gastric; leiomyoma 81%, GIST 16%) arising from the muscularis propria.34 The median tumor size was 2.6 cm (range, 2.0–5.0 cm), and the median resection time was 45 minutes (range, 15–200 minutes). En bloc resection was achieved in 163 of 180 lesions (90.6%). Larger tumor size and irregular shape were associated with piecemeal resection and longer procedure duration. One patient was referred for additional surgery, and 2 were lost to follow-up. Of the remaining 177 patients, all were free of local recurrence or distant metastasis at a median follow-up time of 36 months.
Nonexposed EFTR
The performance of the Plicator device for nonexposed EFTR was evaluated in a single-center retrospective case series. Successful resection of 31 gastric SETs with a mean size of 20.5 mm (range, 8–48 mm) was described, with 3 perforations that were closed with additional sutures.35 The GERDX device has been used in a few patients for the resection of gastric SETs, and additional study is ongoing.15
A prospective multicenter study evaluated the use of the FTRD in 181 colonic lesions including difficult adenomas, early adenocarcinomas, and SETs.36 Resection with the FTRD was technically successful in 89.5% of cases, with an R0 resection rate of 76.9%. The R0 resection rate was higher with lesions ≤2 cm than in those >2 cm (81.2% vs 58.1%, P = .0038). In the 23 patients with an SET, the R0 resection rate was 87%. Adverse events occurred in 18 patients (9.9%) and included perforation (n = 6, 3.3%), delayed bleeding (n = 4, 2.2%), appendicitis, postpolypectomy syndrome, abdominal pain, and small bowel fistula. Emergency surgery was required in 4 patients (2.2%). Earlier case series reported similar outcomes with the FTRD for colorectal lesions.37, 38
The FTRD achieved full-thickness colonic resections with a mean diameter of 21 mm in 4 patients with suspected neuromuscular intestinal disorders without adverse events.39 Histologic analysis was diagnostic in 3 patients, identifying aganglionosis, hypoganglionosis, and eosinophilic leiomyositis. Use of the FTRD to resect duodenal SETs (≤2 cm) and nonlifting duodenal adenomas has been reported in a small case series.14 Dilation of the esophagus was required to permit passage of the device, which is CE marked and FDA cleared for use in the colon.
. A case series of 16 patients described the use of an OTSC to facilitate subsequent resection of mucosal neoplasia (n = 9) or SETs (n = 7) in both the upper- and the lower-GI tract, with a mean lesion size of 22.7 mm (range, 10–25 mm).40 Complete resection was achieved in all patients, and full-thickness resection was observed in 69%, with a deep muscle margin in the other cases. No adverse events were reported.
Comparative studies
A few studies have directly compared different resection and closure techniques in the management of SETs. A retrospective single-center Chinese study of 52 gastric GISTs treated with exposed, nontunneled EFTR (n = 32) or STER (n = 20) identified no difference in en bloc resection rates (96.9% vs 95%) or total procedure time (69 ± 27 minutes vs 75 ± 32 minutes).41 Exposed EFTR required more time to achieve closure and used a greater number of clips. No recurrences were noted in either group during mean follow-up periods of 11 months (STER) and 24 months (exposed EFTR). A retrospective study from a different Chinese tertiary hospital evaluated 68 gastric GISTs (<2 cm) managed with either exposed EFTR (n = 35) or laparoscopic wedge resection (n = 33).42 Procedure time was shorter in the EFTR group (91 ± 63 minutes) than in the laparoscopy group (155 ± 37 minutes, P < .05), as was hospital stay (6.74 ± 0.85 days vs 7.79 ± 1.29 days, P < .05).
The submucosal tunnel created during STER is akin to a POEM tunnel; a retrospective comparison of POEM tunnel closure techniques found no significant difference in efficacy, cost, or procedure duration between clips (n = 62) and endoscopic suturing (n = 61).9 A randomized controlled trial of closure techniques for 18-mm gastrotomies in 20 live swine found OTSC closure to have a lower procedure time relative to TTS clip closure.43 One of the animals in the TTS clip group was noted to have major leakage during leak testing and was killed early because of sepsis. In a retrospective series of 21 patients with iatrogenic colonic perforations, 5 defects were closed with TTS clips and 16 defects were closed with the Overstitch suturing device.8 Four of the 5 patients with clip closures later required laparoscopy because of clinical deterioration, compared with 2 of 16 patients who underwent sutured closures.
Challenges and limitations
. Challenges complicating exposed EFTR persist.44 Leakage of CO2 may limit luminal insufflation and visualization, and leakage of GI contents may promote inflammation or infection. The inability to visualize the external (seromuscular) surface of the intestinal wall limits the ability to avoid and control serosal bleeding.44 The lack of lymph node resection makes EFTR therapeutically inadequate for tumors with lymph node involvement. Finally, reliable secure closure may be difficult to attain with large nonlinear defects.
Ongoing challenges with STER include the limited size of subepithelial lesions (typically <4 cm) that can be removed through the tunnel. Performing dissection and resection within the limited confines of the tunnel while maintaining the lesion’s capsular integrity may also be technically difficult. In addition, specific anatomic locations such as the distal esophagus, gastric cardia, lesser curve of the gastric body, and greater curve of the gastric antrum are more amenable to a tunneled approach than are other sites.45
Limitations of nonexposed EFTR include a paucity of available devices, limitations on the size and anatomic location of the lesions that can be resected, limited maneuverability of current devices, and risk of unintended capture of adjacent strictures. The FTRD is the first FDA-cleared device for nonexposed EFTR in the colon.
Lesions >4 cm remain challenging with any endoscopic approach. A combined endoscopic and laparoscopic approach has been suggested to overcome some of these challenges16 and to facilitate lymph node sampling.17
Ease of use
Training in ESD, POEM, and now EFTR remains a process in evolution. The Asian paradigm of developing ESD skills via mentored resection of early gastric cancer, with possible progression to POEM and EFTR, is not a viable training model in the United States and Europe, given the limited prevalence of early gastric cancer and of ESD expertise. As such, alternative training strategies that include animal models and observerships at expert centers have been described.27 However, upper intestinal and rectal SETs are relatively commonly encountered by Western endoscopists, and this may potentially facilitate the adoption and dispersion of EFTR as technologies evolve and safe and effective techniques become standardized. It is anticipated that the learning curve for nonexposed EFTR will be much shorter than for exposed EFTR or STER, which require ESD and submucosal endoscopic skills and experience. Procedures are typically performed with the patients under general anesthesia variably in the endoscopy suite or operating room. The use of CO2 is imperative. Currently there are no guidelines on postprocedural care. In cases that result in full-thickness defects, many endoscopists favor overnight observation, intravenous antibiotics, and gradual resumption of diet. The current absence of Current Procedural Terminology codes for EFTR limit reimbursement and may impair institutional support for incorporation of these procedures.
Areas for future research
Additional data regarding outcomes of efficacy, infection, bleeding, reliability of closure, complete resection, and tumor seeding are needed for variable lesion types, sizes, locations, and stages to optimize patient and technique selection. The degree to which endoscopic resection can be used in place of oncologic surgical resection remains to be determined.46 Data from centers throughout Asia and Western countries are needed to reflect a variety of patient populations and endoscopists’ experience. Continued refinement of dedicated tools for nonexposed and exposed EFTR and closure may make EFTR more accessible to more of endoscopists.
Summary
Exposed and nonexposed EFTR and STER are emerging as less invasive alternatives to surgical resection of SETs that involve the muscularis propria or mucosal neoplasia with associated fibrosis. The refinement of techniques and devices for resection and defect closure may expand the use of these procedures, although challenges with training and reimbursement may limit their adoption to academic and tertiary centers in the near future.
Disclosure
H. Aslanian: consultant for Olympus, Boston Scientific; speaker for GI Supply; M. Bhutani: advisory board for Medi-Globe (to Feb 2016); A. Goodman: consultant for Invendo Medical; D. Lichtenstein: consultant for Olympus; J. Melson: independent investigator, grant support from Boston Scientific; medical advisory board for Clinical Genomics; U. Navaneethan: consultant for Takeda, AbbVie, and Janssen; R. Pannala: consultant for Boston Scientific; research funding/support from Fujifilm and Apollo Endosurgery; M. Parsi: consultant for Boston Scientific; S. Sullivan: contracted research for Aspire Bariatrics, Allurion, Obalon, Elira, BARANova, USGI Medical, and GI Dynamics; consultant for Aspire Bariatrics, Obalon, Elira, USGI Medical, and GI Dynamics; stock warrants with Elira; N. Thosani: consultant for Boston Scientific, Medtronic, and Mederi; speaker for Boston Scientific and AbbVie; G. Trikudanathan: advisory Board for AbbVie; R. Watson: consultant for Apollo Endosurgery. All other authors disclosed no financial relationships relevant to this publication.
Footnotes
This document was reviewed and approved by the Governing Board of the American Society for Gastrointestinal Endoscopy (ASGE).
Correspondence to: John T. Maple, DO, FASGE, ASGE Technology Committee Chair, Email: John-Maple@ouhsc.edu.
Supplementary data
Nontunneled exposed endoscopic full-thickness resection of a gastric GI stromal tumor. (Courtesy of Dr S. Stavropoulos. https://endoscopedia.com/2014/08/12/endoscopic-full-thickness-resection-for-gi-stromal-tumors/.
Loop and clip closure after rectal endoscopic submucosal dissection (courtesy of Uzma D. Siddiqui, MD, FASGE, Center for Endoscopic Research and Therapeutics, University of Chicago.)
Submucosal endoscopic tunnel resection of a subepithelial tumor in the gastric cardia. (Courtesy of Author Amrita Sethi.)
Nonexposed endoscopic full-thickness resection of a duodenal neuroendocrine tumor by use of a full-thickness resection device. (From Schmidt A, Meier B, Cahyadi O, et al. Duodenal endoscopic full-thickness resection (with video). Gastrointest Endosc 2015;82:728-33.)
References
- 1.Kashab M.A., Pasricha P.J. Conquering the third space: challenges and opportunities for diagnostic and therapeutic endoscopy. Gastrointest Endosc. 2013;77:146–148. doi: 10.1016/j.gie.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Modayil R., Stavropoulos S. A Western prospective on new NOTES: from POEM to full thickness resection and beyond. Gastrointest Endosc Clin N Am 26. 2016;26:413–432. doi: 10.1016/j.giec.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 3.ASGE Technology Committee. Pannala R., Abu Dayyeh B.K., Aslanian H.R. Per-oral endoscopic myotomy (with video) Gastrointest Endosc. 2016;83:1051–1060. doi: 10.1016/j.gie.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 4.ASGE Technology Committee. Kantsevoy S.V., Adler D.G., Chand B. Natural orifice translumenal endoscopic surgery. Gastrointest Endosc. 2008;68:617–620. doi: 10.1016/j.gie.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 5.ASGE Technology Committee. Maple J.T., Abu Dayyeh B.K., Chauhan S.S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311–1325. doi: 10.1016/j.gie.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Stavropoulos S.N., Modayil R., Friedel D. Endoscopic full-thickness resection for GI stromal tumors. Gastrointest Endosc. 2014;80:334–335. doi: 10.1016/j.gie.2014.05.300. [DOI] [PubMed] [Google Scholar]
- 7.Ye L.P., Yu Z., Mao X.L. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978–1983. doi: 10.1007/s00464-014-3421-1. [DOI] [PubMed] [Google Scholar]
- 8.Kantsevoy S.V., Bitner M., Hajiyeva G. Endoscopic management of colonic perforations: clips versus suturing closure (with videos) Gastrointest Endosc. 2016;84:487–492. doi: 10.1016/j.gie.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 9.Stavropoulos S.N., Modayil R., Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc. 2015;7:777–789. doi: 10.4253/wjge.v7.i8.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J., Sun B., Sun S. Endoscopic puncture suture device to close gastric wall defects after full thickness resection: a porcine study. Gastrointest Endosc. 2016;85:447–450. doi: 10.1016/j.gie.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H., Ikeda H., Hosoya T. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–230. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X., Chen J.Y. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524–530. doi: 10.1007/s00464-013-3197-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Y.S., Chen W.F., Hu J.W. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos) Gastrointest Endosc. 2012;75:195–199. doi: 10.1016/j.gie.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt A., Meier B., Cahyadi O. Duodenal endoscopic full-thickness resection (with video) Gastrointest Endosc. 2015;82:728–733. doi: 10.1016/j.gie.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Bauder M., Schmidt A., Caca K. Non-exposure device assisted endoscopic full-thickness resection. Gastrointest Endosc Clin N Am. 2016;26:297–312. doi: 10.1016/j.giec.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Kim C.G., Yoon H.M., Lee J.Y. Nonexposure endolaparoscopic full-thickness resection with simple suturing technique. Endoscopy. 2015;47:1171–1174. doi: 10.1055/s-0034-1392271. [DOI] [PubMed] [Google Scholar]
- 17.Abe N., Mori T., Takeuchi H. Successful treatment of early stage gastric cancer by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Gastrointest Endosc. 2008;68:1220–1224. doi: 10.1016/j.gie.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 18.Goto O., Mitsui T., Fujishiro M. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183–187. doi: 10.1007/s10120-011-0014-8. [DOI] [PubMed] [Google Scholar]
- 19.Nunobe S., Hiki N., Gotoda T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338–342. doi: 10.1007/s10120-012-0146-5. [DOI] [PubMed] [Google Scholar]
- 20.Monkemuller K., Sarker S., Kabir Baig K.R. Endoscopic creation of an omental patch with an over-the-scope clip system after endoscopic excavation and resection of a large gastrointestinal stromal tumor of the stomach. Endoscopy. 2014;46:E451–E452. doi: 10.1055/s-0034-1377495. [DOI] [PubMed] [Google Scholar]
- 21.Hashiba K., Carvalho A., Diniz G. Experimental endoscopic repair of gastric perforations with an omental patch and clips. Gastrointest Endosc. 2001;54:500–504. doi: 10.1067/mge.2001.118444. [DOI] [PubMed] [Google Scholar]
- 22.Guo J., Liu Z., Sun S. Endoscopic full- thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356–3362. doi: 10.1007/s00464-015-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarker S., Gutierrez J.P., Concil L. Over-the scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014;46:758–761. doi: 10.1055/s-0034-1365513. [DOI] [PubMed] [Google Scholar]
- 24.Al-Bawardy B., Rajan E., Wong Kee Song L.M. Over the scope clip assisted full thickness resection of epithelial and subepithelial lesions. Gastrointest Endosc. 2017;85:1087–1092. doi: 10.1016/j.gie.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Fahndrich M., Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76–79. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 26.Rothstein R., Filipi C., Caca K. Endoscopic full-thickness plication for the treatment of GERD: a randomized, sham-controlled trial. Gastroenterology. 2006;131:704–712. doi: 10.1053/j.gastro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 27.ASGE Technology Committee. Banerjee S., Barth B., Bhat S. Endoscopic closure devices. Gastrointest Endosc. 2012;76:244–251. doi: 10.1016/j.gie.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Full thickness resection device (FTRD) for endoluminal removal of large bowel tumours: development of the instrument and related experimental studies. Minim Invasive Ther Allied Technol. 2001;10:301–309. doi: 10.1080/136457001753337357. [DOI] [PubMed] [Google Scholar]
- 29.Kaehler G., Grobholz R., Langner C. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy. 2006;38:86–89. doi: 10.1055/s-2005-921181. [DOI] [PubMed] [Google Scholar]
- 30.Rajan E., Gostout C.J., Burgart L.J. First endoluminal system for transmural resection of colorectal tissue with a prototype full-thickness resection device in a porcine model. Gastrointest Endosc. 2002;55:915–920. doi: 10.1067/mge.2002.124099. [DOI] [PubMed] [Google Scholar]
- 31.Lu J., Jiao T., Li Y. Facilitating retroflexed EFTR through loop mediated or retroflexed countertraction. Gastrointest Endosc. 2016;83:223–228. doi: 10.1016/j.gie.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 32.Ye Li-Ping, Zhang Yu, Yuo Li-Ping. Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am J Gastroenterol. 2016;111:788–796. doi: 10.1038/ajg.2015.426. [DOI] [PubMed] [Google Scholar]
- 33.Lv H.X., Wang C.H., Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017;31:49. doi: 10.1007/s00464-016-4978-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen T., Zhou P., Chu Y. Long term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. 2017;265:363–369. doi: 10.1097/SLA.0000000000001650. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt A., Bauder M., Riecken B. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015;47:154–158. doi: 10.1055/s-0034-1390786. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt A., Benya T., Schumacher B. Colonoscopic full thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67:1280–1289. doi: 10.1136/gutjnl-2016-313677. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt A., Bauerfeind P., Gubler C. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719–725. doi: 10.1055/s-0034-1391781. [DOI] [PubMed] [Google Scholar]
- 38.Andrisani G., Pizzicannella M., Martino M. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: a single-centre study. Dig Liver Dis. 2017;49:1009–1013. doi: 10.1016/j.dld.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Valli P.V., Pohl D., Fried M. Diagnostic use of endoscopic full-thickness wall resection (eFTR): a novel minimally invasive technique for colonic tissue sampling in patients with severe gastrointestinal motility disorders. Neurogastroenterol Motil. 2018;30(1) doi: 10.1111/nmo.13153. [DOI] [PubMed] [Google Scholar]
- 40.Fahndrich M., Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76–79. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 41.Tan Y., Tang X., Guo T. Comparison between submucosal tunneling endoscopic resection and endoscopic full thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376–3382. doi: 10.1007/s00464-016-5350-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Feng X., Ye S. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg Endosc. 2016;30:3357–3361. doi: 10.1007/s00464-015-4612-0. [DOI] [PubMed] [Google Scholar]
- 43.von Renteln D., Vassiliou M.C., Rothstein R.I. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy. 2009;41:1056–1061. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 44.Goto O., Takeuchi H., Kitagawa Y. Endoscopic submucosal dissection and related techniques as precursors of “new NOTES” resection methods for gastric neoplasms. Gastrointest Endosc Clin N Am. 2016;26:313–322. doi: 10.1016/j.giec.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Li Q.L., Chen W.F., Zhang C. Clinical impact of submuocsal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer. Surg Endosc. 2015;29:3640–3646. doi: 10.1007/s00464-015-4120-2. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt A., Meier B., Caca K. Endoscopic full thickness resection: current status. World J Gastroenterol. 2015;21:9273–9285. doi: 10.3748/wjg.v21.i31.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nontunneled exposed endoscopic full-thickness resection of a gastric GI stromal tumor. (Courtesy of Dr S. Stavropoulos. https://endoscopedia.com/2014/08/12/endoscopic-full-thickness-resection-for-gi-stromal-tumors/.
Loop and clip closure after rectal endoscopic submucosal dissection (courtesy of Uzma D. Siddiqui, MD, FASGE, Center for Endoscopic Research and Therapeutics, University of Chicago.)
Submucosal endoscopic tunnel resection of a subepithelial tumor in the gastric cardia. (Courtesy of Author Amrita Sethi.)
Nonexposed endoscopic full-thickness resection of a duodenal neuroendocrine tumor by use of a full-thickness resection device. (From Schmidt A, Meier B, Cahyadi O, et al. Duodenal endoscopic full-thickness resection (with video). Gastrointest Endosc 2015;82:728-33.)