ABSTRACT
Background
Patients with cancer are highly concerned about food choices and dietary supplements that may affect their treatment outcomes. Excess folic acid (synthetic folate) from supplements or fortification can lead to accumulation of unmetabolized folic acid in the systemic circulation and urine and may promote cancer growth, especially among those with neoplastic alterations.
Objective
We investigated the prospective association between synthetic compared with natural folate intake and clinical outcomes in non–muscle-invasive bladder cancer (NMIBC), which is a highly recurrent disease.
Design
In a cohort of 619 NMIBC patients, folate intake at diagnosis was assessed with a previously validated food-frequency questionnaire and categorized according to tertiles. After a median follow-up of 5.2 y, 303 tumor recurrence and 108 progression events were documented from medical record review. Multivariable Cox proportional hazards and logistic models were used to estimate adjusted HRs and ORs with 95% CIs.
Results
Synthetic folic acid intake was positively associated with a risk of recurrence among NMIBC patients (medium compared with low intake—HR: 1.72; 95% CI: 1.20, 2.48; P = 0.003; high compared with low intake—HR: 1.80; 95% CI: 1.14, 2.84; P = 0.01). Patients with a higher folic acid intake were more likely to have multifocal tumors at diagnosis (medium or high compared with low—OR: 2.08; 95% CI: 1.08, 4.02; P = 0.03). In contrast, natural folate intake tended to be inversely associated with the risk of progression (medium or high compared with low—HR: 0.68; 95% CI: 0.44, 1.04; P = 0.08).
Conclusions
A high intake of synthetic folic acid, in contrast to the natural forms, is associated with an increased risk of recurrence in NMIBC and multifocal tumors at diagnosis, which suggests that folic acid may be unsafe for NMIBC patients. These findings provide some evidence for nutritional consultation with regard to folate intake among NMIBC patients.
Keywords: dietary factor, folate, folic acid, bladder cancer, non–muscle-invasive bladder cancer, recurrence, progression, clinical outcome, prognosis, survivorship
INTRODUCTION
Bladder cancer, the fifth most common cancer in the United States (1), has one of the highest recurrence rates of all cancers (2). The most important risk factor is smoking, and other factors include occupational exposures, poor diet, alcohol intake, and genetic predisposition (3, 4). Approximately 80% of new cases in the United States present with a disease that is confined to the mucosa or lamina propria [i.e., non–muscle-invasive bladder cancer (NMIBC)] (5). The 5-y cancer-specific survival of NMIBC patients ranges from 88.5% to 99.1% (6). However, >50% of cases will recur and >10% of cases will progress to muscle invasion within 5 y (7–11). The 5-y cancer-specific survival for bladder cancer with muscle invasion decreases to 40% (12, 13). Due to the high rate of recurrence and progression, there is enormous interest in identifying modifiable factors, especially dietary factors, that may affect the risk of recurrence and progression.
Folate plays a central role in DNA methylation, biosynthesis, and repair (14, 15). Natural folate can be found in a variety of fruits and vegetables. Mandatory food fortification with folic acid, the synthetic form of folate, was authorized in 1996 and fully implemented in 1998 in the United States (16–18). Folic acid fortification (∼100–200 μg/d), together with the common use of folic acid–containing supplements [∼400 μg/standard multivitamin (19, 20)], has resulted in a >2-fold increase in blood measurements of folate in the US population (19–23). Among cancer patients and survivors, 26–77% use folic acid–containing multivitamins and 1–12% use single folic acid supplements (24).
High doses of folic acid may speed cell division and increase tumor growth, thus promoting cancer, especially among those with preneoplastic or neoplastic alterations (14, 17, 20, 25–33). Animal studies have suggested that folic acid supplementation may increase cancer risk and accelerate tumor progression if too much is given or if it is provided after preneoplastic or neoplastic alterations are established in the target organ (29). Several secondary analyses from randomized clinical trials suggested that large doses of folic acid supplementation might increase cancer risk (34–37), but a meta-analysis of all available trials showed that folic acid supplementation had no substantial effect on the risk of overall cancer or site-specific cancer (38).
The kidney excretes folic acid, especially after large doses, resulting in appreciable amounts of unmetabolized folic acid in the urine (39, 40). Unmetabolized folic acid comes into close contact with the bladder epithelial cells, which express folate receptor, reduced folate carrier, and proton-coupled folate transporter (41–43). Unmetabolized folic acid may directly exert a cancer promotion effect on potential occult, residual, neoplastic urothelial cells, which may later manifest as disease recurrence or progression among NMIBC patients after resection. Therefore, we investigated the prospective association of intake of folic acid and natural folate at diagnosis with the risk of recurrence and progression among a large clinical cohort of NMIBC patients.
METHODS
Patients and data collection
Study participants were accrued from a large ongoing cohort study that recruited patients with bladder cancer from The University of Texas MD Anderson Cancer Center and Baylor College of Medicine through a daily review of computerized appointment schedules; more details were previously described (44, 45). In the parent study on NMIBC (tumor stage Ta, Tis, and T1) starting in 1995, we included patients who were newly diagnosed (≤1 y before recruitment) with histologic confirmation and who were previously untreated with chemotherapy or radiotherapy. The primary objectives included discovering multidimensional molecular biomarkers in various biospecimens, identifying modifiable risk factors, and building integrative risk prediction models to accurately predict clinical outcomes of NMIBC. At recruitment, patients filled in questionnaires to report information including demographic characteristics, height, weight, smoking history, and dietary intake. By the time we conducted the present study, 995 NMIBC patients had been recruited in the parent study, and we included 649 patients diagnosed between 1997 and 2012 who reported their dietary intake in this study (Supplemental Figure 1). All of the NMIBC patients underwent the same surveillance protocol and were followed with periodic (every 3 mo for 2 y, every 6 mo for 2 y, then annually) cystoscopic examinations and intravesical treatments as indicated, which consisted of bacillus Calmette-Guérin (BCG) for 6 weekly instillations (induction course), BCG induction plus maintenance, or other intravesical cytotoxic agents. Study staff conducted a detailed chart review of medical records to abstract clinical data, including diagnosis date, clinically relevant (5, 46, 47) tumor characteristics at diagnosis (number of tumor foci at diagnosis, tumor size, stage, grade, presence of concomitant carcinoma in situ), and dates of recurrence and progression events. Tumor recurrence was defined as a newly found bladder tumor, and progression was defined as the transition from non–muscle-invasive to invasive or metastatic disease. This study was approved by the MD Anderson Cancer Center Institutional Review Board, and written informed consent was obtained from each participant.
Dietary assessment
We used the same method of dietary assessment as in our previous studies (48, 49). Dietary intake during the year before diagnosis was assessed by using a previously validated food-frequency questionnaire [FFQ; the National Cancer Institute's Health Habits and History Questionnaire (50)]. It has been shown that the FFQ is a valid and reliable food-frequency survey tool across various populations (51, 52). From the dietary information obtained in the FFQ, dietary intake was calculated on the basis of the USDA National Nutrient Database for Standard Reference (53) and the USDA Food and Nutrient Database for Dietary Studies (54). We further excluded those with outlying (i.e., outside the interval delimited by the 25th percentile minus 1.5 times the IQR and the 75th percentile plus 1.5 times the IQR) or implausible (i.e., <500 or >5000 kcal/d) energy intakes (n = 30) (Supplemental Figure 1). The daily dietary intake was energy-adjusted by using the residual method (55).
Due to folic acid fortification, many foods contain both folic acid and the natural folates that are inherent in foods. Folic acid intake was the sum of folic acid consumption from fortified foods and that from multivitamin or individual supplements. Our questionnaire inquired about the brands of multivitamin supplements for patients recruited more recently (30% of the study population). For patients who were taking known brands, we linked the brands to the Dietary Supplement Label Database at the NIH (https://www.dsld.nlm.nih.gov/dsld-mobile/index.jsp) to obtain the amount of folic acid contained in each serving, and the majority (92%) of the brands that contain folic acid contain 400 µg/serving. Multivitamins with unknown brands were assumed to contain 400 µg folic acid/serving (19, 20, 56). For single folic acid supplements, we asked about the amount in each serving. Total folate intake was calculated by summing natural folates and folic acid. Folic acid is generally assumed to be more bioavailable than natural folates in foods. To account for this difference, total folate was expressed as dietary folate equivalents, which were calculated as natural food folate (µg) + 1.67 × folic acid (µg).
Statistical analysis
Intakes of folic acid, natural folate, and total folate were categorized according to tertiles on the basis of the sex-specific distributions in the study population. Patients with different amounts of synthetic folic acid intake were compared for selected characteristics at baseline by the chi-square test for categorical variables and ANOVA for continuous variables. Time-to-event for the analysis of disease recurrence or progression was calculated from the date of diagnosis to the date of disease recurrence or progression, death, or the latest follow-up, whichever came first. The proportionality of Cox proportional hazards model was examined by visual inspection of the log-log survival plots, time-dependent covariates, and Schoenfeld residuals. We used multivariable sex- and treatment-stratified Cox proportional hazards and logistic models to estimate HRs and ORs with 95% CIs, after adjustment of potential confounders based on a priori knowledge. Patients with missing covariates were excluded in multivariable analysis. The Kaplan-Meier failure function was used to plot and compare the risk of tumor recurrence or progression across different amounts of folate intake.
RESULTS
Host characteristics of NMIBC patients
Selected characteristics of the 619 NMIBC patients by 3 groups of synthetic folic acid intake are presented in Table 1. Patients with a higher folic acid intake were more likely to be older, well educated, never or former smokers, nonobese, and multivitamin users. In addition, they had lower consumption of alcohol and higher consumption of total folate. No significant differences between the 3 groups were observed with regard to sex, race/ethnicity, type of treatment, and total energy intake.
TABLE 1.
Selected characteristics of NMIBC patients by synthetic folic acid intake1
| Synthetic folic acid intake2 | ||||
|---|---|---|---|---|
| Characteristics | Low (n = 207) | Medium (n = 207) | High (n = 205) | P 3 |
| Synthetic folic acid, µg/d | 152.7 ± 70.34 | 439.8 ± 111.3 | 818.1 ± 291.9 | <0.001 |
| Age at diagnosis, y | 61.7 ± 11.1 | 64.2 ± 10.5 | 64.8 ± 11.1 | 0.01 |
| Sex, n (%) | 0.98 | |||
| Men | 165 (79.7) | 164 (79.2) | 164 (80.0) | |
| Women | 42 (20.3) | 43 (20.8) | 41 (20.0) | |
| Race/ethnicity, n (%) | 0.08 | |||
| Non-Hispanic white | 185 (89.4) | 193 (93.2) | 195 (95.1) | |
| Other | 22 (10.6) | 14 (6.8) | 10 (4.9) | |
| Education, n (%) | 0.02 | |||
| High school or less | 63 (30.6) | 54 (26.1) | 49 (24.0) | |
| Some college | 61 (29.6) | 72 (34.8) | 47 (23.0) | |
| Completed college or greater | 82 (39.8) | 81 (39.1) | 108 (52.9) | |
| Smoking status, n (%) | <0.001 | |||
| Never smoker | 52 (26.1) | 61 (30.2) | 73 (37.4) | |
| Former smoker | 79 (39.7) | 99 (49.0) | 94 (48.2) | |
| Current smoker | 68 (34.2) | 42 (20.8) | 28 (14.4) | |
| BMI (kg/m2), n (%) | 0.04 | |||
| <25 (normal or underweight) | 47 (22.7) | 48 (23.3) | 67 (32.7) | |
| 25–29.99 (overweight) | 90 (43.5) | 88 (42.7) | 90 (43.9) | |
| ≥30 (obese) | 70 (33.8) | 70 (34.0) | 48 (23.4) | |
| Multivitamin use, n (%) | <0.001 | |||
| No | 191 (92.3) | 67 (32.4) | 9 (4.4) | |
| Yes | 16 (7.7) | 140 (67.6) | 196 (95.6) | |
| Treatment, n (%) | 0.59 | |||
| TUR only | 60 (29.0) | 49 (23.7) | 60 (29.3) | |
| iBCG after TUR | 55 (26.6) | 68 (32.9) | 52 (25.4) | |
| mBCG after TUR | 51 (24.6) | 49 (23.7) | 56 (27.3) | |
| Other intravesical chemotherapy | 41 (19.8) | 41 (19.8) | 37 (18.0) | |
| Dietary intake | ||||
| Total energy, kcal/d | 2283.9 ± 1017.8 | 2355.8 ± 911.3 | 2312.0 ± 894.7 | 0.74 |
| Alcohol, g/d | 17.2 ± 34.0 | 12.0 ± 18.1 | 9.5 ± 15.3 | 0.004 |
| Natural folate, µg/d | 335.0 ± 122.0 | 363.4 ± 144.6 | 343.5 ± 125.1 | 0.08 |
| Total folate, DFEs/d | 590.1 ± 171.0 | 1097.9 ± 244.7 | 1711.5 ± 519.3 | <0.001 |
1Energy-adjusted intake was categorized into tertiles on the basis of the sex-specific distribution in the study population. Among men: low (<228.0 µg/d), medium (228.0–557.4 µg/d), high (>557.4 µg/d); among women: low (<353.4 µg/d), medium (353.4–593.4 µg/d), high (>593.4 µg/d). DFE, dietary folate equivalent; iBCG, induction bacillus Calmette-Guérin; mBCG, maintenance bacillus Calmette-Guérin; NMIBC, non–muscle-invasive bladder cancer; TUR, transurethral resection.
2Numbers may not add up to the total because of missing data.
3Derived by using chi-square test for categorical variables and ANOVA for continuous variables.
4Mean ± SD (all such values).
Folate intake and risk of recurrence and progression
After a median follow-up of 62 mo, 303 tumor recurrence and 108 progression events were recorded. Table 2 shows the multivariable-adjusted associations of intakes of folic acid, natural folate, and total folate intake with the risk of recurrence or progression. Compared with patients with a low intake of synthetic folic acid, those with medium or high intakes of folic acid had a 72% (HR: 1.72; 95% CI: 1.20, 2.48; P = 0.003) or 80% (HR: 1.80; 95% CI: 1.14, 2.84; P = 0.01) increased risk of recurrence, respectively. In contrast, natural folate intake was in nonsignificant inverse association with the risk of progression; compared with low natural folate intake, medium and high intakes were associated with a 33% (HR: 0.67; 95% CI: 0.40, 1.11; P = 0.12) and 31% (HR: 0.69; 95% CI: 0.41, 1.15; P = 0.15) reduced risk of progression, respectively. Compared with low total folate intake, medium total intake was associated with a 67% increased risk of recurrence (HR: 1.67; 95% CI: 1.16, 2.38; P = 0.005) and an 83% increased risk of progression (HR: 1.83; 95% CI: 1.02, 3.26; P = 0.04), whereas a high total intake was not significantly associated with the risk of recurrence or progression, suggesting an upside-down U-shaped association.
TABLE 2.
Folate intake in different forms with risk of recurrence and progression among NMIBC patients1
| Recurrence | Progression | |||||
|---|---|---|---|---|---|---|
| Folate intake | n Events/total n | HR (95% CI)2 | P 2 | n Events/total n | HR (95% CI)2 | P 2 |
| Synthetic folic acid3 | ||||||
| Low | 93/207 | Reference | N/A | 34/207 | Reference | N/A |
| Medium | 113/207 | 1.72 (1.20, 2.48) | 0.003 | 38/207 | 1.17 (0.65, 2.12) | 0.60 |
| High | 97/205 | 1.80 (1.14, 2.84) | 0.01 | 36/205 | 1.33 (0.63, 2.81) | 0.46 |
| Natural folate4 | ||||||
| Low | 110/207 | Reference | N/A | 44/207 | Reference | N/A |
| Medium | 107/207 | 0.96 (0.71, 1.30) | 0.78 | 33/207 | 0.67 (0.40, 1.11) | 0.12 |
| High | 86/205 | 0.82 (0.60, 1.13) | 0.22 | 31/205 | 0.69 (0.41, 1.15) | 0.15 |
| Total folate5 | ||||||
| Low | 98/206 | Reference | N/A | 32/206 | Reference | N/A |
| Medium | 118/208 | 1.67 (1.16, 2.38) | 0.005 | 45/208 | 1.83 (1.02, 3.26) | 0.04 |
| High | 87/205 | 1.23 (0.78, 1.95) | 0.37 | 31/205 | 1.20 (0.57, 2.53) | 0.63 |
1Energy-adjusted intake was categorized into tertiles on the basis of the sex-specific distribution in the study population. DFE, dietary folate equivalent; N/A, not applicable; NMIBC, non–muscle-invasive bladder cancer.
2Derived by using sex- and treatment-stratified Cox models adjusted for age, race/ethnicity, education, smoking status, BMI, multivitamin use, total energy intake, alcohol consumption, tumor stage, and tumor grade.
3Intakes among men: low (<228.0 µg/d), medium (228.0–557.4 µg/d), high (>557.4 µg/d); among women: low (<353.4 µg/d), medium (353.4–593.4 µg/d), high (>593.4 µg/d).
4Intakes among men: low (<283.5 µg/d), medium (283.5–365.6 µg/d), high (>365.6 µg/d) intakes; among women: low (<296.7 µg/d), medium (296.7–408.3 µg/d), high (>408.3 µg/d).
5Intakes among men: low (<697.0 DFEs/d), medium (697.0–1265.9 DFEs/d), high (>1265.9 DFEs/d); among women: low (<1001.8 DFEs/d), medium (1001.8–1402.7 DFEs/d), high (>1402.7 DFEs/d).
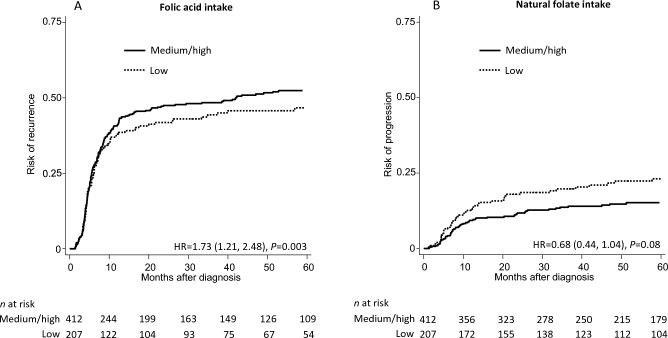
Figure 1 shows the risk of recurrence along time after diagnosis by synthetic folic acid intake (Figure 1A) and the risk of progression by natural folate intake (Figure 1B). As shown in Figure 1A, the risk of recurrence after diagnosis was higher among patients with medium or high synthetic folic acid intake than among those with low folic acid intake. As shown in Figure 1B, the risk of progression tended to be lower among patients with medium or high natural folate intake than among those with low natural folate intake.
FIGURE 1.
Risk of recurrence along time after diagnosis by synthetic folic acid intake (A) and risk of progression along time after diagnosis by natural folate intake (B). For synthetic folic acid intake, among men: low (<228.0 µg/d), medium (228.0–557.4 µg/d), and high (>557.4 µg/d) intakes; among women: low (<353.4 µg/d), medium (353.4–593.4 µg/d), and high (>593.4 µg/d) intakes. For natural folate intake, among men: low (<283.5 µg/d), medium (283.5–365.6 µg/d), and high (>365.6 µg/d) intakes; among women: low (<296.7 µg/d), medium (296.7–408.3 µg/d), and high (>408.3 µg/d) intakes. HRs (medium or high compared with low) and P values were based on sex- and treatment-stratified Cox models adjusted for age, race/ethnicity, education, smoking status, BMI, multivitamin use, total energy intake, alcohol consumption, tumor stage, and tumor grade.
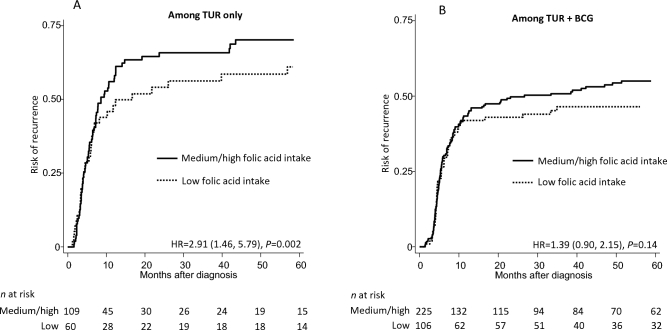
Synthetic folic acid and risk of recurrence stratified by cancer treatment
Figure 2 shows the risk of recurrence by synthetic folic acid intake among patients who received transurethral resection (TUR) only (Figure 2A) and patients who received BCG treatment after TUR (Figure 2B). Medium or high folic acid intake was positively associated with an increased risk of recurrence in both subgroups, but the association was only significant among those who received TUR only (HR: 2.91; 95% CI: 1.46, 5.79; P = 0.002) and was not significant among those who received BCG treatment after TUR (HR: 1.39; 95% CI: 0.90, 2.15; P = 0.14). We were not able to estimate the stratified associations for progression due to the relatively small number of patients with progression in each treatment subgroup.
FIGURE 2.
Risk of recurrence by synthetic folic acid intake among patients who received TUR only (A) and patients who received BCG treatment after TUR (B). For synthetic folic acid intake, among men: low (<228.0 µg/d), medium (228.0–557.4 µg/d), and high (>557.4 µg/d) intakes; among women: low (<353.4 µg/d), medium (353.4–593.4 µg/d), and high (>593.4 µg/d) intakes. HRs (medium or high compared with low) and P values were based on sex-stratified Cox models adjusted for age, race/ethnicity, education, smoking status, BMI, multivitamin use, total energy intake, alcohol consumption, tumor stage, and tumor grade. BCG, bacillus Calmette-Guérin; TUR, transurethral resection.
Folic acid and tumor characteristics
We further assessed whether synthetic folic acid intake was associated with 5 tumor characteristics at diagnosis: focality, size, stage, grade, and concomitant carcinoma in situ. As shown in Table 3, compared with patients with low folic acid intake, those with higher intakes were more likely to have multifocal tumors at diagnosis (medium or high compared with low—OR: 2.08; 95% CI: 1.08, 4.02; P = 0.03). The associations between folic acid intake and the 4 other tumor characteristics were not significant.
TABLE 3.
Synthetic folic acid intake and clinically significant tumor characteristics of NMIBC1
| Synthetic folic acid intake | |||
|---|---|---|---|
| Tumor characteristics | Low | Medium/high | P 2 |
| Multifocal tumors | 0.03 | ||
| Multiple/single, n | 59/81 | 155/142 | |
| OR (95% CI) | Reference | 2.08 (1.08, 4.02) | |
| Larger size | 0.35 | ||
| >2/≤2 cm, n | 62/29 | 128/54 | |
| OR (95% CI) | Reference | 1.57 (0.61, 4.03) | |
| More-advanced stage | 0.20 | ||
| T1/Ta or Tis, n | 96/109 | 214/194 | |
| OR (95% CI) | Reference | 1.40 (0.84, 2.33) | |
| Higher grade | 0.53 | ||
| G3/G1 or G2, n | 119/81 | 251/148 | |
| OR (95% CI) | Reference | 1.20 (0.69, 2.08) | |
| Concomitant CIS | 0.73 | ||
| Yes/no, n | 66/123 | 137/241 | |
| OR (95% CI) | Reference | 1.10 (0.64, 1.89) | |
1Energy-adjusted intake was categorized into tertiles on the basis of the sex-specific distribution in the study population. Among men: low (<228.0 µg/d), medium (228.0–557.4 µg/d), high (>557.4 µg/d); among women: low (<353.4 µg/d), medium (353.4–593.4 µg/d), high (>593.4 µg/d). CIS, carcinoma in situ; NMIBC, non–muscle-invasive bladder cancer.
2Derived by using logistic models adjusted for age, race/ethnicity, education, smoking status, BMI, multivitamin use, total energy intake, and alcohol consumption. No adjustment for multiple comparisons was made.
DISCUSSION
Given the increasing concern over food choices and dietary supplements that may affect cancer treatment outcomes, we investigated the prospective association of folic acid and natural folate intake with clinical outcomes of NMIBC patients. We found that a high intake of folic acid during the year before diagnosis was significantly associated with increased risk of recurrence, whereas a high intake of natural folate tended to be associated with a decreased risk of progression. Furthermore, the increased risk of recurrence associated with high synthetic folic acid was more pronounced among patients who received TUR only than among patients who received BCG treatment after TUR. Finally, high folic acid intake was linked to an increase in multifocal tumors.
Previous epidemiologic studies that examined the association between folate intake and bladder cancer risk yielded inconsistent results (57–69). According to a meta-analysis of these studies (70), a significant 16% (RR: 0.84; 95% CI: 0.72, 0.96) decreased risk of developing bladder cancer was observed when comparing the highest with the lowest amounts of total folate intake, and the reduction was more evident with dietary natural folate (RR: 0.82; 95% CI: 0.65, 0.99) than with synthetic folic acid (RR: 0.91; 95% CI: 0.58, 1.25). Meanwhile, our study provides the first evidence, to our knowledge, that folic acid, in contrast to natural folate, is associated with an increased risk of recurrence in NMIBC patients, and this association is further supported by our observation that a high intake of folic acid was associated with an increase in multifocal tumors. Our findings, together with those from previous studies, suggest a dual modulatory effect (28, 30) of folate on bladder cancer depending on the context.
There are several possible explanations for the observed positive association between folic acid intake and the risk of recurrence in NMIBC. First, high doses of folic acid, which is more bioavailable than natural folate found in foods, may speed cell division and increase tumor growth, especially among those with preneoplastic or neoplastic alterations (14, 29, 32). Second, unlike natural folate, folic acid needs to be reduced enzymatically to functional form by dihydrofolate reductase, and this process takes place mainly in the liver (71). However, dihydrofolate reductase activity in the human liver is extremely low and variable (72), and the capacity of this enzyme can be exceeded with large doses of folic acid, resulting in the presence of unmetabolized folic acid in the systemic circulation and urine (40). There has been no evidence of unmetabolized natural folate in urine. Unmetabolized folic acid can be transported into cells via the folate receptor and several other transporters (73), but the intracellular effects of folic acid are unknown. Interestingly, natural killer cell cytotoxicity, which plays an important role in immune surveillance against cancer and killing of tumor cells (74), is reduced with a high intake of folic acid in aged mice (75) and with the presence of unmetabolized folic acid in plasma among postmenopausal women (76). To the best of our knowledge, no previous study has specifically investigated the effect of natural folate on natural killer cell cytotoxicity. Finally, excess folic acid significantly increases genomic and site-specific methylation, and theoretically, hypermethylation of the promoter regions in tumor suppressor or mismatch repair genes could lead to inactivation of these genes, thus promoting tumor growth and progression (29, 77).
We found that, among patients who received BCG treatment after TUR, the increased HR of recurrence comparing high or medium with low folic acid intake was smaller than that among patients who received TUR only. BCG treatment may mitigate the effect of folic acid on recurrence for 2 reasons: first, BCG treatment results in reduced cell proliferation, cell cycle arrest, and cell death of bladder cancer cells (78); second, the antitumor effect of BCG treatment is mediated through activation of the immune system (78), and after BCG instillation, a marked increase in the number of immune cells, including natural killer cells, can be detected in the urine and the bladder wall (79). Therefore, BCG treatment may mitigate the effects of folic acid (as stated above: folic acid acts by increasing cell proliferation and decreasing the cytotoxicity of natural killer cells).
It is uncertain why high folic acid intake is associated with an increased risk of recurrence but not progression of NMIBC. One potential reason is that high folic acid intake may speed cell division and increase tumor growth, but it may have no significant effect on the aggressiveness of the tumor cells. This reasoning is consistent with our observation that a high intake of folic acid was in association with multifocal tumors but not with stage, grade, and concomitant carcinoma in situ, which are related to the aggressiveness of the tumor cells (47).
Reducing the recurrence of bladder cancer is of significant importance from both a patient and economic point of view. It has been shown that recurrence at follow-up cystoscopy after TUR is one of the most important prognostic factors for progression (47, 80, 81). Due to the high rate of recurrence, lifelong, frequent, and painful monitoring procedures are needed to identify and remove recurrent tumors, making bladder cancer the most expensive cancer to manage per patient, with an estimated cost of $96,000–$187,000 from diagnosis to death in the United States (82, 83).
There are several strengths of our study. For the first time to our knowledge, we investigated the prospective association of folate intake in different forms and clinical outcomes of NMIBC patients and showed that folic acid and natural folate had differential impacts on clinical outcomes in NMIBC patients. Second, we systematically followed a large clinical cohort of NMIBC patients for a sufficient period of time to observe tumor recurrence and progression events that are the most relevant to NMIBC patients and physicians but for which data are commonly unavailable in population-based cohorts. Third, we used a previously validated FFQ to collect dietary data in our study. A final strength is that we were able to adjust for potential epidemiologic and clinical covariates, which helps to minimize the possibility of confounded associations.
Our study is not without limitations. First, the dietary data were self-reported and were subject to measurement errors. Nevertheless, it is unlikely that measurement errors were differential on the basis of clinical outcomes. Second, as for any observational study, we could not exclude the possibility of uncontrolled confounding, even though we adjusted for a long list of potential confounders. Third, the number of progression events is relatively small, with limited statistical power. Fourth, non-Hispanic whites accounted for the majority of the study population; therefore, the study results may not be directly generalizable to other racial/ethnic groups. Fifth, multivitamins with unknown brands were assumed to contain 400 µg folic acid/serving, which is the amount found in 92% of the known brands in our study, but some uncommon brands may contain a different amount. Finally, dietary intake was only evaluated in the year before diagnosis and cancer patients may change their diet after diagnosis. In particular, cancer survivors may initiate dietary supplement use after diagnosis (24). Therefore, in our study, the intake of folic acid might have increased after diagnosis among those who did not use dietary supplements before diagnosis. This change in supplement use after diagnosis may have led to an underestimation of the positive association between folic acid intake and recurrence. Meanwhile, as shown in Figure 1, most recurrence and progression events occurred within 1 y postdiagnosis, so the dietary intake assessed at diagnosis may be more relevant than that assessed several years after diagnosis.
Our study provides the first evidence, to our knowledge, that although a high natural folate intake tends to be associated with decreased risk of progression in NMIBC patients, a high folic acid intake is associated with an increased risk of recurrence (particularly in those who only receive TUR) and multifocal tumors at diagnosis. These findings cast doubt on the safety of the common use of folic acid–containing supplements among NMIBC patients in the context of universal folic acid fortification of the food supply. However, given that our study is an observational cohort study, no causal inference could be made and more studies are needed to confirm these novel and clinically relevant findings. If confirmed, these findings may have great implications for clinical management and nutritional consultation for NMIBC patients.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—HT and XW: designed the research; XW, CPD, YY, HBG, and SPL: conducted the research; HT, XW, and YY: analyzed the data and interpreted the results; HT: wrote the manuscript; XW: had primary responsibility for the final content; and all authors: critically revised the manuscript for important intellectual content and read and approved the final manuscript. None of the authors had a conflict of interest.
Notes
Supported in part by a grant from the National Cancer Institute at the NIH (P50 CA 91846; to XW). Additional funding was provided by the Center for Translational and Public Health Genomics at MD Anderson Cancer Center.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- BCG
bacillus Calmette-Guérin
- FFQ
food-frequency questionnaire
- NMIBC
non–muscle-invasive bladder cancer
- TUR
transurethral resection.
REFERENCES
- 1. American Cancer Society Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2. Vinata BL, Merseburger AS, Hautmann SH. Bladder tumors: molecular aspects and clinical management. 1st ed.New York:Humana Press;2010. [Google Scholar]
- 3. Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, Lotan Y. Bladder cancer. Nat Rev Dis Primers 2017;3:17022. [DOI] [PubMed] [Google Scholar]
- 4. Letasiova S, Medve'ova A, Sovcikova A, Dusinska M, Volkovova K, Mosoiu C, Bartonova A. Bladder cancer, a review of the environmental risk factors. Environ Health 2012;11(Suppl 1):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 2016;196(4):1021–9. [DOI] [PubMed] [Google Scholar]
- 6. Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC, Oosterlinck W, Prescott S, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guerin. Eur Urol 2016;69(1):60–9. [DOI] [PubMed] [Google Scholar]
- 7. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49(3):466–5. [DOI] [PubMed] [Google Scholar]
- 8. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2008;54(2):303–14. [DOI] [PubMed] [Google Scholar]
- 9. Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66(6 Suppl 1):4–34. [DOI] [PubMed] [Google Scholar]
- 10. Kiemeney LA, Witjes JA, Verbeek AL, Heijbroek RP, Debruyne FM; Dutch South-East Cooperative Urological Group. The clinical epidemiology of superficial bladder cancer. Br J Cancer 1993;67(4):806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS Jr., Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178(6):2314–30. [DOI] [PubMed] [Google Scholar]
- 12. Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176(6 Part 1):2414–22; discussion 22. [DOI] [PubMed] [Google Scholar]
- 13. van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60(3):493–500. [DOI] [PubMed] [Google Scholar]
- 14. Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003;3(8):601–14. [DOI] [PubMed] [Google Scholar]
- 15. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130(2):129–32. [DOI] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration : Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid: final rule. Fed Regist 1996;61(44):8781–97. [Google Scholar]
- 17. Drake BF, Colditz GA. Assessing cancer prevention studies—a matter of time. JAMA 2009;302(19):2152–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients 2011;3(3):370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol 2004;160(4):339–49. [DOI] [PubMed] [Google Scholar]
- 20. Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev 2006;15(2):189–93. [DOI] [PubMed] [Google Scholar]
- 21. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999;340(19):1449–54. [DOI] [PubMed] [Google Scholar]
- 23. Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr 2005;82(2):442–50. [DOI] [PubMed] [Google Scholar]
- 24. Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol 2008;26(4):665–73. [DOI] [PubMed] [Google Scholar]
- 25. Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen 2004;44(1):10–25. [DOI] [PubMed] [Google Scholar]
- 26. Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet 2006;367(9519):1352–61. [DOI] [PubMed] [Google Scholar]
- 27. Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr 2008;87(3):517–33. [DOI] [PubMed] [Google Scholar]
- 28. Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr 2007;86(2):271–3. [DOI] [PubMed] [Google Scholar]
- 29. Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80(5):1123–8. [DOI] [PubMed] [Google Scholar]
- 30. Kim YI. Role of folate in colon cancer development and progression. J Nutr 2003;133(11 Suppl 1):3731S–9S. [DOI] [PubMed] [Google Scholar]
- 31. Kim YI. Folic acid fortification and supplementation–good for some but not so good for others. Nutr Rev 2007;65(11):504–11. [DOI] [PubMed] [Google Scholar]
- 32. Ulrich CM, Potter JD. Folate and cancer—timing is everything. JAMA 2007;297(21):2408–9. [DOI] [PubMed] [Google Scholar]
- 33. Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006;55(10):1387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charles D, Ness AR, Campbell D, Davey Smith G, Hall MH. Taking folate in pregnancy and risk of maternal breast cancer. BMJ 2004;329(7479):1375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst 2009;101(6):432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297(21):2351–9. [DOI] [PubMed] [Google Scholar]
- 37. Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njolstad I, Refsum H, Nilsen DW, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009;302(19):2119–26. [DOI] [PubMed] [Google Scholar]
- 38. Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, Manson JE, Hankey GJ, Spence JD, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013;381(9871):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooperman JM, Pesci-Bourel A, Luhby AL. Urinary excretion of folic acid activity in man. Clin Chem 1970;16(5):375–81. [PubMed] [Google Scholar]
- 40. Zempleni J, Suttie JW, Gregory JF III, Stover PJ. Handbook of vitamins. 5th ed.Boca Raton (FL): CRC Press Taylor & Francis Group;2013. [Google Scholar]
- 41. Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338(2):284–93. [DOI] [PubMed] [Google Scholar]
- 42. Abdel-Haleem AM, El-Zeiry MI, Mahran LG, Abou-Aisha K, Rady MH, Rohde J, Mostageer M, Spahn-Langguth H. Expression of RFC/SLC19A1 is associated with tumor type in bladder cancer patients. PLoS One 2011;6(7):e21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. Proteomics: t issue-based map of the human proteome. Science 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 44. Chen M, Hildebrandt MA, Clague J, Kamat AM, Picornell A, Chang J, Zhang X, Izzo J, Yang H, Lin J, et al. Genetic variations in the sonic hedgehog pathway affect clinical outcomes in non-muscle-invasive bladder cancer. Cancer Prev Res (Phila) 2010;3(10):1235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, Gu J, Dong Q, Wu X. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J Clin Oncol 2005;23(24):5746–56. [DOI] [PubMed] [Google Scholar]
- 46. Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71(3):447–61. [DOI] [PubMed] [Google Scholar]
- 47. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49(3):466–5; discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 48. Tu H, Heymach JV, Wen CP, Ye Y, Pierzynski JA, Roth JA, Wu X. Different dietary patterns and reduction of lung cancer risk: a large case-control study in the U.S. Sci Rep 2016;6:26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schabath MB, Spitz MR, Lerner SP, Pillow PC, Hernandez LM, Delclos GL, Grossman HB, Wu X. Case-control analysis of dietary folate and risk of bladder cancer. Nutr Cancer 2005;53(2):144–51. [DOI] [PubMed] [Google Scholar]
- 50. Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol 1994;139(12):1190–6. [DOI] [PubMed] [Google Scholar]
- 51. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 52. Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 1992;92(6):686–93. [PubMed] [Google Scholar]
- 53. Food Surveys Research Group , Agricultural Research Service, US Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 27. Beltsville, MD: US Department of Agriculture; 2014. [Google Scholar]
- 54. Food Surveys Research Group, Agricultural Research Service, USDA USDA Food and Nutrient Database for Dietary Studies, 4.1. Beltsville (MD): USDA; 2010. [Google Scholar]
- 55. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124(1):17–27. [DOI] [PubMed] [Google Scholar]
- 56. Stevens VL, McCullough ML, Sun J, Jacobs EJ, Campbell PT, Gapstur SM. High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer. Gastroenterology 2011;141(1):98–105, e1. [DOI] [PubMed] [Google Scholar]
- 57. Bruemmer B, White E, Vaughan TL, Cheney CL. Nutrient intake in relation to bladder cancer among middle-aged men and women. Am J Epidemiol 1996;144(5):485–95. [DOI] [PubMed] [Google Scholar]
- 58. Zeegers M, Goldbohm R, Van Den Brandt P. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer 2001;85(7):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu J, Cross A, Baris D, Ward M, Karagas M, Johnson A, Schwenn M, Cherala S, Colt J, Cantor K. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer 2012;106(11):1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schabath MB, Spitz MR, Lerner SP, Pillow PC, Hernandez LM, Delclos GL, Grossman HB, Wu X. Case-control analysis of dietary folate and risk of bladder cancer. Nutr Cancer 2005;53(2):144–51. [DOI] [PubMed] [Google Scholar]
- 61. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjønneland A. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol 2009;56(5):764–70. [DOI] [PubMed] [Google Scholar]
- 62. Park S-Y, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr 2013;143(8):1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci E. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol 2000;152(12):1145–53. [DOI] [PubMed] [Google Scholar]
- 64. Michaud D, Pietinen P, Taylor P, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer 2002;87(9):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle Study. J Urol 2011;185(4):1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holick CN, De Vivo I Feskanich D, Giovannucci E, Stampfer M, Michaud DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes Control 2005;16(10):1135–45. [DOI] [PubMed] [Google Scholar]
- 67. García-Closas R, García-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, Tardón A, Carrato A, Castaño-Vinyals G, Dosemeci M. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer 2007;43(11):1731–40. [DOI] [PubMed] [Google Scholar]
- 68. Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control 2010;21(4):609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aune D, Deneo-Pellegrini H, Ronco A, Boffetta P, Acosta G, Mendilaharsu M, De Stefani E. Dietary folate intake and the risk of 11 types of cancer: a case-control study in Uruguay. Ann Oncol 2010;22(2):444–51. [DOI] [PubMed] [Google Scholar]
- 70. He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr 2014;65(3):286–92. [DOI] [PubMed] [Google Scholar]
- 71. Rogers LM, Pfeiffer CM, Bailey LB, Gregory JF. A dual-label stable-isotopic protocol is suitable for determination of folate bioavailability in humans: evaluation of urinary excretion and plasma folate kinetics of intravenous and oral doses of [13C5] and [2H2]folic acid. J Nutr 1997;127(12):2321–7. [DOI] [PubMed] [Google Scholar]
- 72. Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 2009;106(36):15424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006;127(5):917–28. [DOI] [PubMed] [Google Scholar]
- 74. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16(1):7–19. [DOI] [PubMed] [Google Scholar]
- 75. Sawaengsri H, Wang J, Reginaldo C, Steluti J, Wu D, Meydani SN, Selhub J, Paul L. High folic acid intake reduces natural killer cell cytotoxicity in aged mice. J Nutr Biochem 2016;30:102–7. [DOI] [PubMed] [Google Scholar]
- 76. Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 2006;136(1):189–94. [DOI] [PubMed] [Google Scholar]
- 77. Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev 2004;13(4):511–9. [PubMed] [Google Scholar]
- 78. Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol 2014;11(3):153–62. [DOI] [PubMed] [Google Scholar]
- 79. Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, Jocham D, Ratliff TL, Bohle A. NK cells are essential for effective BCG immunotherapy. Int J Cancer 2001;92(5):697–702. [DOI] [PubMed] [Google Scholar]
- 80. van der Heijden AG, Witjes JA. Recurrence, progression, and follow-up in non–muscle-invasive bladder cancer. Eur Urol Suppl 2009;8(7):556–62. [Google Scholar]
- 81. Fernandez-Gomez J, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, Hernandez R, Madero R, Ojea A, Pertusa C, Rodriguez-Molina J, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol 2008;53(5):992–1001. [DOI] [PubMed] [Google Scholar]
- 82. Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 2003;21(18):1315–30. [DOI] [PubMed] [Google Scholar]
- 83. Messing EM. Why should we increase public awareness of bladder cancer, and how can we do it? Nat Clin Pract Urol 2008;5(3):117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.