ABSTRACT
Background
The Dietary Approaches to Stop Hypertension (DASH) dietary pattern is recommended for cardiovascular disease risk reduction. Assessment of dietary intake has been limited to subjective measures and a few biomarkers from 24-h urine collections.
Objective
The aim of the study was to use metabolomics to identify serum compounds that are associated with adherence to the DASH dietary pattern.
Design
We conducted untargeted metabolomic profiling in serum specimens collected at the end of 8 wk following the DASH diet (n = 110), the fruit and vegetables diet (n = 111), or a control diet (n = 108) in a multicenter, randomized clinical feeding study (n = 329). Multivariable linear regression was used to determine the associations between the randomized diets and individual log-transformed metabolites after adjustment for age, sex, race, education, body mass index, and hypertension. Partial least-squares discriminant analysis (PLS-DA) was used to identify a panel of compounds that discriminated between the dietary patterns. The area under the curve (C statistic) was calculated as the cumulative ability to distinguish between dietary patterns. We accounted for multiple comparisons with the use of the Bonferroni method (0.05 of 818 metabolites = 6.11 × 10−5).
Results
Serum concentrations of 44 known metabolites differed significantly between participants randomly assigned to the DASH diet compared with both the control diet and the fruit and vegetables diet, which included an amino acid, 2 cofactors and vitamins (n = 2), and lipids (n = 41). With the use of PLS-DA, component 1 explained 29.4% of the variance and component 2 explained 12.6% of the variance. The 10 most influential metabolites for discriminating between the DASH and control dietary patterns were N-methylproline, stachydrine, tryptophan betaine, theobromine, 7-methylurate, chiro-inositol, 3-methylxanthine, methyl glucopyranoside, β-cryptoxanthin, and 7-methylxanthine (C statistic = 0.986).
Conclusions
An untargeted metabolomic platform identified a broad array of serum metabolites that differed between the DASH diet and 2 other dietary patterns. This newly identified metabolite panel may be used to assess adherence to the DASH dietary pattern. This trial was registered at http://www.clinicaltrials.gov as NCT03403166.
Keywords: dietary intake, biomarkers, blood pressure, metabolism, metabolomics
INTRODUCTION
The Dietary Approaches to Stop Hypertension (DASH) diet is a dietary pattern that is rich in fruit, vegetables, and low-fat dairy products; moderate in meat, fish, poultry, nuts, and beans; and low in sugar-sweetened beverages, sweets, and red meat. In the original DASH feeding study, the reduction in systolic blood pressure was 2.8 mm Hg for the fruit and vegetables only diet and 5.5 mm Hg for the DASH diet compared with the control diet, with even greater blood pressure reductions among individuals with hypertension (1). Epidemiologic studies have subsequently shown that higher adherence to the DASH diet was associated with a multitude of favorable health outcomes, including a reduced risk of hypertension, cardiovascular disease, kidney disease, and mortality (2–5). The DASH diet has been recommended as a healthy dietary pattern for the general population by the US Department of Health and Human Services and the US Food and Drug Administration in the US Dietary Guidelines for Americans as well as by the American Heart Association for the prevention of cardiovascular disease (6, 7).
Biomarkers of dietary intake are useful as objective measures of adherence to a dietary pattern that are not influenced by recall bias, social desirability bias, accuracy of databases used to analyze dietary data, and other types of systematic error that accompany self-reported measures of dietary intake (8, 9). Currently available biomarkers use 24-h urine collections and assess individual nutrients (e.g., urea nitrogen to estimate dietary intake of protein) (10). Metabolomic profiling is the detection of small molecules as a representation of the overall biological system that is influenced by dietary intake (11, 12). Thus, global, untargeted metabolomics can be leveraged to identify novel and established biomarkers of dietary intake, including overall dietary patterns, as well as to characterize the range of metabolic changes attributed to dietary intake (13–15).
The objective of this study was to identify metabolites associated with the DASH dietary pattern by conducting untargeted metabolomic profiling in serum specimens collected from participants randomly assigned to the DASH diet, the fruit and vegetables diet, or a control diet. The main appeal and novelty of our study are that we aimed to identified candidate biomarkers of an overall dietary pattern, which is a more relevant exposure given that nutrients do not act in isolation and given that specific dietary patterns, including the DASH diet, are recommended for health promotion (6, 7).
METHODS
Study design and population
The DASH trial was a multicenter, randomized feeding study designed to test the effect of overall dietary patterns (rather than individual nutrients) on blood pressure (1). The trial design and methods have been previously published (16). In brief, after a 3-wk run-in period with the control diet, participants were randomly assigned to 1 of 3 diet interventions for 8 wk: the DASH diet, the fruit and vegetables diet, or a control diet. Eligible participants were men and women (≥22 y of age) with systolic blood pressure <160 mm Hg and diastolic blood pressure of 80–95 mm Hg. Participants provided written informed consent. The present study was approved by a Johns Hopkins Institutional Review Board, and procedures were followed in accordance with the ethical standards of the institutional review board.
In this study, we obtained stored serum specimens from the National Health, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) (17–19). To characterize the metabolome in response to the diet interventions, we used serum specimens collected at the end of the 8-wk intervention. Among the 459 participants randomly assigned to the DASH feeding study, 3 participants were not included in the repository, 113 participants did not provide informed consent for further use of their biological specimens, 10 participants did not attend the week 8 visit, and 4 participants did not have a sufficient volume of serum available in the repository (Supplemental Figure 1). Therefore, specimens from 329 participants were analyzed in the present study. This trial was registered at http://www.clinicaltrials.gov as NCT03403166.
Dietary exposures
The DASH diet consisted of a high intake of fruit, vegetables, and low-fat dairy products (1, 16). It included a wide range of sources of protein, such as meat, fish, poultry, nuts, and beans. Sugar-sweetened beverages, desserts, and red meat were restricted. In terms of nutrients, the DASH diet had a high amount of fiber and protein; low amounts of saturated fat, total fat, and cholesterol; and intakes of potassium, magnesium, and calcium at amounts close to the 75th percentile of US consumption based on national survey data from the 1970s–1980s (20, 21). The fruit and vegetables diet was similar to the DASH diet with respect to consisting of a high amount of fiber and amounts of potassium and magnesium close to the 75th percentile of US consumption. Relative to the control diet, the fruit and vegetables diet contained more fruit and vegetables and fewer carbohydrate-rich sweet desserts and snacks. Otherwise, the fruit and vegetables diet was similar to the control diet with regard to amounts of calcium (from dairy products), protein, and fat as well as being similar in terms of fat composition. For the control diet, macronutrient intake was similar to average US consumption and intakes of potassium, magnesium, and calcium were similar to the 25th percentile of US consumption. Fat intake (saturated fat, in particular, as well as monounsaturated fat) was lower in the DASH diet relative to both the control diet and the fruit and vegetables diet. Sodium intake was 3 g/d in each diet for all 3 diets. Food was provided at 1 of 4 calorie amounts (1600, 2100, 2600, or 3100 kcal/d), and meals were standardized across centers. The nutrient composition of the diets derived from chemical analyses of menus prepared during the trial are presented at the 2100-kcal intake amount (Table 1).
TABLE 1.
Daily nutritional composition of the randomized diet interventions1
| Nutrient | Control diet | Fruit and vegetables diet | DASH diet |
|---|---|---|---|
| Energy intake, kcal | 2084.7 | 2105.6 | 2094.4 |
| Carbohydrate, % of energy | 49.8 | 52.3 | 58.2 |
| Protein, % of energy | 14.1 | 15.2 | 18.2 |
| Fat, % of energy | 36.8 | 36.7 | 27.3 |
| SFAs, % of energy | 14.4 | 13.0 | 7.4 |
| MUFAs, % of energy | 12.6 | 14.0 | 10.5 |
| PUFAs, % of energy | 7.1 | 6.9 | 7.6 |
| Sodium, mg | 2922.5 | 2834.3 | 2880.9 |
| Calcium, mg | 446.0 | 467.9 | 1220.1 |
| Magnesium, mg | 169.2 | 416.4 | 464.7 |
| Potassium, mg | 1742.8 | 4433.5 | 4589.1 |
| Phosphorus, mg | 939.7 | 1007.1 | 1481.1 |
| Fiber, g/1000 kcal | 5.1 | 14.7 | 14.3 |
| Cholesterol, mg/1000 kcal | 118.0 | 89.4 | 67.1 |
| Vitamin A, IU | 6192.3 | 14,409.1 | 14,020.0 |
| Thiamin (vitamin B-1), mg | 1.8 | 1.7 | 1.5 |
| Riboflavin (vitamin B-2), mg | 1.5 | 1.3 | 1.9 |
| Niacin (vitamin B-3), mg | 23.1 | 22.4 | 22.6 |
| Pantothenic acid (vitamin B-5), mg | 3.0 | 3.8 | 4.7 |
| Vitamin B-6, mg | 1.4 | 2.7 | 2.5 |
| Vitamin B-12, μg | 2.9 | 3.1 | 4.2 |
| Vitamin C, mg | 132.8 | 201.8 | 266.2 |
| Vitamin E | 7.6 | 10.8 | 12.7 |
| Folate, μg | 168.2 | 348.2 | 390.3 |
| Iron, mg | 15.6 | 17.8 | 20.2 |
| Zinc, mg | 7.6 | 9.9 | 10.4 |
| Caffeine, mg | 2.3 | 0.0 | 0.0 |
1Nutrients at the 2100-kcal intake amount were derived from chemical analyses of menus prepared during the trial. DASH, Dietary Approaches to Stop Hypertension.
Participants ate either lunch or dinner onsite during weekdays and were provided with all other meals to be consumed off site. With regard to beverages, those containing alcohol were limited to 2 drinks/d and caffeinated beverages were limited to 3 servings/d.
Metabolomic profiling
Global, untargeted metabolomic profiling was performed by using an untargeted, gas chromatography–mass spectrometry and liquid chromatography–mass spectrometry protocol with Thermo Scientific Orbitrap mass spectrometers by Metabolon (22). Metabolite identification was achieved by matching retention times, m/z, and related fragment spectra to reference compounds that were included in each sample queue and characterized in an extensive chemical library. The known metabolites identified by this metabolomic panel are highly reproducible due to the validation of the metabolites with the use of reference standards to confirm compound structure. Samples were run in a single batch and in random order and were not ordered by intervention group. Laboratory technicians worked with de-identified samples, which lacked any indication of intervention group or other sample characteristics. The median CV for this platform was 18.9% (25th–75th percentile: 12.3–32.9%). The number of metabolites for which the CV was <10% was 179, and the number of metabolites for which the CV was <20% was 592.
A total of 1238 metabolites were identified by the untargeted metabolomic panel. Metabolites with >80% missing in the serum specimens were excluded (n = 21). For the remaining metabolites, missing values were imputed to the minimum detected level. Metabolites were then re-scaled to a median of 1 and log transformed. Metabolites with a variance <0.01 on the log scale were excluded (n = 11). Values were capped at 5 SDs. After this data-cleaning process, we further excluded unknown compounds (n = 388). The present study focused on the remaining 818 standardized known metabolites.
Statistical analysis
Baseline characteristics of the study population are presented with the use of descriptive statistics according to randomized diet intervention group. Linear regression was used to assess the association between the randomized diet intervention groups (exposure) and the individual standardized metabolites (outcome). Crude regression models as well as multivariable regression models adjusted for age (continuous), sex (male or female), race (minority or nonminority), total energy intake (continuous), and BMI (continuous) were conducted. The primary analysis was conducted by randomization arm, allowing for approximately equal distribution of known and unknown confounders across groups. Because the analytic sample for the present study was a subset of the randomly assigned participants in the DASH trial, we adjusted for baseline covariates in order to increase precision (23).
In addition to analyzing the individual metabolites, partial least-squares discriminant analysis was used to detect a panel of metabolites representative of the DASH diet relative to the other 2 diet intervention groups. As a measure of the cumulative ability to distinguish between diets, we calculated the AUC (C statistic) for the addition of the panel of 10 metabolites to participant characteristics (age, sex, race, total energy intake, and BMI) in a logistic regression model with randomly assigned diet group as the outcome (24). We calculated C statistics after fitting the model on a random sample of two-thirds of the analytic sample and then validated in the remaining one-third of the sample. Bonferroni correction was used as a conservative method to account for multiple testing due to the large number of metabolites (α-level = 0.05/818 metabolites = 6.11 × 10−5) (25).
RESULTS
In the analytic study population (n = 329), approximately half of the participants were women (47%), approximately half were from a minority racial group (57%), and approximately one-quarter had hypertension (26%) (Table 2). The majority of study participants were aged 31–55 y (69%). The mean BMI (in kg/m2) was 28 and the mean blood pressure was 130/84 mm Hg. Baseline characteristics were generally similar for those randomly assigned to the control diet, the fruit and vegetables diet, and the DASH diet.
TABLE 2.
Baseline characteristics according to randomized diet interventions1
| Control diet (n = 108) | Fruit and vegetables diet (n = 111) | DASH diet (n = 110) | Total (n = 329) | |
|---|---|---|---|---|
| Age category, % (n) | ||||
| 18–30 y | 14.8 (16) | 11.7 (13) | 9.1 (10) | 11.9 (39) |
| 31–55 y | 63.0 (68) | 68.5 (76) | 75.5 (83) | 69.0 (227) |
| ≥56 y | 22.2 (24) | 19.8 (22) | 15.5 (17) | 19.2 (63) |
| Female sex, % (n) | 42.6 (46) | 45.1 (50) | 52.7 (58) | 46.8 (154) |
| Minority race, % (n) | 54.6 (59) | 55.0 (61) | 60.9 (67) | 56.8 (187) |
| Household income,2 % (n) | ||||
| <$29,999 | 34.9 (37) | 30.6 (33) | 30.9 (34) | 32.1 (104) |
| $30,000–$59,999 | 43.4 (46) | 38.0 (41) | 47.3 (52) | 42.9 (139) |
| ≥$60,000 | 21.7 (23) | 31.5 (34) | 21.8 (24) | 25.0 (81) |
| Employment status,3 % (n) | ||||
| Full time | 76.6 (82) | 70.9 (78) | 80.0 (88) | 75.8 (248) |
| Part time | 7.5 (8) | 8.2 (9) | 5.5 (6) | 7.0 (23) |
| Retired | 7.5 (8) | 9.1 (10) | 3.6 (4) | 6.7 (22) |
| Other | 8.4 (9) | 11.8 (13) | 10.9 (12) | 10.4 (34) |
| Educational level, % (n) | ||||
| High school graduate or less | 19.4 (21) | 19.8 (22) | 10.9 (12) | 16.7 (55) |
| Some college | 31.5 (34) | 31.5 (35) | 40.9 (45) | 34.7 (114) |
| College graduate | 25.0 (27) | 21.6 (24) | 31.8 (35) | 26.1 (86) |
| Postgraduate work/degree | 24.1 (26) | 27.0 (30) | 16.4 (18) | 22.5 (74) |
| Current smoker,4 % (n) | 26.8 (11) | 34.0 (18) | 15.6% (7) | 25.9 (36) |
| Weight, kg | 82.4 ± 15.0 | 81.3 ± 13.2 | 82.6 ± 14.7 | 82.1 ± 14.3 |
| BMI, kg/m2 | 28.0 ± 3.9 | 27.9 ± 4.0 | 28.3 ± 3.9 | 28.1 ± 3.9 |
| SBP, mm Hg | 130.0 ± 12.5 | 130.6 ± 13.5 | 129.9 ± 11.9 | 130.1 ± 12.6 |
| DBP, mm Hg | 85.2 ± 6.8 | 84.3 ± 7.0 | 83.7 ± 7.0 | 84.4 ± 6.9 |
| Ever used BP medication,5 % (n) | 46.9 (23) | 62.0 (31) | 46.2 (24) | 48.3 (73) |
| Hypertension status, % (n) | 26.9 (29) | 27.9 (31) | 24.6 (27) | 26.4 (87) |
1Values are percentages (n) for categorical variables and means ± SDs for continuous variables. BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure.
2Five study participants had missing information on household income.
3Two study participant had missing information on employment status.
4One hundred ninety study participants had missing information on cigarette smoking status.
5One hundred seventy-eight study participants had missing information on BP medication use.
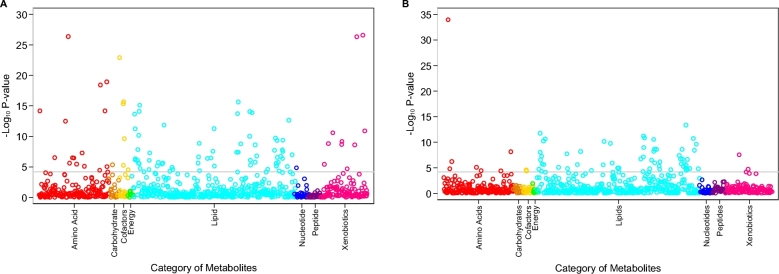
Serum concentrations of 97 known metabolites differed significantly between participants who were randomly assigned to the DASH diet compared with participants randomly assigned to the control diet at the Bonferroni-corrected threshold and after adjustment for age, sex, race, education, BMI, and hypertension (Table 3). The majority of these 97 significant metabolites were lipids (n = 64; 66%). The other categories of metabolites that were significantly different between those randomly assigned to the DASH compared with the control diet included amino acids (n = 15); xenobiotics, which include food components (n = 10); cofactors and vitamins (n = 6); carbohydrate (n = 1); and nucleotides (n = 1). The majority of the amino acids had positive coefficients (12 out of 15), representing higher serum concentrations of amino acid metabolites in the DASH diet compared with the control diet. In contrast, the majority of the lipids had negative coefficients (54 out of 64), indicating lower concentrations of lipid metabolites in participants randomly assigned to the DASH diet relative to the control diet. The smallest P values for the association with the DASH compared with the control diet were observed for amino acids, vitamins and cofactors, and xenobiotics (food components) (Figure 1A).
TABLE 3.
Full list of 97 metabolites significantly associated with the DASH diet relative to the control diet1
| Category and Metabolic Pathway | Metabolite | β2 | SE | P |
|---|---|---|---|---|
| Amino acid | ||||
| Histidine metabolism | N-acetyl-1-methylhistidine | −0.45867 | 0.087168 | 3.51 × 10−7 |
| Leucine, isoleucine and valine metabolism | 2,3-Dihydroxy-2-methylbutyrate | 0.595027 | 0.070507 | 5.19 × 10−15 |
| Leucine, isoleucine, and valine metabolism | β-Hydroxyisovalerate | −0.26521 | 0.05604 | 4.07 × 10−6 |
| Lysine metabolism | Pipecolate | 0.694017 | 0.070502 | 4.84 × 10−19 |
| Methionine, cysteine, SAM, and taurine metabolism | S-methylmethionine | 0.782472 | 0.150277 | 4.57 × 10−7 |
| Phenylalanine metabolism | Phenylalanine | 0.068449 | 0.016297 | 3.94 × 10−5 |
| Tryptophan metabolism | Tryptophan betaine | 1.616693 | 0.1609 | 1.20 × 10−19 |
| Tyrosine metabolism | Dopamine 3-O-sulfate | 0.410064 | 0.072556 | 5.14 × 10−8 |
| Tyrosine metabolism | Gentisate | 0.573119 | 0.126856 | 1.04 × 10−5 |
| Urea cycle; arginine and proline metabolism | N-methylproline | 1.943721 | 0.115198 | <1.00 × 10−40 |
| Urea cycle; arginine and proline metabolism | trans-4-Hydroxyproline | −0.32266 | 0.038192 | 4.90 × 10−15* |
| Urea cycle; arginine and proline metabolism | N-δ-acetylornithine | 0.550054 | 0.070972 | 3.87 × 10−13 |
| Urea cycle; arginine and proline metabolism | Argininate | 0.309273 | 0.058899 | 3.70 × 10−7 |
| Urea cycle; arginine and proline metabolism | N2,N5-diacetylornithine | 0.369682 | 0.076189 | 2.38 × 10−6 |
| Urea cycle; arginine and proline metabolism | Urea | 0.148103 | 0.032773 | 1.04 × 10−5 |
| Carbohydrate | ||||
| Fructose, mannose and galactose metabolism | Galactonate | 0.891876 | 0.187847 | 3.80 × 10−6 |
| Cofactors and vitamins | ||||
| Nicotinate and nicotinamide metabolism | Trigonelline (N'-methylnicotinate) | 0.511047 | 0.120622 | 3.39 × 10−5 |
| Tocopherol metabolism | γ-Tocopherol/β-tocopherol | −0.43779 | 0.065436 | 1.99 × 10−10 |
| Vitamin A metabolism | β-Cryptoxanthin | 1.069524 | 0.093892 | 1.02 × 10−23 |
| Vitamin A metabolism | Carotene diol (2) | 0.557063 | 0.06252 | 2.47 × 10−16* |
| Vitamin A metabolism | Carotene diol (1) | 0.572143 | 0.065107 | 5.50 × 10−16* |
| Vitamin A metabolism | Carotene diol (3) | 0.318296 | 0.068018 | 5.15 × 10−6 |
| Lipid | ||||
| Carnitine metabolism | Carnitine | −0.10746 | 0.02037 | 3.29 × 10−7 |
| Ceramides | Glycosyl-N-tricosanoyl-sphingadienine (d18:2/23:0) | −0.41433 | 0.046622 | 2.87 × 10−16* |
| Ceramides | Glycosyl-N-stearoyl-sphingosine (d18:1/18:0) | −0.32315 | 0.03929 | 2.03 × 10−14* |
| Ceramides | Ceramide (d18:1/17:0, d17:1/18:0) | −0.34967 | 0.059958 | 2.05 × 10−8 |
| Ceramides | Glycosyl-N-behenoyl-sphingadienine (d18:2/22:0) | −0.23 | 0.040415 | 4.21 × 10−8* |
| Ceramides | Glycosyl ceramide (d18:1/20:0, d16:1/22:0) | −0.21128 | 0.037143 | 4.27 × 10−8* |
| Ceramides | N-stearoyl-sphingosine (d18:1/18:0) | −0.25309 | 0.047169 | 2.12 × 10−7 |
| Ceramides | N-palmitoyl-sphingosine (d18:1/16:0) | −0.1422 | 0.029282 | 2.34 × 10−6 |
| Ceramides | Glycosyl-N-palmitoyl-sphingosine (d18:1/16:0) | −0.13029 | 0.031797 | 5.96 × 10−5 |
| Diacylglycerol | Linoleoyl-docosahexaenoyl-glycerol (18:2/22:6) [1] | 0.750572 | 0.090322 | 1.18 × 10−4* |
| Diacylglycerol | Linoleoyl-linolenoyl-glycerol (18:2/18:3) [2] | 0.874337 | 0.129321 | 1.33 × 10−10* |
| Diacylglycerol | Linoleoyl-linolenoyl-glycerol (18:2/18:3) [1] | 0.496667 | 0.097542 | 7.87 × 10−7* |
| Diacylglycerol | Linoleoyl-linoleoyl-glycerol (18:2/18:2) [1] | 0.293617 | 0.061658 | 3.57 × 10−6* |
| Fatty acid, branched | 17-Methylstearate (i19:0) | −0.22335 | 0.049733 | 1.17 × 10−5 |
| Fatty acid, branched | 15-Methylpalmitate (i17:0) | −0.25101 | 0.059751 | 3.93 × 10−5 |
| Fatty acid, dicarboxylate | Heptenedioate (C7:1-DC) | −0.49793 | 0.079246 | 1.88 × 10−9* |
| Fatty acid, dicarboxylate | Octadecenedioate (C18:1-DC) | 0.407662 | 0.074999 | 1.51 × 10−7 |
| Fatty acid metabolism (acyl carnitine) | Margaroylcarnitine (C17) | −0.39482 | 0.04743 | 1.08 × 10−14* |
| Fatty acid metabolism (acyl carnitine) | Stearoylcarnitine (C18) | −0.33796 | 0.043196 | 2.47 × 10−13* |
| Fatty acid metabolism (acyl carnitine) | Arachidoylcarnitine (C20) | −0.30085 | 0.058364 | 5.85 × 10−7* |
| Fatty acid metabolism (acyl carnitine) | Myristoylcarnitine (C14) | −0.30075 | 0.060395 | 1.33 × 10−6* |
| Fatty acid metabolism (acyl carnitine) | Palmitoylcarnitine (C16) | −0.15729 | 0.037251 | 3.60 × 10−5 |
| Fatty acid metabolism (acyl carnitine) | Adipoylcarnitine (C6-DC) | −0.36867 | 0.087552 | 3.77 × 10−5* |
| Fatty acid, monohydroxy | 2-Hydroxydecanoate | 0.266559 | 0.062563 | 3.08 × 10−5* |
| Inositol metabolism | Chiro-inositol | 1.221824 | 0.166378 | 4.51 × 10−12 |
| Lysophospholipid | 1-Oleoyl-GPC (18:1) | −0.11774 | 0.021036 | 6.76 × 10−8 |
| Lysophospholipid | 1-Arachidonoyl-GPE (20:4n–6) | −0.13501 | 0.030852 | 1.90 × 10−5 |
| Lysoplasmalogen | 1-(1-Enyl-stearoyl)-GPE (P-18:0) | −0.23956 | 0.049415 | 2.43 × 10−6 |
| Lysoplasmalogen | 1-(1-Enyl-palmitoyl)-GPC (P-16:0) | −0.16751 | 0.03463 | 2.55 × 10−6* |
| PC | 1-Stearoyl-2-oleoyl-GPC (18:0/18:1) | −0.22556 | 0.030072 | 1.76 × 10−12 |
| PC | 1-Palmitoyl-2-stearoyl-GPC (16:0/18:0) | −0.12019 | 0.024556 | 1.96 × 10−6 |
| PC | 1-Stearoyl-2-docosahexaenoyl-GPC (18:0/22:6) | 0.15169 | 0.031927 | 3.75 × 10−6* |
| PC | 1-Palmitoyl-2-oleoyl-GPC (16:0/18:1) | −0.10351 | 0.022223 | 5.67 × 10−6 |
| PC | 1-Myristoyl-2-palmitoyl-GPC (14:0/16:0) | −0.25447 | 0.059347 | 2.75 × 10−5 |
| PE | 1-Oleoyl-2-docosahexaenoyl-GPE (18:1/22:6) | 0.567689 | 0.112733 | 1.02 × 10−6 |
| PE | 1-Stearoyl-2-docosahexaenoyl-GPE (18:0/22:6) | 0.301512 | 0.062704 | 2.90 × 10−6* |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-linoleoyl-GPE (P-18:0/18:2) | −0.37684 | 0.043281 | 9.29 × 10−16 |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-oleoyl-GPE (P-18:0/18:1) | −0.38933 | 0.046662 | 9.55 × 10−15 |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) | −0.2533 | 0.030894 | 2.39 × 10−14* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-linoleoyl-GPE (P-16:0/18:2) | −0.30174 | 0.041238 | 5.29 × 10−12* |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4) | −0.27658 | 0.039892 | 4.98 × 10−11* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-oleoyl-GPE (P-16:0/18:1) | −0.23614 | 0.040243 | 1.70 × 10−8* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-arachidonoyl-GPC (P-16:0/20:4) | −0.16259 | 0.031136 | 4.25 × 10−7* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-oleoyl-GPC (P-16:0/18:1) | −0.16479 | 0.032647 | 9.67 × 10−7* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-arachidonoyl-GPE (P-16:0/20:4) | −0.1884 | 0.037447 | 1.05 × 10−6 |
| Sphingolipid metabolism | Sphingomyelin (d18:0/18:0, d19:0/17:0) | −0.41936 | 0.06287 | 2.22 × 10−10* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/18:1, d18:2/18:0) | −0.18252 | 0.028076 | 5.71 × 10−10* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/18:1) | −0.25827 | 0.039801 | 6.11 × 10−10* |
| Sphingolipid metabolism | N-stearoyl-sphinganine (d18:0/18:0) | −0.67919 | 0.107103 | 1.37 × 10−9* |
| Sphingolipid metabolism | Myristoyl dihydrosphingomyelin (d18:0/14:0) | −0.21392 | 0.034377 | 2.61 × 10−9* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/23:0, d18:1/23:1, d17:1/24:1) | −0.17172 | 0.029436 | 2.03 × 10−8* |
| Sphingolipid metabolism | Sphingomyelin (d17:1/16:0, d18:1/15:0, d16:1/17:0) | −0.148 | 0.025569 | 2.56 × 10−8* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/21:0, d16:2/23:0) | −0.19818 | 0.034674 | 3.72 × 10−8* |
| Sphingolipid metabolism | Tricosanoyl sphingomyelin (d18:1/23:0) | −0.16936 | 0.030439 | 7.99 × 10−8* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/17:0, d17:1/18:0, d19:1/16:0) | −0.1602 | 0.029845 | 2.10 × 10−7* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/14:0, d18:1/14:1) | −0.20796 | 0.03884 | 2.24 × 10−7* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/16:0, d18:1/16:1) | −0.09578 | 0.018582 | 5.86 × 10−7* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/19:0, d19:1/18:0) | −0.1705 | 0.03531 | 2.64 × 10−6* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/14:0, d16:1/16:0) | −0.11207 | 0.023445 | 3.29 × 10−6 |
| Sphingolipid metabolism | Sphingomyelin (d17:2/16:0, d18:2/15:0) | −0.17955 | 0.038941 | 6.97 × 10−6 |
| Sphingolipid metabolism | Stearoyl sphingomyelin (d18:1/18:0) | −0.13337 | 0.029426 | 9.78 × 10−6* |
| Sphingolipid metabolism | Sphingomyelin (d18:0/20:0, d16:0/22:0) | −0.30627 | 0.06768 | 1.01 × 10−5* |
| Sphingolipid metabolism | Palmitoyl sphingomyelin (d18:1/16:0) | −0.06746 | 0.016341 | 5.27 × 10−5* |
| Sterol | Cholesterol | −0.13843 | 0.030192 | 7.79 × 10−6* |
| Nucleotide | ||||
| Pyrimidine metabolism, uracil containing | 3-Ureidopropionate | 0.238775 | 0.053182 | 1.18 × 10−5 |
| Xenobiotics | ||||
| Benzoate metabolism | Catechol sulfate | 0.347247 | 0.080657 | 2.56 × 10−5 |
| Chemical | 2-Aminophenol sulfate | 0.525099 | 0.109775 | 3.24 × 10−6 |
| Food component/plant | Stachydrine | 1.839321 | 0.103578 | <1.00 × 10−40 |
| Food component/plant | Methyl glucopyranoside (α + β) | 1.065195 | 0.085152 | 3.39 × 10−27 |
| Food component/plant | Homostachydrine | −0.31801 | 0.051036 | 2.49 × 10−9 |
| Xanthine metabolism | Theobromine | −1.48162 | 0.206626 | 1.25 × 10−11 |
| Xanthine metabolism | 3-Methylxanthine | −1.08958 | 0.154741 | 2.67 × 10−11 |
| Xanthine metabolism | 7-Methylxanthine | −0.93264 | 0.144281 | 7.01 × 10−10 |
| Xanthine metabolism | 3,7-Dimethylurate | −0.79681 | 0.126677 | 1.81 × 10−9 |
| Xanthine metabolism | 7-Methylurate | −1.37944 | 0.22019 | 2.08 × 10−9 |
1Significance was determined at the Bonferroni-adjusted threshold (P < 6.11 × 10−5). *Significant for both comparisons (DASH diet compared with the control diet and DASH diet compared with the fruit and vegetables diet). DASH, Dietary Approaches to Stop Hypertension; GPC, glycerophosphorylcholine; GPE, glycerophosphorylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SAM, S-Adenosyl methionine.
2β-Coefficients represent the serum metabolite concentration associated with the DASH diet compared with the control diet in the multivariable linear regression model adjusted for age, sex, race, education, BMI, and hypertension. Positive β-coefficients indicate that the metabolite was higher among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. Conversely, negative β-coefficients indicate that the metabolite was lower among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. Metabolites are sorted by category and metabolic pathway.
FIGURE 1.
Plot of −log10P values for the adjusted association between serum known metabolites and the DASH diet compared with the control diet (A) and fruit and vegetables diet (B). P values were calculated from multivariable regression models adjusted for age, sex, minority race, educational level, BMI, and hypertension status. The analysis was conducted in the 110 participants randomly assigned to the DASH diet and the 108 participants randomly assigned to the control diet in panel A and among 110 participants randomly assigned to the DASH diet and 111 participants randomly assigned to the fruit and vegetables diet in panel B. DASH, Dietary Approaches to Stop Hypertension.
There were a total of 67 serum metabolites that were significantly different between participants randomly assigned to the DASH diet compared with participants randomly assigned to the fruit and vegetables diet at the Bonferroni-corrected threshold and after adjustment for age, sex, race, education, BMI, and hypertension, the majority of which were lipids (n = 56; 84%) followed by amino acids (n = 7), xenobiotics (n = 2), and cofactors and vitamins (n = 2) (Table 4). All of the amino acids, xenobiotics, and cofactors and vitamins had positive coefficients representing higher serum concentrations in the DASH diet than in the fruit and vegetables diet. Although a majority of lipids had negative coefficients, representing lower serum concentrations with the DASH diet relative to the fruit and vegetables diet, 8 lipids had positive coefficients, including diacylglycerol, phosphotidylcholine, phosphotidylethanolamine, and a metabolite of phospholipid metabolism (trimethylamine N-oxide). The smallest P value for the association between the DASH diet compared with the fruit and vegetables diet was observed for an amino acid, 2-methylserine (Figure 1B).
TABLE 4.
Full list of 67 metabolites significantly associated with the DASH diet relative to the fruit and vegetables diet1
| Category and metabolic pathway | Metabolite | β2 | SE | P |
|---|---|---|---|---|
| Amino acid | ||||
| Glutamate metabolism | Carboxyethyl-GABA | −0.26855 | 0.063499 | 3.48 × 10−5 |
| Glycine, serine, and threonine metabolism | 2-Methylserine | 0.775158 | 0.052307 | 1.29 × 10−34 |
| Leucine, isoleucine, and valine metabolism | 3-Methylglutarylcarnitine (2) | −0.48628 | 0.094783 | 6.49 × 10−7 |
| Leucine, isoleucine, and valine metabolism | 3-Hydroxy-2-ethylpropionate | −0.21344 | 0.04753 | 1.16 × 10−5 |
| Methionine, cysteine, SAM, and taurine metabolism | S-methylcysteine sulfoxide | −0.24825 | 0.054271 | 8.11 × 10−6 |
| Methionine, cysteine, SAM, and taurine metabolism | Methionine sulfone | −0.20213 | 0.048863 | 5.07 × 10−5 |
| Urea cycle; arginine and proline metabolism | trans-4-Hydroxyproline | −0.26211 | 0.043879 | 9.64 × 10−9* |
| Cofactors and vitamins | ||||
| Vitamin A metabolism | Carotene diol (2) | 0.261687 | 0.060962 | 2.68 × 10−5* |
| Vitamin A metabolism | Carotene diol (1) | 0.27648 | 0.066347 | 4.48 × 10−5* |
| Lipid | ||||
| Ceramides | Glycosyl-N-tricosanoyl-sphingadienine (d18:2/23:0) | −0.32621 | 0.045899 | 1.76 × 10−11* |
| Ceramides | Glycosyl-N-stearoyl-sphingosine (d18:1/18:0) | −0.25529 | 0.039805 | 9.04 × 10−10* |
| Ceramides | Glycosyl-N-behenoyl-sphingadienine (d18:2/22:0) | −0.22449 | 0.040319 | 7.72 × 10−8* |
| Ceramides | Glycosyl ceramide (d18:1/20:0, d16:1/22:0) | −0.20899 | 0.038724 | 1.80 × 10−7* |
| Ceramides | Ceramide (d18:1/17:0, d17:1/18:0) | −0.27873 | 0.060998 | 8.28 × 10−6* |
| Diacylglycerol | Linoleoyl-docosahexaenoyl-glycerol (18:2/22:6) [1] | 0.684538 | 0.096634 | 2.01 × 10−11* |
| Diacylglycerol | Linoleoyl-linolenoyl-glycerol (18:2/18:3) [2] | 0.80546 | 0.127795 | 1.66 × 10−9* |
| Diacylglycerol | Linoleoyl-linolenoyl-glycerol (18:2/18:3) [1] | 0.62017 | 0.10245 | 6.32 × 10−9* |
| Diacylglycerol | Linoleoyl-linoleoyl-glycerol (18:2/18:2) [1] | 0.327509 | 0.066506 | 1.69 × 10−6* |
| Fatty acid, amino | 2-Aminooctanoate | −0.35515 | 0.073398 | 2.51 × 10−6 |
| Fatty acid, dicarboxylate | Heptenedioate (C7:1-DC) | −0.64989 | 0.089294 | 6.42 × 10−12* |
| Fatty acid metabolism (acyl carnitine) | Stearoylcarnitine (C18) | −0.29912 | 0.042352 | 2.28 × 10−11* |
| Fatty acid metabolism (acyl carnitine) | Adipoylcarnitine (C6-DC) | −0.55124 | 0.079592 | 5.04 × 10−11* |
| Fatty acid metabolism (acyl carnitine) | Arachidoylcarnitine (C20) | −0.40633 | 0.060272 | 1.45 × 10−10* |
| Fatty acid metabolism (acyl carnitine) | Margaroylcarnitine (C17) | −0.32726 | 0.052189 | 1.97 × 10−9* |
| Fatty acid metabolism (acyl carnitine) | Suberoylcarnitine (C8-DC) | −0.61373 | 0.099598 | 3.54 × 10−9 |
| Fatty acid metabolism (acyl carnitine) | Lignoceroylcarnitine (C24) | −0.2794 | 0.053731 | 4.67 × 10−7 |
| Fatty acid metabolism (acyl carnitine) | Octadecanedioylcarnitine (C18-DC) | −0.32593 | 0.063603 | 6.68 × 10−7 |
| Fatty acid metabolism (acyl carnitine) | Myristoylcarnitine (C14) | −0.28495 | 0.060824 | 4.99 × 10−6* |
| Fatty acid metabolism (acyl carnitine) | 5-Dodecenoylcarnitine (C12:1) | −0.32105 | 0.075642 | 3.27 × 10−5 |
| Fatty acid, monohydroxy | 2-Hydroxyoctanoate | −0.35607 | 0.058764 | 6.13 × 10−9 |
| Lysoplasmalogen | 1-(1-Enyl-palmitoyl)-GPC (P-16:0) | −0.13628 | 0.033048 | 5.34 × 10−5* |
| PC | 1-Stearoyl-2-docosahexaenoyl-GPC (18:0/22:6) | 0.180569 | 0.031036 | 2.16 × 10−8* |
| PC | 1-Palmitoyl-2-docosahexaenoyl-GPC (16:0/22:6) | 0.101766 | 0.022672 | 1.17 × 10−5 |
| PE | 1-Stearoyl-2-docosahexaenoyl-GPE (18:0/22:6) | 0.277821 | 0.062915 | 1.6 × 10−5* |
| Phospholipid metabolism | Trimethylamine N-oxide | 0.418681 | 0.093599 | 1.25 × 10−5 |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) | −0.22599 | 0.030174 | 1.81 × 10−12* |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-oleoyl-GPE (P-18:0/18:1) | −0.32627 | 0.046166 | 2.22 × 10−11 |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-linoleoyl-GPE (P-18:0/18:2) | −0.30648 | 0.044454 | 6.05 × 10−11 |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-oleoyl-GPE (P-16:0/18:1) | −0.2358 | 0.039634 | 1.09 × 10−8* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-oleoyl-GPC (P-16:0/18:1) | −0.16364 | 0.029343 | 7.38 × 10−8* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-linoleoyl-GPE (P-16:0/18:2) | −0.21172 | 0.040592 | 4.33 × 10−7* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-palmitoyl-GPC (P-16:0/16:0) | −0.13749 | 0.028368 | 2.42 × 10−6 |
| Plasmalogen | 1-(1-Enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4) | −0.19195 | 0.03993 | 2.89 × 10−6* |
| Plasmalogen | 1-(1-Enyl-palmitoyl)-2-arachidonoyl-GPC (P-16:0/20:4) | −0.14101 | 0.032593 | 2.33 × 10−5* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0,d19:1/24:0) | −0.37356 | 0.046177 | 4.50 × 10−14 |
| Sphingolipid metabolism | Tricosanoyl sphingomyelin (d18:1/23:0) | −0.18925 | 0.028603 | 2.93 × 10−10* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/17:0, d17:1/18:0, d19:1/16:0) | −0.17426 | 0.028035 | 2.65 × 10−9* |
| Sphingolipid metabolism | Sphingomyelin (d17:1/16:0, d18:1/15:0, d16:1/17:0) | −0.14147 | 0.024534 | 2.82 × 10−8* |
| Sphingolipid metabolism | Sphingomyelin (d18:0/18:0, d19:0/17:0) | −0.34152 | 0.06282 | 1.48 × 10−7* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/18:1, d18:2/18:0) | −0.13973 | 0.025814 | 1.66 × 10−7* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/23:0, d18:1/23:1, d17:1/24:1) | −0.14678 | 0.027995 | 3.80 × 10−7* |
| Sphingolipid metabolism | Behenoyl sphingomyelin (d18:1/22:0) | −0.10655 | 0.021701 | 1.81 × 10−6 |
| Sphingolipid metabolism | Sphingomyelin (d18:0/20:0, d16:0/22:0) | −0.32968 | 0.067794 | 2.24 × 10−6* |
| Sphingolipid metabolism | N-stearoyl-sphinganine (d18:0/18:0) | −0.51683 | 0.106722 | 2.46 × 10−6* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0) | −0.15608 | 0.03224 | 2.48 × 10−6 |
| Sphingolipid metabolism | Sphingomyelin (d18:2/16:0, d18:1/16:1) | −0.08372 | 0.017642 | 3.82 × 10−6* |
| Sphingolipid metabolism | Sphingomyelin (d18:1/19:0, d19:1/18:0) | −0.17133 | 0.036743 | 5.50 × 10−6* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/14:0, d18:1/14:1) | −0.16539 | 0.037094 | 1.33 × 10−5* |
| Sphingolipid metabolism | Lignoceroyl sphingomyelin (d18:1/24:0) | −0.12772 | 0.028749 | 1.43 × 10−5 |
| Sphingolipid metabolism | Stearoyl sphingomyelin (d18:1/18:0) | −0.12459 | 0.028233 | 1.62 × 10−5* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/18:1) | −0.15205 | 0.034619 | 1.77 × 10−5* |
| Sphingolipid metabolism | Palmitoyl sphingomyelin (d18:1/16:0) | −0.06346 | 0.014721 | 2.48 × 10−5* |
| Sphingolipid metabolism | Sphingomyelin (d18:2/21:0, d16:2/23:0) | −0.14808 | 0.034627 | 2.87 × 10−5* |
| Sphingolipid metabolism | Myristoyl dihydrosphingomyelin (d18:0/14:0) | −0.14976 | 0.035167 | 3.09 × 10−5* |
| Sterol | Cholesterol | −0.13444 | 0.030218 | 1.39 × 10−5* |
| Xenobiotics | ||||
| Food component/plant | 4-Allylphenol sulfate | 0.567194 | 0.098491 | 2.93 × 10−8 |
| Food component/plant | S-allylcysteine | 0.813047 | 0.189377 | 2.67 × 10−5 |
1Significance was determined at the Bonferroni-adjusted threshold (P < 6.11 × 10−5). *Significant for both comparisons (DASH diet compared with the control diet and DASH diet compared with the fruit and vegetables diet). DASH, Dietary Approaches to Stop Hypertension; GABA, γ-aminobutyric acid; GPC, glycerophosphorylcholine; GPE, glycerophosphorylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SAM, S-Adenosyl methionine.
2β-Coefficients represent the serum metabolite concentration associated with the DASH diet compared with the control diet in the multivariable linear regression model adjusted for age, sex, race, education, BMI, and hypertension. Positive β-coefficients indicate that the metabolite was higher among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. Conversely, negative β-coefficients indicate that the metabolite was lower among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. Metabolites are sorted by category and metabolic pathway.
A total of 44 metabolites differed significantly between the DASH diet and control diet (Table 3) as well as the DASH diet and the fruit and vegetables diet (Table 4), including an amino acid (trans-4-hydroxyproline), vitamin A metabolites (2 isomers of carotene diol), and lipids (ceramides, diacylglycerols, a fatty acid, acyl carnitines, lysoplasmalogen, phosphotidylcholine, phosphotidylethanolamine, plasmalogens, sphingolipids, and cholesterol).
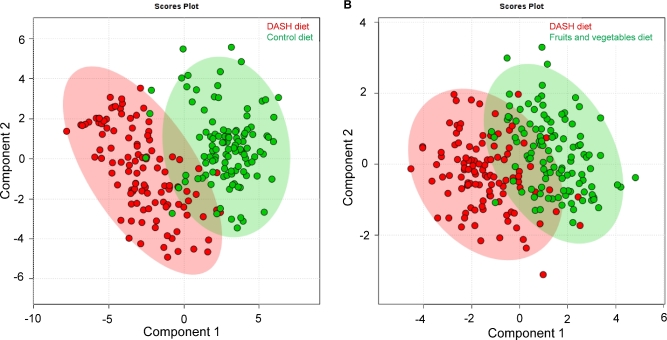
There was a clear differentiation in the serum metabolome between the DASH diet and the control diet (Figure 2A). Component 1 explained 29.4% of the variance and component 2 explained 12.6% of the variance. The differentiation in the serum metabolome for the DASH diet compared with the fruit and vegetables diet was less clear (Figure 2B). The first 2 components explained a slightly smaller proportion of the variance (21.9% and 11.2%, respectively).
FIGURE 2.
Scores plot of principal components 1 and 2 for discriminating between the DASH diet and the control diet (A) and between the DASH diet and the fruit and vegetables diet (B). Plots were created from a partial least-squares discriminant analysis of 110 participants randomly assigned to the DASH diet and the 108 participants randomly assigned to the control diet in panel A and among 110 participants randomly assigned to the DASH diet and 111 participants randomly assigned to the fruit and vegetables diet in panel B. DASH, Dietary Approaches to Stop Hypertension.
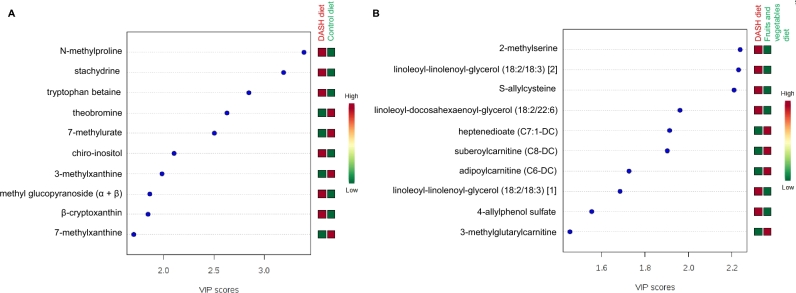
According to Variable Importance in Projection scores, the 10 most influential metabolites distinguishing the DASH diet from the control diet were as follows: N-methylproline, stachydrine, tryptophan betaine, theobromine, 7-methylurate, chiro-inositol, 3-methylxanthine, methyl glucopyranoside (α and β), β-cryptoxanthin, and 7-methylxanthine (Figure 3A). Serum concentrations of N-methylproline, stachydrine, tryptophan betaine, chiro-inositol, methyl glucopyranoside (α and β), and β-cryptoxanthin were higher among those randomly assigned to the DASH diet than those randomly assigned to the control diet. In contrast, serum concentrations of theobromine, 7-methylurate, 3-methylxanthine, and 7-methylxanthine were lower among those randomly assigned to the DASH diet than in those randomly assigned to the control diet. These 10 compounds represent a broad array of metabolic pathways and categories of metabolites, including amino acids (metabolism of arginine, proline, and tryptophan), xanthine metabolism, vitamin A metabolism, lipids, and xenobiotics or food components.
FIGURE 3.
VIP scores for the top 10 serum metabolites for discriminating between the DASH diet and the control diet (A) and between the DASH diet and the fruit and vegetables diet (B). VIP scores were calculated from a partial least-squares discriminant analysis among 110 participants randomly assigned to the DASH diet and the 108 participants randomly assigned to the control diet in panel A and among 110 participants randomly assigned to the DASH diet and 111 participants randomly assigned to the fruit and vegetables diet in panel B. Red boxes for the DASH diet (and green boxes for the control diet) indicate that serum concentrations of the metabolite were higher among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. Green boxes for the DASH diet (and red boxes for the control diet) indicate that serum concentrations of the metabolite were lower among those randomly assigned to the DASH diet compared with those randomly assigned to the control diet. DASH, Dietary Approaches to Stop Hypertension; VIP, Variable Importance in Projection.
The C statistic for the cumulative ability of these 10 metabolites and participant characteristics (age, sex, race, total energy intake, and BMI) to predict the DASH diet or the control diet was 0.986; when the model was fit on a two-thirds random sample and validated in the other one-third of the sample, the C statistics were 0.994 and 0.961, respectively.
The correlation between the 10 most influential metabolites discriminating between the DASH diet and the control diet ranged from –0.24 to 0.94 (Table 5). The strongest correlations were observed between N-methylproline and stachydrine (P = 0.94), between 3-methylxanthine and 7-methylxanthine (P = 0.94), and between theobromine and 3-methylxanthine (P = 0.91). There was an approximately equal distribution between negative (P < 0; 24 of 45 = 53%) and positive (P > 0; 21 of 45 = 47%) correlation coefficients.
TABLE 5.
Correlation matrix for 10 significant and influential serum metabolites for discriminating between the DASH diet and the control diet1
| N-methylproline | Stachydrine | Tryptophan betaine | Theobromine | 7-Methylurate | Chiro-inositol | 3-Methylxanthine | Methyl glucopyranoside | β-Cryptoxanthin | 7-Methylxanthine | |
|---|---|---|---|---|---|---|---|---|---|---|
| N-methylproline | 1 | |||||||||
| Stachydrine | 0.94*** | 1 | ||||||||
| Tryptophan betaine | 0.46*** | 0.49*** | 1 | |||||||
| Theobromine | −0.34*** | −0.33*** | −0.15** | 1 | ||||||
| 7-Methylurate | −0.28*** | −0.27*** | −0.17** | 0.77*** | 1 | |||||
| Chiro-inositol | 0.67*** | 0.62*** | 0.26*** | −0.21*** | −0.16** | 1 | ||||
| 3-Methylxanthine | −0.31*** | −0.30*** | −0.11 | 0.91*** | 0.81*** | -0.17*** | 1 | |||
| Methyl glucopyranoside | 0.71*** | 0.71*** | 0.44*** | −0.28*** | −0.26*** | 0.58*** | −0.26*** | 1 | ||
| β-Cryptoxanthin | 0.55*** | 0.56*** | 0.32*** | −0.28*** | −0.26*** | 0.32*** | −0.25*** | 0.48*** | 1 | |
| 7-Methylxanthine | −0.28*** | −0.28*** | −0.11* | 0.90*** | 0.82*** | −0.16** | 0.94*** | −0.25*** | −0.25*** | 1 |
1Values are Pearson's correlation coefficients. *P < 0.05; **P < 0.01; ***P < 0.001. DASH, Dietary Approaches to Stop Hypertension.
The 10 most influential metabolites for distinguishing between the DASH diet and the fruit and vegetables diet included 6 metabolites that were higher among those randomly assigned to the DASH diet [2-methylserine, S-allylcysteine, 4-allylphenol sulfate, linoleoyl-linolenoyl-glycerol (18:2/18:3) [1], linoleoyl-linolenoyl-glycerol (18:2/18:3) [2], and linoleoyl-docosahexaenoyl-glycerol (18:2/22:6)] and 4 metabolites that were lower among those randomly assigned to the DASH diet [heptenedioate (C7:1-DC), suberoylcarnitine (C8-DC), adipoylcarnitine (C6-DC), and 3-methylglutarylcarnitine] compared with those randomly assigned to the fruit and vegetables diet (Figure 3B). These compounds represented metabolism of amino acids, diacylglycerols, fatty acids, acylcarnitines, and xenobiotics. For the 10 most influential metabolites for discriminating between the DASH diet and the fruit and vegetables diet, the correlations ranged from –0.25 to 0.76 (Table 6).
TABLE 6.
Correlation matrix for 10 significant and influential serum metabolites for discriminating between the DASH diet and the fruit and vegetables diet1
| 2-Methylserine | Linoleoyl-linolenoyl-glycerol [2] | S-allylcysteine | Linoleoyl-docosahexaenoyl-glycerol | Heptenedioate | Suberoyl-carnitine | Adipoyl-carnitine | Linoleoyl-linolenoyl-glycerol [1] | 4-Allylphenol sulfate | 3-Methylglutaryl carnitine | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2-Methylserine | 1 | |||||||||
| Linoleoyl-linolenoyl-glycerol [2] | 0.09 | 1 | ||||||||
| S-allylcysteine | 0.07 | 0.20** | 1 | |||||||
| Linoleoyl-docosahexaenoyl-glycerol | −0.03 | 0.51*** | 0.04 | 1 | ||||||
| Heptenedioate | −0.06 | −0.18** | −0.02 | −0.25*** | 1 | |||||
| Suberoylcarnitine | 0.02 | −0.07 | −0.01 | −0.18** | 0.47** | 1 | ||||
| Adipoylcarnitine | 0.05 | 0.05 | 0.09 | −0.14* | 0.45*** | 0.76*** | 1 | |||
| Linoleoyl-linolenoyl-glycerol [1] | 0.07 | 0.65*** | 0.26*** | 0.61*** | −0.16* | −0.06 | 0.11 | 1 | ||
| 4-Allylphenol sulfate | 0.26*** | 0.19** | 0.21** | 0.14* | −0.08 | 0.06 | 0.12 | 0.19** | 1 | |
| 3-Methylglutarylcarnitine | −0.05 | 0.13 | 0.08 | −0.04 | 0.24*** | 0.19** | 0.31*** | 0.16* | −0.08 | 1 |
1Values are Pearson's correlation coefficients. *P < 0.05; **P < 0.01; ***P < 0.001. DASH, Dietary Approaches to Stop Hypertension.
DISCUSSION
In this feeding trial that enrolled adults with pre- or stage 1 hypertension, an untargeted metabolomic platform identified a broad array of 44 serum metabolites that were significantly different between the DASH and control dietary patterns and between the DASH and the fruit and vegetables dietary patterns after adjustment for participant characteristics and accounting for multiple comparisons. The metabolites that were significantly associated with the DASH diet represented a wide range of compounds, including lipids, amino acids, food components and other xenobiotics, cofactors and vitamins, carbohydrates, and nucleotides. The top 10 metabolites that were most influential with a high cumulative ability to predict the DASH diet compared with the control diet, which constitute the panel of candidate biomarkers of the DASH diet, were as follows: N-methylproline, stachydrine, tryptophan betaine, theobromine, 7-methylurate, chiro-inositol, 3-methylxanthine, methyl glucopyranoside, β-cryptoxanthin, and 7-methylxanthine. The most influential metabolites for discriminating between the DASH diet and the fruit and vegetables diet were 2-methylserine, S-allylcysteine, 4-allylphenol sulfate, linoleoyl-linolenoyl-glycerol (2 isomers), linoleoyl-docosahexaenoyl-glycerol, heptenedioate, suberoylcarnitine, adipoylcarnitine, and 3-methylglutarylcarnitine.
Our study findings address a pressing need in nutritional epidemiology for objective biomarkers of dietary intake without the type of error that threatens the validity of estimated dietary intake assessed by using food-frequency questionnaires, 24-h dietary recalls, and diet records. It has been proposed that biomarkers of dietary intake, identified by metabolomic profiling and other methods, could replace or be combined with self-reported dietary assessment tools to improve the ascertainment of diet exposures and increase the precision of diet-disease estimates of association in nutrition studies (26–28). The few available biomarkers of dietary intake are recovery biomarkers that rely on 24-h urine collections and reflect limited aspects of the mineral content of the diet—namely, sodium, potassium, and urea nitrogen as indicators of sodium, potassium, and protein intake, respectively (29, 30).
To the best of our knowledge, only 1 study has examined alterations in the metabolomic profile in response to the DASH diet (31). In this feeding trial, 13 participants with both hypertension and heart failure received a low-sodium (50 mmol Na/d) DASH diet for 21 d. With the use of a targeted metabolomic panel that identified 152 compounds, the investigators found an increase in diglycerides, short-chain fatty acids (acetate, butyrate, valerate, and heptanoate), total carnitine, and short-chain carnitines (acetyl, butryl, and propionyl), and a decrease in triglycerides, cholesterol esters, saturated long-chain fatty acids, propionate, and isovalerate from the beginning to the end of the low-sodium DASH diet intervention. The authors postulated that the increase in short-chain acyl residues with the low-sodium DASH diet resulted from intestinal production of short-chain fatty acids due to an increase in dietary intake of fiber or resulted from the oxidation of branched-chain amino acids.
Similarly, in our study, we observed significantly higher concentrations of diglycerides (also known as diacylglycerols) and lower concentrations of long-chain fatty acids and cholesterol with the DASH diet compared with both the control diet and the fruit and vegetables diet. Despite substantial alterations in the lipid profile when the metabolites were analyzed individually in our study, chiro-inositol was the only lipid that was included in the panel of 10 most influential metabolites and was found to be at higher serum concentrations with the DASH diet than with the control diet. Inositol is a component of structural lipids (phosphatidylinositol) of cell membranes. A derivative of inositol with 6 phosphate groups, called phytic acid, is found in fruit, beans, grains, nuts, and seeds (32–36). Six lipid-related metabolites were among the most influential metabolites for differentiating between the DASH diet and the fruit and vegetables diet, including diacylglycerols [2 isomers of linoleoyl-linolenoyl-glycerol (18:2/18:3), linoleoyl-docosahexaenoyl-glycerol (18:2/22:6)], a fatty acid [heptenedioate (C7:1-DC)], and acyl carnitines [adipoylcarnitine (C6-DC), suberoylcarnitine (C8-DC)]. This observation reflects the main difference between these 2 diet interventions, which was the lower amount of fat with the DASH diet (saturated fat, in particular, as well as monounsaturated fat) compared with the fruit and vegetables diet. In a metabolomic analysis of the Prevención con Dieta Mediterránea study, higher concentrations of acylcarnitines (we observed higher concentrations with the fruit and vegetables diet compared with the DASH diet) were associated with a higher risk of cardiovascular disease and stroke (37).
Our findings are consistent with previous studies that detected biomarkers of dietary intake, including those which related metabolites to self-reported dietary intake in observational studies (38, 39). We found that serum concentrations of N-methylproline, stachydrine, tryptophan betaine, and methyl glucopyranoside (α and β) were higher among those who consumed the DASH diet than those who consumed the control diet. In 2 cancer case-control studies with metabolomic profiling, serum concentrations of N-methylproline were found to be positively associated with dietary intake of citrus fruit and juice as assessed on a food-frequency questionnaire (38, 39). N-methylproline and other proline derivatives were detected in citrus fruit samples (40). Stachydrine, also known as proline betaine, is another proline derivative that has been proposed as a biomarker for citrus fruit intake (41–43). Tryptophan betaine, which is also referred to as lenticin or hypaphorine, has been identified in extracts of lentils and, as such, has been proposed as a biomarker of legume consumption (44, 45). Glucopyranoside is a component of cereals and cereal products (46). In a metabolomics study of 5 cancer case-control studies nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study that were pooled together, methyl-β-glucopyranoside was positively associated with total fruit intake estimated by using a food-frequency questionnaire (13).
One of the candidate biomarkers of the DASH dietary pattern was in the cofactors and vitamins category, and more specifically involved in the vitamin A metabolic pathway: β-cryptoxanthin. β-Cryptoxanthin is a type of a provitamin A carotenoid and xanthophyll with a natural red pigment that is found in fruit and vegetables such as red peppers, corn, and citrus (47–50). In the body, β-cryptoxanthin is converted to the bioactive form of vitamin A. We also observed that 2 isomers of carotene diols (carotenoids found in fruit and vegetables) were higher with the DASH diet than with the control diet as well as the fruit and vegetables diet. Previous research suggests that multiple biomarkers would be appropriate to use to represent dietary intake of fruit and vegetables (51, 52).
Four compounds involved in the xanthine metabolic pathway were significantly lower in the DASH diet relative to the control diet: 7-methylxanthine, 3-methylxanthine, 7-methylurate, and theobromine. These metabolites were highly correlated with each other in our study. Methylated purines (methylxanthine and methylurate) are derived from the metabolism of theobromine, theophylline, and caffeine (53, 54). The lower serum concentrations of caffeine metabolism byproducts detected by the metabolomic platform among those following the DASH diet are consistent with the lack of caffeine in chemical analyses of the DASH diet administered during the trial. In a metabolomic study conducted in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, serum concentrations of theobromine were associated with chocolate consumption and lower diet quality score, as assessed by using a food-frequency questionnaire (39). In the PLCO Cancer Screening Trial and another cancer case-control study, 1-methylxanthine was positively associated with coffee consumption and 3-methylxanthine and 7-methylxanthine were positively associated with desserts (38, 39). This case-control study also reported that serum concentrations of 3-methylglutarylcarnitine (which we observed at lower concentrations in the DASH diet compared with the fruit and vegetables diet) were positively associated with sugar-sweetened beverages (38).
The main strength of the present study is the use of stored specimens collected from a well-designed and rigorously conducted randomized feeding study. Because food was provided to the study participants with meals provided onsite and the remaining meals sent home with participants, one can be relatively certain that participants followed the assigned diet intervention. Another strength of the design is the random assignment of participants to diet interventions, which allows for equal distribution of known and unknown confounders across groups. The present study was conducted in a subset of trial participants. As such, it is possible that there was some imbalance in confounders between groups. However, there were no substantial differences in baseline characteristics, and we adjusted for key explanatory factors in the multivariable regression model. Last, a key feature of our study design is that metabolomic profiling for the identification of biomarkers was conducted in serum specimens, the collection of which is less burdensome for study participants than the collection of multiple urine specimens over 24-h periods of time. The serum metabolome is an indirect measure of dietary intake, but it captures a physiologically relevant internal dose and suggests metabolic pathways that may mediate the health benefits of the DASH dietary pattern. Furthermore, it has been previously shown that the metabolomic profile of usual dietary intake in serum is similar to that in urine specimens (38).
A limitation of the present study is the lack of an independent population for the replication of our results. Further research is necessary to validate this proposed panel of 10 metabolites as biomarkers of adherence to the DASH diet. However, we used the conservative Bonferroni method to adjust for multiple comparisons and to reduce the likelihood of false-positive findings. The global metabolomic platform obtained relative estimates of metabolites. Future studies should use targeted assays to obtain quantitative results for these novel biomarkers. Another limitation is the use of biospecimens that were stored for an extended period of time (20 y). However, degradation of metabolites over time would be expected to be nondifferential by randomized diet group. Given the relatively short duration of the DASH trial (8 wk), we were unable to assess the stability of the plasma biomarker concentrations over time. The stability of these metabolites over an extended period of time, as diet varies, is uncertain and would be a worthwhile future research direction. It is warranted to conduct metabolomic profiling at multiple time points over an extended period of time in a study with repeated assessments of dietary intake and with a wider range of adherence to the DASH dietary pattern. Our findings are limited to the dietary patterns investigated in the DASH trial. Further research is necessary to examine the specificity of the candidate biomarkers for the DASH diet compared with dietary patterns that vary in terms of macronutrients (protein, carbohydrate, fat).
In summary, conducting untargeted metabolomic profiling on serum specimens collected during a randomized, controlled feeding study showed that the DASH diet was characterized by altered serum concentrations of compounds from a spectrum of metabolic pathways relative to both the control diet and the fruit and vegetables diet. We detected 10 metabolites that were able to distinguish between the DASH diet (representing a heart-healthy dietary pattern) and the control diet (typical of the US diet), which are candidate biomarkers for assessing adherence to the DASH diet in future nutrition research studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—CMR and JC: designed the research study; LJA and JC: provided essential materials; CMR and ZZ: performed the statistical analysis; CMR: wrote the manuscript and had responsibility for the content of the final product; AHL: provided critical input on the interpretation of the results; and all authors: read and approved the final manuscript. The authors had no relevant conflicts of interest to declare.
Notes
CMR is supported by a Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). This research was supported in part by a pilot and feasibility grant (Principal Investigator: CMR) from the Mid-Atlantic Nutrition and Obesity Research Center (NORC), which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK072488). This publication was also made possible by a Nexus Award (Principal Investigator: CMR), a program supported by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by the National Center for Advancing Translational Sciences (NCATS; UL1 TR001079), a component of the NIH and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH. JC is partially supported by the Chronic Kidney Disease Biomarkers Consortium funded by the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK085689).
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
REFERENCES
- 1. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.; DASH Collaborative Research Group A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 2. Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, Appel LJ, Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis 2016;68:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009;302:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 5. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800, e5. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE et al.. 2013. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 7. USDA; US Department of Health and Human Services Dietary guidelines for Americans, 2015–2020. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 8. Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kipnis V, Midthune D, Freedman L, Bingham S, Day NE, Riboli E, Ferrari P, Carroll RJ. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr 2002;5:915–23. [DOI] [PubMed] [Google Scholar]
- 10. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet 2009;125:507–25. [DOI] [PubMed] [Google Scholar]
- 11. Tzoulaki I, Ebbels TM, Valdes A, Elliott P, Ioannidis JP. Design and analysis of metabolomics studies in epidemiologic research: a primer on -omic technologies. Am J Epidemiol 2014;180:129–39. [DOI] [PubMed] [Google Scholar]
- 12. Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr 2012;32:183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Playdon MC, Moore SC, Derkach A, Reedy J, Subar AF, Sampson JN, Albanes D, Gu F, Kontto J, Lassale C et al.. Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr 2017;105:450–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, Hansen T, Beckmann M, Pedersen O, Elliott P et al.. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol 2017;5:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Gorman A, Gibbons H, Brennan L. Metabolomics in the identification of biomarkers of dietary intake. Comput Struct Biotechnol J 2013;4:e201301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA et al.. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 17. Giffen CA, Carroll LE, Adams JT, Brennan SP, Coady SA, Wagner EL. Providing contemporary access to historical biospecimen collections: development of the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Biopreserv Biobank 2015;13:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giffen CA, Wagner EL, Adams JT, Hitchcock DM, Welniak LA, Brennan SP, Carroll LE. Providing researchers with online access to NHLBI biospecimen collections: the results of the first six years of the NHLBI BioLINCC program. PLoS One 2017;12:e0178141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shea KE, Wagner EL, Marchesani L, Meagher K, Giffen C. Efficiently maintaining a national resource of historical and contemporary biological collections: the NHLBI biorepository model. Biopreserv Biobank 2017;15:17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc 1992;92:969–77. [PubMed] [Google Scholar]
- 21. National Center for Health Statistics Carroll MD, Abraham S, Dresser CM. Dietary intake source data: United States, 1976–80 0. Vital Health Stat. Series 11, No. 231, DHHS Pub. No. PHS-83-1681. Public Health Service. Washington: U.S. Government Printing Office, March1983. [PubMed] [Google Scholar]
- 22. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- 23. Colantuoni E, Rosenblum M. Leveraging prognostic baseline variables to gain precision in randomized trials. Stat Med 2015;34:2602–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion: 207–12. [DOI] [PubMed] [Google Scholar]
- 25. Curtin F, Schulz P. Multiple correlations and Bonferroni's correction. Biol Psychiatry 1998;44:775–7. [DOI] [PubMed] [Google Scholar]
- 26. Prentice RL, Willett WC, Greenwald P, Alberts D, Bernstein L, Boyd NF, Byers T, Clinton SK, Fraser G, Freedman L et al.. Nutrition and physical activity and chronic disease prevention: research strategies and recommendations. J Natl Cancer Inst 2004;96:1276–87. [DOI] [PubMed] [Google Scholar]
- 27. Prentice RL, Sugar E, Wang CY, Neuhouser M, Patterson R. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr 2002;5:977–84. [DOI] [PubMed] [Google Scholar]
- 28. Dragsted LO. Relying on biomarkers for intake assessment in nutrition. Am J Clin Nutr 2017;105:8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutr J 2012;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 2003;133(Suppl 3):921S–4S. [DOI] [PubMed] [Google Scholar]
- 31. Mathew AV, Seymour EM, Byun J, Pennathur S, Hummel SL. Altered metabolic profile with sodium-restricted dietary approaches to stop hypertension diet in hypertensive heart failure with preserved ejection fraction. J Card Fail 2015;21:963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clements RS Jr., Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am J Clin Nutr 1980;33:1954–67. [DOI] [PubMed] [Google Scholar]
- 33. Larner J. D-chiro-inositol—its functional role in insulin action and its deficit in insulin resistance. Int J Exp Diabetes Res 2002;3:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silva EO, Bracarense AP. Phytic acid: from antinutritional to multiple protection factor of organic systems. J Food Sci 2016;81:R1357–62. [DOI] [PubMed] [Google Scholar]
- 35. Horbowicz M, Brenac P, Obendorf RL. Fagopyritol B1, O-alpha-D-galactopyranosyl-(1→2)-D-chiro-inositol, a galactosyl cyclitol in maturing buckwheat seeds associated with desiccation tolerance. Planta 1998;205:1–11. [DOI] [PubMed] [Google Scholar]
- 36. Steadman KJ, Burgoon MS, Schuster RL, Lewis BA, Edwardson SE, Obendorf RL. Fagopyritols, D-chiro-inositol, and other soluble carbohydrates in buckwheat seed milling fractions. J Agric Food Chem 2000;48:2843–7. [DOI] [PubMed] [Google Scholar]
- 37. Guasch-Ferre M, Zheng Y, Ruiz-Canela M, Hruby A, Martinez-Gonzalez MA, Clish CB, Corella D, Estruch R, Ros E, Fito M et al.. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr 2016;103:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Playdon MC, Sampson JN, Cross AJ, Sinha R, Guertin KA, Moy KA, Rothman N, Irwin ML, Mayne ST, Stolzenberg-Solomon R et al.. Comparing metabolite profiles of habitual diet in serum and urine. Am J Clin Nutr 2016;104:776–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, Cross AJ. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr 2014;100:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Servillo L, Giovane A, Balestrieri ML, Cautela D, Castaldo D. Proline derivatives in fruits of bergamot (Citrus bergamia Risso et Poit): presence of N-methyl-L-proline and 4-hydroxy-L-prolinebetaine. J Agric Food Chem 2011;59:274–81. [DOI] [PubMed] [Google Scholar]
- 41. Atkinson W, Downer P, Lever M, Chambers ST, George PM. Effects of orange juice and proline betaine on glycine betaine and homocysteine in healthy male subjects. Eur J Nutr 2007;46:446–52. [DOI] [PubMed] [Google Scholar]
- 42. Gibbons H, Michielsen CJR, Rundle M, Frost G, McNulty BA, Nugent AP, Walton J, Flynn A, Gibney MJ, Brennan L. Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol Nutr Food Res 2017;61:1700037. [DOI] [PubMed] [Google Scholar]
- 43. Heinzmann SS, Brown IJ, Chan Q, Bictash M, Dumas ME, Kochhar S, Stamler J, Holmes E, Elliott P, Nicholson JK. Metabolic profiling strategy for discovery of nutritional biomarkers: proline betaine as a marker of citrus consumption. Am J Clin Nutr 2010;92:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keller BO, Wu BT, Li SS, Monga V, Innis SM. Hypaphorine is present in human milk in association with consumption of legumes. J Agric Food Chem 2013;61:7654–60. [DOI] [PubMed] [Google Scholar]
- 45. Tsopmo A, Muir AD. Chemical profiling of lentil (Lens culinaris Medik.): cultivars and isolation of compounds. J Agric Food Chem 2010;58:8715–21. [DOI] [PubMed] [Google Scholar]
- 46. Klausen K, Mortensen AG, Laursen B, Haselmann KF, Jespersen BM, Fomsgaard IS. Phenolic compounds in different barley varieties: identification by tandem mass spectrometry (QStar) and NMR; quantification by liquid chromatography triple quadrupole-linear ion trap mass spectrometry (Q-Trap). Nat Prod Commun 2010;5:407–14. [PubMed] [Google Scholar]
- 47. Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr 2012;96(Suppl):1214S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Granado F, Olmedilla B, Blanco I, Rojas-Hidalgo E. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. Eur J Clin Nutr 1996;50:246–50. [PubMed] [Google Scholar]
- 49. Capocchi A, Bottega S, Spano C, Fontanini D. Phytochemicals and antioxidant capacity in four Italian traditional maize (Zea mays L.) varieties. Int J Food Sci Nutr 2017;68:515–24. [DOI] [PubMed] [Google Scholar]
- 50. Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, Mattisson I, Wirfalt E, Galasso R, Palli D et al.. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 2005;59:1387–96. [DOI] [PubMed] [Google Scholar]
- 51. Couillard C, Lemieux S, Vohl MC, Couture P, Lamarche B. Carotenoids as biomarkers of fruit and vegetable intake in men and women. Br J Nutr 2016;116:1206–15. [DOI] [PubMed] [Google Scholar]
- 52. Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev 1994;3:493–500. [PubMed] [Google Scholar]
- 53. Desiraju RK, Sugita ET, Mayock RL. Determination of theophylline and its metabolites by liquid chromatography. J Chromatogr Sci 1977;15:563–8. [DOI] [PubMed] [Google Scholar]
- 54. Tarka SM Jr., Arnaud MJ, Dvorchik BH, Vesell ES. Theobromine kinetics and metabolic disposition. Clin Pharmacol Ther 1983;34:546–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.