Abstract
The current dataset follows the published article [1]. The dataset provides preliminary phytochemical analysis and antioxidant activity of selected invasive alien plant used by Bapedi Traditional Health Practitioners to treat sexually transmitted infections (STIs). It was evident that seven STIs are treated with herbal remedies of the documented plant species. Informational on the medicinal plant uses and the use categories of sexually transmitted infections are presented on table 1. Table 2 shows the yield of plant extracts. Detailed data on phytochemical analysis and antioxidant activity are presented on Fig 1 and 2 respectively. Rf values of separated compounds are provided in Table 3. The data contains both qualitative and quantitative information.
Keywords: Sexually transmitted infections, Medicinal plants, Phytochemical analysis, Antioxidant activity
Specifications Table
| Subject area | Botany, pharmacology |
|---|---|
| More specific subject area | Ethnobotany, phytochemistry, antioxidant activity |
| Type of data | Tables and figures |
| How data was acquired | Semi-structured questionnaire, guided field survey and experimental design |
| Data format | Raw and processed |
| Experimental factors | Plant species were selected based on previous ethnobotanical survey [1]. Plants with high fidelity level were selected for phytochemical analysis and antioxidant activity |
| Experimental features | Quantitative data analysis of selected plants, plant material collections, extraction of plant materials, phytochemical analysis and antioxidant activity using qualitative analysis. |
| Data source location | Waterberg District, South Africa (23°10′−24°20′S and 28°10'−29°10′E) for collected plant materials. |
| Data accessibility | The data is with the article. |
| Related research article | [1] |
| Value of the Data | |
| |
1. Data
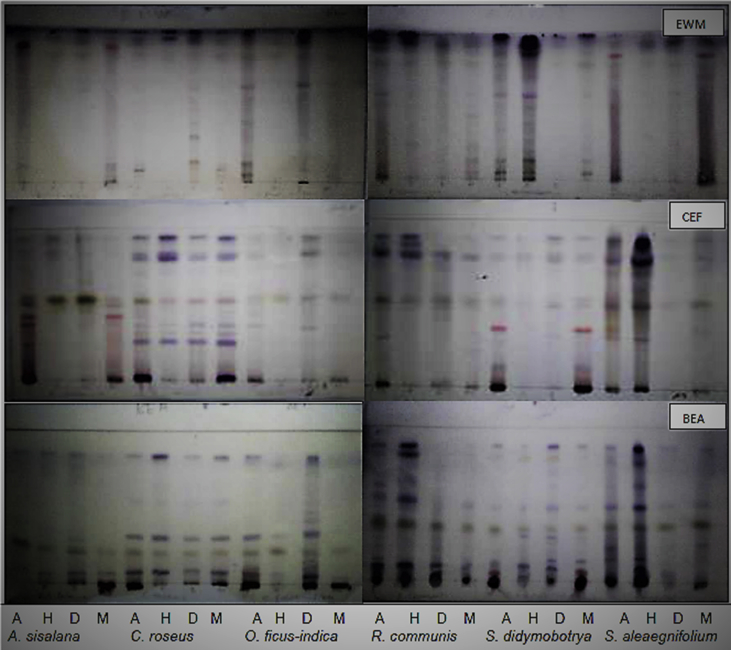
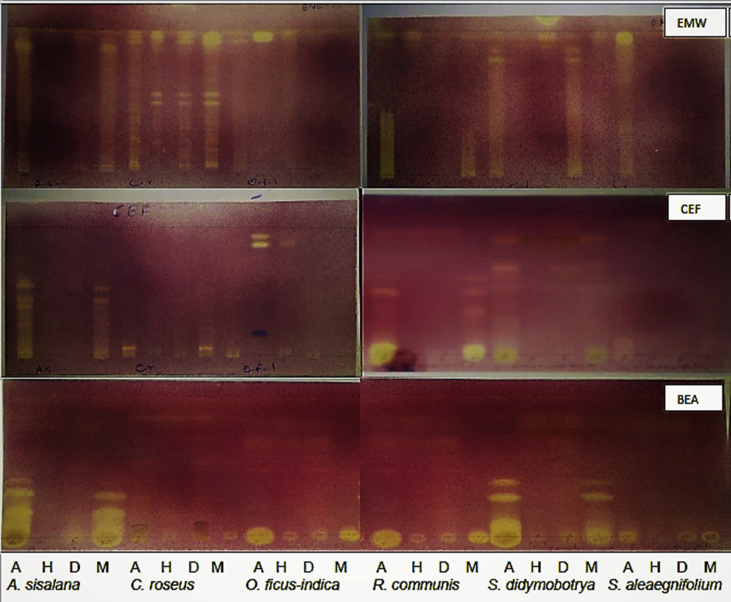
The data shared entails information on medicinal plants used to treat sexually transmitted infection (STIs) (Table 1). The plant materials were extracted using solvents of varying polarities. The yield of crude extracts from roots of selected plant species were calculated as presented on Table 2. Plants were selected because of high number of FL values (Table 1). Moreover, preliminary phytochemical analysis of plant crude extracts was analysed using a thin layer chromatography (TLC) (Fig. 1). The antioxidant activity of plant extracts is portrayed on the TLC plate with a yellow band against purple background (Fig. 2). Retention factor (Rf) values for each separated compound were calculated, including compounds with antioxidant activity (Table 3).
Table 1.
Medicinal plants most frequently used to treat sexually transmitted infections.
| Plant species | STIs treated | Frequency of report | Fidelity level |
|---|---|---|---|
| Catharanthus roseus (L.) G.Don | Gonorrhoea | 11 | 57.9 |
| Chlamydia | 2 | 10.5 | |
| Syphilis | 3 | 15.8 | |
| HIV/AIDS | 1 | 5.3 | |
| Genital warts | 2 | 10.5 | |
| Agave sisalana Perrine ex Engelm. | Gonorrhoea | 4 | 33.3 |
| Chlamydia | 4 | 33.3 | |
| Syphilis | 2 | 16.7 | |
| Makgoma | 1 | 8.3 | |
| Genital warts | 1 | 8.3 | |
| Opuntia ficus-indica Mill. | Gonorrhoea | 7 | 63.6 |
| Chlamydia | 1 | 9.1 | |
| Syphilis | 1 | 9.1 | |
| Makgoma | 1 | 9.1 | |
| Genital warts | 1 | 9.1 | |
| Ricinus communis L. | Gonorrhoea | 3 | 33.3 |
| Chlamydia | 2 | 22.2 | |
| Syphilis | 1 | 11.1 | |
| Makgoma | 3 | 33.3 | |
| Senna didymobotrya (Fresen.) H.S.Irwin & Barneby | Gonorrhoea | 3 | 30.0 |
| Chlamydia | 2 | 20.0 | |
| Syphilis | 1 | 10.0 | |
| Mokabe | 3 | 30.0 | |
| Genital warts | 1 | 10.0 | |
| Solanum elaeagnifolium Cav. | Gonorrhoea | 6 | 37.5 |
| Chlamydia | 3 | 18.8 | |
| Syphilis | 3 | 18.8 | |
| HIV/AIDS | 1 | 6.3 | |
| Makgoma | 3 | 18.8 | |
| Genital warts | 2 | 12.5 |
Table 2.
Percentage yield from powdered roots material of different plant species using different extraction solvents: acetone [A], hexane [H], dichloromethane [D] and methanol [M].
| Plant species | % of plant material extracted (mg) |
Average | |||
|---|---|---|---|---|---|
| A | H | D | M | ||
| Agave sisalana | 3.9 | 1.3 | 1.4 | 9.9 | 4.1 |
| Catharanthus roseus | 9.5 | 1.4 | 2.5 | 9.5 | 5.7 |
| Ricinus communis | 1.9 | 1.5 | 0.4 | 9.1 | 3.2 |
| Opuntia ficus-indica | 0.7 | 0.3 | 0.3 | 0.8 | 0.5 |
| Senna didymobotrya | 7.6 | 0.5 | 1.2 | 12.1 | 5.4 |
| Solanum elaeagnifolium | 0.9 | 1.9 | 0.3 | 4.3 | 1.8 |
| Average | 4.1 | 1.2 | 1 | 7.6 | 3.5 |
Fig. 1.
Thin layer chromatography sprayed with vanillin-sulphuric acid showing phytochemical constituents of six plants extracted with four solvents (A: acetone, H: hexane, D: dichloromethane, M: methanol) separated with three solvent systems (BEA, CEF, EMW).
Fig. 2.
Thin layer chromatography showing antioxidant activity of six plants extracted with four solvents (A: acetone, H: hexane, D: dichloromethane, M: methanol) separated with three solvent systems (BEA, CEF, EMW).
Table 3.
Phytoconstituets profiles and antioxidants of roots extracted with acetone, hexane, dichloromethane and methanol using three solvent systems.
| Solvent system | A. sisalana |
C. roseus |
O. ficus-indica |
R. communis |
S. didymobotrya |
S. eleaegnifolium |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMW | CEF | BEA | EMW | CEF | BEA | EMW | CEF | BEA | EMW | CEF | BEA | EMW | CEF | BEA | EMW | CEF | BEA | |
| Acetone | 0.12 | 0.13 | 0.06 | 0.05 | 0.04 | 0.03 | 0.06 | 0.37 | 0.03 | 0.06 | 0.29 | 0.06 | 0.03 | 0.13 | 0.09 | 0.37 | 0.28 | 0.06 |
| 0.51 | 0.24 | 0.09 | 0.09 | 0.24 | 0.06 | 0.09 | 0.56 | 0.06 | 0.09 | 0.43 | 0.09 | 0.13 | 0.20 | 0.13 | 0.52 | 0.29 | 0.09 | |
| 0.83 | 0.29 | 0.13 | 0.13 | 0.29 | 0.24 | 0.13 | 0.87 | 0.09 | 0.29 | 0.51 | 0.13 | 0.51 | 0.27 | 0.27 | 0.60 | 0.37 | 0.13 | |
| 0.87 | 0.37 | 0.20 | 0.62 | 0.37 | 0.29 | 0.34 | 0.96 | 0.24 | 0.37 | 0.77 | 0.37 | 0.60 | 0.37 | 0.37 | 0.8 | 0.51 | 0.20 | |
| 0.91 | 0.43 | 0.29 | 0.87 | 0.56 | 0.56 | 0.62 | 0.29 | 0.53 | 0.83 | 0.53 | 0.69 | 0.46 | 0.53 | 0.87 | 0.69 | 0.24 | ||
| 0.51 | 0.37 | 0.92 | 0.83 | 0.83 | 0.87 | 0.37 | 0.60 | 0.87 | 0.60 | 0.81 | 0.51 | 0.83 | 0.96 | 0.77 | 0.37 | |||
| 0.56 | 0.40 | 0.95 | 0.87 | 0.95 | 0.73 | 0.80 | 0.87 | 0.87 | 0.56 | 0.87 | 0.91 | 0.43 | ||||||
| 0.87 | 0.87 | 0.96 | 0.97 | 0.79 | 0.91 | 0.96 | 0.77 | 0.87 | ||||||||||
| 0.97 | 0.83 | 0.96 | 0.87 | |||||||||||||||
| Hexane | 0.73 | 0.56 | 0.06 | 0.43 | 0.24 | 0.06 | 0.73 | 0.56 | 0.06 | 0.24 | 0.51 | 0.09 | 0.03 | 0.51 | 0.03 | 0.93 | 0.28 | 0.06 |
| 0.91 | 0.87 | 0.24 | 0.54 | 0.29 | 0.20 | 0.86 | 0.92 | 0.20 | 0.80 | 0.77 | 0.37 | 0.13 | 0.77 | 0.09 | 0.96 | 0.29 | 0.09 | |
| 0.97 | 0.37 | 0.87 | 0.37 | 0.29 | 0.95 | 0.87 | 0.24 | 0.96 | 0.83 | 0.53 | 0.51 | 0.83 | 0.29 | 0.37 | 0.24 | |||
| 0.87 | 0.95 | 0.56 | 0.56 | 0.93 | 0.29 | 0.87 | 0.87 | 0.60 | 0.91 | 0.37 | 0.52 | 0.37 | ||||||
| 0.83 | 0.83 | 0.77 | 0.91 | 0.69 | 0.53 | 0.69 | 0.87 | |||||||||||
| 0.87 | 0.81 | 0.83 | 0.77 | |||||||||||||||
| 0.96 | 0.87 | 0.87 | 0.91 | |||||||||||||||
| 0.96 | ||||||||||||||||||
| dichloromethane | 0.60 | 0.56 | 0.06 | 0.60 | 0.24 | 0.21 | 0.08 | 0.37 | 0.06 | 0.43 | 0.51 | 0.06 | 0.81 | 0.32 | 0.09 | 0.64 | 0.51 | 0.09 |
| 0.83 | 0.87 | 0.20 | 0.09 | 0.29 | 0.24 | 0.62 | 0.56 | 0.09 | 0.51 | 0.77 | 0.09 | 0.87 | 0.48 | 0.13 | 0.80 | 0.24 | ||
| 0.91 | 0.97 | 0.24 | 0.14 | 0.37 | 0.34 | 0.86 | 0.87 | 0.13 | 0.80 | 0.13 | 0.96 | 0.51 | 0.24 | 0.93 | 0.37 | |||
| 0.29 | 0.17 | 0.56 | 0.62 | 0.8 | 0.96 | 0.20 | 0.96 | 0.25 | 0.77 | 0.29 | 0.96 | |||||||
| 0.83 | 0.21 | 0.83 | 0.87 | 0.95 | 0.24 | 0.29 | 0.83 | 0.37 | ||||||||||
| 0.24 | 0.87 | 0.92 | 0.97 | 0.37 | 0.37 | 0.87 | 0.53 | |||||||||||
| 0.34 | 0.91 | 0.95 | 0.43 | 0.91 | 0.91 | 0.83 | ||||||||||||
| 0.54 | 0.96 | 0.47 | 0.87 | |||||||||||||||
| 0.62 | 0.69 | |||||||||||||||||
| 0.87 | 0.77 | |||||||||||||||||
| 0.95 | 0.83 | |||||||||||||||||
| Methanol | 0.03 | 0.24 | 0.06 | 0.05 | 0.04 | 0.03 | 0.06 | 0.56 | 0.06 | 0.03 | 0.20 | 0.04 | 0.03 | 0.13 | 0.09 | 0.37 | 0.37 | 0.06 |
| 0.09 | 0.29 | 0.13 | 0.09 | 0.24 | 0.06 | 0.73 | 0.87 | 0.24 | 0.13 | 0.32 | 0.06 | 0.13 | 0.27 | 0.13 | 0.52 | 0.520.77 | 0.09 | |
| 0.13 | 0.37 | 0.20 | 0.10 | 0.29 | 0.09 | 0.87 | 0.96 | 0.24 | 0.43 | 0.13 | 0.60 | 0.37 | 0.27 | 0.60 | 0.91 | 0.20 | ||
| 0.87 | 0.43 | 0.29 | 0.37 | 0.37 | 0.13 | 0.37 | 0.51 | 0.25 | 0.81 | 0.46 | 0.37 | 0.8 | 0.24 | |||||
| 0.91 | 0.51 | 0.87 | 043 | 0.56 | 0.15 | 0.43 | 0.54 | 0.29 | 0.87 | 0.51 | 0.53 | 0.87 | 0.37 | |||||
| 0.56 | 0.51 | 0.83 | 0.20 | 0.60 | 0.77 | 0.37 | 0.56 | 0.83 | 0.96 | 0.87 | ||||||||
| 0.54 | 0.87 | 0.29 | 0.80 | 0.91 | 0.77 | 0.87 | ||||||||||||
| 0.87 | 0.96 | 0.53 | 0.87 | 0.87 | ||||||||||||||
| 0.91 | 0.83 | 0.96 | ||||||||||||||||
| 0.95 | ||||||||||||||||||
Note: bolded Rf values are phytoconstituents with antioxidant activity.
2. Experimental design, materials, and methods
2.1. Ethnobotanical survey and plant extraction
An ethnobotanical survey was conducted as elaborated in the published article [1]. Based on ethnobotanical information provided by traditional health practitioners, plant materials (roots) were collected, dried, and grinded. Separate aliquots of finely ground plant material (5 g) were extracted with 50 ml of solvents of increasing polarities: hexane, dichloromethane, acetone and methanol for at least 72 h with frequent shaking on a shaking incubator. The samples were filtered through Whatman No.1 filter paper and filtrates were used for phytochemical screening. Second extraction procedure was executed, and filtrates were pre-weighed in the glass vials and air-dried under a stream of cold air. The quantity of plant material extracted was determined by comparing the amount of extract with the original plant material. The extracts were stored in airtight glass vials in the dark until used for antioxidant and phytochemical assays. The dry plant extracts were reconstituted into acetone making 100 mg/ml stock solution used for biological assays.
2.2. Qualitative phytochemical analysis
Chemical constituents of the extracts were analysed using aluminium-backed Thin Layer Chromatography (TLC) plates (ALIGRAM_SIL g/UV 254-MACHEREY-NAGEL, Merck), that was developed with three eluent systems developed in the botany laboratory (UNIVEN). Ethylacetate: methanol: water: 40:5:0.4 [EMW] (polar) Chloroform: ethylacetate: formic acid: 5:4:1 [CEF] (intermediate polarity) Benzene: ethanol: ammonia hydroxide: 90:10:1 [BEA] (non-polar/basic) [2].
The stock solution (100 mg/ml) of the extracts were re-dissolved to the concentration of 10mg/ml in acetone. Acetone was selected due to its extraction capability. Development of the chromatograms was under eluent-saturated conditions. Approximately 100 μg aliquot (10 mg/ml) was applied on the TLC plates in a 1 cm band and developed without delay to minimize the possibility of photo-oxidative change. The separated components were visualized under visible and UV light (254 and 360 nm, Lamina flow). For the detection of chemical compounds not visible under UV light, vanillin-sulphuric acid reagent (0.1 g vanillin, 28 ml methanol (MeOH); 1 ml sulphuric acid) was sprayed on the chromatogram and heated at 110 °C for colour development.
2.3. Antioxidant compounds analysis
The antioxidant compounds of each plant extract were determined by using a qualitative 2, 2-diphenyl-1-picrylhydrazyl (DPPH). This assay is preferred because it is used to provide stable free radicals. A solution of 0.2% DPPH in MeOH was prepared and then sprayed on the plates (until they became wet) and allowed to dry in a fume cupboard. The presence of yellow zones against a purple background on chromatograms indicated the presence of the scavenging activity of free radicals by compounds present in the plant extracts.
2.4. Data analysis
Data were captured in Microsoft Excel 2016 programme and were later analysed by descriptive statistics. Quantitative tool such as Fidelity Level (FL), was used to analyse the importance of medicinal plants and informants' knowledge about categories of STIs [3]. Compound bands on the TLC were then used to calculate Rf values with the formula Rf = distance moved by the compounds/distance moved by the solvents front.
Acknowledgments
Traditional health practitioner are highly acknowledged for participating in the research project. Dr Ndivhaleni A. Masevhe is thanked for assisting with TLC techniques. The National Research foundation of South Africa is acknowledged for financial support (Grant no: 100528, 2016].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104281.
Contributor Information
Lesibana Petrus Maema, Email: plesibana@gmail.com.
Martin Johannes Potgieter, Email: martin.potgieter@ul.ac.za.
Amidou Samie, Email: samieamidou@yahoo.com.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Maema L.P., Potgieter M.J., Samie A. Ethnobotanical survey of invasive alien plant species used in thetreatment of sexually transmitted infections in Waterberg District, South Africa. South Afr. J. Bot. 2019 doi: 10.1016/j.sajb.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotze M., Eloff J.N., Houghton P.J. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae) South Afr. J. Bot. 2002;68:62–67. [Google Scholar]
- 3.Friedman J., Yaniv Z., Dafni A., Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986;16:275–287. doi: 10.1016/0378-8741(86)90094-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.