Abstract
This scientific commentary refers to ‘Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline’ by Ayton et al., (doi:10.1093/brain/awx137).
This scientific commentary refers to ‘Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline’ by Ayton et al., (doi:10.1093/brain/awx137).
The association of brain iron and Alzheimer’s disease is frustratingly enigmatic. Iron can participate in so many critical and pathological processes that it is difficult not to ascribe an aetiological role in Alzheimer’s disease, yet direct evidence of its participation remains elusive and indirect evidence through therapeutic targeting contradictory. The function and chronology of iron accumulation are not well understood in part because of difficulty in measurement. Prior work by Ayton et al. showed that the concentration of ferritin, an iron storage protein, in the CSF was positively correlated with cognitive decline (Ayton et al., 2015). Apropos of treatments targeting pathological iron, it has been proposed that developing suitable clinical treatments for Alzheimer’s disease will likely require better patient and disease stratification (Huang and Mucke, 2012). In this issue of Brain, Ayton and co-workers report the use of iron-sensitive quantitative susceptibility mapping magnetic resonance imaging (QSM-MRI) in tandem with amyloid-β-PET as a potential means of addressing the challenge of access with a non-invasive, high spatial resolution technique (Ayton et al., 2017).
In this latest work by Ayton et al., 100 subjects were evaluated for cognitive function on an 18-month basis for 6 years. Of the 100 individuals in the study, 64 were cognitively normal, 17 had mild cognitive impairment, and 19 were previously diagnosed with Alzheimer’s disease as defined by the NINCDS-ADRDA criteria. Amyloid-β-PET scans were performed using the 11C Pittsburgh compound B (11C-PiB) followed by MRI acquisition with T1-weighted and QSM modes. An important distinguishing factor in this study is the stratification into groups delineating the presence (Aβ+) or absence (Aβ−) of PET-determined amyloid-β pathology. As the authors explain, MRI data from the Aβ+ groups were most predictive of cognitive decline. For instance, hippocampal QSM was positively correlated with Z-score change in Aβ− individuals, but negatively correlated in Aβ+ individuals. In Aβ− patients, QSM of the temporal lobe was weakly correlated with Z-score change, while that of the frontal lobe was negatively correlated. The association of QSM was region specific but generally showed a positive correlation in Aβ+ individuals that the authors hope may predict future cognitive function loss. A key point in the report is that MRI-measured iron was not necessarily associated with cognitive decline unless the individual already had mild cognitive impairment: in individuals with mild cognitive impairment, a higher iron load often correlated with greater cognitive decline. The predictive power of QSM-MRI may be applicable to clinical trials where the diverse patient pool has been scrutinized as a possible explanation for the poor outcomes of promising studies (Huang and Mucke, 2012). The authors conclude that this technique can demonstrate an iron load-dependent decline in cognitive function and the capability to better stratify patients into risk categories. The authors also acknowledge that a full understanding of the association of iron with proteins related to the pathology of Alzheimer’s disease will likely require additional studies using ligands to other proteins associated with Alzheimer’s disease, such as amyloid oligomer and tau-specific PET labels. We agree with the authors on both the promise of the technique as well as the need for additional studies in larger populations and consideration of the association of iron with multiple Alzheimer’s disease-related mechanisms.
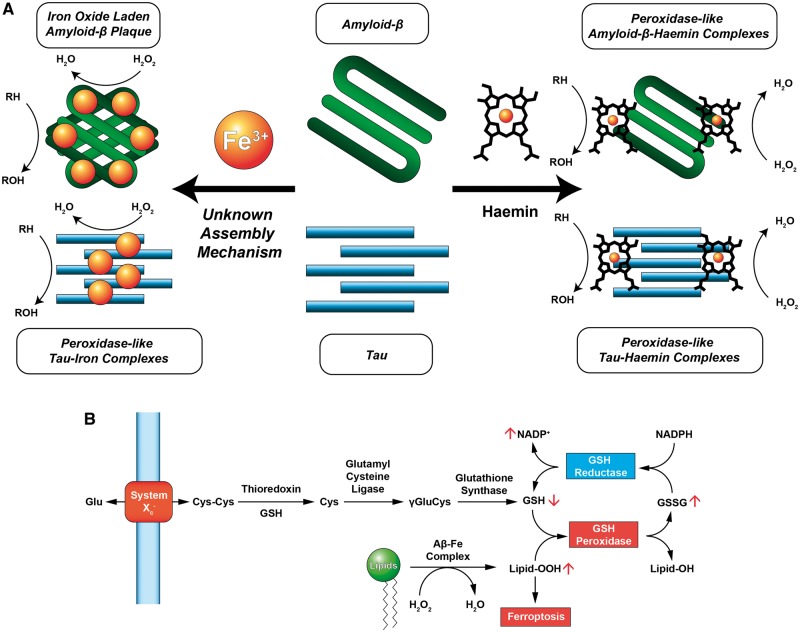
Previously, iron deposits have been observed in both amyloid plaques and tau neurofibrillary tangles (Sayre et al., 2001; Everett et al., 2014) and both structures are known to oxidize reduced substrates (Fig. 1A). Work by Sayre et al. (2001) showed that Alzheimer’s disease lesions in fixed cortical tissue possess peroxidase-like properties, by demonstrating the catalytic polymerization of diaminobenzidine (DAB) with hydrogen peroxide by both senile plaques and neurofibrillary tangles (Sayre et al., 2001). Removal of iron and copper from the fixed tissue by chelation with deferoxamine eliminated the oxidative capacity. Replacing the iron and copper resulted in a return of DAB oxidation in spatially identical locations (Sayre et al., 2001). Everett et al. (2014) reported that iron in amyloid plaques was of the Fe(II) oxidation state and that amyloid plaques were capable of reducing Fe(III) to Fe(II) to form a metal oxide core. These metal oxide particles are composed of wüstite and magnetite and have two properties relevant to this discussion: first, they can generate reactive oxygen species, and second, the magnetic susceptibilities of these oxides are different.
Figure 1.
Iron interacts with Alzheimer’s disease-related peptides through haem and non-haem mechanisms and can participate in generating reactive oxygen species that affect the ferroptosis pathway. (A) Iron binds to tau and amyloid-β either through the deposition of iron(III) or through the formation of a peptide-haemin complex. In both instances, peroxidase-like activity is observed in which reduced substrates are oxidized (RH → ROH) in the presence of hydrogen peroxide (H2O2) in a catalytic fashion. Detection of iron deposits in amyloid or tau aggregates via QSM-MRI may be a valuable tool in following the progress of Alzheimer’s disease and differentiating patient pools for clinical trials, although iron in different conditions may complicate quantitation. (B) Lipid peroxidation is an important contributor to the ferroptotic pathway. Glutathione is synthesized from cystine (CysCys), an amino acid dimer, and is subsequently reduced by thioredoxin to form cysteine (Cys), and then by glutamyl cysteine ligase to form γ-glutamyl-l-cysteine (γGluCys). Finally, γGluCys is reduced by glutathione synthase to form glutathione (GSH). GSH is oxidized by GSH peroxidase to form glutathione disulfide (GSSG) and quench hydrogen peroxide. GSH reductase reduces GSSG to GSH at the expense of NADPH. Elevated peroxidase activity by amyloid-β-iron or tau-iron complexes may deplete endogenous antioxidant stores and trigger ferroptosis not by blockade of System Xc−, but by increasing the formation of oxidizer species such as lipid peroxides (Lipid-OOH). Adapted from Conrad et al. (2016).
Glossary
Ferroptosis: An iron-mediated cell death pathway whereby iron catalyses membrane lipid peroxidation. First described by Dixon et al. (2012) as a distinct cell death mechanism.
Magnetic susceptibility: A dimensionless value linked to the number of unpaired electrons in a substance that determines the magnetization of a material within an external magnetic field.
Quantitative susceptibility mapping: A magnetic resonance imaging technique that generates a linearly proportional, volumetric image based on the magnetic susceptibility of the subject tissue.
One issue not considered by Ayton et al. is that iron may be found in different states in Alzheimer’s disease, and that many different iron-containing compounds exist in the brain; there may therefore be multiple iron-containing species that interact with amyloid-β or tau (Fig. 1A). In addition to the iron oxides wüstite and magnetite, haemin, the oxidized form of haem B, has been shown to not only complex with amyloid-β and reduce aggregation but also imbue peroxidase-like functionality (Atamna and Boyle, 2006). It is not clear to us whether QSM is equally sensitive and quantitative for iron in different states and when bound to different molecules, as each species of iron has a different intrinsic molar magnetic susceptibility. This complexity may result in experimental variability in quantitation of iron content and could explain some of the seemingly contradictory findings in the different populations in this report.
One particularly interesting aspect of iron-containing tangles and plaques is their ability to perform oxidative chemistry in a catalytic fashion (Sayre et al., 2001). In other words, trace amounts of complexed iron are capable of causing a proportionally large amount of oxidative stress. Because of the intrinsic reactivity of tangles and plaques towards reduced substrates, catching aggregates early with the help of imaging methods such as QSM and PET may provide the opportunity to intervene early to mitigate oxidative damage in Alzheimer’s disease and reduce cognitive decline if appropriate drugs can be identified.
The possible role of iron in mediating neurodegeneration received a boost when viewed through the lens of ferroptosis (Conrad et al., 2016) (Fig. 1B). Dixon et al. (2012) demonstrated that by inhibiting System Xc−, a glutamate-cystine antiporter, intracellular cystine was depleted and an oxidative crisis could be established in BJeLR cells. Several hallmark indicators of oxidative stress in Alzheimer’s disease appear as effects in the ferroptosis pathway, namely elevated lipid peroxidation and glutathione depletion (Dixon et al., 2012). Depletion of endogenous antioxidants through inhibition of System Xc− may be mirrored by an increase in the amount of reactive iron, or an increase in reactive oxygen species from a secondary source leading to a similar activation of the ferroptotic pathway.
The discussion of ferroptosis usually assumes a strong role in oxidative stress by labile iron or iron in the cytosol not bound by haem or ferritin. Chelation of labile iron by deferoxamine was shown by Dixon et al. (2012) to inhibit the ferroptotic death pathway. The early study conducted by Crapper McLachlan with deferoxamine in individuals with Alzheimer’s disease showed an ∼50% reduction in the rate of cognitive decline that the authors originally attributed to a reduction in iron-mediated oxidative stress (McLachlan et al., 1991), but which has not been replicated or confirmed. Ayton et al. suggest that their technique could guide a fresh look at this treatment.
It is evident that the role of iron in Alzheimer’s disease has not been completely resolved. Perhaps the coupling of QSM-MRI and PET can answer some long-standing questions about the role of iron in the pathogenesis of Alzheimer’s disease and its presymptomatic antecedents.
Funding
The contents of this publication do not reflect the views of the United States Department of Veterans Affairs or the United States Government. Supported by NIH R01NS094535 and the Padfield Foundation.
References
- Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci USA 2006; 103: 3381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Faux NG, Bush AI, Weiner MW, Aisen P, Petersen R, , et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 2015; 6: 6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Yen Ying L, , et al. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain 2017; 140: 2112–9. [DOI] [PubMed] [Google Scholar]
- Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2016; 15: 348–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, , et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149: 1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J, Céspedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, , et al. Evidence of redox-active iron formation following aggregation of ferrihydrate and the Alzheimer's disease peptide β-amyloid. Inorg Chem 2014; 53: 2803–9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 2012; 148: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan DRC, Kruck TPA, Kalow W, Andrews DF, Dalton AJ, Bell MY, , et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991; 337: 1304–8. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Harris PLR, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease. J. Neurochem. 2001; 74: 270–279. [DOI] [PubMed] [Google Scholar]