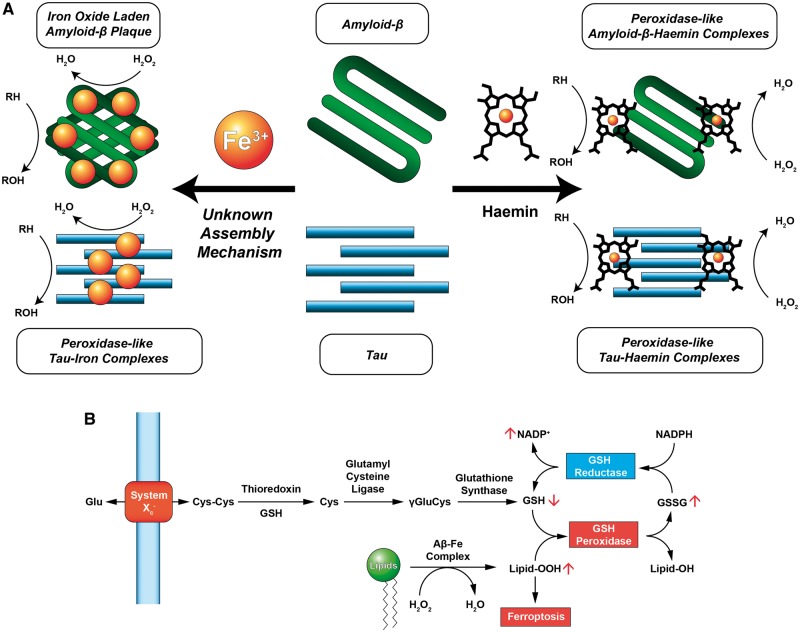
Figure 1.
Iron interacts with Alzheimer’s disease-related peptides through haem and non-haem mechanisms and can participate in generating reactive oxygen species that affect the ferroptosis pathway. (A) Iron binds to tau and amyloid-β either through the deposition of iron(III) or through the formation of a peptide-haemin complex. In both instances, peroxidase-like activity is observed in which reduced substrates are oxidized (RH → ROH) in the presence of hydrogen peroxide (H2O2) in a catalytic fashion. Detection of iron deposits in amyloid or tau aggregates via QSM-MRI may be a valuable tool in following the progress of Alzheimer’s disease and differentiating patient pools for clinical trials, although iron in different conditions may complicate quantitation. (B) Lipid peroxidation is an important contributor to the ferroptotic pathway. Glutathione is synthesized from cystine (CysCys), an amino acid dimer, and is subsequently reduced by thioredoxin to form cysteine (Cys), and then by glutamyl cysteine ligase to form γ-glutamyl-l-cysteine (γGluCys). Finally, γGluCys is reduced by glutathione synthase to form glutathione (GSH). GSH is oxidized by GSH peroxidase to form glutathione disulfide (GSSG) and quench hydrogen peroxide. GSH reductase reduces GSSG to GSH at the expense of NADPH. Elevated peroxidase activity by amyloid-β-iron or tau-iron complexes may deplete endogenous antioxidant stores and trigger ferroptosis not by blockade of System Xc−, but by increasing the formation of oxidizer species such as lipid peroxides (Lipid-OOH). Adapted from Conrad et al. (2016).