Abstract
Purpose
Results of a study to determine whether obesity is associated with acute kidney injury (AKI) among patients receiving combination therapy with piperacillin–tazobactam and vancomycin are reported.
Methods
A retrospective, single-center cohort study of patients who received combination therapy for at least 48 hours was conducted using data from the University of Kentucky Center for Clinical and Translational Science’s Enterprise Data Trust. Patients with chronic kidney disease, baseline creatinine clearance of less than 30 mL/min, cystic fibrosis, or missing height or weight information were excluded.
Results
A total of 8,125 patients were included in the cohort. Among the variables evaluated, total body weight of 91 kg or more was the variable most predictive of AKI. Patients with a weight of 91 kg or higher were more likely than lower-weight patients to have diabetes (39% versus 21%, p < 0.00001), hypertension (64% versus 47%, p < 0.00001), and heart failure (15% versus 13%, p = 0.007). The median daily vancomcyin dose was lower in patients with a weight of less than 91 kg (2,000 mg versus 3,000 mg, p < 0.00001); however, weight-based doses were lower in patients weighing 91 kg or more (25.5 mg/kg/day versus 27.9 mg/kg/day, p < 0.00001). AKI was more common in patients weighing 91 kg or more (24% versus 18%, p < 0.00001; adjusted odds ratio, 1.46 [95% confidence interval, 1.28–1.66]).
Conclusion
Increased total body weight increased the rate of AKI among patients concurrently treated with piperacillin–tazobactam and vancomycin independent of clinically important confounders, with an important breakpoint occurring at 91 kg.
Keywords: acute kidney injury, obesity, piperacillin–tazobactam, total body weight, vancomycin
KEY POINTS.
A total body weight of 91 kg or more was associated with acute kidney injury in patients receiving vancomycin with concomitant piperacillin–tazobactam.
Several therapeutic alternatives to both vancomycin and piperacillin–tazobactam have been shown to be noninferior in prospective randomized controlled trials.
Prescribing of both vancomycin and piperacillin–tazobactam to patients with a weight of 91 kg or more may unnecessarily risk the development of acute kidney injury.
The use of broad-spectrum anti- microbial agents in U.S. hospitalized patients has increased significantly in recent years.1 From 2006 to 2012, β-lactam/β-lactamase inhibitor use increased by 18.0 per 1,000 patient-days, with use of glycopeptides increasing by 22.4 per 1,000 patient-days; among all classes of antimicrobials, these 2 classes had the largest absolute increases in levels of use over that period. Both piperacillin–tazobactam (a β-lactam/β-lactamase inhibitor) and vancomycin (a glycopeptide) were historically considered well tolerated, with nephrotoxicity rates of 0 to 5% in prospective randomized clinical trials.2–4 However, the use of a standard definition of acute kidney injury (AKI) has shown that the combined use of piperacillin–tazobactam and vancomycinis associated with the development of AKI more so than use of either medication as monotherapy or use of other combination antimicrobial regimens.5,6
Increased body size (obesity) affects 40% of U.S. adults, which complicates many aspects of care, including medication dosing and pharmacokinetics and pharmacodynamics.7 Previous investigations have shown that a total body weight of 100 kg or greater is associated with the development of AKI in patients receiving vancomycin monotherapy for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia.8–10 Several studies in patients with gram-negative infections have also found an association between AKI and increased body size, whether defined as a continuous variable or as a cut point (i.e., >80 kg).11–13
It is unclear whether obesity influences the rate of AKI associated with combined piperacillin–tazobactam and vancomycin therapy. Therefore, the study described here evaluated that research question and determined the weight most predictive of AKI.
Materials and methods
The retrospective, single-center cohort study utilized clinical data collected from the University of Kentucky Center for Clinical and Translational Science’s Enterprise Data Trust (EDT) for the period September 2007 through September 2015. The EDT contains clinical data from the inpatient population at University of Kentucky Medical Center from 2006 to the present. Details of the data stored in the EDT have been described elsewhere.14 The University of Kentucky institutional review board reviewed and approved the protocol for this analysis.
The cohort included patients 18 years of age or older who received the combination of piperacillin–tazobactam and vancomycin for at least 48 hours. The primary piperacillin–tazobactam regimen used during the study period was 4.5 g administered every 6 hours over a 30-minute infusion period. Vancomycin dosing, therapeutic drug monitoring, and dosing adjustments were guided by the institutional clinical pharmacokinetics service and anticoagulation guidelines.15 Reasons for exclusion of patients from the cohort included pregnancy, cystic fibrosis, chronic kidney disease, baseline creatinine clearance of less than 30 mL/min, and missing height or weight data.
Data extracted for analysis included demographic data, length of hospitalization, admitting and primary diagnosis codes, Charlson Comorbidity Index (CCI) score,16 all serum creatinine concentrations during hospitalization, receipt of other nephrotoxic agents (listed in Table 1), and any receipt of i.v. contrast agents. Any patient who received at least 1 dose of a nephrotoxic drug within 24 hours of the period from initiation to discontinuation of piperacillin–tazobactam and vancomycin was considered to have received a nephrotoxic agent. The initial serum creatinine concentration was used as a patient’s baseline value. We used an adjusted Cockcroft-Gault equation that assumes a weight of 72 kg for all patients and removes the number 72 from the denominator.17 This approach results in a formula that considers only age and serum creatinine concentration in calculating creatinine clearance. Hypotension was defined as a mean arterial pressure of less than 65 mm Hg, a systolic blood pressure of less than 90 mm Hg, or vasopressor exposure during treatment.
Table 1.
Patient Demographics and Clinical Characteristics, Overall and by Weight Groupa
| Characteristic or Variableb | Overall (n = 8,125) | Low Weight (<91 kg) (n = 5,673 [69.8%]) | High Weight (≥91 kg) (n = 2,452 [30.2%]) | p |
|---|---|---|---|---|
| Male | 4,731 (58.2) | 3,062 (54.0) | 1,669 (68.1) | <0.00001 |
| Age range, yr | <0.00001 | |||
| 18–44 | 2,543 (31.3) | 1,745 (30.8) | 798 (32.5) | |
| 45–64 | 3,657 (45.0) | 2,425 (42.7) | 1,232 (50.2) | |
| 65–79 | 1,565 (19.3) | 1,183 (20.8) | 382 (15.6) | |
| ≥80 | 360 (4.4) | 320 (5.6) | 40 (1.6) | |
| Caucasian | 7,316 (90.0) | 5,099 (89.9) | 2,217 (90.4) | 0.48537 |
| Median (IQR) weight, kg | 80.0 (67.0–95.5) | 72.5 (62.1–81.6) | 106.5 (97.5–120.0) | <0.00001 |
| Median (IQR) BMI, kg/m2 | 26.8 (22.9–31.9) | 24.5 (21.6–27.4) | 34.6 (31.2–39.7) | <0.00001 |
| BMI classification | <0.00001 | |||
| Underweight | 493 (6.1) | 493 (8.7) | 0 | |
| Normal | 2,576 (31.7) | 2,565 (45.2) | 11 (0.4) | |
| Overweight | 2,357 (29.0) | 1,978 (34.9) | 379 (15.5) | |
| Obese I | 1,451 (17.9) | 554 (9.8) | 897 (36.6) | |
| Obese II | 643 (7.9) | 67 (1.2) | 576 (23.5) | |
| Obese III | 605 (7.4) | 16 (0.3) | 589 (24.0) | |
| Baseline CLcr, mL/min | 0.00003 | |||
| 30–59 | 1,326 (16.3) | 910 (16.0) | 416 (17.0) | |
| 60–89 | 2,308 (28.4) | 1,537 (27.1) | 771 (31.4) | |
| ≥90 | 4,491 (55.3) | 3,226 (56.9) | 1,265 (51.6) | |
| Median (IQR) CCI score | 2 (0–3) | 2 (0–3) | 2 (1–3) | 0.86332 |
| Hypertension | 4,209 (51.8) | 2,652 (46.7) | 1,557 (63.5) | <0.00001 |
| Diabetes mellitus | 2,137 (26.3) | 1,183 (20.8) | 954 (38.9) | <0.00001 |
| Heart failure | 1,073 (13.2) | 711 (12.5) | 362 (14.8) | 0.00714 |
| Hypotension | 4,810 (59.2) | 3,391 (59.8) | 1,419 (57.9) | 0.11462 |
| Nephrotoxin exposures | ||||
| ACE inhibitor | 1,570 (19.3) | 944 (16.6) | 626 (25.5) | <0.00001 |
| Aminoglycoside | 1,156 (14.2) | 816 (14.4) | 340 (13.9) | 0.56287 |
| Amphotericin B | 116 (1.4) | 96 (1.7) | 20 (0.8) | 0.00312 |
| ARB | 237 (2.9) | 131 (2.3) | 106 (4.3) | <0.00001 |
| Calcineurin inhibitor | 272 (3.3) | 196 (3.4) | 76 (3.1) | 0.45298 |
| I.V. radiocontrast dye | 477 (5.9) | 330 (5.8) | 147 (6.0) | 0.79329 |
| Loop diuretic | 2,679 (33.0) | 1,811 (31.9) | 868 (35.4) | 0.00241 |
| NSAID | 1,337 (16.4) | 945 (16.7) | 392 (16.0)) | 0.47393 |
| Vasopressor | 821 (10.1) | 562 (9.9) | 259 (10.6) | 0.389334 |
| Median (IQR) vancomycin and/or piperacillin–tazobactam DOT, days | 5 (4–8) | 5 (4–9) | 5 (4–8) | 0.08061 |
| Median (IQR) combination therapy DOT, days | 3 (3–5) | 3 (3–5) | 3 (2–5) | 0.00173 |
| Median (IQR) vancomycin dose, mg/day | 2,000 (1,500–3,000) | 2,000 (1,250–2,500) | 3,000 (2,000–3,500) | <0.00001 |
| Median (IQR) vancomycin dose, mg/kg/day | 27.4 (18.2–33.2) | 27.9 (18.7–34.2) | 25.5 (16.3–31.5) | <0.00001 |
aIQR = interquartile range, BMI = body mass index, CLcr = creatinine clearance (calculated via adjusted Cockcroft-Gault equation), CCI = Charlson Comorbidity Index, ACE = angiotensin-converting enzyme, ARB = angiotensin II receptor blocker, NSAID = nonsteroidal antiinflammatory drug, DOT = duration of therapy.
bAll values are no. (%) unless otherwise noted.
Any patient receiving an imaging procedure in which contrast agent use was indicated via Healthcare Common Procedure Coding System codes was categorized as having received an i.v. contrast agent. Piperacillin–tazobactam and vancomycin doses, frequency, and duration were collected for each patient in addition to serum vancomycin concentrations. A day of therapy was defined as receipt of at least 1 dose of piperacillin–tazobactam or vancomycin per day.
Weights between 70 and 120 kg were analyzed via bivariable logistic regression to determine what cutoff was most predictive of AKI through minimization of the Akaike information criterion (AIC).18 The AIC was defined by the model and the maximum likelihood estimates of the parameters that gave the minimum AIC, as follows: AIC = (–2)log – (maximum likelihood) + 2(number of independently adjusted parameters within the model). We determined that the model with the lowest AIC value provided the value for total body weight most closely associated with AKI. In other words, we divided the cohort into high- and low-weight groups using the model with the lowest AIC value. The difference in the rates of AKI between the high- and low-weight groups was the primary study outcome. Development of AKI was evaluated using the RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) criteria.19 The study evaluated only AKI severity classes based on glomerular filtration rate due to the lack of data regarding long-term outcomes.
Patient demographics were analyzed using a Student’s t test or Wilcoxon rank sum test for continuous variables; nominal variables were analyzed using a chi-square or Fisher’s exact test, as appropriate. Multivariable logistic regression including all variables with significant associations with AKI in univariate regressions was performed. All statistical analyses were completed with the RStudio (v0.98) program running R (v3.1.2) software (R Foundation for Statistical Computing, Vienna, Austria).20,21 The model fit was assessed by using the standardized Hosmer-Lemeshow test and the C statistic,22 with an a priori level of significance of 0.05.
Results
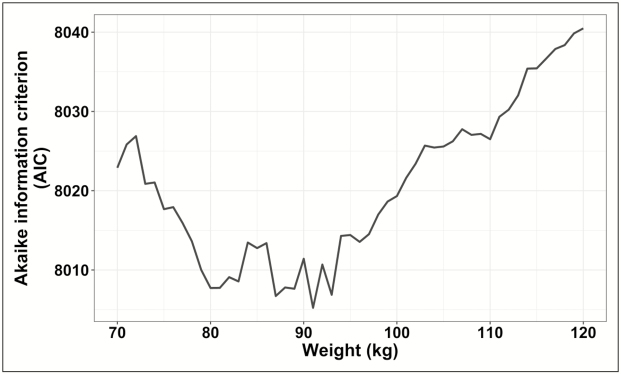
In total, 8,125 patients were included in the cohort after application of the inclusion and exclusion criteria. The bivariable logistic regression determined that a weight cutoff of 91 kg was the most predictive of AKI (Figure 1); that value was used to categorize the groups for the subsequent analyses. A total of 2,452 patients (30.2%) weighed 91 kg or more (Table 1). In comparison to patients with weights at or above the cutoff, those weighing less than 91 kg were less likely to receive concomitant nephrotoxins and had a higher median baseline creatinine clearance value (97.3 [interquartile range, 70.1–128.1] mL/min versus 91.8 [interquartile range, 68.1–116.5] mL/min, p < 0.00001). Baseline severity of illness was similar between the high- and low-weight groups; however, patients weighing 91 kg or more had higher rates of diabetes (38.9% versus 20.8%, p < 0.00001), hypertension (63.5% versus 46.7%, p < 0.00001), and heart failure (14.8% versus 12.5%, p = 0.007). The median daily vancomycin dose was lower among sub—91 kg patients (2,000 [interquartile range, 1,250–2,500] mg versus 3,000 [interquartile range, 2,000–3,500] mg, p < 0.00001); however, the median weight-based dose was lower in the group weighing 91 kg or more (25.5 [interquartile range, 16.3–31.5] mg/kg/day versus 27.9 [interquartile range, 18.7–34.2] mg/kg/day, p < 0.00001). AKI was more common in the patients weighing 91 kg or more than in those with weights below the cutoff (frequency, 23.8% versus 17.8%, p < 0.00001; adjusted odds ratio, 1.46 [95% confidence interval, 1.28–1.66]) (Table 2).
Figure 1.
Determination of the optimal total body weight cut point by analysis of Akaike information criterion (AIC) values. The lowest point on the graph occurs at 91 kg, representing the total body weight cut point (as measured by AIC value) that was the best predictor of acute kidney injury within the cohort.
Table 2.
Results of Logistic Regression Analyses of Association of Variables With Acute Kidney Injury During Concomitant Use of Vancomycin and Piperacillin–Tazobactama
| Variable | Bivariable Models | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| ORb | 95% CI | p | Adjusted ORb | 95% CI | p | |
| Weight ≥91 kg | 1.44 | 1.28–1.62 | <0.001 | 1.46 | 1.28–1.67 | <0.001 |
| Hypertension | 1.17 | 1.05–1.31 | 0.004 | 1.03 | 0.90–1.17 | 0.72 |
| Diabetes mellitus | 1.32 | 1.17–1.49 | <0.001 | 1.24 | 1.07–1.43 | 0.003 |
| Heart failure | 1.85 | 1.60–2.14 | <0.001 | 1.08 | 0.90–1.29 | 0.393 |
| CCI score | 1.15 | 1.12–1.17 | <0.001 | 1.1 | 1.07–1.13 | <0.001 |
| Combination therapy DOT | 1.10 | 1.09–1.12 | <0.001 | 1.06 | 1.04–1.08 | <0.001 |
| Caucasian | 0.87 | 0.73–1.04 | 0.124 | 0.82 | 0.68–0.99 | 0.04 |
| Male sex | 1.50 | 1.33–1.68 | <0.001 | 1.58 | 1.39–1.79 | <0.001 |
| Age group, yr | ||||||
| 18–44 (reference) | . . .c | . . . | . . . | . . . | . . . | . . . |
| 45–64 | 1.14 | 1.00–1.29 | 0.050 | 1.02 | 0.87–1.19 | 0.828 |
| 65–79 | 1.08 | 0.92–1.26 | 0.357 | 1.17 | 0.95–1.44 | 0.142 |
| ≥80 | 0.92 | 0.68–1.22 | 0.551 | 1.51 | 1.06–2.13 | 0.022 |
| Baseline CLcr, mL/min | ||||||
| 30–59 (reference) | . . . | . . . | . . . | . . . | . . . | . . . |
| 60–89 | 1.15 | 0.95–1.38 | 0.147 | 1.63 | 1.33–2.02 | <0.001 |
| ≥90 | 1.65 | 1.40–1.95 | <0.001 | 3.95 | 3.20–4.90 | <0.001 |
| Nephrotoxin exposures | ||||||
| Aminoglycoside | 1.92 | 1.67–2.21 | <0.001 | 1.47 | 1.26–1.72 | <0.001 |
| Amphotericin B | 3.54 | 2.44–5.11 | <0.001 | 2.03 | 1.34–3.07 | <0.001 |
| NSAID | 1.20 | 1.04–1.38 | 0.013 | 1.28 | 1.09–1.49 | 0.003 |
| Calcineurin inhibitor | 1.59 | 1.21–2.08 | <0.001 | 1.42 | 1.04–1.92 | 0.024 |
| Loop diuretic | 2.70 | 2.41–3.02 | <0.001 | 2.08 | 1.82–2.39 | <0.001 |
| Vasopressor | 3.69 | 3.17–4.29 | <0.001 | 2.42 | 2.01–2.90 | <0.001 |
| Ionotropic | 4.11 | 2.75–6.15 | <0.001 | 2.14 | 1.37–3.35 | <0.001 |
| ACE inhibitor | 1.14 | 1.00–1.31 | 0.053 | 0.93 | 0.80–1.10 | 0.408 |
| Hypotension | 1.93 | 1.72–2.18 | <0.001 | 1.30 | 1.13–1.48 | <0.001 |
| Vancomycin dose, mg/kg/day | ||||||
| <18.2 (reference) | . . . | . . . | . . . | . . . | . . . | . . . |
| 18.2–27.3 | 0.73 | 0.63–0.85 | <0.001 | 0.70 | 0.59–0.82 | <0.001 |
| 27.4–33.1 | 0.68 | 0.58–0.79 | <0.001 | 0.59 | 0.50–0.70 | <0.001 |
| ≥33.2 | 0.64 | 0.55–0.74 | <0.001 | 0.64 | 0.54–0.77 | <0.001 |
aOR = odds ratio, CI = confidence interval, CCI = Charlson Comorbidity Index, DOT = duration of therapy, CLcr = creatinine clearance (calculated via adjusted Cockcroft-Gault equation), NSAID = nonsteroidal antiinflammatory drug, ACE = angiotensin-converting enzyme.
bAs applicable, OR is for comparison with reference value.
cNot applicable.
Discussion
Patients weighing 91 kg or more had significantly higher odds of AKI associated with combined use of piperacillin–tazobactam and vancomycin than those weighing less than 91 kg after controlling for multiple confounders, including vancomycin dose and other nephrotoxin exposures.
Our results are in agreement with those of previous studies showing an association between increased body size and AKI.8–13 The vast majority of these studies have evaluated total body weight as the body size descriptor. These studies have found different weight breakpoints for increased risk depending on the patient population studied and the weight distribution of patients. Studies focused on AKI associated with vancomycin use have identified a cut point of 100 kg as being associated with increased risk relative to an 80-kg cut point for patients treated with various antimicrobials for gram-negative bacteremia. However, very few patients in the gram-negative bacteremia cohorts had a weight greater than 110 kg, and this limited the generalizability of the findings to obese patients. Our cohort of patients receiving piperacillin–tazobactam and vancomycin was a more generalizable population than those assessed in previous studies. One study evaluated the association of body mass index and AKI in patients receiving colistin.12 The study found that a body mass index of 30 kg/m2 or higher was associated with AKI.
The 1,594 cases of AKI in our cohort dwarfed the case counts discussed in previous publications on the impact of total body weight on AKI in patients receiving vancomycin.8–10 Lodise and colleagues9 evaluated a random sample of patients from Albany Medical Center Hospital who received vancomycin (n = 246) or linezolid (n = 45) from January 2005 to December 2006. A total of 36 patients developed AKI. A follow-up study by Lodise et al. of patients with an initial serum vancomycin trough concentration within the first 96 hours of therapy (n = 166) included 21 cases of AKI.10 In our previous study in patients with MRSA bacteremia, there were 78 cases of AKI. These previous studies may have suffered from model underfitting given the low number of events.8 The study described here was able to accommodate more variables into the multivariable logistic regression model without risking overfitting. The study also provided a more detailed analysis of the impact of individual nephrotoxic agents than was achieved in previous evaluations. Other studies evaluating the impact of total body weight on AKI risk have used the consensus definition of vancomycin-associated AKI, but that approach has not provided information regarding the extent of AKI experienced.8–10
On the other hand, the 20% rate of AKI in our cohort was lower than the rate of 30% found in previous studies evaluating concomitant vancomycin and piperacillin–tazobactam use.23–26 This difference can be partially explained by the fact that approximately 15% of patients in our study were in the intensive care unit, as compared with 35% to 100% of those in other studies.23–25 An evaluation of the medication combination discussed here by Moenster et al.26 involved patients with osteomyelitis. In that study the average duration of therapy was over 2 weeks, as compared with a median of 5 days in our cohort. Each of the previous studies also used a definition of AKI different from the one we used, which could have had an impact on the AKI rates reported.
Our findings are clinically relevant because they identify a group of patients at increased risk for AKI. Potentially nephrotoxic agents should be avoided in these patients unless the benefit clearly outweighs the risk. There are several therapeutic alternatives to both vancomycin and piperacillin–tazobactam that have been shown to be noninferior in prospective randomized controlled trials. Prescribing of both piperacillin–tazobactam and vancomycin to patients weighing 91 kg or more may unnecessarily risk the development of AKI. However, data on AKI risk associated with other antimicrobial combinations are needed in order to determine whether the increased risk is unique to the concomitant use of vancomycin and piperacillin–tazobactam.
Our study had several limitations, including its retrospective design and the inclusion of data from a single center. Our results are not generalizable to all patients receiving other combinations of antimicrobials. However, our cohort was much larger than those in any previous evaluation seeking to define the impact of body size on AKI risk. In addition, our study evaluated a more generalizable patient population than those evaluated in previous studies. The weight cut point derived from our cohort has not been externally validated. Validation of this cut point is an important step before applying it broadly in clinical practice. Our analysis did not specifically evaluate the impact of infusion type (e.g., short versus extended). However, we have previously shown that for standalone piperacillin–tazobactam therapy, infusion type is not associated with AKI risk.27 As in many other evaluations of AKI risk, we did not analyze the cohort via a time-to-event analysis and did not account for the duration of therapy with other nephrotoxic agents.
Conclusion
Increased total body weight increased the rate of AKI among patients concurrently treated with piperacillin–tazobactam and vancomycin in a manner independent of clinically important confounders, with an important breakpoint occurring at 91 kg.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant number UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Disclosures
Dr. Hall has received grant funding from Merck. The other authors have declared no potential conflicts of interest.
References
- 1. Baggs J, Fridkin SK, Pollack LA et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016; 176(11):1639-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brun-Buisson C, Sollet JP, Schweich H et al. Treatment of ventilator-associated pneumonia with piperacillin-tazobactam/amikacin versus ceftazidime/amikacin: a multicenter, randomized controlled trial. VAP study group. Clin Infect Dis. 1998; 26(2):346-54. [DOI] [PubMed] [Google Scholar]
- 3. Marra F, Reynolds R, Stiver G et al. Piperacillin/tazobactam versus imipenem: a double-blind, randomized formulary feasibility study at a major teaching hospital. Diagn Microbiol Infect Dis. 1998; 31(2):355-68. [DOI] [PubMed] [Google Scholar]
- 4. Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010; 123(2):182.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammond DA, Smith MN, Li C et al. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis. 2017; 64(5):666-74. [DOI] [PubMed] [Google Scholar]
- 6. Giuliano CA, Patel CR, Kale-Pradhan PB. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury? A meta-analysis. Pharmacotherapy. 2016; 36(12):1217-28. [DOI] [PubMed] [Google Scholar]
- 7. Hales CM, Fryar CD, Carroll MD et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. Jama. 2018; 319(16):1723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall RG 2nd, Hazlewood KA, Brouse SD et al. Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013; 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008; 52(4):1330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lodise TP, Patel N, Lomaestro BM et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009; 49(4):507-14. [DOI] [PubMed] [Google Scholar]
- 11. Hall RG 2nd, Yoo E, Faust A et al. Impact of total body weight on acute kidney injury in patients with gram-negative bacteremia. Expert Rev Clin Pharmacol. 2018; 11(6):651-4. [DOI] [PubMed] [Google Scholar]
- 12. Gauthier TP, Wolowich WR, Reddy A et al. Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob Agents Chemother. 2012; 56(5):2392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubin CJ, Ellman TM, Phadke V et al. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect. 2012; 65(1):80-7. [DOI] [PubMed] [Google Scholar]
- 14. Rutter WC, Cox JN, Martin CA et al. Nephrotoxicity during vancomycin therapy in combination with piperacillin-tazobactam or cefepime. Antimicrob Agents Chemother. 2017; 61(2):e02089–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pharmacy Services, UK HealthCare. Clinical pharmacokinetics service and anticoagulation guidelines https://ukhealthcare.uky.edu/sites/default/files/clinical-pks-anticoagulation-manual.pdf (accessed 2018 Sep 12).
- 16. Quan H, Li B, Couris CM et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173(6):676-82. [DOI] [PubMed] [Google Scholar]
- 17. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011; 31(7):658-64. [DOI] [PubMed] [Google Scholar]
- 18. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974; 19:716–23. [Google Scholar]
- 19. Bellomo R, Ronco C, Kellum JA et al. , for the Acute Dialysis Quality Initiative Workgroup Acute renal failure–-definition, outcome, measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. RStudio Team. RStudio: integrated development for R. Boston, MA: RStudio Team; 2015. [Google Scholar]
- 21. R Foundation for Statistical Computing. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 22. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013; 32(1):67-80. [DOI] [PubMed] [Google Scholar]
- 23. Gomes DM, Smotherman C, Birch A et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014; 34(7):662-9. [DOI] [PubMed] [Google Scholar]
- 24. Hammond DA, Smith MN, Painter JT et al. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy. 2016; 36(5):463-71. [DOI] [PubMed] [Google Scholar]
- 25. Mullins BP, Kramer CJ, Bartel BJ et al. Comparison of the nephrotoxicity of vancomycin in combination with cefepime, meropenem, or piperacillin/tazobactam: a prospective, multicenter study. Ann Pharmacother. 2018; 52(7):639-44. [DOI] [PubMed] [Google Scholar]
- 26. Moenster RP, Linneman TW, Finnegan PM et al. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014; 20(6):O384-9. [DOI] [PubMed] [Google Scholar]
- 27. Cotner SE, Rutter WC, Burgess DR et al. Influence of β-lactam infusion strategy on acute kidney injury. Antimicrob Agents Chemother. 2017; 61:e00871–17. [DOI] [PMC free article] [PubMed] [Google Scholar]