Abstract
Non-invasive imaging of the coronary arteries is an enterprise in rapid development. From the research perspective, there is great demand for in vivo techniques that can reliably identify features of high-risk plaque that may offer insight into pathophysiological processes and act as surrogate indicators of response to therapeutic intervention. Meanwhile, there is clear clinical need for greater accuracy in diagnosis and prognostic stratification. Fortunately, ongoing technological improvements and emerging data from randomized clinical trials are helping make these elusive goals a reality. This review provides an update on the current status of non-invasive coronary imaging with computed tomography, magnetic resonance, and positron emission tomography with a focus on current clinical applications and future research directions.
Keywords: Coronary heart disease, Computed tomography, Positron emission tomography, Magnetic resonance imaging
Introduction
Despite remarkable diagnostic and therapeutic advances in recent decades, coronary heart disease remains the largest single cause of death in Europe.1 In order to address this residual burden of morbidity and mortality, clinicians need greater access to imaging techniques that can improve the detection and prognostic classification of patients at risk of cardiovascular events.
Non-invasive imaging of the coronary arteries holds genuine promise that these hopes may be realized via both well-established diagnostic technologies, such as computed tomography coronary angiography (CTCA), and exciting new developments such as positron emission tomography (PET) and magnetic resonance coronary angiography (MRCA). Indeed, the results of large multicentre clinical trials are now emerging which will help clarify the optimal strategies for incorporating such tools into clinical practice.
To better understand the role and relative merits of the various options for non-invasive imaging, it is helpful to appreciate the processes of plaque biology and pathophysiology. Such background knowledge holds relevance to clinicians as it informs the rationale for imaging coronary atherosclerosis and the histological processes that we endeavour to identify through these investigations.
Plaque biology as it relates to non-invasive imaging
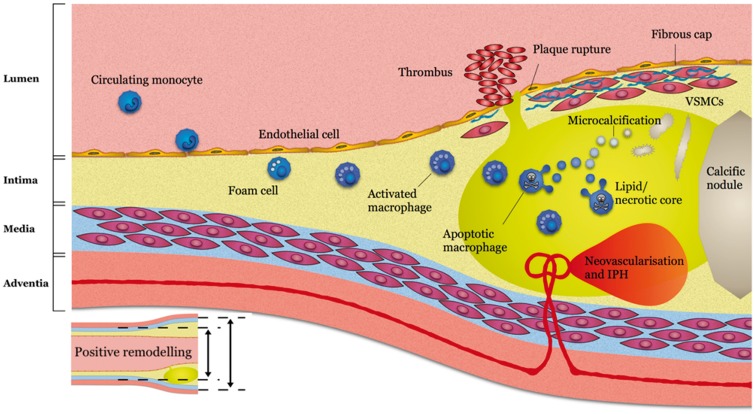
Atherosclerosis is a chronic inflammatory disease characterized by the formation of lipid-rich plaques. Intimal thickening is a near universal development by early adulthood and begins with an increase in vascular smooth muscle cells within the subintimal space. This is associated with concurrent insudation of circulating lipoproteins across the vascular endothelium where they are bound by an extracellular proteoglycan rich matrix. Oxidation of these lipoproteins initiates an inflammatory cascade as endothelial and smooth muscle cells express cellular adhesion molecules that promote migration and differentiation of circulating monocytes. The resultant macrophages, particularly the M1-subtype, act to promote a persistent maladaptive response leading to the development of the archetypal high-risk plaque; the thin-cap fibroatheroma. The hallmarks associated with high-risk plaque can be loosely categorized as those either related to the macroscopic structure of the plaque or to the biological processes occurring within it. Indeed, histological and imaging data have consistently demonstrated that culprit plaques responsible for myocardial infarction have the following characteristics: a large plaque volume, a lipid-rich necrotic core, positive remodelling, peripheral neovascularization, micro-calcification, intra-plaque haemorrhage, chronic inflammation, and a thin fibrous cap. Each of these characteristics represents a potential imaging target for in vivo identification of high-risk plaques and for guiding subsequent therapeutic modification (Figure 1).2
Figure 1.
Imaging targets of high-risk plaque. Reused from Adamson et al.2 Circulating monocytes migrate into early intimal thickening where they phagocytose lipid becoming foam cells and activated macrophages detectable on 68Ga-DOTATATE positron emission tomography. Vascular remodelling can be detected on computed tomography coronary angiography prior to luminal stenosis developing. As the lipid core develops this can be detected as low-density signal on computed tomography coronary angiography. The resulting hypoxic environment prompts neovascularization with friable vessels prone to intraplaque haemorrhage, both of which can be detected on magnetic resonance coronary angiography. A necrotic core develops with microvesicles arising from apoptotic macrophages and vascular smooth muscle cells giving rise to microcalcifications detectable on 18F-fluoride positron emission tomography before coalescing into more stable calcific nodules detectable on computed tomography calcium scans. Rupture of the fibrous cap may result in intraluminal thrombosis detectable on magnetic resonance coronary angiography.
Aims of non-invasive coronary imaging
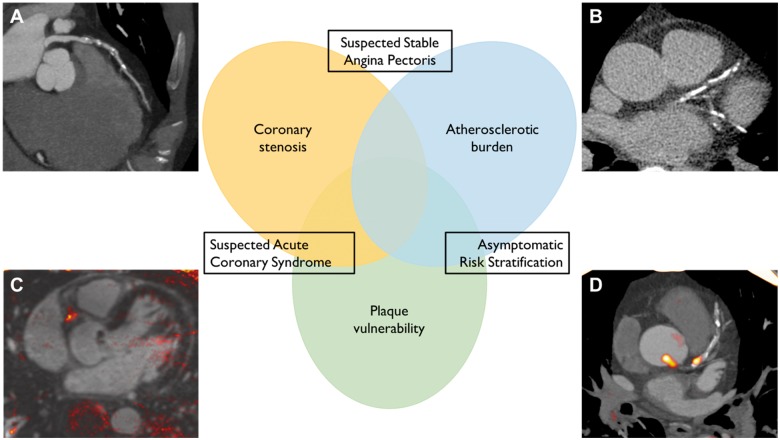
In evaluating the clinical role of non-invasive imaging, it is important to distinguish between patients based on the presence or absence of symptoms likely to be related to myocardial ischaemia. The former group comprises individuals with suspected stable angina pectoris or possible acute coronary syndromes. Here, the diagnostic objective is to identify or to exclude the presence of obstructive plaque causing sufficient luminal compromise that myocardial blood flow may be insufficient to meet metabolic demand. In contrast, coronary imaging in the asymptomatic population is largely targeted at estimating the risk of future events through the identification of atherosclerotic burden including non-obstructive disease and high-risk plaque (Figure 2). This review focusses on non-invasive imaging of coronary plaque structure and pathophysiology and will not describe the additional utility of these modalities in the assessment of coronary flow and myocardial ischaemia.
Figure 2.
Complementary roles of non-invasive coronary imaging. The use of non-invasive coronary imaging can be considered in three contexts, each with specific objectives that may inform the choice of imaging modality. (A) Computed tomography angiography provides accurate assessment of coronary stenosis that can guide management of patients with suspected stable angina and rule out Type 1 myocardial infarction in patients with potential acute coronary syndrome. (B) Coronary artery calcium scanning is able to reliably quantify overall atherosclerotic burden and improve risk stratification in asymptomatic individuals. (C) T1-weighted magnetic resonance coronary angiography can identify features of atherosclerotic instability including intracoronary thrombus and intraplaque haemorrhage and may be of value in suspected acute coronary syndrome or asymptomatic risk stratification. (D) Positron emission tomography can employ specific tracers designed to target markers of plaque vulnerability that may improve prognostic assessment or act as a surrogate of therapeutic response in asymptomatic patients.
The four most developed non-invasive coronary imaging modalities include computed tomography coronary artery calcium (CAC) scoring, CTCA, MRCA, and positron PET—usually employed in combination with either computed tomography or magnetic resonance imaging (Table 1).
Table 1.
Comparison of non-invasive coronary imaging modalities
| Imaging parameters | CT calcium scan | CT coronary angiography | MRCA | PET |
|---|---|---|---|---|
| Image acquisition | ||||
| Scan duration | 0.5–10 s | 0.5–10 s | 10–20 min | 60–90 min (tracer uptake)15–30 min/PET bed |
| Spatial resolution | 1.5–3.0 mm | 0.5–1.0 mm | 1.0–2.0 mm | 4.0–10.0 mm (tracer dependent) |
| Temporal resolution | 240–420 ms | 240–420 ms (65 ms with dual-source CT) | <60 ms | Minutes |
| Radiation exposure | <1 mSv | 1–10 mSv (protocol dependent) | Nil | 6–15 mSv (less in PET-MR) |
| Advantages |
|
|
|
Tracers can be developed to target almost any structural or pathophysiological process of interest |
| Limitations | Limited spatial resolution Non-calcified (potentially high-risk) plaque not detectable |
|
|
|
| Indications | Risk stratification in primary prevention for individuals at low-intermediate risk of cardiovascular events | Non-invasive assessment of suspected stable angina in patients with intermediate pre-test probability of coronary artery disease |
|
Research purposes only at present |
CT, computed tomography; MRCA, magnetic resonance coronary angiography; PET, positron-emission tomography.
Coronary artery calcium scoring
Atherosclerotic calcification is a well-described process, in part occurring as a healing response to pathological inflammation within the plaque. In its earliest stages, extracellular debris acts as a nidus for calcium deposition and the resultant microcalcifications have been shown to increase the likelihood of rupture of the surface of fibroatheromas. In more advanced disease—by which stage calcium is detectable on computed tomography—these microcalcifications coalesce into large, calcific nodules.
The assessment of coronary artery calcification, is one of the most enduring applications for non-invasive coronary artery imaging. The relationship between coronary calcification and obstructive coronary artery disease was initially determined from chest radiography and confirmed with findings from in vivo invasive coronary angiography and ex vivo histology.3,4 The subsequent introduction of electron-beam computed tomography substantially improved diagnostic sensitivity, and scans are now commonly performed on non-contrast images obtained from multi-detector computed tomography scanners at sub-millisevert radiation doses.5 The CAC scan consists of a non-contrast, gated computed tomography of the heart acquired during a short period of held inspiration. Arterial calcium is defined as the presence of a lesion with a density >130 Hounsfield units across an area of at least 1 mm2. Atherosclerotic calcification can be reported by volume or mass, but most commonly is described in Agatston units (AU), a semi-quantitative measure that incorporates aspects of calcium density and distribution. Coronary calcium scoring has been evaluated both in patients with suspected angina and asymptomatic populations.
Uncertainty persists regarding the utility of CAC scoring amongst symptomatic individuals. In these patients, a CAC score of >0 has a diagnostic sensitivity for identifying a coronary stenosis of ≥50% of between 0.89 and 0.99.6–8 However, the corresponding specificity is poor, ranging from 0.40 to 0.59. Consequently, in low-risk populations, such as patients with atypical symptoms in the outpatient clinic, the low positive predictive value will necessitate additional diagnostic imaging in many cases. Conversely, in situations with high pre-test probability of disease, for example, amongst troponin positive patients in the Emergency Department, the test will have an unacceptably high ‘false-negative’ rate. This is a particular concern as non-calcified plaques often exhibit additional high-risk features on alternative imaging modalities and are therefore more likely to rupture with resultant myocardial infarction. Consequently, CAC scoring is not recommended in the diagnostic assessment of symptomatic chest pain patients.
Accepting that not all plaques contain calcium detectable on computed tomography, the total CAC score has been demonstrated in histopathological and intravascular ultrasound studies to offer an acceptable approximation of the overall plaque burden for an individual. Given the association between CAC and both plaque burden and coronary obstruction, it is perhaps unsurprising that numerous reports have confirmed the prognostic value of such scores. The risk stratification provided is in addition to established clinical and biochemical risk factors, and the St Francis Heart Study found an improvement in the c-statistic for clinical events from 0.69 to 0.79 when added to the Framingham risk score9; a finding that has been confirmed in several larger cohort studies including the MESA (Multi-Ethnic Study of Atherosclerosis),10 and HNR (Heinz Nixdorf Recall—Risk Factors, Evaluation of Coronary Calcium and Lifestyle)11 trials. This improvement is of most value in intermediate risk patients without established cardiovascular disease who may be considering whether to commence primary prevention therapies. In this context, both the 2013 ACC/AHA and 2016 ESC guidelines on the prevention of cardiovascular disease give a Class IIb (may be considered) recommendation to CACS.12,13
Computed tomography coronary angiography
The clinical application of CTCA was long delayed by the problems of cardiac motion and high radiation exposure. Fortunately, advances in scanner technology and the introduction of prospective electrocardiographic (ECG)-gating have largely overcome these challenges and diagnostic image quality can now be obtained in 95% of scans in unselected populations.14 Where image quality remains poor, it is commonly related to motion artefacts at high heart rates, dense coronary calcification, or coronary stents. Such limitations can be minimized through appropriate patient selection and preparation, including judicious use of beta-blockers as part of the scanning protocol. Clinical imaging is performed on ≥64-slice computed tomography scanners, using intravenously administered contrast media and can be performed with radiation exposures in the range of 3–5 mSv.
Computed tomography coronary angiography as a diagnostic tool for coronary obstruction
One of the earliest proposed uses for CTCA related to improving the selection of patients presenting with suspected stable angina who required invasive coronary angiography and initial studies on this imaging modality focused on diagnostic accuracy with regards to detecting or excluding obstructive CAD. A meta-analysis published in 2007 described a sensitivity of 93% and specificity of 96% on a per-segment basis for the detection of coronary stenoses >50%,15 and in symptomatic patients with an intermediate pre-test probability of obstructive coronary artery disease, the negative predictive value of a negative CTCA is reported to be >95%.16 Based on the results of these diagnostic accuracy studies, CTCA has gained endorsement by international guidelines as a reasonable choice of non-invasive test in appropriately selected patients.17,18
Computed tomography coronary angiography for prognosis
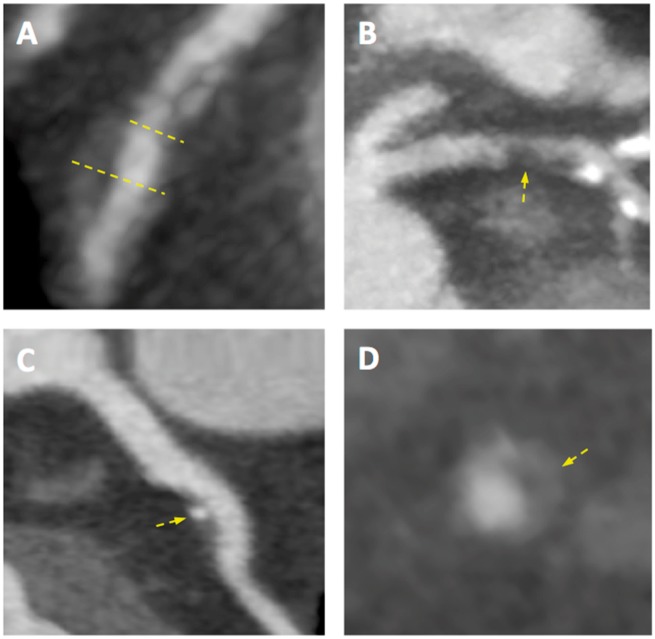
In addition to diagnosing coronary obstruction, the ability of CTCA to accurately quantify the anatomical distribution and severity of coronary atherosclerosis enables it to provide valuable prognostic information. As for invasive imaging, there is a clear stepwise worsening of prognosis associated with increasing numbers of diseased vessels.19 Indeed, due to its sensitivity in the detection of non-obstructive coronary atheroma and ability to identify additional adverse plaque characteristics, risk stratification is a particular strength of CTCA. As previously mentioned, histopathological and invasive coronary imaging studies have identified a number of features that are commonly present in plaques at risk of coronary rupture.20 Correlates of these features have been described for non-invasive imaging with CTCA and include the presence of positive remodelling, low attenuation plaque, spotty calcification, and the ‘napkin ring’ sign (Figure 3).21 More recently, changes in the composition of perivascular adipose tissue have also been described that are detectable on CTCA, correlate with histological evidence of plaque inflammation, and may arise in response to paracrine signalling.22 Numerous studies have reported an association between such adverse plaque characteristics and an increased risk of subsequent cardiovascular events.19,21,23,24 Furthermore, as for quantification of coronary calcification, a variety of prognostic scores have been described. At the simplest level, the segment involvement score (SIS) sums the number of diseased coronary segments, whilst the stenosis severity score (SSS) also incorporates a weighting factor for stenotic severity.19 More recently, the computed tomography-adapted Leaman score, combining stenotic severity, myocardium at risk, and high-risk plaque features, appears to improve risk stratification further.25
Figure 3.
Adverse coronary plaque characteristics identified on computed tomography coronary angiography. Coronary atherosclerotic plaque features detected using computed tomography coronary angiography including (A) positive remodelling—defined as an outer vessel diameter (large yellow line) 10% greater than the mean diameter of the segments immediately proximal (small yellow line) and distal to the plaque; (B) low attenuation plaque—defined as a focal central area of plaque with an attenuation density of <30 Hounsfield Units (yellow arrow); (C) spotty calcification—defined as focal calcification within the coronary artery wall <3 mm in maximum diameter (yellow arrow); and (D) the ‘napkin ring’ sign—defined as a central area of low attenuation plaque with a peripheral rim of high attenuation (yellow arrow).
Randomized controlled trials testing computed tomography coronary angiography
In contrast with most radiological investigations used in medical practice, the clinical utility of CTCA has been rigorously determined in a series of randomized clinical trials. In a comparison with exercise ECG testing, the CAPP (Cardiac CT for the Assessment of Pain and Plaque) trial (n = 500) demonstrated an improvement in angina-related quality of life, with the use of CTCA.26 This trial was underpowered for hard clinical events but identified a corresponding reduction in unplanned hospital admissions amongst those in the CTCA intervention arm. More recently, the much larger PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) and SCOT-HEART (Scottish Computed Tomography of the HEART) trials have reported their findings. PROMISE (n = 10 003) randomized intermediate-risk symptomatic patients being evaluated for the presence of coronary heart disease to CTCA or non-invasive functional testing (67% nuclear stress imaging, 23% stress echocardiography, 10% exercise ECG).27 The median duration of follow-up was 25 months and no difference in the primary composite endpoint (death, myocardial infarction, hospitalization for unstable angina, or major procedural complication) was demonstrated despite a downstream reduction in the rate of unnecessary invasive coronary angiograms and apparent reductions in death or myocardial infarction at 12 months. The SCOT-HEART trial (n = 4146) investigated the utility of adding CTCA to standard care (predominantly exercise ECG) in a broad population of patients seen in rapid access chest pain clinics across Scotland.14 The primary endpoint of diagnostic certainty at 6-weeks was increased with CTCA. Recently, the 5-year composite clinical outcome of coronary death or non-fatal myocardial infarction has been reported, with a marked 40% relative risk reduction in the CTCA arm of the trial.28 In aggregate, these trials provide powerful evidence of benefit for a CT first approach in the assessment of stable chest pain.29
Randomized trials of CTCA have also been conducted in the Emergency Department setting amongst patients with suspected acute coronary syndromes. Examples include the CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment, n = 1370), ROMICAT-II (Rule Out Myocardial Infarction/ischaemia Using Computer Assisted Tomography, n = 1000), and ACRIN-PA (CT Angiography for Safe Discharge of Patients with Possible Acute Coronary Syndromes, n = 699) trials.30–32 Compared with standard care, CTCA reduced both the time required to establish a diagnosis and the overall length of stay, albeit with no difference in hard clinical outcomes. However, these trials were performed prior to the introduction of high-sensitivity cardiac troponin assays, which allow more rapid rule-out of myocardial infarction in the Emergency Department setting.33,34 The more contemporary BEACON (Better Evaluation of Acute Chest Pain with Coronary Computed Tomography Angiography, n = 500) trial employed such assays and perhaps consequently was unable to demonstrate any benefit regarding length of stay with the use of CTCA.35 This finding has been corroborated by a post hoc analysis of the ROMICAT I and II trials where stored samples were used for the measurement of high-sensitivity cardiac troponin I.36
Less evidence exists to inform the role of CTCA in the management of asymptomatic patients at risk of cardiovascular disease, and such an approach has been investigated in only a single trial to date. The FACTOR-64 (Screening For Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64) trial randomized 900 patients with established diabetes mellitus but no prior history of cardiovascular disease to standard care or CTCA.37 Intensive treatment of co-morbid vascular risk factors was strongly encouraged in those identified with coronary atheroma on non-invasive imaging. Due to excellent use of preventative therapies in the standard care arm, CTCA was associated with only a small incremental reduction in cholesterol profiles and event rates were low in both treatment groups; less than one quarter of that expected. Overall, despite a numerical trend, no improvement in the primary composite cardiovascular endpoint was identified (hazard ratio 0.69, 95% confidence interval 0.41–1.16; P = 0.16).
Future research directions for computed tomography coronary angiography
Having demonstrated the benefits of CTCA in the diagnosis of suspected stable angina, further work is underway to test further roles for this technique in clinical practice. The RAPID-CTCA (Rapid Assessment of Potential Ischaemic Heart Disease with CTCA) trial is a multicentre study recruiting patients with suspected acute coronary syndrome and an additional risk factor; such as positive cardiac troponin, ischaemic changes on the ECG, or an established history of CAD. Patients will be randomized to early CTCA or standard care—in many cases likely to include invasive coronary angiography. The trial is powered with regards to the primary endpoint of all-cause death or recurrent non-fatal myocardial infarction at 1-year and plans to recruit 2500 patients.38 Another ongoing study of CTCA in the acute coronary syndrome setting is the TARGET-CTCA (Troponin in Acute chest pain to Risk stratify and Guide EffecTive use of Computed Tomography Coronary Angiography) trial. This study exploits the potential of high-sensitivity troponin to identify an at risk subgroup of the suspected ACS population where the peak troponin concentration is mildly increased but remains below the 99th centile upper reference limit. The trial is based on the premise that many of these patients are currently discharged without a definitive aetiology for their symptoms being established and that CTCA, by providing diagnostic clarification, may allow better therapeutic targeting.
Magnetic resonance coronary angiography
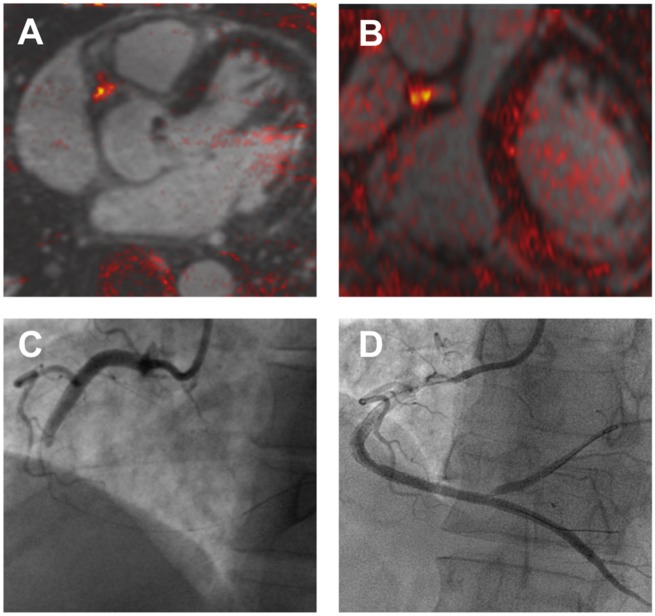
Magnetic resonance angiography is a widely accepted technique for imaging larger conduit vessels, particularly the carotid arteries and abdominal aorta. However, due to limitations related to spatial resolution and long-scan times, coronary imaging with MRCA currently has limited indications in clinical practice, except in the assessment of anomalous coronary arteries, coronary aneurysms, and coronary bypass grafts. Nevertheless, MRCA has important potential strengths that have maintained ongoing research interest. These include the ability for luminal visualization in the presence of dense calcification, the absence of ionizing radiation, the possibility for concomitant functional imaging of the myocardium, and the potential for detailed tissue characterization. A recently described example of the latter, is the identification of high-intensity plaque on non-contrast T1-weighted imaging (Figure 4).39 T1-weighted imaging targets methaemoglobin, a component of thrombus and intraplaque haemorrhage, and high-intensity plaque appears to be an MRCA analogue for low-density plaque on CTCA that is associated with high-risk features on invasive imaging with intravascular ultrasound or optical coherence tomography.40,41 The presence of high-intensity plaque also correlates with increased risk of procedure-related myocardial injury during percutaneous coronary intervention.39
Figure 4.
Coronary atherosclerosis T1-weighted characterization with integrated anatomical reference (CATCH). T1-weighted magnetic resonance coronary angiogram of a patient who presented with an inferior myocardial infarction shows evidence of a focal high intensity lesion (arrows) in the right coronary artery on magnetic resonance imaging (A and B). Subsequent coronary angiogram demonstrated occlusion of the mid-right coronary artery (C) with restoration of flow following thrombus aspiration (D).
Stepwise advances in imaging, moving from single-slice breath-hold sequences, through free-breathing whole-heart scanning, and the introduction of 3 Tesla magnets have brought a broader clinical role for MRCA closer. Nevertheless, in the assessment of suspected stable angina, MRCA currently has only modest diagnostic accuracy, with a recent meta-analysis of 24 studies reporting a pooled sensitivity and specificity for the detection of >50% stenosis on invasive coronary angiography of 89% and 72%, respectively.42 Whilst diagnostic performance can be increased with the use of gadolinium-based intravascular contrast agents, MRCA undoubtedly remains in its infancy.
Positron emission tomography
Positron emission tomography is a non-invasive technique that is underpinned by molecularly targeted probes, conjugated to a radioactive isotope that undergoes beta decay. The probes vary widely in regards to structural complexity but each is chosen in order to bind with very high sensitivity to important components of a specific pathophysiological process of interest. After binding, the emitted positron travels a short distance in vivo before interacting with an electron. The resultant annihilation releases two photons in opposing directions which exit the body and are identified as coincident events by the encircling detector ring.43 Even more so than with magnetic resonance imaging, PET imaging of the coronary arteries has traditionally been challenged by the problems of prolonged scan times, spatial resolution and limited availability.44 Hybrid PET-CT and PET-MR scanners have begun to address these challenges and coronary PET imaging now appears to be a viable proposition. Although the scope to manufacture probes for molecular imaging targets is near-limitless, to date the majority of clinical research has related to three tracers of interest (Table 2).
Table 2.
PET radiotracers for coronary atherosclerosis
| Target | Ligand | Radiotracer | Application to date | Selected ongoing clinical trials |
|---|---|---|---|---|
| Macrophage activation | GLUT (1 and 3) and conversion by hexokinase to 18F-FDG-6-phosphate | 18F-FDG |
|
Vascular Inflammation in Psoriasis (NCT02187172, NCT03082729) |
| Somatostatin receptor subtype 2 | 68Ga-DOTATATE |
|
||
| Translocator protein 18-kDa | 11C-PK11195 | Prospective in vivo study in carotid stenosis | ||
| Translocator protein 18-kDa | 11C-PBR28 | Clinical studies in healthy controls and multiple sclerosis | Cardiac Sarcoidosis (NCT02017522) | |
| Mannose receptor | 18F-FDM | Preclinical cell culture model | ||
| Choline kinase phosphorylated to Phosphatidylcholine | 18F-choline | Preclinical murine model | ESCAPPE (NCT02640313) | |
| Apoptosis | Phosphatidylserine | 68Ga-Annexin A5 | Preclinical murine model | |
| Hypoxia | Reduction to amine derivative in low O2 environment | 18F-FMISO | Preclinical murine model | |
| Reduction to amine derivative in low O2 environment | 18F-HX4 | Proof of concept in carotid atherosclerosis | ||
| Microcalcification | Hydroxyapatite | 18F-fluoride | Prospective in vivo studies in coronary and extracardiac atherosclerosis |
|
| Angiogenesis | αVβ3 and αVβ5 integrin | 18F-Fluciclatide | Prospective in vivo studies in cardiac and extracardiac atherosclerosis | |
| αVβ3 integrin | 18F-RGD-K5 | Ex vivo human carotid studies | Carotid plaque imaging study NCT01968226 |
Adapted from Moss et al.45
18F-Fluorodeoxyglucose
18F-Fluorodeoxyglucose (18F-FDG), a non-specific marker of cellular inflammation was the first tracer to be investigated for coronary imaging with preclinical models showing a correlation between tracer uptake and increased macrophage activity.46 When used in vivo to image the carotid arteries, 18F-FDG uptake correlates with high-risk plaque features on CT and histological specimens. Carotid uptake has also been demonstrated to identify a reduction in atherosclerotic inflammation in response to treatment with simvastatin.47 Similar associations within the coronary vasculature are likely, but imaging in this location is made challenging by the intense myocardial uptake, often overwhelming the coronary signal.48
18F-Fluoride
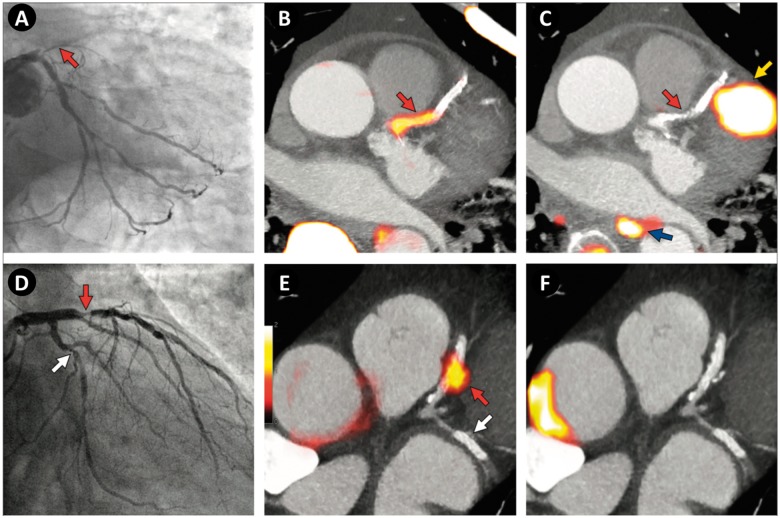
18F-Fluoride binds with high affinity to the exposed surface of hydroxyapatite, a key mineral component of vascular calcification. Initially developed for the detection of bony metastases, it is now recognized to enable the detection of early microscopic atherosclerotic calcification prior to the development of macroscopic calcification on CT imaging.49 In this context, tracer binding demonstrates intense signal in areas of active mineralization where large numbers of microcalcific deposits are present throughout the plaque, increasing strain on the fibrous cap, thereby potentially provoking plaque rupture. In contrast to 18F-FDG, 18F-fluoride is not limited by myocardial uptake and identifies the culprit artery in patients diagnosed with acute myocardial infarction (Figure 5).48 The ability of coronary imaging with 18F-fluoride PET-CT to improve risk stratification following myocardial infarction is currently being investigated in a prospective multicentre trial (NCT02278211).
Figure 5.
Focal 18F-fluoride and 18F-fluorodeoxyglucose uptake in patients with myocardial infarction and stable angina. (Top row, A–C) Patient with acute ST-segment elevation myocardial infarction with (A) proximal occlusion (red arrow) of the left anterior descending artery on invasive coronary angiography and (B) intense focal 18F-fluoride uptake (yellow-red) at the site of the culprit plaque (red arrow) on the combined positron emission and computed tomography coronary angiography (PET-CTCA). Corresponding 18F-fluorodeoxyglucose PET-CT image (C) showing no uptake at the site of the culprit plaque. Note the significant myocardial uptake overlapping with the coronary artery (yellow arrow) and uptake within the oesophagus (blue arrow). (Bottom row) Patient with anterior non-ST-segment elevation myocardial infarction with (D) culprit (red arrow; left anterior descending artery) and bystander non-culprit (white arrow; circumflex artery) lesions on invasive coronary angiography that were both stented during the index admission. Only the culprit lesion had increased 18F-NaF uptake on PET-CT (E) after percutaneous coronary intervention. Corresponding 18F-fluorodeoxyglucose PET-CT (F) showing no uptake either at the culprit or the bystander stented lesion. Note intense uptake within the ascending aorta. Adapted from Joshi et al.47
68Ga-DOTATATE
68Gallium-labelled DOTATATE binds to the somatostatin receptor subtype 2 (SSTR2) found on the surface of macrophages, particularly the proinflammatory M1 subtype. Preclinical studies suggest it may be a superior marker of coronary macrophage activity than 18F-FDG and importantly myocardial uptake is minimal. A recent report demonstrates increased 68Ga-DOTATATE uptake in culprit coronary and carotid plaques and correlation with CT and histological evidence of high-risk plaque.50 Whether this information can be used to inform patient management remains to be determined.
Conclusions
Non-invasive imaging of the coronary arteries is an enterprise in rapid development. From the research perspective, there is great demand for in vivo techniques that can reliably identify features of high-risk plaque that may offer insight into pathophysiological processes and act as surrogate indicators of response to therapeutic intervention. Meanwhile, there is clear clinical need for greater accuracy in symptom diagnosis and prognostic stratification. Fortunately, constant incremental enhancements in scanner technology and image post-processing are helping make these elusive goals a reality. To date, computed tomography, remains the most clinically applicable technique due to its broad availability and the strength of its evidence base. Coronary calcium scoring appears to be a useful technique for improving risk assessment in primary prevention, whilst CTCA is a valuable diagnostic test that improves diagnostic certainty and optimizes downstream management in symptomatic patients. At present, MRCA and PET remain largely investigational imaging modalities but landmark trials are now underway that will inform their future clinical role.
Acknowledgements
The BHF Centre for Cardiovascular Science is supported by the British Heart Foundation.
Funding
This work was supported by the British Heart Foundation (CH/09/002, RE/13/3/30183 to D.E.N.) and is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA); and the Heart Diseases Research Trust, UK (to P.D.A.).
Conflict of interest: D.E.N. has received honoraria and consultancy from Toshiba Medical Systems. Other author declared no conflict of interest.
References
- 1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M.. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 2. Adamson PD, Dweck MR, Newby DE.. The vulnerable atherosclerotic plaque: in vivo identification and potential therapeutic avenues. Heart 2015;101:1755–1766. [DOI] [PubMed] [Google Scholar]
- 3. Pyke D, Symons C.. Calcification of the aortic valve and of the coronary arteries. Br Heart J 1951;13:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rifkin RD, Parisi AF, Folland E.. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol 1979;44:141–147. [DOI] [PubMed] [Google Scholar]
- 5. Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV.. Ionizing radiation in cardiac imaging: a science advisory. Circulation 2009;119:1056–1065. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (update) Clinical Guideline 95. London: NICE; 2016. [PubMed] [Google Scholar]
- 7. Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K.. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–688. [DOI] [PubMed] [Google Scholar]
- 8.The CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–2540. [DOI] [PubMed] [Google Scholar]
- 9. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD.. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166–172. [DOI] [PubMed] [Google Scholar]
- 10. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA.. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 11. Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker-Preuss M, Mann K, Siegrist J, Jöckel K-H.. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–1406. [DOI] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF.. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 13.The Sixth Joint Task Force of the European Society of Cardiology. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 15. Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MGM.. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology 2007;244:419–428. [DOI] [PubMed] [Google Scholar]
- 16. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK.. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–1732. [DOI] [PubMed] [Google Scholar]
- 17.American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 18.European Society of Cardiology Task Force. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 19. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ.. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 20. Virmani R, Burke AP, Farb A, Kolodgie FD.. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 21. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J.. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–346. [DOI] [PubMed] [Google Scholar]
- 22. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, Petrou M, Sayeed R, Krasopoulos G, Psarros C, Ciccone P, Brophy CM, Digby J, Kelion A, Uberoi R, Anthony S, Alexopoulos N, Tousoulis D, Achenbach S, Neubauer S, Channon KM, Antoniades C.. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. [DOI] [PubMed] [Google Scholar]
- 23. Thomsen C, Abdulla J.. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2016;17:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, Bax JJ.. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;49:62–70. [DOI] [PubMed] [Google Scholar]
- 25. Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, Pontone G, Bartorelli AL, Bertella E, Campos CM, Pepi M, Serruys PW, Andreini D.. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging 2015;8:e002332. [DOI] [PubMed] [Google Scholar]
- 26. McKavanagh P, Lusk L, Ball PA, Verghis RM, Agus AM, Trinick TR, Duly E, Walls GM, Stevenson M, James B, Hamilton A, Harbinson MT, Donnelly PM.. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. Eur Heart J Cardiovasc Imaging 2015;16:441–448. [DOI] [PubMed] [Google Scholar]
- 27. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, Ejr V. B, Williams MC; on behalf of The SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 29. Adamson PD, Hunter A, Williams MC, Shah ASV, McAllister DA, Pawade TA, Dweck MR, Mills NL, Berry C, Boon NA, Clark E, Flather M, Forbes J, McLean S, Roditi G, van Beek EJR, Timmis AD, Newby DE.. Diagnostic and prognostic benefits of computed tomography coronary angiography using the 2016 National Institute for Health and Care Excellence guidance within a randomised trial. Heart 2018;104:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE.. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
- 31.The Romicat-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O'Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL.. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011;58:1414–1422. [DOI] [PubMed] [Google Scholar]
- 33. Shah ASV, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, Chapman AR, Langdon T, Sandeman D, Vaswani A, Strachan FE, Ferry A, Stirzaker AG, Reid A, Gray AJ, Collinson PO, McAllister DA, Apple FS, Newby DE, Mills NL.. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman AR, Lee KK, McAllister DA, Cullen L, Greenslade JH, Parsonage W, Worster A, Kavsak PA, Blankenberg S, Neumann J, Sorensen NA, Westermann D, Buijs MM, Verdel GJE, Pickering JW, Than MP, Twerenbold R, Badertscher P, Sabti Z, Mueller C, Anand A, Adamson PD, Strachan FE, Ferry A, Sandeman D, Gray A, Body R, Keevil B, Carlton E, Greaves K, Korley FK, Metkus TS, Sandoval Y, Apple FS, Newby DE, Shah ASV, Mills NL.. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA 2017;318:1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dedic A, Lubbers MM, Schaap J, Lammers J, Lamfers EJ, Rensing BJ, Braam RL, Nathoe HM, Post JC, Nielen T, Beelen D, Le Cocq d'Armandville MC, Rood PP, Schultz CJ, Moelker A, Ouhlous M, Boersma E, Nieman K.. Coronary CT angiography for suspected ACS in the era of high-sensitivity troponins: randomized multicenter study. J Am Coll Cardiol 2016;67:16–26. [DOI] [PubMed] [Google Scholar]
- 36. Ferencik M, Mayrhofer T, Lu MT, Woodard PK, Truong QA, Peacock WF, Bamberg F, Sun BC, Fleg JL, Nagurney JT, Udelson JE, Koenig W, Januzzi JL, Hoffmann U.. High-sensitivity cardiac troponin I as a gatekeeper for coronary computed tomography angiography and stress testing in patients with acute chest pain. Clin Chem 2016;63:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muhlestein JB, Lappe DL, Lima JA, Rosen BD, May HT, Knight S, Bluemke DA, Towner SR, Le V, Bair TL, Vavere AL, Anderson JL.. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014;312:2234–2243. [DOI] [PubMed] [Google Scholar]
- 38. Gray AJ, Roobottom C, Smith JE, Goodacre S, Oatey K, O'Brien R, Storey RF, Na L, Lewis SC, Thokala P, Newby DE.. The RAPID-CTCA trial (Rapid Assessment of Potential Ischaemic Heart Disease with CTCA)—a multicentre parallel-group randomised trial to compare early computerised tomography coronary angiography versus standard care in patients presenting with suspected or confirmed acute coronary syndrome: study protocol for a randomised controlled trial. Trials 2016;17:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoshi T, Sato A, Akiyama D, Hiraya D, Sakai S, Shindo M, Mori K, Minami M, Aonuma K.. Coronary high-intensity plaque on T1-weighted magnetic resonance imaging and its association with myocardial injury after percutaneous coronary intervention. Eur Heart J 2015;36:1913–1922. [DOI] [PubMed] [Google Scholar]
- 40. Kawasaki T, Koga S, Koga N, Noguchi T, Tanaka H, Koga H, Serikawa T, Orita Y, Ikeda S, Mito T, Goto Y, Shintani Y, Tanaka A, Fukuyama T.. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging 2009;2:720–728. [DOI] [PubMed] [Google Scholar]
- 41. Kanaya T, Noguchi T, Otsuka F, Asaumi Y, Kataoka Y, Morita Y, Miura H, Nakao K, Fujino M, Kawasaki T, Nishimura K, Inoue T, Narula J, Yasuda S, Optical coherence tomography-verified morphological correlates of high-intensity coronary plaques on non-contrast T1-weighted magnetic resonance imaging in patients with stable coronary artery disease. Eur Heart J Cardiovasc Imaging 2018; doi: 10.1093/ehjci/jey035 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Di Leo G, Fisci E, Secchi F, Ali M, Ambrogi F, Sconfienza LM, Sardanelli F.. Diagnostic accuracy of magnetic resonance angiography for detection of coronary artery disease: a systematic review and meta-analysis. Eur Radiol 2016;26:3706–3718. [DOI] [PubMed] [Google Scholar]
- 43. Adamson PD, Newby DE, Dweck MR.. Translational coronary atherosclerosis imaging with PET. Cardiol Clin 2016;34:179–186. [DOI] [PubMed] [Google Scholar]
- 44. Adamson PD, Williams MC, Newby DE.. Cardiovascular PET-CT imaging: a new frontier? Clin Radiol 2016;71:647–659. [DOI] [PubMed] [Google Scholar]
- 45. Moss AJ, Adamson PD, Newby DE, Dweck MR.. Positron emission tomography imaging of coronary atherosclerosis. Future Cardiol 2016;12:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, JohnströM P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL.. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–2711. [DOI] [PubMed] [Google Scholar]
- 47. Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T.. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–1831. [DOI] [PubMed] [Google Scholar]
- 48. Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE.. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–713. [DOI] [PubMed] [Google Scholar]
- 49. Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP.. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, Starks LT, Martin-Garrido A, Manavaki R, Yu E, Kuc RE, Grassi L, Kreuzhuber R, Kostadima MA, Frontini M, Kirkpatrick PJ, Coughlin PA, Gopalan D, Fryer TD, Buscombe JR, Groves AM, Ouwehand WH, Bennett MR, Warburton EA, Davenport AP, Rudd JH.. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]