See Jellinger (doi:10.1093/awx190) for a scientific commentary on this article.
Monoamine oxidase-B is a drug target in Parkinson’s disease and putative biomarker of astrogliosis. Using quantitative immunoblotting in post-mortem tissue, Tong et al. provide evidence to support the use of monoamine oxidase-B as a biomarker of astroglial activation, and distinguish astrocyte status in Parkinson’s disease from that in Parkinson-plus conditions.
Keywords: monoamine oxidase, gliosis, Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy
Abstract
See Jellinger (doi:10.1093/awx190) for a scientific commentary on this article.
The enzyme monoamine oxidases (B and A subtypes, encoded by MAOB and MAOA, respectively) are drug targets in the treatment of Parkinson’s disease. Inhibitors of MAOB are used clinically in Parkinson’s disease for symptomatic purposes whereas the potential disease-modifying effect of monoamine oxidase inhibitors is debated. As astroglial cells express high levels of MAOB, the enzyme has been proposed as a brain imaging marker of astrogliosis, a cellular process possibly involved in Parkinson’s disease pathogenesis as elevation of MAOB in astrocytes might be harmful. Since brain monoamine oxidase status in Parkinson’s disease is uncertain, our objective was to measure, by quantitative immunoblotting in autopsied brain homogenates, protein levels of both monoamine oxidases in three different degenerative parkinsonian disorders: Parkinson’s disease (n = 11), multiple system atrophy (n = 11), and progressive supranuclear palsy (n = 16) and in matched controls (n = 16). We hypothesized that if MAOB is ‘substantially’ localized to astroglial cells, MAOB levels should be generally associated with standard astroglial protein measures (e.g. glial fibrillary acidic protein). MAOB levels were increased in degenerating putamen (+83%) and substantia nigra (+10%, non-significant) in multiple system atrophy; in caudate (+26%), putamen (+27%), frontal cortex (+31%) and substantia nigra (+23%) of progressive supranuclear palsy; and in frontal cortex (+33%), but not in substantia nigra of Parkinson’s disease, a region we previously reported no increase in astrocyte protein markers. Although the magnitude of MAOB increase was less than those of standard astrocytic markers, significant positive correlations were observed amongst the astrocyte proteins and MAOB. Despite suggestions that MAOA (versus MAOB) is primarily responsible for metabolism of dopamine in dopamine neurons, there was no loss of the enzyme in the parkinsonian substantia nigra; instead, increased nigral levels of a MAOA fragment and ‘turnover’ of the enzyme were observed in the conditions. Our findings provide support that MAOB might serve as a biochemical imaging marker, albeit not entirely specific, for astrocyte activation in human brain. The observation that MAOB protein concentration is generally increased in degenerating brain areas in multiple system atrophy (especially putamen) and in progressive supranuclear palsy, but not in the nigra in Parkinson’s disease, also distinguishes astrocyte behaviour in Parkinson’s disease from that in the two ‘Parkinson-plus’ conditions. The question remains whether suppression of either MAOB in astrocytes or MAOA in dopamine neurons might influence progression of the parkinsonian disorders.
Introduction
For over half a century levodopa dopamine substitution therapy has remained the mainstay, most efficacious, pharmacological treatment for Parkinson’s disease (Birkmayer and Hornykiewicz, 1961). However, interest has also focused on the enzyme monoamine oxidase B (MAOB) as a secondary drug target in Parkinson’s disease (Birkmayer et al., 1975; Youdim et al., 2006; Finberg, 2014; Li et al., 2014; Miklya, 2016) and also as a brain glial biomarker (Rodriguez-Vieitez et al., 2016). MAOB is one of the two subtypes (MAOA and MAOB) of the major monoamine metabolizing enzyme that oxidizes the neurotransmitter dopamine and breaks down other amines (Shih et al., 1999; Youdim et al., 2006; Finberg and Rabey, 2016). MAOB is considered to be localized primarily (but not exclusively, e.g. serotonin neuron cell bodies) to astrocytes whereas MAOA is located largely to neurons in the brain (Levitt et al., 1982; Westlund et al., 1985, 1993; Shih et al., 1999; Sader-Mazbar et al., 2013, Finberg and Rabey, 2016; but see Youdim and Bakhle, 2006; Harris et al., 2015). Inhibitors of MAOB (e.g. selegiline, rasagiline) are used clinically to provide symptomatic benefit in Parkinson’s disease because of the elevation of brain dopamine by inhibiting its breakdown (Riederer and Youdim, 1986; Youdim and Bakhle, 2006; Youdim et al., 2006). More speculatively, MAOB inhibitors have been used as potential neuroprotective agents (Olanow et al., 2009; Rascol et al., 2011), with the hope that MAOB inhibition might prevent formation of damaging dopamine-derived oxidation products (Jenner, 2004; Rascol et al., 2011; Finberg, 2014; Finberg and Rabey, 2016) or possible conversion of an endogenous/environmental MPTP-like compound to a neurotoxic substance (Heikkila et al., 1984; Youdim et al., 2006). Although inhibitors of MAOA are used clinically mainly for the treatment of mood disorders, MAOA suppression might also be neuroprotective (Naoi et al., 2011; Goldstein, 2013; Fitzgerald et al., 2014; Goldstein et al., 2016). Indeed, in studies of induced pluripotent stem-cells (iPSCs)-derived dopamine neurons from patients with triplicated α-synuclein (SNCA) gene (Byers et al., 2011), with parkin (PRKN) (Jiang et al., 2012; but see Imaizumi et al., 2012) or LRRK2 (Nguyen et al., 2011) mutations, enhanced expression/activities of MAOA and/or MAOB were observed in differentiated dopamine neurons leading to oxidative stress.
More recent attention has focused on the involvement of MAOB in astroglial cells. Astrocytes, in particular reactive astrocytes (Ekblom et al., 1993) such as those in brains of patients with Alzheimer’s disease (Nakamura et al., 1990; Saura et al., 1994; Gulyas et al., 2011), express high levels of MAOB but not MAOA (Levitt et al., 1982; Westlund et al., 1985, 1993; Sader-Mazbar et al., 2013); thus, the enzyme has been proposed as a brain PET biomarker of astrogliosis, a typical consequence of toxic brain damage, and PET with the MAOB ligand 11C-L-deprenyl-D2 (see ‘Discussion’ section) has been examined in several neurological conditions with astrocytosis including epilepsy (Kumlien et al., 1995, 2001; Bergstrom et al., 1998), traumatic brain injury (Fowler et al., 1999), Creutzfeldt–Jakob disease (Engler et al., 2003), and amyotrophic lateral sclerosis (Johansson et al., 2007), with particular recent emphasis on using this imaging modality for the early detection of astroglial activation in prodromal Alzheimer’s disease (Carter et al., 2012; Choo et al., 2014; Rodriguez-Vieitez et al., 2015, 2016; Scholl et al., 2015). Although the function of astrocytes activated during neurodegeneration continues to be debated [i.e. beneficial versus detrimental; A2 versus A1 phenotypes (Verkhratsky et al., 2013; Liddelow et al., 2017)], some experimental findings show that elevated MAOB in astrocytes can induce Parkinson’s-like pathologies (Mallajosyula et al., 2008), suggesting that astrocytic MAOB might exacerbate the parkinsonian degenerative process and that MAOB inhibitors might help suppress presumably harmful astroglial activation and neuroinflammation accompanying neurodegeneration.
Given the above considerations, we felt that it might be helpful to know whether MAOB levels are abnormal in brain of patients with Parkinson’s disease, and for comparison, in the different ‘Parkinson-plus’ disorders of multiple system atrophy (MSA) and progressive supranuclear palsy (PSP), which have more widespread pathological changes but have not been examined for MAOs. Early reports of MAO status in Parkinson’s disease were non-specific for subtypes (Lloyd et al., 1975; Schneider et al., 1981) whereas results of measurements of MAOB activity were problematic and variable (normal in substantia nigra and putamen: Yong and Perry, 1986; Gargalidis-Moudanos et al., 1997; moderate increase in substantia nigra and putamen: Riederer and Jellinger, 1983; increase only in putamen: Jellinger and Riederer, 1984; moderate increase in substantia nigra: Riederer et al., 1989) (cf.Strolin Benedetti and Dostert, 1989; Oreland, 1991). A neuropathological investigation (Damier et al., 1996) reported above-normal number of MAOB immunoreactive ‘glial cells’ in Parkinson’s disease substantia nigra; however, measurement of total MAOB in the nigra was not determined. With regard to MAOA in Parkinson’s disease, anecdotal reports of MAOA activities from the same laboratory were inconsistent, ranging from no change to +90% in substantia nigra (Riederer and Jellinger, 1983; Jellinger and Riederer, 1984; Riederer et al., 1989). Methodologically, enzyme activity and immunohistochemical assays of MAOs were not absolutely specific for the two isozymes, which may have contributed to literature inconsistency; in contrast, by using highly specific and potent antibodies, we recently established quantitative immunoblotting assays that distinguish MAOB and MAOA proteins unequivocally (Tong et al., 2013). The present study was therefore undertaken to measure protein levels of MAOB and MAOA in autopsied brain of patients with Parkinson’s disease, PSP and MSA. Because of the evolving interest (Rodriguez-Vieitez et al., 2016) in use of MAOB as a brain imaging marker of astrogliosis in humans, and the paucity of supportive quantitative investigations in different brain disorders in which astroglial activation is expected, we compared MAOB levels to those of other astroglial markers including glial fibrillary acidic protein (GFAP), vimentin and heat shock protein-27 (Hsp27) (Tong et al., 2015). We hypothesized that the degree and regional specificity of any MAOB increase would generally be associated with those of other astroglial markers reported previously in the parkinsonian conditions. Given the presumed localization of MAOA to dopamine neurons, we also hypothesized that levels of MAOA in substantia nigra would be decreased in the dopamine deficiency disorders.
Materials and methods
Subjects
Autopsied brains were obtained from patients with Parkinson’s disease (n = 11), MSA (n = 11), and PSP (n = 16) and matched controls (n = 16). No significant difference was found in post-mortem interval (h) (control: 12 ± 1; Parkinson’s disease: 14 ± 2; MSA: 13 ± 2; PSP: 11 ± 1; mean ± SEM) or age (years) (control: 71 ± 2; Parkinson’s disease: 77 ± 3; MSA: 65 ± 3; PSP: 73 ± 3) among the four groups. One half-brain was used for neuropathological examination, whereas the other half was frozen for neurochemical analyses. The characteristics of the patients were previously reported (Tong et al., 2010, 2015, see Supplementary Table 1). All levodopa treated patients with Parkinson’s disease had good response to the therapy whereas levodopa treatment in MSA and PSP produced only varying, at most moderate response. No detailed information on the neuropsychological or mental function of the patients was available. The causes of death for the controls were cardiovascular illnesses (n = 10), bronchopneumonia (n = 2), pulmonary oedema (n = 2), breast cancer (n = 1), and natural death (n = 1).
Neuropathological assessment
Neuropathological findings (qualitative only) of neuronal loss by routine haematoxylin and eosin stain and assessments of the pathological hallmarks including Lewy bodies, glial cytoplasmic inclusions and neurofibrillary tangles in brains of patients with Parkinson’s disease, MSA and PSP were previously reported (Tong et al., 2010, 2015; see Supplementary Table 1 for a summary). Lewy bodies (haematoxylin and eosin stain) were confirmed in all Parkinson’s disease patients (idiopathic), with significant Lewy body pathology restricted to substantia nigra, locus coeruleus and other brainstem areas and with the anterior cingulate, hippocampus including transentorhinal cortex, and frontal and temporal neocortices showing no obvious Lewy body pathology. Biochemical analyses confirmed accumulation of high molecular weight (HMW) α-synuclein species in substantia nigra in all Parkinson’s disease cases, in putamen in four cases but in caudate and frontal cortex of only one case, confirming that the Parkinson’s disease cases had brainstem-predominant α-synuclein accumulation. Lewy bodies were absent in all MSA cases. The presence of glial cytoplasmic inclusions (by Bielschowsky silver impregnation and α-synuclein immunohistochemistry) was confirmed in five MSA patients whereas the analysis of the remaining MSA patients was conducted prior to the consensus statement on the diagnosis of MSA (Gilman et al., 1999), which incorporated assessment of the neuropathology of glial cytoplasmic inclusions. However, all MSA cases showed characteristic regional pattern of degenerative changes in the striato-nigral and pontocerebellar brain regions with marked cell loss with gliosis in substantia nigra, putamen, globus pallidus, locus ceruleus, the olives, pontine nuclei, cerebellum and autonomic nuclei. Further, characteristic heterogeneous accumulation of HMW α-synuclein species in the widespread brain regions and white matters of all MSA cases was confirmed (Supplementary Table 1). For all PSP cases, pathological examination confirmed absence of Lewy bodies and the presence of neuronal loss in substantia nigra, globus pallidus, subthalamic nucleus, brainstem, and cerebellar dentate nucleus together with tau-positive neurofibrillary tangles (by Bielschowsky silver impregnation and tau immunohistochemistry). No systematic or quantitative assessment of gliosis (microglia and astrocytes) was performed during the neuropathological examination.
SDS-PAGE and western blotting
Brain dissection followed published procedures (Kish et al., 1988) using the atlas of Riley (Riley, 1943). Brain regions examined in this study included substantia nigra pars compacta (referred to as ‘substantia nigra’ for simplicity only), putamen, caudate, and frontal cortex (Brodmann area 9). Substantia nigra samples (20–30 mg wet weight) were taken from slices at the level of red nucleus, corresponding roughly to plates T4-1308 and T4-1443 of Riley (1943), (cf. slices #10–12 of Kish et al., 1988), with pars reticulata excluded as much as possible from the excised pigmented pars compacta area; caudate and putamen samples were taken from the representative intermediate (along a dorsoventral gradient) subdivision of the middle (along a rostrocaudal gradient) portion of both nuclei, i.e. slices #5 or #6 for caudate and slices #7 or #8 for putamen as described in Kish et al. (1988). Dissected tissue was homogenized (10×, vol/vol) by sonication in ice-cold 50 mM Tris-HCl, 2 mM EGTA, pH 7.4, containing 1% (vol/vol) protease inhibitors (cat# P8340, Sigma-Aldrich). Aliquoted homogenates were used for the quantitative immunoblotting assays of MAOA and MAOB with a five-point tissue standards composed of a pooled human striatal sample (see Tong et al., 2013 for details and antibodies used). Briefly, after probing for MAOA, the PVDF membranes were stripped and reprobed for MAOB. The antibodies used were rabbit polyclonal antibodies from Santa Cruz Biotechnology (sc-20156, H-70, against C-terminal amino acids 458–527 of human MAOA) and Abcam (ab67297, against amino acids 448–466 of human MAOB), respectively. [It should be noted that the H-70 MAOA antibody reacts non-specifically with a soluble plasma protein in both human and rat that is slightly larger in molecular weight (∼70 kDa) than that of MAOA (65 kDa)]. Levels of MAOs (in ng/µg protein) in the tissue standard were calibrated by using recombinant human MAOA (M7316) and MAOB (M7441) from Sigma-Aldrich (Tong et al., 2013). Levels of GFAP, vimentin, and Hsp27, including the low molecular weight (LMW) and HMW species, were previously reported (Tong et al., 2015). Concentrations of the ‘control’ proteins neuron specific enolase (NSE) and α-tubulin were determined by the same immunoblotting technique in the SDS-PAGE samples. For simplicity only, ‘immunoreactivity’ of the proteins examined will be referred to as ‘levels’.
Statistical analyses
Statistical analyses on differences in MAO levels among controls and parkinsonian conditions were performed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests. Correlations were examined by Pearson product-moment or Spearman rank order correlation analyses as indicated in the text. The criterion of statistical significance was P < 0.05.
Results
Characteristics of MAOB and MAOA in autopsied human brain and detection of protein fragments
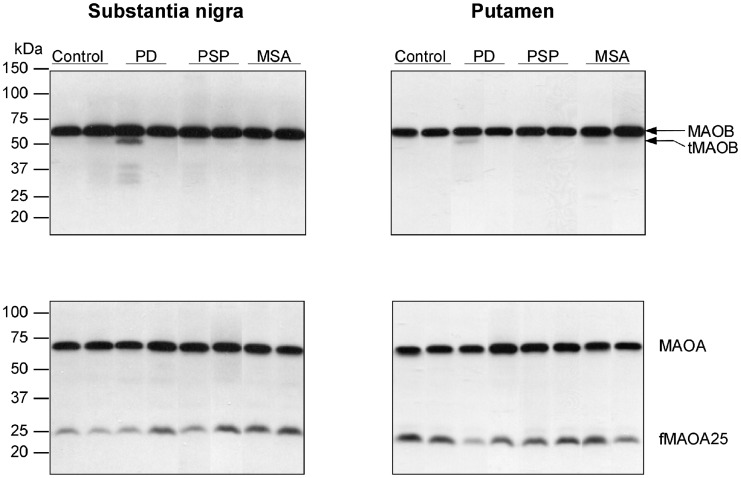
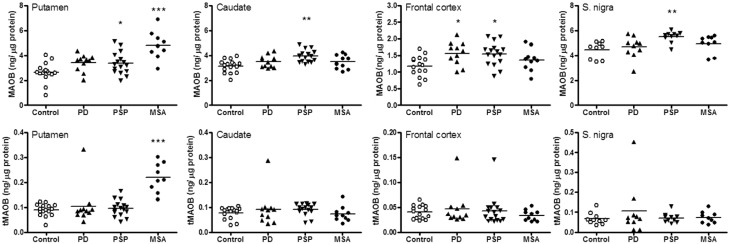
As expected (Tong et al., 2013), the MAOB antibody detected the major intact MAOB protein at the expected molecular weight position (64 kDa). In addition, the antibody detected in many, but not all, samples a slightly smaller, faint protein band of ∼62 kDa (Fig. 1), especially in aged brain. This truncated form of MAOB was also detected in purified recombinant MAOB (Tong et al., 2013) and in rat brain (data not shown). Similar to intact MAOB (Tong et al., 2013), truncated MAOB was membrane-bound and there was a significant age-related increase in truncated MAOB levels in the frontal cortex of autopsied human brain (Supplementary Fig. 1); truncated MAOB was detected in frontal cortex of only 1 of 34 subjects under 18 years old, 4 of 15 aged 18–50, but 16 of 21 above 50 years of age. Overall, truncated MAOB levels were positively correlated with those of intact MAOB. However, levels of truncated MAOB were generally low and comprised <5% of the intact protein with the exception of putamen of MSA, frontal cortex of 1 of 16 patients with PSP and across examined brain regions of 1 of 11 patients with Parkinson’s disease (Table 1 and Fig. 2).
Figure 1.
Substantia nigra and putamen. Representative immunoblots of monoamine oxidase (MAOB and MAOA) in controls and in patients with Parkinson's disease (PD), PSP and MSA. Protein bands for the intact proteins (MAOB, 64 kDa and MAOA, 65 kDa) and the partially proteolysed species, i.e. truncated MAOB (tMAO-B, 62 kDa) and a 25 kDa fragment of MAOA (fMAOA25) are identified.
Table 1.
Levels of MAOB and MAOA in brain of patients with Parkinson’s disease, PSP, and MSA
| Control | PD | PSP | MSA | P | |
|---|---|---|---|---|---|
| (n = 10–16) | (n = 10–11) | (n = 10–16) | (n = 9–10) | ||
| Substantia nigra pars compacta | |||||
| MAOB | 4.46 ± 0.20 | 4.67 ± 0.28 | 5.50 ± 0.14** | 4.91 ± 0.24 | 0.011 |
| (+23%) | |||||
| tMAOB | 0.067 ± 0.009 | 0.105 ± 0.041 | 0.072 ± 0.008 | 0.073 ± 0.010 | 0.61 |
| tMAOB/MAOB | 1.5 ± 0.2% | 2.1 ± 0.8% | 1.3 ± 0.1% | 1.5 ± 0.2% | 0.60 |
| MAOA | 0.64 ± 0.03 | 0.67 ± 0.03 | 0.63 ± 0.04 | 0.61 ± 0.03 | 0.61 |
| fMAOA25 | 0.095 ± 0.005 | 0.127 ± 0.009* | 0.126 ± 0.009** | 0.133 ± 0.009** | 0.008 |
| (+33%) | (+32%) | (+40%) | |||
| fMAOA25/MAOA | 15.4 ± 1.2% | 19.2 ± 1.7% | 20.5 ± 1.6% | 22.1 ± 1.7%* | 0.028 |
| (+44%) | |||||
| Putamen | |||||
| MAOB | 2.64 ± 0.19 | 3.41 ± 0.20 | 3.37 ± 0.22* | 4.82 ± 0.38*** | <0.0001 |
| (+27%) | (+83%) | ||||
| tMAOB | 0.089 ± 0.006 | 0.104 ± 0.024 | 0.095 ± 0.008 | 0.220 ± 0.019*** | <0.0001 |
| (+147%) | |||||
| tMAOB/MAOB | 3.5 ± 0.2% | 3.1 ± 0.6% | 2.8 ± 0.2% | 4.6 ± 0.2%* | 0.0047 |
| (+32%) | |||||
| MAOA | 0.41 ± 0.03 | 0.53 ± 0.04* | 0.45 ± 0.02 | 0.31 ± 0.04* | 0.002 |
| (+29%) | (-23%) | ||||
| fMAOA25 | 0.144 ± 0.012 | 0.103 ± 0.016 | 0.153 ± 0.013 | 0.106 ± 0.017 | 0.031 |
| fMAOA25/MAOA | 39.6 ± 6.6% | 19.9 ± 2.9% | 34.6 ± 2.5% | 37.8 ± 7.2% | 0.057 |
| Caudate | |||||
| MAOB | 3.14 ± 0.13 | 3.51 ± 0.15 | 3.95 ± 0.12** | 3.48 ± 0.18 | 0.0005 |
| (+26%) | |||||
| tMAOB | 0.077 ± 0.006 | 0.092 ± 0.021 | 0.091 ± 0.006 | 0.072 ± 0.010 | 0.50 |
| tMAOB/MAOB | 2.5 ± 0.2% | 2.5 ± 0.4% | 2.3 ± 0.1% | 2.1 ± 0.3% | 0.78 |
| MAOA | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.47 ± 0.02* | 0.36 ± 0.02 | 0.0013 |
| (+19%) | |||||
| fMAOA25 | 0.065 ± 0.008 | 0.075 ± 0.010 | 0.081 ± 0.012 | 0.077 ± 0.013 | 0.71 |
| fMAOA25/MAOA | 17.2 ± 2.4% | 18.2 ± 2.3% | 18.6 ± 3.2% | 23.2 ± 4.9% | 0.62 |
| Frontal cortex | |||||
| MAOB | 1.18 ± 0.08 | 1.56 ± 0.10* | 1.55 ± 0.09* | 1.36 ± 0.11 | 0.010 |
| (+33%) | (+31%) | ||||
| tMAOB | 0.041 ± 0.003 | 0.047 ± 0.011 | 0.043 ± 0.007 | 0.033 ± 0.004 | 0.65 |
| tMAOB/MAOB | 3.5 ± 0.3% | 3.0 ± 0.5% | 2.8 ± 0.5% | 2.5 ± 0.2% | 0.35 |
| MAOA | 0.49 ± 0.03 | 0.56 ± 0.02 | 0.48 ± 0.02 | 0.58 ± 0.05 | 0.075 |
| fMAOA25 | 0.061 ± 0.014 | 0.071 ± 0.016 | 0.047 ± 0.007 | 0.059 ± 0.012 | 0.60 |
| fMAOA25/MAOA | 13.2 ± 3.2% | 13.0 ± 3.1% | 10.6 ± 2.2% | 11.3 ± 3.0% | 0.89 |
Data, expressed in mean ± SEM (% change versus control in cases with significant changes), are in ng/µg protein. tMAOB = a truncated form of MAOB; PD = Parkinson’s disease.
***P < 0.001, **P < 0.01, *P < 0.05, Parkinsons’ disease, PSP, or MSA versus controls (one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests).
Figure 2.
MAOB. Scatter plots of levels of MAOB and the truncated form (tMAOB) in brain regions of patients with Parkinson's disease (PD), PSP and MSA. ***P < 0.001, **P < 0.01, and *P < 0.05, PD, PSP, or MSA versus controls (one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests).
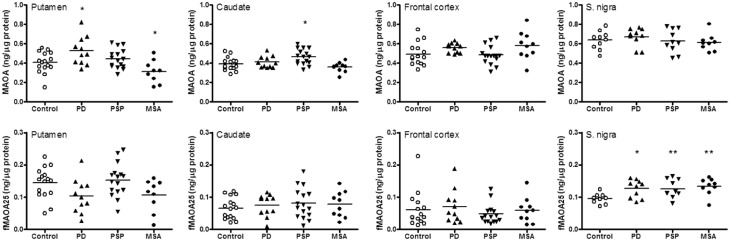
The MAOA H-70 antibody, as reported previously (Tong et al., 2013), detected a major 65 kDa band as well as a small fragment of ∼25 kDa (fMAOA25) across the examined brain regions (Fig. 1) and in recombinant MAOA. Similar to intact MAOA, this fragment was membrane-bound and was also detected in rat brain by H-70 (data not shown). Unlike MAOA, which is highly expressed in brain of infants to teenagers (Tong et al., 2013), fMAOA25 was detected in frontal cortex of only 5 of 34 subjects under 18 years old but in variable levels in all adults. Therefore, although a significant positive correlation with age was observed when including all age groups, fMAOA25 levels were not changed during adulthood (Supplementary Fig. 1), a finding similar to that of the intact protein. Levels of fMAOA25 were highly variable and were not correlated with that of intact MAOA. The putamen had higher levels of the MAOA fragment and larger ratios of fMAOA25 versus MAOA than other brain regions examined (Table 1 and Fig. 3; see also Fig. 1).
Figure 3.
MAOA. Scatter plots of levels of MAOA and the 25 kDa fragment (fMAOA25) in brain regions of patients with Parkinson's disease (PD), PSP and MSA. **P < 0.01, and *P < 0.05, PD, PSP, or MSA versus controls (one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests).
MAOB and MAOA in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy
As shown in Table 1, levels of (intact) MAOB were significantly increased in putamen of MSA (+83%), in substantia nigra (+23%), caudate (+26%), putamen (+27%) and frontal cortex (+31%) of PSP, and in frontal cortex (+33%) of Parkinson’s disease. Analysis of the individual data (Fig. 2) disclosed that the MSA putamen values had the least overlap between control and neurological subject ranges. Significant differences in levels of the truncated form of MAOB were limited to a marked increase (+147%) in the MSA putamen, with no overlap between control and MSA patient values. A single patient ‘outlier’ with Parkinson’s disease showed high levels of truncated MAOB across brain regions examined (Fig. 1) and one PSP patient had a high level of truncated MAOB in frontal cortex although levels of intact MAOB in these patients were not outstanding.
As shown in Table 1 and Fig. 3, increased levels of MAOA were only observed in caudate of PSP (+19%) and putamen of Parkinson’s disease (+29%). Mean levels of MAOA were below normal in putamen of MSA (−23%). Levels of fMAOA25 in the parkinsonian conditions were generally not significantly different from those of the controls, with the exception of the substantia nigra, in which levels of the MAOA fragment were significantly higher in MSA (+40%), PSP (+32%) and Parkinson’s disease (+33%), and with the ratios of fMAOA25 versus MAOA significantly above normal in MSA (+44%) (see also Fig. 1).
NSE and α-tubulin in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy
As expected, MSA patients had marked losses of NSE and α-tubulin in the putamen (−54% and −54%, respectively) and in substantia nigra (−33% and −27%, respectively). PSP patients had significant losses of NSE and α-tubulin in the substantia nigra (−47% and −27%, respectively). Levels of NSE and α-tubulin were otherwise normal in other brain regions examined in MSA and PSP and in all brain regions examined in Parkinson’s disease.
MAOB versus conventional astroglial markers in progressive supranuclear palsy nigra and multiple system atrophy putamen
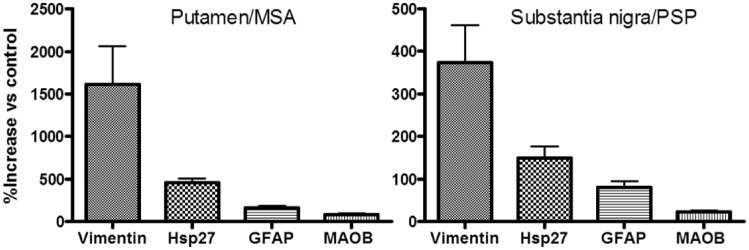
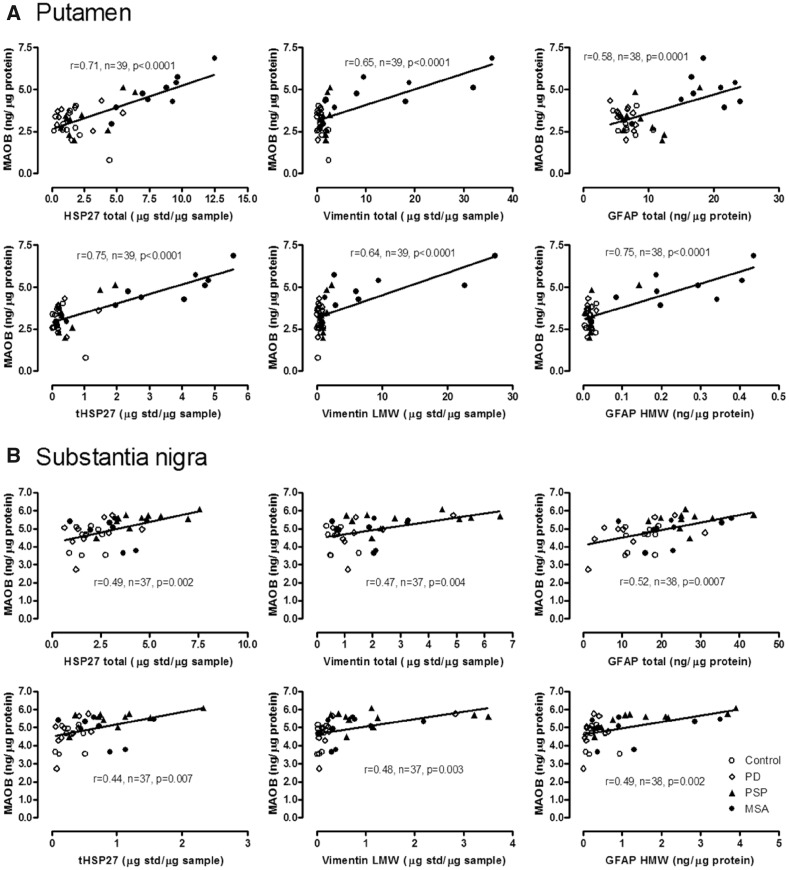
As shown in Fig. 4, in MSA putamen and PSP substantia nigra, the two brain areas with the most marked increase in astroglial marker proteins (Tong et al., 2015), the magnitude of MAOB increase was less than that of the other astroglial markers examined, with concentrations (% increase) of vimentin >> Hsp27 > GFAP > MAOB. However, as shown in Table 2 (see also Fig. 5), significant positive correlations were observed between levels of MAOB and those of Hsp27 (total and tHsp27), vimentin (total and LMW) and GFAP (HMW) in MSA putamen (r = 0.71–0.91, P < 0.05); those of Hsp27 (total and tHsp27), vimentin (LMW) and GFAP (HMW) in PSP putamen (r = 0.63–0.82, P < 0.05); and those of Hsp27 (total) and GFAP (HMW) in PSP substantia nigra (r = 0.67 and 0.73, respectively, P < 0.05). No consistent correlations were observed between levels of MAOA and those of the astroglial markers with the exception of the MSA putamen where levels of fMAOA25 were negatively correlated with those of MAOB (r = −0.68, P = 0.042), Hsp27 (total: r = −0.71, P = 0.034; tHsp27: r = −0.70, P = 0.036), vimentin (total: r = −0.88, P = 0.002; LMW: r = −0.90, P = 0.001) and GFAP (HMW: r = −0.63, P = 0.069).
Figure 4.
MAOB versus other astrocyte markers. Comparison of changes (% above control) in levels of the astroglial marker proteins in putamen of patients with MSA and in substantia nigra of patients with PSP. Data for Hsp27, vimentin and GFAP were previously published (Tong et al., 2015).
Table 2.
Correlations between levels of MAOB and MAOA and levels (total, LMW, and HMW) of the astroglial markers (GFAP, vimentin, and Hsp27) in individual brain regions of patients with PSP and MSA
| GFAP | Vimentin | Hsp27 | |||||
|---|---|---|---|---|---|---|---|
| Total | LMW | HMW | Total | LMW | Total | tHsp27 | |
| MSA: putamen (n = 9) | |||||||
| MAOB | 0.39 | 0.34 | 0.73 (0.026) | 0.73 (0.024) | 0.71 (0.033) | 0.91 (0.001) | 0.89 (0.001) |
| MAOA | −0.44 | −0.46 | −0.30 | −0.28 | −0.10 | 0.02 | −0.15 |
| PSP: putamen (n = 10) | |||||||
| MAOB | 0.21 | 0.15 | 0.63 (0.05) | 0.43 | 0.75 (0.013) | 0.70 (0.024) | 0.82 (0.003) |
| MAOA | 0.40 | 0.37 | 0.59 (0.07) | 0.24 | 0.50 | 0.62 (0.06) | 0.79 (0.01) |
| PSP: substantia nigra (n = 10) | |||||||
| MAOB | 0.17 | -0.34 | 0.73 (0.016) | 0.34 | 0.18 | 0.67 (0.03) | 0.58 (0.08) |
| MAOA | 0.27 | 0.14 | 0.09 | −0.23 | −0.21 | 0.08 | −0.15 |
| MSA: substantia nigra (n = 8–9) | |||||||
| MAOB | 0.32 | 0.29 | 0.30 | 0.06 | 0.39 | −0.33 | −0.28 |
| MAOA | 0.79 (0.011) | 0.75 (0.02) | 0.15 | 0.40 | 0.26 | 0.42 | 0.24 |
Shown are Pearson product-moment correlation coefficients r and the P-values in parentheses where there is a significant correlation (in bold) or only a trend. tHsp27 = truncated Hsp27. Note mostly positive correlations between levels the astroglial markers and those of MAOB but not of MAOA. Data for Hsp27, vimentin and GFAP were previously published (Tong et al., 2015).
Figure 5.
Correlations in putamen and substantia nigra. Correlations (Pearson) between levels of MAOB and those of the astroglial marker proteins in putamen (A) and substantia nigra (B) of patients with Parkinson's disease (PD), PSP and MSA and control subjects. tHsp27 = truncated Hsp27. Data for Hsp27, vimentin and GFAP were previously published (Tong et al., 2015).
MAOB versus astroglial markers in the entire group of controls and parkinsonian patients
We also examined whether MAOB levels were correlated with those of the three astroglial markers within the entire group of controls and parkinsonian patients. Significant positive correlations were observed between levels of the astroglial markers (total, LMW, and/or HMW species of GFAP, vimentin and Hsp27) and those of MAOB (intact or truncated form) in the putamen (MAOB: r = 0.58–0.75, n = 38–39, P < 0.0001; Fig. 5A; truncated MAOB: r = 0.56–0.71, n = 38–39, P < 0.0002) and substantia nigra (MAOB: r = 0.44–0.52, n = 37–38, P < 0.007; Fig. 5B). In caudate and frontal cortex, in which astroglial changes were more limited in the parkinsonian conditions, the correlations between levels of MAOB and the astrocyte markers were much milder or absent (Supplementary Fig. 2).
MAOB and MAOA versus α-synuclein, and serotonin and dopamine ‘metabolism’
Similar to results of our previous report of positive correlations between levels of astroglial markers and α-synuclein accumulation in brain of MSA patients (Tong et al., 2015), nigral MAOB levels of these patients were also correlated positively with that of membrane bound α-synuclein (17 kDa intact: r = 0.73, n = 9, P = 0.027; HMW species: r = 0.70, n = 9, P = 0.037).
In the striatum (putamen and caudate) of the parkinsonian patients, the ratio of 5-hydroxyindoleacetic acid (5-HIAA) versus serotonin, an index of serotonin metabolism, correlated significantly and positively with levels of total MAOA (MAOA plus fMAOA25: r = 0.49, n = 83, P < 0.0001) but not with that of MAOB (MAOB plus truncated MAOB: r = −0.09, n = 83, P = 0.40) (Supplementary Fig. 3). Dopamine metabolism in the striatum as indexed by the ratio of homovanillic acid (HVA) versus dopamine was not correlated with levels of either MAOB or MAOA.
Discussion
The major finding of our study is that brain protein levels of MAOB are normal or elevated in the three parkinsonian conditions—with MAOB increase generally associated with elevations in levels of astrocyte markers. Brain MAOA concentrations were, somewhat surprisingly, not decreased in Parkinson’s disease, PSP, or MSA, with the exception of the atrophic putamen in MSA, despite loss of dopamine neurons that presumably contain this enzyme (see below). Our data, unequivocally distinguishing by immunoblotting the two MAO subtypes—and for the first time across different parkinsonian disorders—provide some support to the use of MAOB as an astrocyte activation marker and might also be relevant for speculations in the literature that brain MAOB (e.g. that localized to astrocytes) and MAOA (e.g. that expressed by dopamine neurons) might be harmful.
MAOB and MAOA in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy versus present findings
To our knowledge, brain MAOB and MAOA have not yet been measured in either PSP or MSA. In Parkinson’s disease, the only previous investigation of either MAOB or MAOA protein appears to be that of Damier et al. (1996) who reported in substantia nigra a marked (+200%) increase in number of MAOB-positive ‘glia’ which was accompanied by a loss (by 54%) of MAOB-positive neurons; however, as the investigators noted, no assessment of total nigral MAOB protein was made. As mentioned in the ‘Introduction’ section, the results of brain MAOB and MAOA activity measurements in Parkinson’s disease were conflicting, sparse, or problematic. An in vivo PET imaging study with the first generation irreversible MAOB ligand 11C-deprenyl found normal brain (substantia nigra not examined) MAOB availability in Parkinson’s disease (Fowler et al., 1993, 1994) although interpretation of the findings is uncertain as only a small number (four to six) of Parkinson’s disease subjects were assessed and the time-activity curves of 11C-deprenyl binding, as well as that of the later deuterated 11C-L-deprenyl-D2 (see below), in brain also include a contribution from the radiolabelled metabolite, N-11C-methamphetamine which is present in brain tissue as well as plasma (Fowler et al., 1988; Cumming et al., 1999). The limited MAOB and MAOA literature in parkinsonian conditions makes comparison with our new data difficult.
MAOB versus astroglial markers
We expected that if, as suggested by Nordberg and colleagues and others (Carter et al., 2012; Choo et al., 2014; Rodriguez-Vieitez et al., 2015, 2016; Scholl et al., 2015), MAOB is an astrogliosis ‘biomarker’, albeit not entirely specific, protein levels of the enzyme should be higher than normal in degenerating areas of human brain in which astrogliosis would be expected to be present. Our post-mortem brain findings in PSP and in MSA generally support this possibility. Thus, in the two brain areas in PSP and MSA, substantia nigra and putamen, respectively, which we previously found to contain the highest concentration of the three examined (standard) astroglial markers (Tong et al., 2015), levels of MAOB (but not those of MAOA) were increased. Amongst the three dopamine deficiency conditions, PSP had the most marked increase of MAOB in substantia nigra and also increased MAOB in every brain region examined, an observation consistent with generally more severe nigral pathology and gliosis in PSP as compared with Parkinson’s disease and MSA and widespread pathology, e.g. tau, and astroglial activation, in this condition (Tong et al., 2015). In frontal cortex in Parkinson’s disease, the mild increase in MAOB levels is consistent with a trend for increased levels of aggregates (GFAP) and fragments (vimentin and Hsp27) of other astroglial markers. Further, in several brain areas in PSP (substantia nigra, caudate, putamen) and MSA (putamen, substantia nigra), positive correlations were observed between MAOB (but not MAOA) and each of the astroglial markers (vimentin, GFAP, Hsp27), whereas in Parkinson’s disease substantia nigra, in which levels of the astroglial markers were normal (GFAP, Hsp27) or only a trend for an increase was observed (vimentin), MAOB protein concentration was normal. Our inability to find increased level of MAOB in substantia nigra [and also in striatum (putamen and caudate] of Parkinson’s disease is consistent with findings of others suggesting at most limited astrogliosis in the dopamine depleted striatum and substantia nigra in Parkinson’s disease (Banati et al., 1998; Mirza et al., 2000; Song et al., 2009; Saal et al., 2017; but see Liddelow et al., 2017) (for reviews see Halliday and Stevens, 2011; Tong et al., 2015; Bruck et al., 2016).
When including, for the correlations, values from all subject groups, generally significant positive correlations were observed between MAOB versus the astroglial markers in substantia nigra, putamen, and caudate, but not in the cerebral cortical area examined. These associations between MAOB and astroglial markers add to the literature evidence supporting the localization of MAOB to astrocytes and the possible use of MAOB as a astroglial biomarker in imaging studies of living brain (Nakamura et al., 1990; Ekblom et al., 1993; Saura et al., 1994; Kumlien et al., 1995; Gulyas et al., 2011; Carter et al., 2012; Choo et al., 2014; Rodriguez-Vieitez et al., 2015, 2016; Scholl et al., 2015). Our findings also add to the evolving literature distinguishing the astroglial response in PSP and MSA from that of Parkinson’s disease (Song et al., 2009; Halliday and Stevens, 2011; Tong et al., 2015; Bruck et al., 2016). Although the absolute extent of localization of MAOB to astroglial versus neurons in human brain is not yet established, MAOB is likely expressed to some degree by different types of neurons (e.g. histamine, acetylcholine) (Lin et al., 1993; Vitalis et al., 2002), and in particular, by serotonin neurons in the brainstem (Levitt et al., 1982; Westlund et al., 1985, 1988). This means, for example, that in brain neurodegenerative conditions having loss of monoaminergic neurons, any MAOB increase resulting from astroglial changes could be ‘confounded’ to some extent by loss of MAOB containing neurons in the same brain areas. Thus, in the present study, increased levels of MAOB in MSA were only observed in putamen but not in other brain regions where significant elevation of other astroglial markers was observed (Tong et al., 2015). This might be explained by loss, in MSA, of serotonin neurons enriched in MAOB (Adams et al., 1964; Sawada et al., 1985; but see Benarroch et al., 2007).
A recent finding in developing MAOB PET imaging as a biomarker of brain astrocytosis has been the observation of increased 11C-L-deprenyl-D2 MAOB binding in prodromal but not in early symptomatic Alzheimer’s patients, indicating that astroglial activation might be an early feature of the dementia. However, the negative finding in symptomatic Alzheimer’s patients is somewhat surprising, given pathological evidence of marked astrogliosis and increased MAOB levels in post-mortem brain of Alzheimer patients (Nakamura et al., 1990; Jossan et al., 1991; Saura et al., 1994; Gulyas et al., 2011), with the explanation for the ‘discrepancy’ uncertain. One possibility could be inadequate sensitivity of 11C-L-deprenyl-D2 binding relative to the magnitude of disease effect. In principle, a phenotype switch from MAOB-rich to MAOB-poor astrocytes (Ekblom et al., 1993; John Lin et al., 2017) during chronic neurodegeneration could be an explanation for negative findings in early symptomatic Alzheimer’s disease (Carter et al., 2012; Choo et al., 2014; Rodriguez-Vieitez et al., 2015, 2016) and in Parkinson’s disease (this study). To improve sensitivity for detecting astrogliosis, one approach to avoid pitfalls of early MAOB radiotracer development would be use of a newer MAOB radiotracer e.g. 11C-SL25.1188 with no brain-penetrating metabolites and improved reversibility (Saba et al., 2010; Rusjan et al., 2014). Radiotracers with such characteristics might be better astrogliosis biomarkers for disease monitoring and it will be interesting in future to compare findings in Alzheimer’s disease with these second-generation radiotracers. Notwithstanding the above limitations and uncertainties, our post-mortem brain findings do add to the support for the possibility that MAOB PET imaging could be a useful biomarker for brain astrogliosis and possibly disease progression related to gliosis.
MAOA versus degenerative neuronal changes in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy
Although the literature has been aware of the existence of MAOA for 50 years (Johnston, 1968), there still remains, surprisingly, much uncertainty regarding the actual localization (e.g. neurons versus glia; neuronal cell body versus axonal terminals; dopamine versus non-dopamine neurons) of the enzyme in human brain—making interpretation of our present MAOA findings difficult. Based on suggestions, or perhaps assumptions, that MAOA is the MAO subtype primarily responsible for dopamine metabolism inside dopamine neurons (Stenstrom et al., 1985; Fagervall and Ross, 1986; O’Carroll et al., 1987; Butcher et al., 1990; Wachtel and Abercrombie, 1994; Finberg et al., 1995; Di Monte et al., 1996; Fornai et al., 1999) (for reviews see Youdim et al., 2006; Finberg, 2014), loss of dopamine neuron innervation to striatum in the three parkinsonian conditions should have caused a significant loss of MAOA protein in this dopamine-rich brain region (see also below). However, in animal models of parkinsonism with lesions of nigrostriatal dopamine neurons, most studies did not observe a loss of striatal MAOA (Carlsson et al., 1981; Van der Krogt et al., 1983; Francis et al., 1987; Sader-Mazbar et al., 2013; but see Agid et al., 1973; Demarest et al., 1980; Stenstrom et al., 1985), suggesting that MAOA might not be highly localized in dopamine neurons in striatum; these (‘negative’) experimental data are consistent with our findings of generally preserved MAOA in putamen and caudate of Parkinson’s disease and PSP and in caudate of MSA. Indeed, slightly increased levels of MAOA were observed in Parkinson’s disease putamen (+29%) and PSP caudate (+19%) and the magnitude of MAOA loss in MSA putamen, the brain structure with severe atrophy and gliosis in this condition, was also less than expected from NSE loss (−23% versus −56%). Possibly, loss of dopamine innervation, e.g. in the putamen of Parkinson’s disease, might have induced sprouting of serotonin terminals that are suggested to contain MAOA (Sader-Mazbar et al., 2013), which could offset to some extent any enzyme reduction caused by loss of dopamine neurons.
Given the assumption that MAOA is localized at least to some extent in dopamine neurons (e.g. see Fig. 1 in Youdim et al., 2006), we were somewhat surprised to discover that in all three parkinsonian conditions there was no loss of MAOA protein in the substantia nigra (pars compacta), in which dopamine neurons (melanin-containing) account for a vast majority of total number of neurons (Pakkenberg et al., 1991; Gibb, 1992; McRitchie et al., 1996). One possibility is that the enzyme is not highly localized in dopamine neuronal cell bodies in the substantia nigra in the human. In this regard, previous enzyme histochemical (Willoughby et al., 1988; Konradi et al., 1989; Arai et al., 1998; Hida et al., 1999), immunohistochemical (Westlund et al., 1985, 1988, 1993; Konradi et al., 1988) and in situ hybridization (Luque et al., 1995; Jahng et al., 1997; Vitalis et al., 2002) studies, as compared to autoradiography and immunoblotting findings (Richards et al., 1992; Saura et al., 1992, 1996; Tong et al., 2013), have shown sometimes inconsistent levels of nigral MAOA. To our knowledge, there has been no examination of MAOA protein in substantia nigra in lesioned animal models of Parkinson’s disease. Alternatively, the lack of any loss of nigral MAOA in the parkinsonian conditions can be explained by upregulation of MAOA expression in remaining dopamine neurons, possible hyperinnervation by other neurotransmitter terminals, e.g. serotonin, as shown in animal models (Zhou et al., 1991; Gaspar et al., 1993) but apparently not in Parkinson’s disease (Guttman et al., 2007; Azmitia and Nixon, 2008; Cheshire et al., 2015; Maillet et al., 2016), and/or ectopic expression of MAOA in glial cells. Some contamination of the nigral compacta samples by zona reticulata in our dissection might also have contributed to the lack of MAOA change; however, patients with PSP have been reported to have additional marked loss of reticulata (GABA) neurons (Hardman et al., 1997).
A ‘small’ fragment of MAOA, fMAOA25, could be detected in human brain samples. We suspect that this fragment likely has no intrinsic function, but possibly could provide an index, for example, of MAOA ‘turnover’ or metabolism. Interestingly, we observed increased levels of fMAOA25 in substantia nigra in Parkinson’s disease, PSP and MSA, supporting the idea that MAOA expression and turnover might be elevated in dopamine neurons of the parkinsonian conditions, explained as a genetic predisposing factor for dopamine neuron vulnerability (Byers et al., 2011; Jiang et al., 2012) or possibly as a response to the compensatory increased dopaminergic activity in surviving dopamine neurons (Sacher et al., 2012). In this regard, levodopa exposure could be a confounding factor, i.e., have contributed to changes or lack of changes in MAOA protein (Sacher et al., 2012), especially in Parkinson’s disease; however, the extent of MAOA regulation by dopamine availability in human brain is still uncertain; most of the patients with PSP and MSA did not receive regular levodopa treatment. To our knowledge, only limited information is available regarding brain MAOA expression regulation, e.g. by stress and oxidative stress (Raitsin et al., 2017) and little is known about turnover of the enzyme. An alternative explanation for increased MAOA fragment levels might be generally compromised protein degradation (Pan et al., 2008) and/or stalled axonal mitochondrial protein trafficking (Abeliovich and Gitler, 2016) [e.g. fMAOA25 levels are high in white matter tracts (Tong et al., 2013)] in parkinsonian conditions, resulting in incomplete processing/accumulation of fMAOA25 although brain areas outside of the nigra are also affected by neurodegeneration in MSA and PSP. Unlike truncated MAOB, levels of fMAOA25 are independent of intact protein levels, either during brain development and ageing or among different brain areas. Further studies are required to replicate these preliminary findings and to understand better MAOA expression and metabolism.
MAOB and MAOA as disease-modifying drug targets?
Our post-mortem brain findings may have some clinical relevance, given that both MAO enzymes are drug targets in human brain disorders and that protein levels of the enzymes were generally normal or elevated in the parkinsonian conditions (i.e. the targets are not substantially diminished in concentration despite loss of monoamine neurons). Some experimental data suggest that elevated MAOB expression in astrocytes and ensuing astrocytosis might promote dopamine neurodegeneration by increasing oxidative stress and inhibiting mitochondrial complex-I (Mallajosyula et al., 2008). Assuming that there is some validity to this speculation in the human, our data suggest that, of the three parkinsonian conditions, MAOB inhibition might possibly be more helpful as a neuroprotective agent in PSP, in which an MAOB increase was observed in all examined brain regions, and in MSA, in which a marked (+83%; +147% in truncated MAOB) increase in enzyme concentration was present in the degenerating putamen. In this regard, the MAOB inhibitor deprenyl was found to suppress oxidative stress and glial activation and rescue the MAOB-astrocyte overexpression animal model (Mallajosyula et al., 2008). In Parkinson’s disease, clinical evidence supporting a neuroprotective role of MAOB inhibitors is controversial (Ahlskog and Uitti, 2010; Olanow and Rascol, 2010). There is also much uncertainty regarding the status of astrocytes and ‘neuroinflammation’ in Parkinson’s disease (Ghadery et al., 2017; Liddelow et al., 2017; see Tong et al., 2015 for a review). Given the limited availability of drug history information in our post-mortem study, future in vivo studies using the new generation of MAOB PET radiotracer, e.g. 11C-SL25.1188 (Rusjan et al., 2014) would help clarify the question of astroglial status, in particular in drug-naïve early stage and progression of Parkinson’s disease, and might provide a new imaging approach in differentiating Parkinson’s disease from Parkinson-plus movement disorders.
Regarding MAOA as a potential drug target, much evidence from brain imaging and post-mortem brain investigations suggests that globally elevated brain MAOA is associated with mood/sadness in a variety of psychiatric illnesses and prodromal states and therefore represents a potential target for pharmacological intervention to correct the mood disturbance (Meyer et al., 2006, 2009; Sacher et al., 2010; Rekkas et al., 2014; Kolla et al., 2016). A limitation of our study is that subject information on presence of mood problems, which are commonly observed in Parkinson’s disease, could not be obtained; however, our data do suggest that a global increase in brain MAOA might not be a characteristic of any of the three parkinsonian conditions. Further investigation, e.g. by 11C-harmine PET, is needed to establish whether brain MAOA might be elevated in subgroups of depressed patients with the movement disorders. On the other hand, given some evidence suggesting that MAOA inhibition can be neuroprotective, our finding of no loss of MAOA and increased MAOA turnover in surviving dopamine neurons might provide a rationale for the clinical use of reversible MAOA inhibitors (without cheese reaction), although there still remains the as yet unanswered question of the net effect of MAOA inhibition on production of intraneuronal toxic dopamine-derived oxidation products (overall helpful or harmful? Goldstein et al., 2016).
In conclusion, our findings on MAOB in Parkinson’s disease, PSP and MSA support the use of MAOB PET binding as a biomarker of astrogliosis in the human brain. Although the preservation or elevation of MAOB and/or MAOA in brain of patients with the parkinsonian conditions may suggest possibility of clinical use of inhibitors of either or both MAO isozymes for symptomatic purposes, it remains to be determined whether such inhibitor drugs possess any disease-modifying (beneficial or detrimental) properties in humans.
Funding
This study was supported in part by the US NIDA/NIH DA07182 and DA040066 and the Centre for Addiction and Mental Health Foundation.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- fMAOA25
a 25 kDa fragment of MAOA
- HMW
high molecular weight
- Hsp27
heat shock protein-27
- LMW
low molecular weight
- MAOA/B
monoamine oxidase A/B
- MSA
multiple system atrophy
- PSP
progressive supranuclear palsy
References
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature 2016; 539: 207–16. [DOI] [PubMed] [Google Scholar]
- Adams RD, Vanbogaert L, Vandereecken H. Striato-Nigral degeneration. J Neuropathol Exp Neurol 1964; 23: 584–608. [PubMed] [Google Scholar]
- Agid Y, Javoy F, Youdim MB. Monoamine oxidase and aldehyde dehydrogenase activity in the striatum of rats after 6-hydroxydopamine lesion of the nigrostriatal pathway. Br J Pharmacol 1973; 48: 175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Uitti RJ. Rasagiline, Parkinson neuroprotection, and delayed-start trials: still no satisfaction? Neurology 2010; 74: 1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai R, Horiike K, Hasegawa Y. Dopamine-degrading activity of monoamine oxidase is not detected by histochemistry in neurons of the substantia nigra pars compacta of the rat. Brain Res 1998; 812: 275–8. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res 2008; 1217: 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord 1998; 13: 221–7. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Sandroni P, Parisi JE, Low PA. Rostral raphe involvement in Lewy body dementia and multiple system atrophy. Acta Neuropathol 2007; 114: 213–20. [DOI] [PubMed] [Google Scholar]
- Bergstrom M, Kumlien E, Lilja A, Tyrefors N, Westerberg G, Langstrom B. Temporal lobe epilepsy visualized with PET with 11C-L-deuterium-deprenyl–analysis of kinetic data. Acta Neurol Scand 1998; 98: 224–31. [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia [in German]. Wien Klin Wochenschr 1961; 73: 787–8. [PubMed] [Google Scholar]
- Birkmayer W, Riederer P, Youdim MB, Linauer W. The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil. J Neural Transm 1975; 36: 303–26. [DOI] [PubMed] [Google Scholar]
- Bruck D, Wenning GK, Stefanova N, Fellner L. Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 2016; 85: 262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J Neurochem 1990; 55: 981–8. [DOI] [PubMed] [Google Scholar]
- Byers B, Cord B, Nguyen HN, Schule B, Fenno L, Lee PC, et al. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS One 2011; 6: e26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Fowler CJ, Magnusson T, Oreland L, Wiberg A. The activities of monoamine oxidase-A and -B, succinate dehydrogenase and acid phosphatase in the rat brain after hemitransection. Naunyn Schmiedebergs Arch Pharmacol 1981; 316: 51–5. [DOI] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med 2012; 53: 37–46. [DOI] [PubMed] [Google Scholar]
- Cheshire P, Ayton S, Bertram KL, Ling H, Li A, McLean C, et al. Serotonergic markers in Parkinson's disease and levodopa-induced dyskinesias. Mov Disord 2015; 30: 796–804. [DOI] [PubMed] [Google Scholar]
- Choo IL, Carter SF, Scholl ML, Nordberg A. Astrocytosis measured by (1)(1)C-deprenyl PET correlates with decrease in gray matter density in the parahippocampus of prodromal Alzheimer's patients. Eur J Nucl Med Mol Imaging 2014; 41: 2120–6. [DOI] [PubMed] [Google Scholar]
- Cumming P, Yokoi F, Chen A, Deep P, Dagher A, Reutens D, et al. Pharmacokinetics of radiotracers in human plasma during positron emission tomography. Synapse 1999; 34: 124–34. [DOI] [PubMed] [Google Scholar]
- Damier P, Kastner A, Agid Y, Hirsch EC. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson's disease? Neurology 1996; 46: 1262–9. [DOI] [PubMed] [Google Scholar]
- Demarest KT, Smith DJ, Azzaro AJ. The presence of the type A form of monoamine oxidase within nigrostriatal dopamine-containing neurons. J Pharmacol Exp Ther 1980; 215: 461–8. [PubMed] [Google Scholar]
- Di Monte DA, DeLanney LE, Irwin I, Royland JE, Chan P, Jakowec MW, et al. Monoamine oxidase-dependent metabolism of dopamine in the striatum and substantia nigra of L-DOPA-treated monkeys. Brain Res 1996; 738: 53–9. [DOI] [PubMed] [Google Scholar]
- Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM. Monoamine oxidase-B in astrocytes. Glia 1993; 8: 122–32. [DOI] [PubMed] [Google Scholar]
- Engler H, Lundberg PO, Ekbom K, Nennesmo I, Nilsson A, Bergstrom M, et al. Multitracer study with positron emission tomography in Creutzfeldt-Jakob disease. Eur J Nucl Med Mol Imaging 2003; 30: 85–95. [DOI] [PubMed] [Google Scholar]
- Fagervall I, Ross SB. A and B forms of monoamine oxidase within the monoaminergic neurons of the rat brain. J Neurochem 1986; 47: 569–76. [DOI] [PubMed] [Google Scholar]
- Finberg JP. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release. Pharmacol Ther 2014; 143: 133–52. [DOI] [PubMed] [Google Scholar]
- Finberg JP, Rabey JM. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front Pharmacol 2016; 7: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg JP, Wang J, Goldstein DS, Kopin IJ, Bankiewicz KS. Influence of selective inhibition of monoamine oxidase A or B on striatal metabolism of L-DOPA in hemiparkinsonian rats. J Neurochem 1995; 65: 1213–20. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JC, Ugun-Klusek A, Allen G, De Girolamo LA, Hargreaves I, Ufer C, et al. Monoamine oxidase-A knockdown in human neuroblastoma cells reveals protection against mitochondrial toxins. FASEB J 2014; 28: 218–29. [DOI] [PubMed] [Google Scholar]
- Fornai F, Chen K, Giorgi FS, Gesi M, Alessandri MG, Shih JC. Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J Neurochem 1999; 73: 2434–40. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Cilento R, Wang GJ, Felder C, Logan J. Comparison of brain glucose metabolism and monoamine oxidase B (MAO B) in traumatic brain injury. Clin Positron Imaging 1999; 2: 71–9. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Schlyer DJ, MacGregor RR, Wang GJ, et al. Monoamine Oxidase B (MAO B) inhibitor therapy in Parkinson's disease: the degree and reversibility of human brain MAO B inhibition by Ro 19 6327. Neurology 1993; 43: 1984–92. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, et al. Slow recovery of human brain MAO B after L-deprenyl (Selegeline) withdrawal. Synapse 1994; 18: 86–93. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wolf AP, MacGregor RR, Dewey SL, Logan J, Schlyer DJ, et al. Mechanistic positron emission tomography studies: demonstration of a deuterium isotope effect in the monoamine oxidase-catalyzed binding of [11C]L-deprenyl in living baboon brain. J Neurochem 1988; 51: 1524–34. [DOI] [PubMed] [Google Scholar]
- Francis A, Whittemore R, Jeffery DR, Pearce LB, Roth JA. Catecholamine-metabolizing enzyme activity in the nigrostriatal system. Biochem Pharmacol 1987; 36: 2229–31. [DOI] [PubMed] [Google Scholar]
- Gargalidis-Moudanos C, Pizzinat N, Javoy-Agid F, Remaury A, Parini A. I2-imidazoline binding sites and monoamine oxidase activity in human postmortem brain from patients with Parkinson's disease. Neurochem Int 1997; 30: 31–6. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Febvret A, Colombo J. Serotonergic sprouting in primate MTP-induced hemiparkinsonism. Exp Brain Res 1993; 96: 100–6. [DOI] [PubMed] [Google Scholar]
- Ghadery C, Koshimori Y, Coakeley S, Harris M, Rusjan P, Kim J, et al. Microglial activation in Parkinson's disease using [18F]-FEPPA. J Neuroinflammation 2017; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR. Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Res 1992; 581: 283–91. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999; 163: 94–8. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Biomarkers, mechanisms, and potential prevention of catecholamine neuron loss in Parkinson disease. Adv Pharmacol 2013; 68: 235–72. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, Sharabi Y. Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-Dihydroxyphenylacetaldehyde in PC12 cells. J Pharmacol Exp Ther 2016; 356: 483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas B, Pavlova E, Kasa P, Gulya K, Bakota L, Varszegi S, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int 2011; 58: 60–8. [DOI] [PubMed] [Google Scholar]
- Guttman M, Boileau I, Warsh J, Saint-Cyr JA, Ginovart N, McCluskey T, et al. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur J Neurol 2007; 14: 523–8. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord 2011; 26: 6–17. [DOI] [PubMed] [Google Scholar]
- Hardman CD, Halliday GM, McRitchie DA, Cartwright HR, Morris JG. Progressive supranuclear palsy affects both the substantia nigra pars compacta and reticulata. Exp Neurol 1997; 144: 183–92. [DOI] [PubMed] [Google Scholar]
- Harris S, Johnson S, Duncan JW, Udemgba C, Meyer JH, Albert PR, et al. Evidence revealing deregulation of the KLF11-MAO A pathway in association with chronic stress and depressive disorders. Neuropsychopharmacology 2015; 40: 1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 1984; 311: 467–9. [DOI] [PubMed] [Google Scholar]
- Hida T, Hasegawa Y, Arai R. Histochemical study of dopamine-degrading monoamine oxidase activity in dopaminergic neurons of rat brain. Brain Res 1999; 842: 491–5. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, et al. Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 2012; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Wessel TC, Chen K, Shih JC, Joh TH. Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse 1997; 25: 30–6. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Riederer P. Dementia in Parkinson’s disease and (pre) senile dementia of Alzheimer type: morphological aspects and changes in the intracerebral MA0 activity. New York, NY: Raven Press; 1984. [PubMed] [Google Scholar]
- Jenner P. Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson's disease. Neurology 2004; 63 (7 Suppl 2): S13–22. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, et al. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 2012; 3: 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci 2007; 255: 17–22. [DOI] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 2017; 20: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 1968; 17: 1285–97. [DOI] [PubMed] [Google Scholar]
- Jossan SS, Gillberg PG, Gottfries CG, Karlsson I, Oreland L. Monoamine oxidase B in brains from patients with Alzheimer's disease: a biochemical and autoradiographical study. Neuroscience 1991; 45: 1–12. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 1988; 318: 876–80. [DOI] [PubMed] [Google Scholar]
- Kolla NJ, Chiuccariello L, Wilson AA, Houle S, Links P, Bagby RM, et al. Elevated monoamine oxidase-A distribution volume in borderline personality disorder is associated with severity across mood symptoms, suicidality, and cognition. Biol Psychiatry 2016; 79: 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Kornhuber J, Froelich L, Fritze J, Heinsen H, Beckmann H, et al. Demonstration of monoamine oxidase-A and -B in the human brainstem by a histochemical technique. Neuroscience 1989; 33: 383–400. [DOI] [PubMed] [Google Scholar]
- Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J. Topographic immunocytochemical mapping of monoamine oxidase-A, monoamine oxidase-B and tyrosine hydroxylase in human post mortem brain stem. Neuroscience 1988; 26: 791–802. [DOI] [PubMed] [Google Scholar]
- Kumlien E, Bergstrom M, Lilja A, Andersson J, Szekeres V, Westerberg CE, et al. Positron emission tomography with [11C]deuterium-deprenyl in temporal lobe epilepsy. Epilepsia 1995; 36: 712–21. [DOI] [PubMed] [Google Scholar]
- Kumlien E, Nilsson A, Hagberg G, Langstrom B, Bergstrom M. PET with 11C-deuterium-deprenyl and 18F-FDG in focal epilepsy. Acta Neurol Scand 2001; 103: 360–6. [DOI] [PubMed] [Google Scholar]
- Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 1982; 79: 6385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang CW, Chen GY, Zhu B, Chai C, Xu QH, et al. A sensitive two-photon probe to selectively detect monoamine oxidase B activity in Parkinson's disease models. Nat Commun 2014; 5: 3276. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017; 541: 481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Kitahama K, Fort P, Panula P, Denney RM, Jouvet M. Histaminergic system in the cat hypothalamus with reference to type B monoamine oxidase. J Comp Neurol 1993; 330: 405–20. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Davidson L, Hornykiewicz O. The neurochemistry of Parkinson's disease: effect of L-dopa therapy. J Pharmacol Exp Ther 1975; 195: 453–64. [PubMed] [Google Scholar]
- Luque JM, Kwan SW, Abell CW, Da Prada M, Richards JG. Cellular expression of mRNAs encoding monoamine oxidases A and B in the rat central nervous system. J Comp Neurol 1995; 363: 665–80. [DOI] [PubMed] [Google Scholar]
- Maillet A, Krack P, Lhommee E, Metereau E, Klinger H, Favre E, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain 2016; 139 (Pt 9): 2486–502. [DOI] [PubMed] [Google Scholar]
- Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson's pathology. PLoS One 2008; 3: e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J Comp Neurol 1996; 364: 121–50. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 2006; 63: 1209–16. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry 2009; 66: 1304–12. [DOI] [PubMed] [Google Scholar]
- Miklya I. The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015). Mol Psychiatry 2016; 21: 1499–503. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience 2000; 95: 425–32. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamata T, Akiguchi I, Kameyama M, Nakamura N, Kimura H. Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol 1990; 80: 419–25. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Inaba-Hasegawa K, Akao Y. Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Int Rev Neurobiol 2011; 100: 85–106. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011; 8: 267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll AM, Tipton KF, Sullivan JP, Fowler CJ, Ross SB. Intra- and extra-neuronal deamination of dopamine and noradrenaline by the two forms of human brain monoamine oxidase. Implications for the neurotoxicity of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biog Amines 1987; 4: 165–78. [Google Scholar]
- Olanow CW, Rascol O. The delayed-start study in Parkinson disease: can't satisfy everyone. Neurology 2010; 74: 1149–50. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med 2009; 361: 1268–78. [DOI] [PubMed] [Google Scholar]
- Oreland L. Monoamine oxidase, dopamine and Parkinson's disease. Acta Neurol Scand Suppl 1991; 136: 60–5. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry 1991; 54: 30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain 2008; 131 (Pt 8): 1969–78. [DOI] [PubMed] [Google Scholar]
- Raitsin S, Tong J, Kish S, Xu X, Magomedova L, Cummins C, et al. Subchronic glucocorticoids, glutathione depletion and a postpartum model elevate monoamine oxidase A activity in the prefrontal cortex of rats. Brain Res 2017; 1666: 1–10. [DOI] [PubMed] [Google Scholar]
- Rascol O, Fitzer-Attas CJ, Hauser R, Jankovic J, Lang A, Langston JW, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol 2011; 10: 415–23. [DOI] [PubMed] [Google Scholar]
- Rekkas PV, Wilson AA, Lee VW, Yogalingam P, Sacher J, Rusjan P, et al. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry 2014; 71: 873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JG, Saura J, Ulrich J, Da Prada M. Molecular neuroanatomy of monoamine oxidases in human brainstem. Psychopharmacology 1992; 106: S21–3. [DOI] [PubMed] [Google Scholar]
- Riederer P, Jellinger K. Neurochemical insights into monoamine oxidase inhibitors, with special reference to deprenyl (selegiline). Acta Neurol Scand Suppl 1983; 95: 43–55. [DOI] [PubMed] [Google Scholar]
- Riederer P, Konradi C, Hebenstreit G, Youdim MB. Neurochemical perspectives to the function of monoamine oxidase. Acta Neurol Scand Suppl 1989; 126: 41–5. [DOI] [PubMed] [Google Scholar]
- Riederer P, Youdim MB. Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem 1986; 46: 1359–65. [DOI] [PubMed] [Google Scholar]
- Riley RA. An atlas of the basal ganglia, brain stem and spinal cord. Baltimore, MD: The Williams & Wilkins Company; 1943. [Google Scholar]
- Rodriguez-Vieitez E, Ni R, Gulyas B, Toth M, Haggkvist J, Halldin C, et al. Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging 2015; 42: 1119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, Almkvist O, Farid K, Scholl M, et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer's disease. Brain 2016; 139 (Pt 3): 922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Miler L, Fan I, Mizrahi R, Houle S, et al. Kinetic modeling of the monoamine oxidase B radioligand [(1)(1)C]SL25.1188 in human brain with high-resolution positron emission tomography. J Cereb Blood Flow Metab 2014; 34: 883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal KA, Galter D, Roeber S, Bahr M, Tonges L, Lingor P. Altered expression of growth associated protein-43 and Rho Kinase in human patients with Parkinson's disease. Brain Pathol 2017; 27: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba W, Valette H, Peyronneau MA, Bramoulle Y, Coulon C, Curet O, et al. [(11)C]SL25.1188, a new reversible radioligand to study the monoamine oxidase type B with PET: preclinical characterisation in nonhuman primate. Synapse 2010; 64: 61–9. [DOI] [PubMed] [Google Scholar]
- Sacher J, Rabiner EA, Clark M, Rusjan P, Soliman A, Boskovic R, et al. Dynamic, adaptive changes in MAO-A binding after alterations in substrate availability: an in vivo [(11)C]-harmine positron emission tomography study. J Cereb Blood Flow Metab 2012; 32: 443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry 2010; 67: 468–74. [DOI] [PubMed] [Google Scholar]
- Sader-Mazbar O, Loboda Y, Rabey MJ, Finberg JP. Increased L-DOPA-derived dopamine following selective MAO-A or -B inhibition in rat striatum depleted of dopaminergic and serotonergic innervation. Br J Pharmacol 2013; 170: 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience 1996; 70: 755–74. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 1992; 12: 1977–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 1994; 62: 15–30. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nagatsu T, Nagatsu I, Ito K, Iizuka R, Kondo T, et al. Tryptophan hydroxylase activity in the brains of controls and parkinsonian patients. J Neural Transm 1985; 62: 107–15. [DOI] [PubMed] [Google Scholar]
- Schneider G, Oepen H, von Wedel HR. Monoamine oxidase activity in brain regions and organs of patients with Parkinson's disease and Huntington's disease and serum MAO activity of patients with Huntington's disease as compared with neurologically healthy individuals (author's transl), [in German]. Arch Psychiatr Nervenkr 1981; 230: 5–15. [DOI] [PubMed] [Google Scholar]
- Scholl M, Carter SF, Westman E, Rodriguez-Vieitez E, Almkvist O, Thordardottir S, et al. Early astrocytosis in autosomal dominant Alzheimer's disease measured in vivo by multi-tracer positron emission tomography. Sci Rep 2015; 5: 16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Ann Rev Neurosci 1999; 22: 197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YJ, Halliday GM, Holton JL, Lashley T, O'Sullivan SS, McCann H, et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol 2009; 68: 1073–83. [DOI] [PubMed] [Google Scholar]
- Stenstrom A, Arai Y, Oreland L. Intra- and extraneuronal monoamineoxidase-A and -B activities after central axotomy (hemisection) on rats. J Neural Transm 1985; 61: 105–13. [DOI] [PubMed] [Google Scholar]
- Strolin Benedetti M, Dostert P. Monoamine oxidase, brain ageing and degenerative diseases. Biochem Pharmacol 1989; 38: 555–61. [DOI] [PubMed] [Google Scholar]
- Tong J, Ang LC, Williams B, Furukawa Y, Fitzmaurice P, Guttman M, et al. Low levels of astroglial markers in Parkinson's disease: relationship to alpha-synuclein accumulation. Neurobiol Dis 2015; 82: 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab 2013; 33: 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain 2010; 133 (Pt 1): 172–88. [DOI] [PubMed] [Google Scholar]
- Van der Krogt JA, Koot-Gronsveld E, Van den Berg CJ. Localization of rat striatal monoamine oxidase activities towards dopamine, serotonin and kynuramine by gradient centrifugation and nigro-striatal lesions. Life Sci 1983; 33: 615–23. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol 2013; 8: 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Fouquet C, Alvarez C, Seif I, Price D, Gaspar P, et al. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J Comp Neurol 2002; 442: 331–47. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Abercrombie ED. L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J Neurochem 1994; 63: 108–17. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science 1985; 230: 181–3. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 1988; 25: 439–56. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Krakower TJ, Kwan SW, Abell CW. Intracellular distribution of monoamine oxidase A in selected regions of rat and monkey brain and spinal cord. Brain Res 1993; 612: 221–30. [DOI] [PubMed] [Google Scholar]
- Willoughby J, Glover V, Sandler M. Histochemical localisation of monoamine oxidase A and B in rat brain. J Neural Transm 1988; 74: 29–42. [DOI] [PubMed] [Google Scholar]
- Yong VW, Perry TL. Monoamine oxidase B, smoking, and Parkinson's disease. J Neurol Sci 1986; 72: 265–72. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol 2006; 147 (Suppl 1): S287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 2006; 7: 295–309. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Murphy J. Serotonergic sprouting is induced by dopamine-lesion in substantia nigra of adult rat brain. Brain Res 1991; 556: 108–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.