Abstract
Objectives
To investigate patterns of programmed death protein-1 (PD-L1) expression in microsatellite instability (MSI)-high intestinal carcinomas and correlate them with pathologic and molecular features.
Methods
One hundred and fifteen MSI-high and 41 microsatellite stable carcinomas were included. Tumor sections were immunohistochemically labeled for PD-L1. The results were correlated with histologic subtypes, MSI, and BRAF status.
Results
As expected, MSI status was associated with PD-L1 expression. Among 115 MSI-high tumors, PD-L1 expression was observed on tumor cells in 28 tumors and on tumor-associated inflammatory cells in 77 tumors. Medullary carcinoma demonstrated more frequent PD-L1 expression on tumor cells than mucinous and typical adenocarcinoma. PD-L1 expression was more frequent in medullary and typical adenocarcinoma than in mucinous adenocarcinoma based on combined positive scores. Tumors with more nucleotide shifts by PCR-based MSI testing were more likely to express PD-L1.
Conclusions
Expression of PD-L1 is different among different histologic subtypes of MSI-high intestinal carcinomas.
Keywords: PD-L1, MSI-high, Intestinal carcinoma, Histologic subtypes
Colorectal cancer (CRC) represents a significant proportion of cancer diagnoses and cancer-related deaths in the United States. Cancer tumorigenesis through the microsatellite instability-high (MSI-H) pathway has been shown to account for approximately 15% of these tumors, including both sporadic and Lynch syndrome-associated CRCs.1 Similar mechanisms underlie MSI-H small intestinal adenocarcinoma. MSI is characterized by expansion or contraction of DNA sequences through the insertion or deletion of repeated DNA sequences, which are routinely screened for by multiplex fluorescent polymerase chain reaction (PCR) assays.2 MSI-H, MSI-low, and microsatellite stable (MSS) are defined as detection of MSI at equal to or greater than 30%, less than 30%, and 0% of the loci analyzed, respectively. There are several different morphologic subtypes of MSI-H intestinal carcinoma, including medullary carcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma.3 However, adenocarcinomas with conventional histomorphology (ie, glandular formation, nuclear pseudostratification, and dirty necrosis), typical adenocarcinomas, can also be MSI-H. Genetically, some MSI-H intestinal adenocarcinomas harbor the BRAF V600E mutation, whereas others have wild-type BRAF.1 Lynch-associated intestinal carcinomas are always BRAF wild type.
Programmed death protein-1 (PD-1) and its ligand, PD-L1, serve a crucial role in the host immune response to cancer. Tumor cell surface expression of PD-L1 inactivates the host immune response to tumor by binding PD-1 on T cells.4,5 This interaction forms the basis for treatment with PD-1 inhibitors, which allows the host immune response to proceed without inhibition. Checkpoint inhibitors using PD-1/PD-L1 pathway blockade are effective in treating some cancers in first- or second-line settings through immune activation.6,7 Additionally, there is growing evidence that the tumor-associated immune cell expression of PD-L1 may also play a role in response to these medications.8-10
MSI-H intestinal carcinomas have been shown to highly express PD-L1,11-13 which in theory allows them to evade host immune response. A recent study14 has reported objective radiographic responses to PD-1 blockage in more than half of patients with advanced MSI-H CRCs. Here, we explored PD-L1 expression patterns in different subtypes of intestinal adenocarcinomas and correlated PD-L1 expression with BRAF mutational status and MSI PCR patterns.
Materials and Methods
Patient Selection and Clinicopathologic Data Collections
One hundred and fifteen MSI-H intestinal carcinomas were identified from 113 patients (six small intestinal carcinomas and 109 CRCs) with resections performed at Vanderbilt University Hospital between January 1, 2004, and March 1, 2018, and for which paraffin-embedded tissue blocks were available and sufficient for PD-L1 immunohistochemical studies. Forty-one MSS colorectal carcinomas (collected from June 1, 2010, to January 31, 2014) were also included. Patient electronic medical records and pathology reports were reviewed for clinicopathologic features, BRAF mutation status, and presence/absence of Lynch syndrome. Pathology slides were also reviewed for histologic subtypes. Histologic subtypes were divided into typical adenocarcinoma, not otherwise specified (NOS), and other variants, including mucinous adenocarcinomas and medullary carcinomas, based on the 2010 World Health Organization classification of tumors of the digestive system.15 This retrospective study was approved by Vanderbilt Institutional Review Board.
PD-L1 Immunohistochemistry
One tumor section containing the deepest invasion was used for immunohistochemistry for each cancer. Four-μm unstained tumor sections from formalin-fixed paraffin-embedded tissue were first deparaffinized by routine methods. After antigen retrieval, the sections were stained with primary antibody PD-L1 (clone E1L3N; Cell Signaling Technology) at 1:200 dilution, followed by antibody localization using Envision+ horseradish peroxidase-labeled polymer (DAKO). Staining was visualized by 5-minute incubation with diaminobenzidine.
Cell surface expression of PD-L1 by tumor cells and cell surface and/or cytoplasmic expression of PD-L1 by tumor-associated immune/inflammatory cells were evaluated by two surgical pathologists (J.R. and C.S.) separately and two surgical pathologists (S.N.S. and C.S.) simultaneously. There were discrepancies between the pathologists, mostly in percentages of cells stained. Consensus was always reached through discussion and re-reviewing the stain. Tumor-associated immune/inflammatory cells included tumor-infiltrating lymphocytes, and intratumoral and peritumoral inflammatory cells (including lymphocytes and macrophages) at invasion front. Tumor-associated immune cells at the invasion front were always within half field of a 20× field from the tumor. Inflammatory cells/lymphoid aggregates distant from the invasion front were not considered tumor associated. The entire tumor area including the invasion front was analyzed. The percentage of PD-L1–positive tumor cells out of the total tumor cells was estimated. Positive expression was defined as membranous staining of 1% or more of the tumor cells expressing PD-L1. For immune cells, the percentage of the tumor’s cross-sectional area (including the invasion front) occupied by PD-L1–expressing inflammatory cells was assessed.16,17 Positive expression was defined as membranous and/or cytoplasmic staining of 1% or more of the area occupied with PD-L1–expressing immune/inflammatory cells. Positive PD-L1 expression by immune cells associated with normal mucosa, adenoma, and ulceration was excluded. Pale cytoplasmic labeling in plasma cells was also excluded. Combined positive scores (CPS) were calculated by dividing the total number of PD-L1–positive cells (including tumor cells and immune cells) by the total number of viable tumor cells in the entire tumor area. A CPS score of 1% or higher was considered positive.
MSI Analysis by PCR
MSI testing on intestinal carcinomas by PCR was performed for clinical purpose at our institution using the MSI Analysis System (catalog number MD1641; Promega), which included five mononucleotide repeat MSI markers (BAT25, BAT26, NR21, NR24, and MONO27) and two pentanucleotide repeat markers (PENTA C and PENTA D) used for confirmation of identity. MSI patterns by PCR were retrospectively analyzed in 60 MSI-H tumors with PCR electropherograms available for review. The absolute nucleotide shift represents the change in the length of mononucleotide repeats of the tumor DNA compared with normal tissue DNA. The data are presented as an average number of nucleotide shifts per marker, as described previously.18
Statistics
Fisher exact test was employed to compare PD-L1 expression on tumor cells, on immune cells, and by CPS using Graphpad Prism Version 5.01 (GraphPad Software) between six different groups: (1) MSI-H vs MSS carcinoma, (2) MSI-H adenocarcinoma, NOS vs MSI-H mucinous carcinoma, (3) MSI-H adenocarcinoma, NOS vs MSI-H medullary carcinoma, (4) MSI-H mucinous vs MSI-H medullary carcinoma, (5) MSI-H BRAF wild-type vs MSI-H BRAF V600E mutant carcinoma, and (6) Lynch-associated vs MSI-H BRAF mutant carcinoma. P values were corrected by Bonferroni effect by multiplying 18. Student t test was used to compare nucleotide shifts by PCR-based MSI testing in cases with positive and negative PD-L1 expression. P < .05 was considered statistically significant.
Results
General Clinicopathologic Features
MSI-H tumors were from 59 females and 54 males, with a mean age of 64 years (range, 30-98 years). As expected, most of the MSI-H CRCs were from the right colon, defined as proximal to the splenic flexure. The majority of MSI-H tumors (79/115, 69%) were typical adenocarcinoma, NOS. There were also 22 mucinous adenocarcinomas and 14 medullary carcinomas. The 41 MSS included 11 females and 30 males, with a mean age of 62 years (range, 30-84 years). BRAF V600E mutational status was available for 101 MSI-H cancers; 60 were BRAF wild type and 41 were mutant. Among 60 BRAF wild- type tumors, 29 were from patients with Lynch syndrome. The clinicopathologic features are shown in Table 1.
Table 1.
Overall Clinicopathologic Features of Microsatellite Instability-High (MSI-H) and Microsatellite Stable (MSS) Intestinal Carcinomas
| Characteristic | MSI-H, No. (%) (n = 115) | MSS, No. (%) (n = 41) | Total, No. (%) (n = 156) |
|---|---|---|---|
| Sex | |||
| Male | 55 (48) | 30 (73) | 85 (54) |
| Female | 60 (52) | 11 (27) | 71 (46) |
| Age (y), mean ± SD | 64 ± 16 | 62 ± 13 | 63 ± 15 |
| Location | |||
| Right colon | 85 (74) | 12 (29) | 97 (62) |
| Left colon | 13 (11) | 15 (37) | 28 (18) |
| Rectum | 11 (10) | 14 (34) | 25 (16) |
| Small bowel | 6 (5) | 0 (0) | 6 (4) |
| Histologic types | |||
| Adenocarcinoma, NOS | 79 (69) | 39 (95) | 118 (76) |
| Medullary | 14 (12) | 0 (0) | 14 (9) |
| Mucinous | 22 (19) | 2 (5) | 24 (15) |
| Tumor stage | |||
| I | 26 (23) | 7 (17) | 33 (21) |
| II | 53 (46) | 17 (42) | 70 (45) |
| III | 29 (25) | 13 (32) | 42 (27) |
| IV | 7 (6) | 3 (7) | 10(6) |
| Unknown | 0 (0) | 1 (2) | 1(1) |
| BRAF | |||
| Wild type | 60 (52) | — | — |
| Lynch | 29 (25) | — | — |
| Unknown | 31 (27) | — | — |
| Mutant (V600E) | 41 (36) | — | — |
| Unknown | 14 (12) | — | — |
NOS, not otherwise specified.
Expression of PD-L1 by Tumor Cells
Tumor cell PD-L1 expression was seen in 28 of 115 (24%) MSI-H tumors Image 1, whereas only one of 41 (2%) MSS tumors had tumor cell expression (P = .02) Figure 1A and Table 2. PD-L1 expression in one positive MSS tumor was low and was only seen in 2% of total tumor cells. Tumor cell expression in positive MSI-H tumors ranged from 1% to 90%, with 13 of the 28 tumors showing expression on 10% or more of the tumor cells. In addition, PD-L1 expression was most prominent on tumor cells at the invasion front.
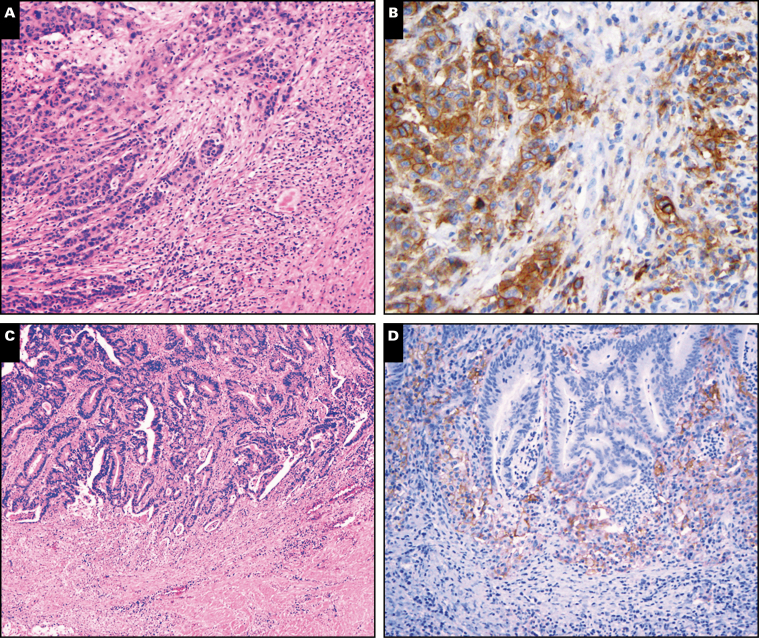
Image 1.
Expression of programmed death protein-1 ligand (PD-L1) by colon cancers. A, An H&E stain shows a medullary carcinoma with prominent intratumoral lymphocytes and peritumoral inflammation (×40). B, The tumor in A shows expression of PD-L1 by tumor cells and peritumoral inflammatory cells (×100). C, A conventional moderately differentiated adenocarcinoma with peritumoral inflammation at the invasive front (H&E, ×100). D, The tumor in C shows expression of PD-L1 by peritumoral inflammatory cells but not by tumor cells (×200).
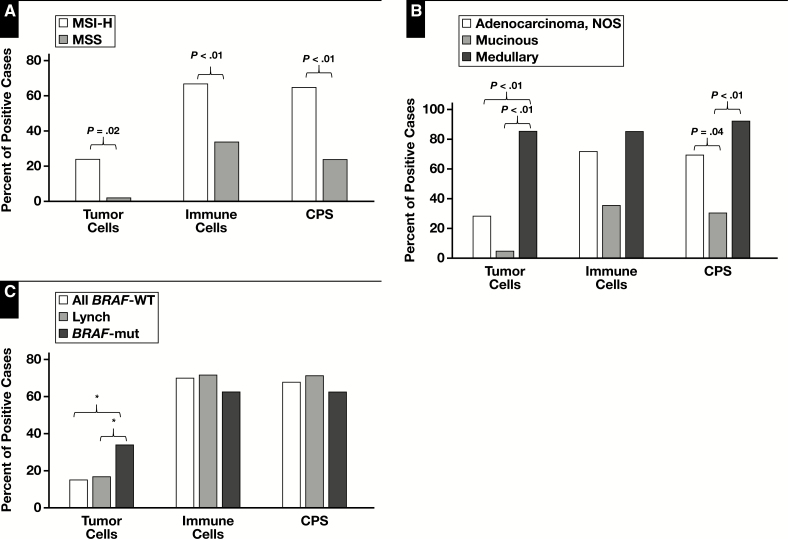
Figure 1.
Expression of programmed death protein-1 ligand (PD-L1) by tumor cells, inflammatory cells, and combined positive score (CPS). A, Microsatellite instability-high (MSI-H) vs microsatellite stable (MSS) intestinal carcinomas. B, Different histologic subtypes of MSI-H intestinal cancer: adenocarcinoma, not otherwise specified (NOS), mucinous, and medullary carcinoma. C, BRAF wild type (BRAF-WT), Lynch-associated, and BRAF mutant (BRAF-mut) MSI-H intestinal carcinomas. *Original P value was <.05 but was >.05 after correction for the Bonferroni effect.
Table 2.
Expression of PD-L1 in Microsatellite Instability-High (MSI-H) and Microsatellite Stable (MSS) Intestinal Carcinoma
| Positivity, % | Tumor Cells | Immune Cells | CPS ≥1% | |||||
|---|---|---|---|---|---|---|---|---|
| Positive, No. (%) | Negative, No. (%) | Positive, No. (%) | Negative, No. (%) | Positive, No. (%) | Negative, No. (%) | |||
|
MSI-H (n = 115) |
28 (24) | 87 (76) | 77 (67) | 38 (33) | 75 (65) | 40 (35) | ||
| Histologic subtypes | ||||||||
| Adenocarcinoma, NOS (n = 79) | ||||||||
| ≥50% | 1 (1) | 2 (3) | 5 (7) | |||||
| ≥10% to <50% | 5 (6) | 21 (26) | 19 (24) | |||||
| ≥1% to <10% | 9 (12) | 34 (43) | 31 (39) | |||||
| Total | 15 (19) | 64 (81) | 57 (72) | 22 (28) | 55 (70) | 24 (30) | ||
| Mucinous carcinoma (n = 22) | ||||||||
| ≥50% | 0 (0) | 0 (0) | 1 (5) | |||||
| ≥10% to <50% | 0 (0) | 1 (5) | 1 (5) | |||||
| ≥1% to <10% | 1 (5) | 7 (31) | 5 (21) | |||||
| Total | 1 (5) | 21 (95) | 8 (36) | 14 (64) | 7 (31) | 15 (68) | ||
| Medullary carcinoma (n = 14) | ||||||||
| ≥50% | 3 (21) | 0 (0) | 4 (29) | |||||
| ≥10% to <50% | 4 (29) | 3 (22) | 5 (35) | |||||
| ≥1% to <10% | 5 (36) | 9 (64) | 4 (29) | |||||
| Total | 12 (86) | 2 (14) | 12 (86) | 2 (14) | 13 (93) | 1 (7) | ||
| BRAF status | ||||||||
| BRAF wild type (n = 60) | ||||||||
| ≥50% | 1 (2) | 1 (2) | 3 (5) | |||||
| ≥10% to <50% | 1 (2) | 18 (30) | 17 (28) | |||||
| ≥1% to <10% | 7 (11) | 23 (38) | 21 (35) | |||||
| Total | 9 (15) | 51 (85) | 42 (70) | 18 (30) | 41 (68) | 19 (32) | ||
| Lynch (n = 29) | ||||||||
| ≥50% | 1 (3) | 0 (0) | 2 (7) | |||||
| ≥10% to <50% | 0 (0) | 11 (38) | 11 (38) | |||||
| ≥1% to <10% | 4 (14) | 10 (36) | 8 (27) | |||||
| Total | 5 (17) | 24 (83) | 21 (72) | 8 (28) | 21 (72) | 8(28) | ||
| BRAF mutant (n = 41) | ||||||||
| ≥50% | 2 (5) | 1 (2) | 5 (12) | |||||
| ≥10% to <50% | 5 (12) | 5 (12) | 6 (15) | |||||
| ≥1% to <10% | 7 (17) | 20 (49) | 15 (36) | |||||
| Total | 14 (34) | 27 (66) | 26 (63) | 15 (37) | 26 (63) | 15 (37) | ||
| MSS (n = 41) | ||||||||
| ≥50% | 0 (0) | 0 (0) | 0 (0) | |||||
| ≥10% to <50% | 0 (0) | 2 (5) | 0 (0) | |||||
| ≥1% to <10% | 1 (2) | 12 (29) | 10 (24) | |||||
| Total | 1 (2) | 40 (98) | 14 (34) | 27 (66) | 10 (24) | 31 (76) |
CPS, combined positive score; NOS, not otherwise specified.
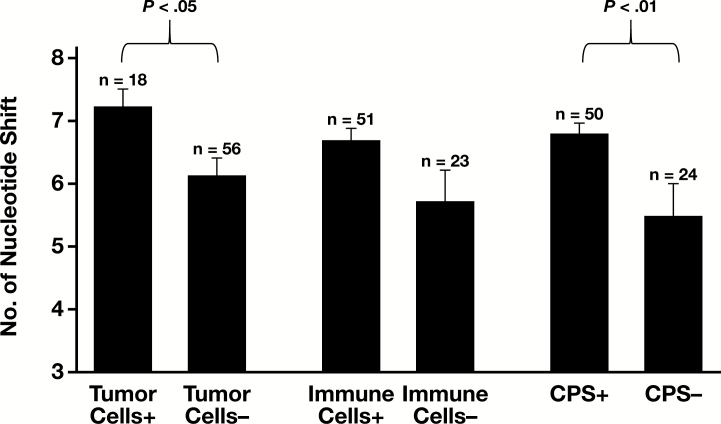
Among MSI-H tumors, medullary carcinomas expressed PD-L1 on tumor cells (Images 1A and 1B) more frequently than typical adenocarcinoma, NOS or mucinous adenocarcinomas (P < .01; Figure 1B and Table 2). However, there was no difference in tumor cell PD-L1 expression between typical adenocarcinomas and mucinous adenocarcinomas. Most of the medullary carcinomas expressed PD-L1 on 10% or more of the tumor cells (Table 2). There was a statistically significant difference in nucleotide shift in MSI PCR electropherograms (P < .05) between tumors with and those without tumor cell PD-L1 expression Figure 2. BRAF-mutated MSI-H tumors tended to more frequently express PD-L1 on tumor cells compared to BRAF wild-type MSI-H tumors (14/41 vs 9/60; Figure 1C); however, statistically the difference was not significant after correcting for the Bonferroni effect. There was no difference in tumor cell PD-L1 expression between Lynch-associated and BRAF-mutated cancers (5/29 vs 14/41; Table 2).
Figure 2.
Number of nucleotide shifts (mean ± SEM) in polymerase chain reaction-based microsatellite instability testing in programmed death protein-1 ligand positive and negative tumors. CPS, combined positive score.
Expression of PD-L1 by Tumor-Associated Immune Cells
Compared to MSS tumors, MSI-H intestinal carcinomas frequently expressed PD-L1 on tumor-associated immune/inflammatory cells (14/41 vs 77/115; P < .01; Table 2 and Figure 1A). The tumor-associated immune cells were composed predominantly of macrophages and lymphocytes.
Among MSI-H tumors, PD-L1 expression by immune cells was not statistically different between typical adenocarcinoma, NOS, mucinous adenocarcinoma, and medullary carcinoma after correcting for the Bonferroni effect (57/79 vs 8/22 vs 12/14; Table 2, Images 1C and 1D, and Figure 1B). However, mucinous adenocarcinomas tended less frequently to express PD-L1 on immune cells, which might have partially contributed to limited numbers of tumor-associated immune cells in these tumors. All 22 mucinous adenocarcinomas in this cohort, however, contained tumor-associated immune cells intratumorally and at the invasion front, but they did not express PD-L1 in 14 negative cases.
There was no difference in PD-L1 expression by immune cells between BRAF mutant and wild type tumors as well as between BRAF mutant and Lynch-associated carcinomas (Figure 1C). Average nucleotide shifts were not significantly different between tumors with and those without immune cell PD-L1 expression (Figure 2).
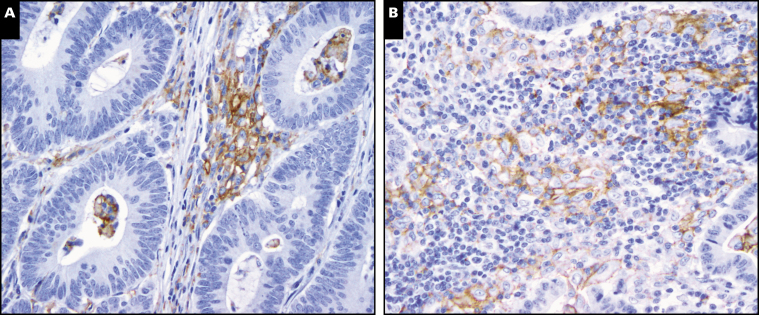
PD-L1 was expressed by immune cells that were intermingled with cancer glands/clusters (intratumoral immune cells) and also at the invasive front of the tumor (peritumoral immune cells). For most cases with immune cell PD-L1 expression, the expression was most prominent at the invasive front (Images 1C and 1D), with only scattered clusters of intratumoral immune cells (mainly macrophages) expressing PD-L1 Image 2A. In three cases, there were sheets of intratumoral immune cells among tumor glands. Most of these cells (mainly macrophages) strongly expressed PD-L1 Image 2B.
Image 2.
Expression of programmed death protein-1 ligand (PD-L1) by immune cells within tumor (×200). A, Small clusters of immune cells among cancer glands expressing PD-L1. B, Sheets of immune cells among cancer glands expressing PD-L1.
Evaluation of PD-L1 Expression by CPS
Overall, 75 of 115 (65%) MSI-H tumors had a CPS of 1% or higher, ranging from 1% to 100%, and 35 of them had a score of 10% or higher (Table 2 and Figure 1A). On the other hand, only 10 of 41 (24%) MSS tumors had a positive CPS (P < .01), all of which were less than 10%.
Among MSI-H tumors, CPS was more likely positive in medullary carcinomas or typical adenocarcinomas than in mucinous carcinomas (P < .01 and P = .04, respectively; Figure 1B), and no statistical difference was observed between medullary carcinomas and typical adenocarcinomas. There was no difference in BRAF mutational status between CPS-positive and CPS-negative tumors. Additionally, no difference in CPS was observed between BRAF mutant and Lynch-associated tumors (Table 2 and Figure 1C). CPS-positive MSI-H tumors had more nucleotide shift in MSI PCR electropherograms compared to CPS-negative MSI-H tumors (6.8 ± 0.2 vs 5.5 ± 0.5; P < .01; Figure 2).
Only two patients with MSI-H tumor in this cohort were treated with checkpoint inhibitors. The first patient was a 65-year-old man who had a stage IV, BRAF wild-type mucinous adenocarcinoma with no PD-L1 expression on tumor cells or immune cells. This patient had disease progression on pembrolizumab. The second patient, a 72-year-old woman, had a stage IV BRAF mutant typical adenocarcinoma and demonstrated a partial response on nivolumab. The tumor from the second patient showed immune cell PD-L1 expression with a CPS of 1%.
Discussion
The next significant revolution in cancer therapy appears to be immune system-related therapies, with PD-1 blockade being the first approved class of agents. This therapy is being utilized for non-small cell lung cancers as second-line and, in some instances, first-line protocol.19,20 While PD-L1 expression has been used as a biomarker for immunotherapy in some tumors, including lung cancers, mismatch repair deficiency predicts response of other solid tumors to PD-1 blockade.1,11,14 Objective radiographic responses to PD-1 inhibition were observed in 53%, and complete responses were achieved in 21% patients, with advanced mismatch repair-deficient cancers across 12 different tumor types, including CRCs.14 On the other hand, mismatch repair-proficient CRCs showed no objective response and only an 11% progression-free survival rate.
Although MSI status is highly predictive of response to PD-1 blockage, PD-1 inhibition showed no significant effect on approximately 20% MSI-H CRCs.14 The question is whether there is variable PD-L1 expression in these MSI-H tumors, which could affect the response. Le et al21 performed PD-L1 immunohistochemistry on CRCs treated with pembrolizumab, an antibody to PD-1, and observed PD-L1 expression only by MSI-H CRCs. However, correlation between the expression and response within MSI-H CRCs was not evaluated, likely due to the small sample size. When comparing PD-L1 expression by MSI-H and MSS CRCs, previous studies also identified an association of PD-L1 expression on tumor cells with medullary morphology,22 which was also observed in this study. In addition, in this study we systemically analyzed PD-L1 expression on both tumor and immune cells among the three main histologic variants of MSI-H intestinal carcinoma using paraffin-embedded tumor blocks. Medullary carcinoma most frequently expressed PD-L1 on tumor cells in comparison to the other two subtypes. Both medullary and typical adenocarcinomas tended to highly expressed PD-L1 on immune cells. Mucinous adenocarcinomas demonstrated the least frequent PD-L1 expression of all the CRCs, and this decreased expression was noted both on tumor cells and immune cells. In this cohort, we had only two patients with MSI-H tumor treated with checkpoint inhibitors. Interestingly the mucinous tumor with negative PD-L1 expression did not respond to the treatment, whereas a partial response was observed in the patient with typical adenocarcinoma showing PD-L1 expression. Large-scale studies correlating treatment response with histologic subtypes and PD-L1 expression are warranted to determine whether they predict treatment response to immunotherapies.
Consistent with previous studies,16,21 PD-L1 expression was observed on both tumor cells and tumor-associated immune/inflammatory cells, and the expression was more prominent on tumor-associated immune cells at the invasive front. In this study, only 24% of the tumors expressed PD-L1 on tumor cells, whereas 67% expressed PD-L1 on tumor-associated immune cells. There is increasing evidence showing that this peritumoral immune cell expression of PD-L1 may also have a significant role in host immune response.12 MSI-H CRCs in Le et al’s series21 showed PD-L1 expression mainly by tumor-associated immune cells, but these tumors responded well to PD-L1 inhibitor therapy. In addition, the CPS, which takes into account PD-L1 expression by both tumor and tumor-associated immune cells, has been developed and refined for gastric and esophageal adenocarcinomas.13
Significant tumor response to PD-1 inhibitors may be due to the increased mutational burden these tumors carry, which may increase the number of potential epitopes for immune activation.23 For that reason, we investigated the association between the MSI patterns generated by PCR and PD-L1 expression in MSI-H intestinal carcinoma. We speculate that more nucleotide shifts in tumor DNA may indicate more DNA replications/cell divisions and potentially the accumulations of more genetic alterations in the tumor. We observed that MSI-H tumors with PD-L1 expression demonstrated more nucleotide shifts in MSI patterns as noted on the electopherograms of the PCR products.
In conclusion, PD-L1 expression is different among histologic variants of MSI-H intestinal adenocarcinoma. Genetic alterations may also be correlated with PD-L1 expression in these tumors. Further studies correlating morphology and efficacy of anti-PD-L1/PD-1 therapies may clarify the significance of different PD-L1 expression in these tumors.
Acknowledgement
This work was supported by NIH/NIDDK (grant No. 5P30 DK058404-13 to C.S.) and NIH/NCI (grant No. 5P50 CA095103-13 to C.S.).
References
- 1. Gelsomino F, Barbolini M, Spallanzani A, et al. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19-26. [DOI] [PubMed] [Google Scholar]
- 2. Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] [Google Scholar]
- 3. Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [DOI] [PubMed] [Google Scholar]
- 5. Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580-6587. [DOI] [PubMed] [Google Scholar]
- 7. Kluger HM, Zito CR, Turcu G, et al. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res. 2017;23:4270-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baine MK, Turcu G, Zito CR, et al. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget. 2015;6:24990-25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen AR, Siu LL. PD-L1 testing in cancer: challenges in companion diagnostic development. JAMA Oncol. 2016;2:15-16. [DOI] [PubMed] [Google Scholar]
- 11. Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22:813-820. [DOI] [PubMed] [Google Scholar]
- 12. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salem ME, Puccini A, Grothey A, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum, In: Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 16. Thota R, Gonzalez RS, Berlin J, et al. Could the PD-1 pathway be a potential target for treating small intestinal adenocarcinoma? Am J Clin Pathol. 2017;148:208-214. [DOI] [PubMed] [Google Scholar]
- 17. Roberts JA, Gonzalez RS, Das S, et al. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: a potential target for anti-PD-1/PD-L1 therapy. Hum Pathol. 2017;70:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Shi C, Eisenberg R, et al. Differences in microsatellite instability profiles between endometrioid and colorectal cancers: a potential cause for false-negative results? J Mol Diagn. 2017;19:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assi HI, Kamphorst AO, Moukalled NM, et al. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer. 2018;124:248-261. [DOI] [PubMed] [Google Scholar]
- 20. Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484-3515. [DOI] [PubMed] [Google Scholar]
- 21. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104-1112. [DOI] [PubMed] [Google Scholar]
- 23. Mehnert JM, Monjazeb AM, Beerthuijzen JMT, et al. The challenge for development of valuable immuno-oncology biomarkers. Clin Cancer Res. 2017;23:4970-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]