Abstract
Introduction: This cross-sectional study aimed to assess the association between cardiovascular disease (CVD) risk factors and dinner consumption in a nationally representative sample of Iranian adolescents.
Methods: The present study was conducted on 5642 adolescents aged 10-18 years old in 27 provinces in Iran. The subjects were included applying by multistage random cluster sampling. Participants who ate ≥5 dinners during a week were considered as a dinner consumer.
Results: Among 5642 subjects, 1412 (25%) did not consume dinner. Dinner consumers were less likely to be overweight or obese (P < 0.001) and abdominally obese (P < 0.001) as well as to have an abnormal level of HDL-C (P = 0.02). Dinner skipper youths had a higher risk for overweight or obesity (odds ratio [OR]: 1.62; 95% CI: 1.39-1.89) and abdominal obesity (OR: 1.59; 95% CI: 1.36-1.85) which remained significant after adjusting confounding factors (P <0001). No relationship was observed between dinner consumption and the rest of the CVD risk factors, neither in crude nor in adjusted models. A higher proportion of dinner-consumer adolescents had no CVD risk factors in comparison to dinner-skipper subjects (31.1% vs. 28%).
Conclusion: Eating dinner might be inversely associated with some CVD risk factors among Iranian adolescents. Further prospective studies will need to prove this theory.
Keywords: Dinner, CVD Risk Factors, Adolescents
Introduction
Cardiovascular diseases (CVDs) have remained high on a list of leading causes of death for more than a decade throughout the world.1 Although CVD mortality rate and CVD risk factors are decreasing in high-income countries, it tends to increase in low and middle-income countries.2-5 Additionally, reports indicated the growing trend for CVD prevalence in future; for example, it is estimated that 40.5% of the US population will have some form of CVD by 2030.6 Middle East countries have highest cardiovascular death rates through the world which possibly Iran has a higher burden in this region.7 Moreover, reports revealed a dramatically increasing rate for CVD risk factors in Iranian adolescents.8 Based on the results of the cohort studies, CVD risks in childhood and adolescence are predictors of CVD in adulthood.9-12
The CVD-diet relationship is assessed in several epidemiological surveys.2 Dietary factors affect CVD events13 and CVD risk factors.14 According to reports diet play an important role in developing or restricting CVD risk factors in adults13,15-17 as well as children and adolescents.18-20 Interventions to reduce CVD risk factors in regard to diet as a modifiable behavior have achieved favorable results.21-23 Earlier studies investigated the effect of a specific nutrient or component of diet like fat, carbohydrate, different fatty acids and salt on CVD risk factors.13,24,25 Whereas recent papers have mostly focused on dietary patterns26 and habits27,28 in relation to CVD risk factors. Eating frequency and main meals consumption have been linked to CVD and CVD risk factors.28-30 A recent paper showed an additional eating occasion was associated with lower levels of CVD risk factors including waist circumference, fasting glucose, fasting insulin, triglycerides (TG), TC, low-density lipoprotein cholesterol (LDL-C).31 An earlier survey on 10 years old children which aimed to investigate dietary variables in relation to CVD risk factors reported that most of the dietary cholesterol was received from breakfast and dinner.32 Another study among adults showed that dinner had the highest density for fat and cholesterol.13 In spite of being one of the main meals, dinner consumption is often neglected and few studies examined dinner consumption in relation to other variables. With regard to CVD risk factors, studies examined the just obesity-dinner relationship. Eating family dinner was negatively associated with being overweight among children and adolescents.33-35 However, one of the studies failed to show this association in longitudinal analysis among adolescents.35 Furthermore, eating family dinner was linked to better dietary intakes and habits33,36 and lower eating disorders.37
We are aware of no study evaluating the association between dinner consumption and CVD risk factors among adolescents. According to findings of different studies, Iranian children and adolescents prone to have abnormal CVD risk factors such as high prevalence of overweight and obesity,38 metabolic syndrome,39 and abnormal lipid profile.40 Therefore, we conducted this study to investigate whether there is any correlation between dinner consumption and each of CVD risk factors among a nationally representative sample of Iranian adolescents.
Materials and Methods
Subjects
The present study was performed using data of the “national survey of school student high-risk behaviors” (2009–2010) as the third survey of the school-based surveillance system titled CASPIAN-III (“Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable disease Study”). In spite of the published methodology in detail,41 a brief explanation is given therein. However, the sample size was calculated based on different variables but we considered the maximum sample size. Using multistage random cluster sampling 5642 Iranian school students aged 10 to 18 years were selected from elementary, intermediate, and high school schools among rural and urban areas of 27 provinces in Iran (with estimated 80% response rate, 20% was added to the sample size). Students with CVD or those on taking medicine were not enrolled in the present study due to exclusion criteria. Protocols of this study were approved by the ethics committees and other relevant national regulatory organizations. After explaining study objectives to students and parents, written informed consent and oral assent were obtained from parents and students, respectively.
Assessment of anthropometric measurements
Weight was measured by the digital scale in barefoot and lightly dressed condition. Standing height of no-shoes-wearing subjects was marked on a stadiometer. Weight and height were measured twice, average to the nearest 0.2 kg and 0.2 cm, respectively. Body mass index (BMI) was calculated as the ratio of body weight in kilogram to height squared in meters. Waist circumference (WC) was measured using a nonelastic tape to the nearest 0.5 cm midway between the lower rib margin and iliac crest. In standing position widest hip was measured to the nearest 0.5 cm. According to the World Health Organization (WHO) guidelines for 10-19 years old adolescents, “underweight, overweight and obesity were defined as BMI <5th, =85th-95th, and >95th percentiles, respectively”.42 Abdominal obesity was predicated on WC (cm) divided by height (cm) >0.5.43
Assessment of lipid profiles and blood pressure
To collect blood samples, participants were referred to the nearest health center to the school. Blood samples were collected from all participants. The samples of venous blood were taken after 12 h fast to assess lipid profiles including TC, LDL-C, HDL-C, TG and fasting plasma glucose (FPG). All biochemical analyses were performed in the Central Provincial Laboratory according to the standards of the National Reference Laboratory, a WHO collaborating center in Tehran. “The borderline and risky cut-off points for this population were defined according to latest recommendation of the American Academy of Pediatrics as followed TC = 170-199 and ≥200 mg/dL, LDL-C = 110-129 and ≥130 mg/dL, HDL-C = 40-45 and <40 mg/dL, TG = 90-129 and ≥130 mg/dL, and FPG = 100-125 and > 125 mg/dL, respectively”.44 Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice with a suitable size of cuff for each student after a 5 minutes rest and in the sitting position. SBP was determined as the first sound which is clear and DBP as the disappearance of sound. The average of two-time measurements was considered for analysis.
Assessment of dinner consumption and sociodemographic variables
The questionnaire applied in this study was designed based on the WHO STEPwise approach to Surveillance (STEPS) approved to non-communicable disease (NCD) (Tools ver 9.5) and WHO Global School Health Survey (GSHS). Some questions in regard to sociodemographic characteristics such as child’s birth weight and family dietary habits were included in the “parents’ questionnaire”.45 The validity and reliability of questionnaires have been confirmed in the first survey of this surveillance system.45 Smoking habits (yes or no), sleeping (hour/day), watching television (TV) (hour/day), and working with a computer (hour/day) were self-reported. To assess dinner-CVD risk factors, a binary variable applied through counting subjects that had ≥ 5 dinners during a week as a dinner consumer and < 5 as a dinner skipper.
Assessment of Socioeconomic status
The method and variables which were used for calculating SES was approved in the previous international study for Iran.46 SES of family was constructed using some variables including parental occupation, parental education, possessing private car, school type (public/private), type of home (private/rented) and having personal computer at home. These variables were summarized in one main component named SES score using principal component analysis. A lower score corresponds to a lower SES.
Statistical analysis
All analyses were done based on dinner consumption (yes/no) using SPSS for Windows software (version 16.0. SPSS, Chicago, IL). Descriptive variables such as age, weight and lipid profiles expressed as mean ± standard deviation (SD) and significant differences between means of 2 groups (dinner consumer or skipper) were examined employing Independent t test. Categorical variables like gender, normal BMI were represented as percentages and between-groups differences assessed by chi-square tests. Furthermore, the distribution of CVD risk factors according to dinner consumption was reported using chi-square tests. To evaluate the association between dinner consumption and CVD risk factors, logistic regression was employed with consideration of dinner consumer as reference and odds ratio (OR) with 95% confidence interval (CI) were reported. Adjusted model for sex, age, SES, physical activity, and smoking 47 was defined. Finally, to observe the overall effect of dinner on CVD risk factors chi-square tests were used.
Results
A total of 2816 girls and 2826 boys aged 10 to 18 years old were examined in the present study. Among these population 4230 adolescents were dinner consumers while 1412 of individuals did not consume dinner; i.e. 25% of the population was not dinner consumers. Mean ± SD of general characteristics and CVD risk factors as well as the distribution of some features according to dinner consumption is shown in Table 1. Dinner consumers spent more hours sleeping, watching television (TV) and doing physical activity and fewer hours working with the computer (P < 0.001).
Table 1. General characteristics of the participants according to dinner consumption .
| Variable | Dinner consumer | P | |
| Yes 1 (n = 4230) | No (n = 1412) | ||
| Age (year) | 14.85 ± 2 .42 | 14.37 ± 2.36 | <0.001 |
| Girls (%) | 51.1 | 46.2 | 0.001 |
| Urban (%) | 70.2 | 67.3 | 0.040 |
|
Level of education (%) Primary school Secondary High school |
30.4 34.4 35.2 |
41.1 31.2 27.6 |
<0.001 |
| Weight (kg) | 47.26 ± 14.93 | 46.80 ± 15.36 | 0.320 |
| Height (cm) | 154.88 ± 14.06 | 152.06 ± 13.48 | <0.001 |
| BMI (kg/m2) | 19.30 ± 3.97 | 19.79 ± 4.42 | <0.001 |
| Normal BMI3 (%) | 66.6 | 62.6 | <0.001 |
| Waist girth (cm) | 68.81 ± 22.85 | 68.52 ± 12.20 | 0.652 |
| Waist to Height ratio | 0.44 ± 0.14 | 0.45 ± 0.07 | 0.151 |
|
Birth weight (kg) (%) <2500 g 2500-4000 g >4000 g |
14.2 76.2 9.6 |
14.8 72.5 12.8 |
0.003 |
| Systolic blood pressure (mm Hg) | 103.42 ± 13.70 | 102.45 ± 14.29 | 0.033 |
| Diastolic blood pressure (mm Hg) | 65.97 ± 10.89 | 65.52 ± 10.72 | 0.191 |
| Sleeping (hour/day) | 9.12 ± 2.15 | 8.64 ± 2.44 | <0.001 |
| Watching TV (hour/day) | 3.58 ± 1.21 | 3.38 ± 1.09 | <0.001 |
| Working with computer (hour/day) | 1.97 ± 1.20 | 2.21 ± 1.18 | <0.001 |
| Physical activity (hour/day) | 2.02 ± 1.21 | 1.60 ± 1.17 | <0.001 |
| Smoker (%) | 12.7 | 12.4 | 0.851 |
| Total cholesterol (mg/dl) | 148.84 ± 32.23 | 147.36 ± 30.42 | 0.162 |
| LDL-C (mg/dl) | 83.98 ± 26.78 | 84.95 ± 28.98 | 0.391 |
| HDL-C (mg/dl) | 46.55 ± 14.47 | 45.24 ± 13.70 | 0.011 |
| Triglycerides (mg/dl) | 92.49 ± 41.49 | 94.41 ± 45.91 | 0.210 |
| Fasting plasma glucose (mg/dl) | 87.84 ± 13.68 | 86.94 ± 14.17 | 0.060 |
| SGOT (U/L) | 25.55 ± 13.49 | 26.35 ± 13.37 | 0.101 |
| SGPT (U/L) | 18.12 ± 11.27 | 18.32 ± 12.16 | 0.630 |
BMI: body mass index; LDL-C: low-density lipoprotein- cholesterol; HDL-C: high-density lipoprotein- cholesterol; SGOT: Aspartate aminotransferase; SGPT: Alanine Aminotransferase.
1Subjects were considered as dinner consumer if they had dinner ≥ 5 days a week.
2Values are mean ± SD, otherwise, it is indicated.
3Normal BMI: according to WHO criteria, 85th >BMI>5th.
Distribution of subjects with regard to CVD risk factors based on dinner consumption is given in Table 2. Dinner consumers were significantly more likely to be underweight (P = 0.004) and less likely to be overweight or obese (P < 0.001) and abdominally obese (P < 0.001) as well as to have an abnormal level of HDL-C (P = 0.02) compared with dinner skipper adolescents.
Table 2. Cardiovascular disease risk factors among dinner consumers and skippers .
| Variable | Dinner consumer | P | |||
| Yes (n = 4230) | No (n = 1412) | ||||
| n | % | n | % | ||
| Underweight1 (%) | 768 | 18.3 | 209 | 15.0 | 0.004 |
| Overweight or obese2 (%) | 634 | 15.1 | 312 | 22.4 | <0.001 |
| Abdominal obese3 (%) | 594 | 14.1 | 289 | 20.7 | <0.001 |
|
Hypertension4 Pre-HTN HTN |
762 234 |
18.8 5.8 |
247 75 |
18.3 5.6 |
0.891 |
|
Total cholesterol5 (mg/dL) Borderline High |
570 209 |
16.4 6.0 |
210 62 |
17.2 5.1 |
0.420 |
|
LDL-C6 (mg/dL) Borderline High |
259 133 |
10.6 5.5 |
104 58 |
12.2 6.8 |
0.131 |
|
HDL-C7 (mg/dL) Borderline Low |
477 1014 |
16.1 34.3 |
186 384 |
18.1 37.4 |
0.021 |
|
Triglycerides8 (mg/dL) Borderline High |
988 474 |
28.7 13.8 |
344 183 |
29.6 15.7 |
0.151 |
|
Fasting plasma glucose9 (mg/dL) Borderline High |
506 20 |
15.0 0.6 |
155 6 |
13.9 0.5 |
0.650 |
HTN: hypertension; LDL-C: Low-density lipoprotein–cholesterol; HDL-C: high-density lipoprotein-cholesterol
Cut-offs for biochemical variables: Total Cholesterol; borderline= 170-199 and high ≥200 mg/dL, LDL-C; borderline= 110-129 and high ≥130 mg/dL, HDL-C; borderline= 40-45 and high <40 mg/dL, TG; borderline= 90-129 and high ≥130 mg/dL, and FPG; borderline= 100-125 and high > 125 mg/dL,
1Defines as BMI<5th according to WHO criteria.
2Defines as according to WHO criteria, 95th >BMI>85th.
3Abdominal obese defined according to waist to height ratio >0.5.
4Pre-HTN: If BP confirmed 120/80-130/85, HTN: If BP confirmed >130/85.
5Total cholesterol: Borderline=170-199 mg/dL and high≥200 mg/dL.
6LDL- C: Borderline=110-129 mg/dL and high≥130 mg/dL.
7HDL- C: Borderline=40-45 mg/dL and low<40 mg/dL.
8Triglycerides: Borderline=90-129 mg/dL and high≥130 mg/dL.
9FPG; borderline: 100-125 mg/dL, high> 125 mg/dL.
Crude and multivariable-adjusted ORs for CVD risk factors according to dinner consumption are represented in Table 3. Dinner skippers were 21% less likely to be underweight than dinner-consumer participants (OR 0.79; 95% CI: 0.67-0.93). However, this relationship disappeared after adjusting for age, sex, physical activity, and smoking (OR 0.86; 95% CI: 0.55-1.12). Dinner skipper youths had a higher risk for overweight or obesity (OR 1.62; 95% CI: 1.39-1.89) and abdominal obesity (OR: 1.59; 95% CI: 1.36-1.85) which remained significant after adjusting (P < 0001). Additionally, dinner skipping was associated with 23 and 21% increased risk for borderline (OR: 1.23; 95% CI: 1.02-1.52) and low (OR: 1.21; 95% CI: 1.04-1.42) levels of HDL-C, respectively, in crude model among adolescents. After adjustments, the relationship disappeared for the low level of HDL-C, but for borderline levels, it remained strongly significant with 43% increased risk (OR: 1.43; 95% CI: 1.11-1.92; P = 0.006).
Table 3. Odds ratio (95% CIs) for having CVD risk factors among dinner consumers and skippers .
| Variable | Dinner consumer | P | |
| Yes | No | ||
|
Underweight (%)
Crude Adjusted1 |
1.00 1.00 |
0.79 (0.67-0.93) 0.87 (0.66-1.14) |
0.005 0.331 |
|
Overweight or obese (%)
Crude Adjusted |
1.00 1.00 |
1.62 (1.39-1.89) 1.53 (1.22-1.94) |
<0.001 <0.001 |
|
Abdominal obese (%)
Crude Adjusted |
1.00 1.00 |
1.59 (1.36-1.85) 1.65 (1.30-2.10) |
<0.001 <0.001 |
|
Prehypertension
Crude Adjusted HTN Crude Adjusted |
1.00 1.00 1.00 1.00 |
1.03 (0.88-1.21) 0.85 (0.67-1.10) 1.04 (0.80-1.37) 0.87 (0.60-1.28) |
0.691 0.209 0.753 0.481 |
|
Cholesterol (mg/dL)
Borderline Crude Adjusted High Crude Adjusted |
1.00 1.00 1.00 1.00 |
0.95 (0.80-1.13) 1.19 (0.89-1.58) 1.18 (0.88-1.58) 1.45 (0.90-2.34) |
0.550 0.221 0.271 0.120 |
|
LDL-C (mg/dL)
Borderline Crude Adjusted High Crude Adjusted |
1.00 1.00 1.00 1.00 |
0.84 (0.66-1.07) 0.98 (0.66-1.45) 0.77 (0.56-1.06) 1.67 (0.86-3.23) |
0.150 0.920 0.111 0.120 |
|
HDL-C (mg/dL)
Borderline Crude Adjusted Low Crude Adjusted |
1.00 1.00 1.00 1.00 |
1.23 (1.02-1.52) 1.43 (1.11-1.92) 1.21 (1.04-1.42) 0.99 (0.77-1.26) |
0.031 0.006 0.021 0.821 |
|
TG (mg/dL)
borderline Crude Adjusted High Crude Adjusted |
1.00 1.00 1.00 1.00 |
0.92 (0.79-1.07) 1.02 (0.81-1.27) 0.83 (0.67-1.01) 0.92 (0.69-1.22) |
0.291 0.889 0.060 0.580 |
|
FPG (mg/dL)
Borderline Crude Adjusted High Crude Adjusted |
1.00 1.00 1.00 1.00 |
1.09 (0.90-1.33) 0.96 (0.72-1.26) 1.12 (0.45-2.79) 1.10 (0.36-3.32) |
0.360 0.746 0.811 0.860 |
HTN: hypertension; LDL-C: Low-density lipoprotein–cholesterol; HDL-C: high-density lipoprotein-cholesterol
Cut-offs for biochemical variables: Total Cholesterol; borderline= 170-199 and high ≥200 mg/dL, LDL-C; borderline= 110-129 and high ≥130 mg/dL, HDL-C; borderline= 40-45 and high <40 mg/dL, TG; borderline= 90-129 and high ≥130 mg/dL, and FPG; borderline= 100-125 and high > 125 mg/dL,
1 Adjusted for sex, age, socioeconomic status, physical activity, and smoking.
Distribution of adolescents regarding overall CVD risk factors based on dinner consumption is shown in Figure 1. As it is obvious, a higher proportion of the population in dinner consumer subjects has none of the risk factors or just 1 risk factors compared with dinner skippers, whereas having 2 or more risk factors simultaneously are more prevalent in dinner skipper than consumer individuals (P = 0.007).
Figure 1.
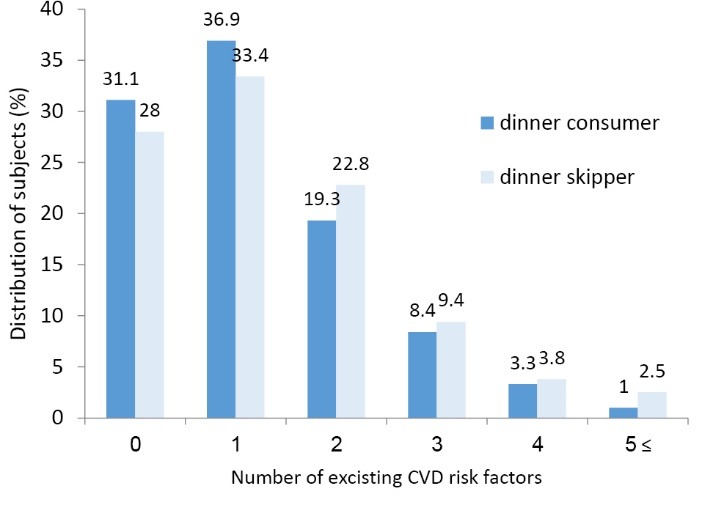
Distribution of adolescents regarding a number of identified CVD risk factors simultaneously according to dinner consumption; P = 0.007; eight CDV risk factors defined as underweight or overweight or obesity, abdominal obesity, high blood pressure, hypercholesterolemia, high LDL- cholesterol, Low HDL-cholesterol, Hypertriglyceridemia, High fasting plasma glucose.
Discussion
In the present study which was performed among a nationally representative sample of 5642 Iranian adolescents, dinner skipping was prevalent and an inverse association was found between dinner consumption and some CVD risk factors including overweight or obesity, abdominal obesity, and HDL-C. Moreover, the results regarding overall CVD risk factors revealed that higher proportion of dinner-consumer adolescents had no or 1 CVD risk factors and a smaller proportion had 2 or more CVD risk factors simultaneously in comparison to dinner-skipper subjects.
To the best of our knowledge, regardless of a few studies examined the dinner-overweight relationship among adolescents,33-35 this study is the first paper trying to investigate the relationship between dinner consumption and CVD risk factors. Therefore, there is little opportunity to compare the findings of this study with similar ones and so inevitably, we review papers on breakfast or meal consumption despite the fact that consuming dinner may have entirely different outcomes.
Lower BMI and a higher proportion of normal BMI reported among subjects who consumed dinner could be attributed to dinner eating due to age-independent nature of BMI. Moreover, an inverse association between BMI and dinner consumption were revealed in this study is consistent with previous studies which were conducted in developing countries.33-35 In a study conducted among Japanese women, individuals who were late dinner consumer or consumed bedtime snakes were more likely to be overweight or obese.48 However, these women tend to skip breakfast and probably they were prone to be obese. Additionally, a recent report with a substantial sample size showed five-meal-a-day pattern strongly was associated with reduced risk of overweight and obesity in boys and girls.49 The direct association between skipping breakfast and increased risk of overweight and obesity is well-established especially in children and adolescents as a recently published systematic review and meta-analysis papers confirmed previous findings.50,51
On the other hand, the results of the present study suggested a novel inverse association between dinner consumption and abdominal obesity as well as low level of HDL-C. One important mechanism is related to energy balance according to the relationship between energy intake and energy expenditure. The previous study has indicated that regular meals consumption could increase energy expenditure and contribute to reducing weight.52 The inverse association between consuming 5 meals per day and abdominal obesity were reported only in boys.49 Also, a study in 13 303 twenty years old individuals revealed that people who eat dinner alone are more likely to be obese than individuals who eat dinner with family.53 However, reduced risk for abdominal obesity and abnormal level of HDL-C might attribute to the other healthy lifestyles. It should be considered that although dinner consumer subjects reported more hours of physical activity and fewer hours working with a computer, they spent more hours sleeping and watching TV. In addition, OR for mentioned risk factors after adjusting potential confounders like physical activity remained significant.
In spite of a higher risk of being underweight in dinner eater, it cannot be deduced that dinner consumption is associated with underweight as the association is disappeared after adjusting some confounders. Previous studies mostly focused on overweight as CVD risk factors and the underweight-meal consumption relationship has not been fully examined. A recent study among 1-7 years old children suggested skipping breakfast was associated with underweight.54
This study failed to reach to a significant association between dinner consumption and other CVD risk factors i.e. hypertension, TC, LDL-C, TG, and FPG levels. Likewise, a population-based survey could not find any association between eating 5 meals daily and other risk factors after adjustment for potential confounders except for overweight and abdominal obesity.49 A recent data revealed a negative association between frequency of eating breakfast and blood glucose, TG and HDL-C levels in obese children and adolescents even after adjusting some confounders.55 In contrast, another study which was conducted in adults reported that eating breakfast regularly was associated with evaluated serum TG.56
Considering the number of CVD risk factors identified in adolescents according to dinner eating led to interesting results. In the present study, a higher percentage of dinner eater subjects had no or 1 risk factors and fewer suffered from 2 or more risk factors compared to adolescents’ skipped dinner.
Although, we considered the frequency of eating dinner, quantity, and quality of dinner also play an important role in developing CVD risk factors as suggested in the recent papers (in 2013). One study among overweight women diagnosed as having metabolic syndrome which compared 2 weight loss isocaloric diet, i.e. high caloric intake in breakfast (700 kcal breakfast, 500 kcal lunch, 200 kcal dinner) versus dinner (200 kcal breakfast, 500 kcal lunch, 700 kcal dinner) for 12 weeks revealed that participants who received high-calorie breakfast had a greater amount of weight loss and reduced waist circumference and lower levels for fasting glucose and triglyceride in comparison to high-calorie dinner consumers.57 Examining the effects of eating carbohydrates and proteins mostly at lunch or dinner among overweight/obese men suggested that eating carbohydrates mostly at lunch and protein mostly at dinner had an unfavorable impact on glucose homeostasis.58
Besides the short-term effects of meal consuming on weight loss, a recent report demonstrated that regular meal frequency could even modify the effect of common genetic variants on BMI in adolescents.59 Previous studies proved that more frequent meals pattern has favorable effects on CVD risk factors which attributed to reduced concentrations of serum insulin.60,61 Therefore, we assumed that eating dinner regularly has similar results through the same mechanism.
Some limitations should be considered regarding this study. The first one may be noted is the cross-sectional nature of this study which could not lead to inferring a causal relationship. Due to incomplete data on some dietary factors, adjusted models for dietary variables could not be used to extract the full effects of dinner frequency on CVD risk factors. Converting dinner frequency to a binary variable might leads to probable misclassification. Maybe using an ordinal variable and comparing the highest versus lowest might result in more significant associations.
A representative sample of the Iranian population and large sample size as well as controlling some potential confounders could be addressed as strength points for this study.
In conclusion, eating dinner is inversely associated with some CVD risk factors among Iranian adolescents. Therefore, more emphasis should be given to dinner consumption of adolescents. However, because of limited investigations on this issue, further studies with the prospective design are needed to confirm our finding.
Ethical approval
The study was approved by Research and Ethics Committee of Isfahan University of Medical Sciences; oral assent and written informed consent were obtained from students and their parents, respectively.
Competing interests
All authors declare no competing financial interests exist.
Funding
This study was conducted as part of a national surveillance program, supported by the Ministry of Health.
Acknowledgements
The authors are thankful for all individuals which participated in this study.
Please cite this article as: Azadbakht L, Akbari F, Qorbani M, Motlagh ME, Ardalan G, Heshmat R, Daneshzad E, Kelishadi R. Dinner consumption and cardiovascular disease risk factors among a nationally representative sample of Iranian adolescents: the CASPIANIII Study. J Cardiovasc Thorac Res 2019;11(2):138-146. doi: 10.15171/jcvtr.2019.24.
References
- 1. The top 10 causes of death. World Health Organization. http://who.int/mediacentre/factsheets/fs310/en/index.html. Updated: July 2013.
- 2. Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries; Fuster V KB, editors. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington (DC): National Academies Press (US); 2010. [PubMed]
- 3.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff. 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM. et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 6.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA. et al. Forecasting the future of stroke in the United States: a policy statement from the american heart association and american stroke association. Stroke. 2013;44:2361–75. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini-Esfahani F, Mousavi Nasl Khameneh A, Mirmiran P, Ghanbarian A, Azizi F. Trends in risk factors for cardiovascular disease among Iranian adolescents: the Tehran Lipid and Glucose Study, 1999-2008. J Epidemiol. 2011;21(5):319–28. doi: 10.2188/jea.JE20100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N. et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 10.Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY. et al. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med. 1993;328:313–8. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]
- 11.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–8. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 12.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 13.Capita R, Alonso-Calleja C. Intake of nutrients associated with an increased risk of cardiovascular disease in a Spanish population. Int J Food Sci Nutr. 2003;54:57–75. doi: 10.1080/096374803/000062001. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L. et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282:1539–46. doi: 10.1001/jama.282.16.1539. [DOI] [PubMed] [Google Scholar]
- 15.Stampfer MJ, Hu FB. et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 16.McCarron DA, Oparil S, Chait A, Haynes RB, Kris-Etherton P, Stern JS. et al. Nutritional management of cardiovascular risk factors A randomized clinical trial. Arch Intern Med. 1997;157:169–77. [PubMed] [Google Scholar]
- 17.Rodríguez-Morán M, Guerrero-Romero F, Rascón-Pacheco RA. Dietary factors related to the increase of cardiovascular risk factors in traditional Tepehuanos communities from Mexico A 10 year follow-up study. Nutr Metab Cardiovasc Dis. 2009;19:409–16. doi: 10.1016/j.numecd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Yee KE, Eisenmann JC, Carlson JJ, Pfeiffer KA. Association between the family nutrition and physical activity screening tool and cardiovascular disease risk factors in 10-year old children. Int J Pediatr Obes. 2011;6:314–20. doi: 10.3109/17477166.2011.590198. [DOI] [PubMed] [Google Scholar]
- 19.Petridou E, Malamou H, Doxiadis S, Pantelakis S, Kanellopoulou G. et al. Blood lipids in Greek adolescents and their relation to diet, obesity, and socioeconomic factors. Ann Epidemiol. 1995;5:286–91. doi: 10.1016/1047-2797(94)00094-a. [DOI] [PubMed] [Google Scholar]
- 20.Truthmann J, Richter A, Thiele S, Drescher L, Roosen J, Mensink GB. Associations of dietary indices with biomarkers of dietary exposure and cardiovascular status among adolescents in Germany. Nutr Metab. 2012;9:92. doi: 10.1186/1743-7075-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahota P, Rudolf MC, Dixey R, Hill AJ, Barth JH, Cade J. Evaluation of implementation and effect of primary school based intervention to reduce risk factors for obesity. BMJ. 2001;323:1027–9. doi: 10.1136/bmj.323.7320.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puska P, Tuomilehto J, Nissinen A, Salonen J. Ten years of the North Karelia project. Acta Med Scand Suppl. 1985;701:66–71. doi: 10.1111/j.0954-6820.1985.tb08891.x. [DOI] [PubMed] [Google Scholar]
- 23.White K, Jacques PH. Combined diet and exercise intervention in the workplace: effect on cardiovascular disease risk factors. AAOHN J. 2007;55:109–14. doi: 10.1177/216507990705500303. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113 Suppl 9B:13S–24S. doi: 10.1016/s0002-9343(01)00987-1. [DOI] [PubMed] [Google Scholar]
- 25.Beilin LJ. Dietary salt and risk factors for cardiovascular disease. Kidney Int Suppl. 1992;37:S90–6. [PubMed] [Google Scholar]
- 26.Lopez EP, Rice C, Weddle DO, Rahill GJ. The relationship among cardiovascular risk factors, diet patterns, alcohol consumption, and ethnicity among women aged 50 years and older. J Am Diet Assoc. 2008;108:248–56. doi: 10.1016/j.jada.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kastorini CM, Milionis HJ, Georgousopoulou E, Kostapanos MS, Yannakoulia M, Nikolaou V. et al. Modelling eating practices in non-fatal acute coronary syndrome or stroke development: a case/case-control study. Nutr Metab Cardiovasc Dis. 2013;23:242–9. doi: 10.1016/j.numecd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Ko GT, Chan JC, Tong SD, Chan AW, Wong PT, Hui SS. et al. Associations between dietary habits and risk factors for cardiovascular diseases in a Hong Kong Chinese working population--the “Better Health for Better Hong Kong” (BHBHK) health promotion campaign. Asia Pac J Clin Nutr. 2007;16:757–65. [PubMed] [Google Scholar]
- 29.Smith KJ, Gall SL, McNaughton SA, Blizzard L, Dwyer T, Venn AJ. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am J Clin Nutr. 2010;92:1316–25. doi: 10.3945/ajcn.2010.30101. [DOI] [PubMed] [Google Scholar]
- 30.Toschke AM, Küchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13:1932–8. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- 31.Smith KJ, Blizzard L, McNaughton SA, Gall SL, Dwyer T, Venn AJ. Daily eating frequency and cardiometabolic risk factors in young Australian adults: cross-sectional analyses. Br J Nutr. 2012;108:1086–94. doi: 10.1017/S0007114511006398. [DOI] [PubMed] [Google Scholar]
- 32.Frank GC, Berenson GS, Webber LS. Dietary studies and the relationship of diet to cardiovascular disease risk factors variables in 10-year-old children--The Bogalusa Heart Study. Am J Clin Nutr. 1978;31:328–40. doi: 10.1093/ajcn/31.2.328. [DOI] [PubMed] [Google Scholar]
- 33.Woodruff SJ, Hanning RM. Associations between family dinner frequency and specific food behaviors among grade six, seven, and eight students from Ontario and Nova Scotia. J Adolesc Health. 2009;44:431–6. doi: 10.1016/j.jadohealth.2008.10.141. [DOI] [PubMed] [Google Scholar]
- 34.Yannakoulia M, Ntalla I, Papoutsakis C, Farmaki AE, Dedoussis GV. Consumption of vegetables, cooked meals, and eating dinner is negatively associated with overweight status in children. J Pediatr. 2010;157:815–20. doi: 10.1016/j.jpeds.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 35.Taveras EM, Rifas-Shiman SL, Berkey CS, Rockett HR, Field AE. et al. Family dinner and adolescent overweight. Obes Res. 2005;13:900–6. doi: 10.1038/oby.2005.104. [DOI] [PubMed] [Google Scholar]
- 36.Gillman MW, Rifas-Shiman SL, Frazier AL, Rockett HR, Camargo CA Jr, Field AE. et al. Family dinner and diet quality among older children and adolescents. Arch Fam Med. 2000;9:235–40. doi: 10.1001/archfami.9.3.235. [DOI] [PubMed] [Google Scholar]
- 37.Haines J1, Gillman MW, Rifas-Shiman S, Field AE, Austin SB. Family dinner and disordered eating behaviors in a large cohort of adolescents. Eat Disord. 2010;18:10–24. doi: 10.1080/10640260903439516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khashayar P, Heshmat R, Qorbani M, Motlagh ME, Aminaee T, Ardalan G. et al. Metabolic syndrome and cardiovascular risk factors in a national sample of adolescent population in the Middle East and North Africa: the CASPIAN III study. Int J Endocrinol. 2013;2013:702095. doi: 10.1155/2013/702095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelishadi R, Hovsepian S, Djalalinia S, Jamshidi F, Qorbani M. A systematic review on the prevalence of metabolic syndrome in Iranian children and adolescents. J Res Med Sci. 2016;21:90. doi: 10.4103/1735-1995.192506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taheri F, Chahkandi T, Kazemi T, Bijari B, Zardast M, Namakin K. Lipid Profiles and Prevalence of Dyslipidemia in Eastern Iranian Adolescents, Birjand, 2012. Iran J Med Sci. 2015;40(4):341–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Kelishadi R, Heshmat R, Motlagh ME, Majdzadeh R, Keramatian K, Qorbani M. et al. Methodology and Early Findings of the Third Survey of CASPIAN Study: A National School-based Surveillance of Students’ High Risk Behaviors. Int J Prev Med. 2012;3:394–401. [PMC free article] [PubMed] [Google Scholar]
- 42. World Health Organization, Expert Committee on Physical Status. Physical Status: the Use and Interpretation of Anthropometry, vol. 420 of WHO Technical Report 854, Geneva, Switzerland: World Health Organization; 1995. [PubMed]
- 43.Knowles KM, Paiva LL, Sanchez SE, Revilla L, Lopez T, Yasuda MB. et al. Waist circumference, body mass index, and other measures of adiposity in predicting cardiovascular disease risk factors among peruvian adults. Int J Hypertens. 2011:1–10. doi: 10.4061/2011/931402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A. et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN Study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caro DH, Cortés D. Measuring family socioeconomic status: An illustration using data from PIRLS 2006 IERI Monograph Series Issues and Methodologies in Large-Scale Assessments. 2012;5:9–33. [Google Scholar]
- 47.Kelishadi R, Noori A, Qorbani M, Rahimzadeh S, Djalalinia S, Shafiee G. et al. Are active and passive smoking associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Paediatr Int Child Health. 2016;36(3):181–8. doi: 10.1179/2046905515Y.0000000039. [DOI] [PubMed] [Google Scholar]
- 48.Okada C, Imano H, Muraki I, Yamada K, Iso H. The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J Obes. 2019;3:2439571. doi: 10.1155/2019/2439571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jääskeläinen A, Schwab U, Kolehmainen M, Pirkola J, Järvelin MR, Laitinen J. Associations of meal frequency and breakfast with obesity and metabolic syndrome traits in adolescents of Northern Finland Birth Cohort. Nutr Metab Cardiovasc Dis. 2013;23(10):1002–9. doi: 10.1016/j.numecd.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Horikawa C, Kodama S, Yachi Y, Heianza Y, Hirasawa R, Ibe Y. et al. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: A meta-analysis. Prev Med. 2011;53:260–7. doi: 10.1016/j.ypmed.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 51.Szajewska H, Ruszczynski M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit Rev Food Sci Nutr. 2010;50:113–9. doi: 10.1080/10408390903467514. [DOI] [PubMed] [Google Scholar]
- 52.Farshchi HR, Taylor MA, Macdonald IA. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr. 2005;81:16–24. doi: 10.1093/ajcn/81.1.16. [DOI] [PubMed] [Google Scholar]
- 53.Rah W, So J, Park EC, Lee SA, Jang SI. Association between family dinner and BMI in adults: data from the 2013 to 2015 Korean National Health and Nutrition Examination Survey. Public Health Nutr. 2018 Oct;30:1–8. doi: 10.1017/S1368980018002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Luo M, Wang Z, Luo J, Shi Y. [Relationship between dietary behaviors and growth-development of 1-7 years old children from seven provinces in Chinese rural areas] Wei Sheng Yan Jiu. 2013;42:375–80. [PubMed] [Google Scholar]
- 55.Freitas Júnior IF, Christofaro DG, Codogno JS, Monteiro PA, Silveira LS, Fernandes RA. The association between skipping breakfast and biochemical variables in sedentary obese children and adolescents. J Pediatr. 2012;161:871–4. doi: 10.1016/j.jpeds.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 56.Min C, Noh H, Kang YS, Sim HJ, Baik HW. et al. Skipping breakfast is associated with diet quality and metabolic syndrome risk factors of adults. Nutr Res Pract. 2011;5:455–63. doi: 10.4162/nrp.2011.5.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21(12):2504–12. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 58.Alves RD, de Oliveira FC, Hermsdorff HH, Abete I, Zulet MA, Martínez JA. et al. Eating carbohydrate mostly at lunch and protein mostly at dinner within a covert hypocaloric diet influences morning glucose homeostasis in overweight/obese men. Eur J Nutr. 2014;53(1):49–60. doi: 10.1007/s00394-013-0497-7. [DOI] [PubMed] [Google Scholar]
- 59.Jääskeläinen A, Schwab U, Kolehmainen M, Kaakinen M, Savolainen MJ, Froguel P. et al. Meal frequencies modify the effect of common genetic variants on body mass index in adolescents of the northern Finland birth cohort 1986. PloS One. 2013;8:e73802. doi: 10.1371/journal.pone.0073802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, Rao AV. et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–34. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 61.Rashidi MR, Mahboob S, Sattarivand R. Effects of nibbling and gorging on lipid profiles, blood glucose and insulin levels in healthy subjects. Saudi Med J. 2003;24:945–8. [PubMed] [Google Scholar]


