Abstract
Knowledge on haemostatic changes in humans infected with Ebola virus is limited due to safety concerns and access to patient samples. Ethical approval was obtained to collect plasma samples from patients in Sierra Leone infected with Ebola virus over time and samples were analysed for clotting time, fibrinogen, and D-dimer levels. Plasma from healthy volunteers was also collected by two methods to determine effect of centrifugation on test results as blood collected in Sierra Leone was not centrifuged. Collecting plasma without centrifugation only affected D-dimer values. Patients with Ebola virus disease had higher PT and APTT and D-dimer values than healthy humans with plasma collected in the same manner. Fibrinogen levels in patients with Ebola virus disease were normal or lower than values measured in healthy people. Clotting times and D-dimer levels were elevated during infection with Ebola virus but return to normal over time in patients that survived and therefore could be considered prognostic. Informative data can be obtained from plasma collected without centrifugation which could improve patient monitoring in hazardous environments.
Keywords: Ebola virus, haemostasis, clotting, D-dimers, fibrinogen, PT, APTT
1. Introduction
Ebola virus disease (EVD) is an ongoing public health issue with a current outbreak in the Democratic Republic of the Congo causing concern. The largest outbreak of EVD to date occurred in West Africa between 2013 and 2016 resulting in over 11,000 deaths [1]. Due to the size of that outbreak and the international response, much knowledge was gained on the diagnostics, transmission, and clinical presentation of EVD in humans and there were also multiple efforts to trial medical interventions. Increased understanding of the pathology of EVD in humans can lead to improved options for therapy or patient management to improve patient outcome. Despite the wealth of knowledge gathered, there remains less information on the coagulopathy in humans infected with Ebola virus. Haemostatic monitoring was a low priority for laboratories that handled samples from Ebola virus-infected patients, although the CDC did state that laboratories handling suspected cases of EVD should be able to test the prothrombin time (PT) as one of a battery of tests to aid in the determination of EVD [2]. Data from haemostatic tests are not reported in several papers describing clinical presentation of EVD in Sierra Leone [3,4], showing the paucity of information about this particular clinical aspect of EVD.
EVD begins with an influenza-like syndrome characterised by fever, malaise, and joint pains following an incubation period of 3–21 days. The early descriptions of EVD mentioned that mild haemorrhage may present as a maculopapular rash and/or petechiae or bleeding from mucous membranes as well as oozing from venepuncture sites [5,6,7]. Although the most common signs of Ebola virus disease during the West Africa outbreak were fever, headache, diarrhoea, and vomiting, fatigue, weakness and dizziness which are not associated with coagulopathy, haemorrhage of some kind was a common complication [3,8].
The aim of this study was to characterise the basic coagulation changes in a group of people infected with Ebola virus during the West Africa Ebola virus outbreak. Four key haemostatic parameters were measured—Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) and fibrinogen and D-dimer levels. As the blood collected in Sierra Leone was not centrifuged to obtain plasma (due to safety reasons), we also collected blood from healthy donors in the UK to control for sample processing and to compare collection methods and determine if centrifugation affected PT, APTT, D-dimer or fibrinogen results.
2. Materials and Methods
2.1. Patients
Adult patients with PCR-confirmed Ebola virus infection were enrolled at the Kerry Town Ebola Treatment Facility in Sierra Leone between February–June 2015 after a research proposal was written, reviewed and given a favourable opinion by Ministry of Defence Research Ethics Committee (MoDREC number 633/MODREC/15). Children under the age of 18, pregnant women, prisoners, patients not undergoing routine blood sampling and patients with malaria were excluded from selection. Blood was only taken from patients who were already having blood drawn for diagnostics; additional blood sampling for the sole purpose of this study was not performed to minimize the risk from additional venepuncture. Patient information sheets were provided to all patients and were available in English, Krio, and Mende. Consent was obtained from patients themselves (if they scored 15/15 on the Glasgow Coma Scale, i.e., eyes open spontaneously, normal verbal and motor responses) or their next of kin or a medical worker as a final option, and in the presence of a witness. The original application for MoDREC approval was for up to 50 patients.
2.2. Blood Sampling and Processing
After routine blood sampling (typically into EDTA tubes), a further 9 mL of whole blood was drawn from participating patients into two 4.5 mL sodium citrate (1.02 M) vacutainer tubes (Becton Dickinson/BD, Wokingham UK). Following a minimum of 3 h storage at 4 °C with samples upright allowing blood to separate naturally without centrifugation (due to the high risk of aerosolisation), 500 µL aliquots of plasma were collected and stored at −20 °C in Sierra Leone. Samples were sent to Dstl, Porton Down, UK for storage at −80 °C prior to analysis.
In addition to the plasma samples collected for this study, upon admission and at various days post-admission, blood chemistries, complete blood cell counts and other blood components were assayed in the onsite laboratory using the Piccolo Express system (Abaxis, CA, USA), Horiba ABX Micros ES60 analyser (Horiba, Montpellier, France) and Hemochron Signature Elite (Accriva Diagnostics, CA, USA). A spreadsheet table of results from this testing was provided. Reverse-transcriptase-PCR (RT-PCR) assays for diagnosis of Ebola virus disease were done with the Altona RealStar Filovirus RT-PCR Kit (Altona, Hamburg, Germany). This PCR targets a conserved region in the L gene.
For establishing a ‘normal’ range for comparison of collection methods, anonymized healthy human donor samples at Dstl Porton Down, UK were collected from adult volunteers (aged 18 and above) under the 2004 Human Tissue Act. Samples were collected as in Sierra Leone and described above or were centrifuged for 15 min at room temperature at 2000 rpm in the AccuSpin 1 centrifuge (Fisher Scientific, Loughborough, UK) as per normal practice in the UK. Samples were stored at −80 °C prior to analysis. Some donors had plasma collected by centrifugation only, some by separation only, and a subset by both methods when the volume of blood to be taken was increased.
2.3. Plasma Analysis
Each sample was analysed for PT, APTT, and Clauss fibrinogen and D-dimer levels. Analysis of samples was performed on the CA-660 automated coagulation analyser (Sysmex, Milton Keynes, UK) held within a rigid half-suit isolator housed in a Containment Level 4 laboratory at Dstl Porton Down. All reagents were produced by DADE/SIEMENS (Marburg, Germany) and used as per manufacturer’s guidelines. Samples were tested in duplicate.
2.4. Statistical Analysis
Collection methods of healthy human plasma from UK donors were compared using an unpaired t-test (GraphPad Prism V6). Mean and standard deviation for the un-centrifuged data set was determined. The mean and 3× standard deviation were then used to create a point below which >99% of normally distributed data was expected to fall. Due to the small number of patients enrolled in Sierra Leone and the variation in days of sampling and unknown day of infection, traditional statistical analysis on the data from the Ebola virus-infected patients was not appropriate and results are better shown graphically as a visual comparison between the EVD patients and the range seen in uninfected individuals.
3. Results
3.1. Effect of Centrifugation on Test Results
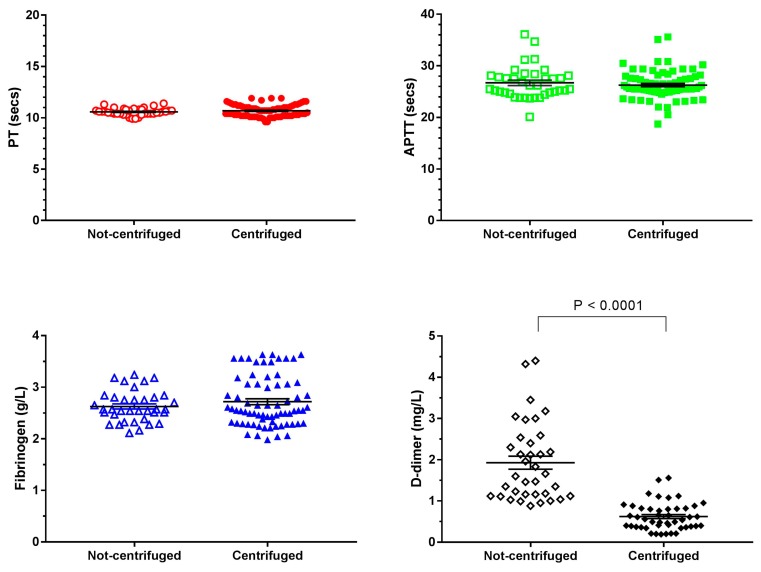
Sample collection methods were compared using naïve human plasma collected from healthy UK volunteers either with or without centrifugation (Table 1 and Figure 1). Data appeared normally distributed. For the PT, APTT, and fibrinogen tests the mean, median and range of values from non-centrifuged samples was similar to the values for centrifuged samples. The mean and median values and range were also similar to the Laboratory Reference Values available online or in [9] which reports a PT range of 11.1–13.1 s and the Fibrinogen range of 1.5–4.0 g/L [9] (no reference value for APTT given in [9] but various online sources gives a range of 25–40 s. D-dimer values showed the most variation with collection method (Figure 1 and Table 1). Un-centrifuged samples gave a wider range of values and an approximately three times higher median and mean than the centrifuged samples. The reference values for adults for D-dimers is < 0.5 mg/L [9]. An unpaired t-test showed there was no significant difference in test results for PT, APTT, and fibrinogen when collection methods were compared but the collection method did cause a significant difference for D-dimer test results (p < 0.0001). The non-centrifuged values were used to create a ‘normal’ range for comparison with the Ebola virus-infected plasma samples which were collected without centrifugation. For normally distributed data, 99.7% of data falls within 3 standard deviations of the mean.
Table 1.
Select parameters for different tests and collection methods.
| PT (sec) | APTT (sec) | Fibrinogen (g/L) | D-Dimers (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Centrifuged | Not centrifuged | Centrifuged | Not centrifuged | Centrifuged | Not centrifuged | Centrifuged | Not centrifuged | |
| Mean (SD) | 11.0 (0.57) | 11.0 (0.35) | 26 (2.7) | 26 (3.1) | 2.7 (0.49) | 2.7 (0.30) | 0.62 (0.34) | 1.9 (0.95) |
| Median | 11.0 | 10.0 | 26 | 25 | 2.6 | 2.7 | 0.55 | 1.6 |
| Range | 11.0–13.0 | 9.6–12.0 | 19–36 | 20–36 | 2.3–3.9 | 2.0–3.6 | 0.9–1.6 | 0.9–4.4 |
SD = Standard Deviation.
Figure 1.
Effect of collection method on four haemostatic parameters. Naïve blood samples were collected from healthy UK volunteers and analysed for Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT), fibrinogen, and D-dimer amounts. Plasma was obtained by centrifugation (standard method) or by non-centrifugation where blood was left to separate over time. Non-centrifuged plasma was collected for comparison with Ebola virus-infected plasma samples collected without centrifugation. Each test was run in duplicate and both values are shown. For some centrifuged samples, multiple aliquots from the same donor were analysed on different occasions, each individual result is also shown. Mean and SEM (coloured lines and bars) are shown for each data set. Unpaired t-tests were performed to compare the results from the two collection methods.
3.2. Changes in Haemostatic Parameters in Patients Infected with Ebola Virus
Samples from five people infected with Ebola virus were acquired at Kerry Town, Sierra Leone (Table 2) over a 5-month period. The data set included 3 males and 2 females, 3 Sierra Leoneans, one person from the United States and one person from Western Europe (data kept vague to preserve anonymity). PT, APTT, fibrinogen, and D-dimer testing was performed on available time-points (Figure 2). As patients were admitted at different times post-infection, sampling time was measured in days after reported onset of symptoms.
Table 2.
Details of Patients and samples available for this study.
| Patient ID | Gender | Age (Years) | Nationality | No. Days Between Symptoms and Sampling | Samples Available (Days Post Symptom Onset) 1 | Diagnostic Ebola Virus PCR Ct Value at Admission | Outcome | Other Comments 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 48 | Sierra Leonean | 7 | Days 7–9 | 18.3 | Died | HIV+, blood culture positive for E. coli. Given 4 × FFP day 8 and 2 × FFP, cryoprecipitate and pRBC day 9. Oozing from CVC day 9 |
| 2 | M | 38 | Sierra Leonean | 7 | Days 7–10 | 32.4 | Survived | |
| 3 | M | 34 | European/American | 1 | Days 1 & 2 | 24.4 | Survived | |
| 4 | F | 26 | European/American | 3 | Days 3 & 4 | 24.1 | Survived | |
| 5 | M | 32 | Sierra Leonean | 4 | Days 4, 6–8 | Not available | Survived | Oozing from CVC day 6 and 7 |
1 Do not correlate to days post-infection 2 FFP: Fresh frozen plasma, pRBC: packed red blood cells, CVC: central venous catheter.
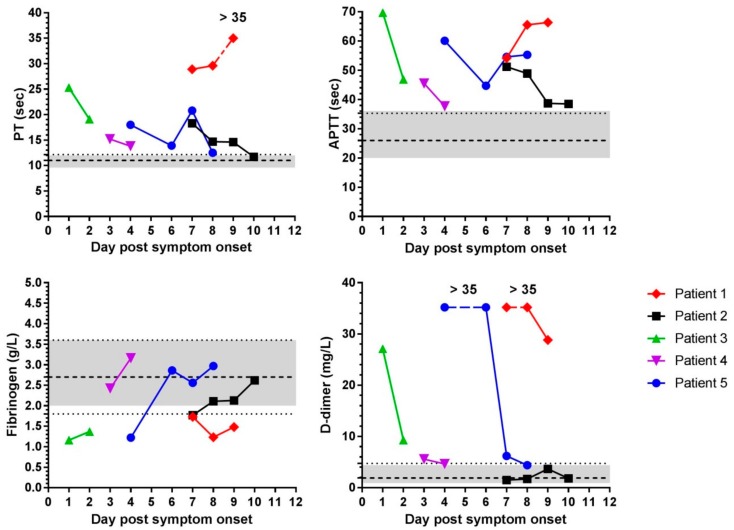
Figure 2.
Changes in haemostasis parameters over time in Ebola virus-infected individuals. Mean values of Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) (top row) and fibrinogen, and D-Dimer values (bottom row) from plasma samples collected without centrifugation from five patients are shown. Each time-point was tested in at least duplicate and mean plotted. Each patient is represented by a different colour and symbol and days of sampling post-symptom onset were different for each patient. Grey shaded area indicates the range of values for each test obtained from naïve ‘normal’ human donor samples also collected without centrifugation (Figure 1). Dashed line is the mean value from the un-centrifuged non-infected samples and the dotted line is the value of the un-centrifuged mean + 3 standard deviations (for fibrinogen—3 standard deviations also shown with a second dotted line). If data distribution is approximately normal, then 99.7 % lies within three standard deviations. One PT result and several D-dimer results were out of range and are plotted at the upper limit of the test and indicated as such for each data set.
Patient 1, the only patient in this study that succumbed to infection, was admitted with developed EVD as indicated by the low Ebola virus PCR Cycle threshold (Ct) value at admission (18.3) suggesting a high viral titre in the blood. (The Ebola virus PCR Ct value is an indicator of prognosis—see [10]). Patient 1 had comorbidities and some blood management was tried (Table 2) which may have affected results. Patient 1’s PT and APTT times were prolonged at around double the mean clotting time obtained in UK donors. For Patient 1, fibrinogen was low and outside the normal range and D-dimer levels were increased on all days (Figure 2). Patient 2 likely presented with less severe EVD as suggested by the higher Ct value of 32.4 at admission. For Patient 2, PT and APTT were prolonged but fibrinogen levels were within the range seen in healthy UK donors. Over time, PT and APTT results returned to normal values seen in non-infected un-centrifuged samples. D-dimer values of Patient 2 were within the range seen in non-infected people (Figure 2). Patients 3 and 4 had samples taken early after symptom onset. Patients 3 and 4 had prolonged PT and APTT, but the values decreased on the second day. Patient 3 had reduced fibrinogen and increased D-dimer amounts which improved on day 2. Patient 4 had fibrinogen levels within, and D-dimer levels just above, the range seen in non-infected individuals (Figure 2). Patient 5 initially had prolonged PT and APTT, reduced fibrinogen and increased D-dimer values. APTT remained elevated throughout and D-dimer levels were high initially and then dropped to near the normal range (Figure 2).
4. Discussion
The purpose of this study was to look at haemostatic changes in EVD patients where currently there is little information available from human cases. Ethical approval was obtained to collect additional blood from EVD patients to obtain plasma to use in this study. By the time ethical approval was in place (February 2015), the number of EVD cases admitted to Kerry Town Ebola Treatment Centre was low. The ethical approval was for up to 50 patients, but in 5 months only 5 patients were enrolled and there were two months where no patients were enrolled. The Kerry Town laboratory was decommissioned in November 2015 after 13 months in operation. From the five patients enrolled in the study we were able to observe that, in general, EVD patients had longer clotting times and higher D-dimer values than plasma processed in the same manner collected from healthy volunteers. In addition, EVD patients had lower or normal fibrinogen levels. The results of high clotting times and D-dimer values indicate consumption of clotting factors and an increased breakdown of clots.
The low numbers of patients in this study make it difficult to draw strong conclusions however, for patients that recovered from Ebola virus infection, plasma analysis showed haemostatic parameters were returning towards normal over time (within 2–3 days) whereas for the one patient who succumbed, all parameters stayed high (or low for fibrinogen). Measurement of these clotting factors may, therefore, be another prognostic marker for survival, but a larger data set would be needed to confirm this.
Many factors can influence coagulation results [11] such as storage duration and temperature [12,13] and centrifugation parameters [14] but centrifugation is typically performed. For safety reasons, blood was not centrifuged in Sierra Leone. To determine whether this had an effect on results, blood from healthy donors was collected by both centrifugation (standard) and non-centrifugation. Ideally, blood would have been collected from healthy individuals in Sierra Leone to obtain values from a genetically similar background to the majority of the EVD samples, but this was not practically possible, and donations were from people living in the UK. There was no significant difference for the PT, APTT and fibrinogen results from the two collection methods. There was a significant difference in D-dimer values between the two collection methods possibly due to the extended time that blood had to separate. The effect of long-term storage will also be investigated using separate aliquots of naïve samples tested over a period of years.
Despite differences in sample collection and the small number of patients available for testing, the results we generated do show observable differences in coagulation in humans with EVD compared to the normal range of values from uninfected individuals. For PT and APTT all values from EVD patients over time were outside the range seen in un-centrifuged non-infected people. For D-dimers, four of the five EVD patients had values higher than the normal range (much higher in three of those patients). Fibrinogen testing showed the least change in patients with EVD compared to the non-infected donors with plasma collected in the same manner. Only two EVD patients had values below the normal range. The difference in EVD patients may be due to the presence of Ebola virus, or could be an indication of non-specific infection, sepsis or multi-organ failure. The other parameters measured during hospitalization for Patient 1 suggested liver and kidney damage (e.g., elevated liver enzymes and urea) and an inflammatory response (elevated CRP) which may not have been solely due to EVD (data not shown). Race has been shown to influence fibrinogen [15] and D-dimer levels [16] and our patient data set was made up of 3 black people and 2 white people. Fibrinogen levels are reported to be generally lower in black people (by up to 0.3 g/L [15]) but there were minimal differences in fibrinogen levels in patients with EVD anyway. D-dimer levels are slightly higher in black people but in healthy individuals, they are still below 6 mg/L [16] whereas in four of the five EVD patients, D-dimer values were >5 mg/L on at least one day of testing.
Haemostatic changes of prolonged clotting times, low fibrinogen and high D-dimers observed during EVD match the criteria for disseminated intravascular coagulation (DIC) [17], sepsis or organ failure. DIC has been observed in other haemorrhagic fever diseases [18]. Liver disease can present like DIC [17] and there is evidence of liver failure during EVD in humans and experimental animals. The array of clinical signs and pathologies that have been reported in humans and experimental animals infected with Ebola virus results in a complex presentation involving many factors and different pathways which ultimately may affect the disease outcome. This makes management or treatment best done on a case-by-case basis with active interventions as early as possible. Management strategies for DIC are based on blood component therapy (not whole blood transfusions) and could include replacement of platelets and coagulation factors, in fresh frozen plasma (FFP), and fibrinogen, in cryoprecipitate, to control severe bleeding [17,19]. Sepsis and organ failure are complex to treat and there is no one solution for these conditions; the many different cascades and pathways involved have to try to be corrected.
There is limited existing data from humans in this area. D-dimer levels were shown to be elevated in patients infected with the related species of Sudan virus, particularly in those that succumbed to infection [20]. For Sudan virus, D-dimer levels in fatal cases were all greater than 50,000 ng/mL (sometimes greater than 150,000 ng/mL) whilst in survivors, numbers were still elevated over the normal value of 400 ng/mL but were generally less than 50,000 ng/mL [20]. The values in our study were mostly over 5 mg/L (5000 ng/mL). Observations during the original 1976 Ebola virus outbreak included elevated PTT levels and presence of fibrin-degradation products [21]. An EVD patient treated in South Africa in 1996 had “abnormalities in activated partial thromboplastin time, D-dimers, and fibrinogen degradation products” but no actual values are given [22]. During the West Africa outbreak, haemostatic measurements were taken from some patients medically evacuated from West Africa. Elevated PT, APTT, and D-dimer were measured in patients after transportation to the US [23] or UK [24]. These studies recorded elevated PT values of >16 s, APTT values of >60 s and D-dimer levels of >1200 ng/mL during the early days of illness before normalizing. Similar elevations were seen in our data. A study of APTT values in Sierra Leonean patients reported elevation in all cases (mean = 68.4 s), but greater elevation in fatal cases (mean = 84.9 s) compared to survivors (mean = 49.6 s) [25] leading to the hypothesis that coagulation parameters “may have value as a potential prognostic indicator of disease severity” in patients with EVD [23]. Our data indicated a similar prognostic possibility. The mean APTT value from all samples from the 5 EVD patients in this study was 51 s with a range of 37–71 s over time. Haemostatic parameters measured in this study were not part of some of the larger studies of clinical features in EVD patients carried out in Sierra Leone [3,4].
Results in this study are also consistent with observations made in animal models of Ebola virus infection. PT and APTT clotting times and D-dimer levels have been observed to increase as animals succumb to infection with Ebola virus [26,27,28,29,30]. As non-human primates and humans show similar haemostatic changes this helps validate animal models as suitable disease models for human infection and means any treatment options targeting haemostasis could be tested in these models. Two studies have already looked at treating coagulation deficiencies during EVD. Recombinant human activated protein C (rhAPC) has been shown to improve survival in sepsis, which is multi-organ dysfunction often caused by microbial infection and has been tested against EVD in rhesus macaques [31]. The rhAPC treated animals showed an increased time to death compared to control animals and 2 of 11 animals survived [31]. Recombinant nematode anticoagulant protein c2 (rNAPc2), which is an inhibitor of tissue factor-initiated blood coagulation has also been tested against EVD in non-human primates with similar results of low-level survival and prolonged time to death [32]. As mentioned above, patients with EVD may develop DIC, organ failure, sepsis or other complications so treatment is complex. In recent years studies providing intensive care levels of supportive care to non-human primates infected with Ebola virus, including fluid and electrolyte management for virus induced sepsis have been attempted [33] and Maj AP. Cardile, personal communication as we continue to try and find ways to manage this complex disease.
This work increases understanding of the underlying pathology of the coagulation system in EVD patients. Further information should be gathered in the future if human samples become available. We have also shown that the process of centrifugation to obtain plasma affects D-dimer test results but does not affect results for PT, APTT, and fibrinogen which is useful information for scenarios where centrifugation is not safe or possible.
Acknowledgments
The authors would like to thank Paul Russell and Paul Rice for guidance and advice. The authors would also like to acknowledge all the staff and patients at the Kerry Town Ebola Treatment Facility who participated in this study.
Author Contributions
Conceptualization, T.W., B.J.H., S.W. and E.K.; methodology, S.J.S., L.M.O., L.E., K.P.; validation, S.J.S., L.M.O., L.E. and K.P.; formal analysis, S.J.S., L.M.O. and L.E.; investigation, S.J.S., L.M.O. and L.E.; resources, M.L.; data curation, S.J.S. and T.F.; writing—original draft preparation, S.J.S.; writing—review and editing, all authors.; supervision, M.L., B.J.H. and E.K.; funding acquisition and ethical approvals, T.W., M.L., B.J.H., S.W. and E.K.
Funding
This work was funded by the UK Ministry of Defence.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Nyakarahuka L., Kankya C., Krontveit R., Mayer B., Mwiine F.N., Lutwama J., Skjerve E. How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence. BMC Infect. Dis. 2016;16:708. doi: 10.1186/s12879-016-2045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Guidance for U.S. Laboratories for Managing and Testing Routine Clinical Specimens When There Is a Concern about Ebola Virus Disease. [(accessed on 23 April 2019)]; Available online: http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/safe-specimen-management.html.
- 3.Schieffelin J.S., Shaffer J.G., Goba A., Gbakie M., Gire S.K., Colubri A., Sealfon R.S., Kanneh L., Moigboi A., Momoh M., et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N. Engl. J. Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt L., Gupta-Wright A., Simms V., Tamba F., Knott V., Tamba K., Heisenberg-Mansaray S., Tamba E., Sheriff A., Conteh S. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: An observational cohort study. Lancet Infect. Dis. 2015;15:1292–1299. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 5.Isaacson M., Sureau P., Courteille G., Pattyn S.R. Clinical aspects of Ebola virus disease at the Ngaliema hospital, Kinshasa, Zaire, 1976. In: Pattyn S.R., editor. Ebola Virus Haemorrhagic Fever. Elsevier/North-Holland Biomedical Press; New York, NY, USA: 1978. pp. 15–20. [Google Scholar]
- 6.Piot P., Sureau P., Breman J.G., Heymann D.L., Kintoki V., Masamba N., Mbuyi M., Miatudila M., Ruppol J.F., van Nieuwenhove S. Clinical aspects of Ebola virus infection in Yambuku area, Zaire, 1976. In: Pattyn S.R., editor. Ebola Virus Haemorrhagic Fever. Elsevier/North-Holland Biomedical Press; New York, NY, USA: 1978. pp. 7–14. [Google Scholar]
- 7.Smith D.H., Francis F., Simpson D.I.H. African haemorrhagic fever in the southern Sudan, 1976: The clinical manifestations. In: Pattyn S.R., editor. Ebola Virus Haemorrhagic Fever. Elsevier/North-Holland Biomedical Press; New York, NY, USA: 1978. pp. 21–26. [Google Scholar]
- 8.Bah E.I., Lamah M.C., Fletcher T., Jacob S.T., Brett-Major D.M., Sall A.A., Shindo N., Fischer W.A., 2nd, Lamontagne F., Saliou S.M., et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N. Engl. J. Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 9.Kratz A., Ferraro M., Sluss P.M., Lewandrowski K.B. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N. Engl. J. Med. 2004;351:1548–1563. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- 10.Crowe S.J., Maenner M.J., Kuah S., Erickson B.R., Coffee M., Knust B., Klena J., Foday J., Hertz D., Hermans V. Prognostic indicators for Ebola patient survival. Emerg. Infect. Dis. 2016;22:217–223. doi: 10.3201/eid2202.151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnette A., Chatelain M., Chatelain B., Ten Cate H., Mullier F. Pre-analytical issues in the haemostasis laboratory: Guidance for the clinical laboratories. Thromb J. 2016;14:49. doi: 10.1186/s12959-016-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L., Zhao Y., Zhao H., Shao Z. Effects of storage time and temperature on coagulation tests and factors in fresh plasma. Sci. Rep. 2014;4:3868. doi: 10.1038/srep03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zürcher M., Sulzer I., Barizzi G., Lämmle B., Alberio L. Stability of coagulation assays performed in plasma from citrated whole blood transported at ambient temperature. Thromb. Haemost. 2008;99:416–426. doi: 10.1160/TH07-07-0448. [DOI] [PubMed] [Google Scholar]
- 14.Lesche D., Geyer R., Lienhard D., Nakas C.T., Diserens G., Vermathen P., Leichtle A.B. Does centrifugation matter? Centrifugal force and spinning time alter the plasma metabolome. Metabolomics. 2016;12:159. doi: 10.1007/s11306-016-1109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook D.G., Cappuccio F.P., Atkinson R.W., Wicks P.D., Chitolie A., Nakandakare E.R., Sagnella G.A., Humphries S.E. Ethnic differences in fibrinogen levels: The role of environmental factors and the beta-fibrinogen gene. Am. J. Epidemiol. 2001;153:799–806. doi: 10.1093/aje/153.8.799. [DOI] [PubMed] [Google Scholar]
- 16.Pieper C.F., Rao K.M., Currie M.S., Harris T.B., Cohen H.J. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:M649–M657. doi: 10.1093/gerona/55.11.M649. [DOI] [PubMed] [Google Scholar]
- 17.Boral B.M., Williams D.J., Boral L.I. Disseminated intravascular coagulation. Am. J. Clin. Pathol. 2016;146:670–680. doi: 10.1093/ajcp/aqw195. [DOI] [PubMed] [Google Scholar]
- 18.Chen J.P., Cosgriff T.M. Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul. Fibrinolysis. 2000;11:461–483. doi: 10.1097/00001721-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Disseminated Intravascular Coagulation Online MSD Manual, Professional Version. [(accessed on 24 April 2019)]; Available online: https://www.msdmanuals.com/en-gb/professional/hematology-and-oncology/coagulation-disorders/disseminated-intravascular-coagulation-dic.
- 20.Rollin P.E., Bausch D.G., Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J. Infect. Dis. 2007;196:S364–S371. doi: 10.1086/520613. [DOI] [PubMed] [Google Scholar]
- 21.WHO Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- 22.Richards G.A., Murphy S., Jobson R., Mer M., Zinman C., Taylor R., Swanepoel R., Duse A., Sharp G., de la Rey I.C., et al. Unexpected Ebola virus in a tertiary setting: Clinical and epidemiologic aspects. Crit. Care Med. 2000;28:240–244. doi: 10.1097/00003246-200001000-00041. [DOI] [PubMed] [Google Scholar]
- 23.McElroy A.K., Harmon J.R., Flietstra T.D., Campbell S., Mehta A.K., Kraft C.S., Lyon M.G., Varkey J.B., Ribner B.S., Kratochvil C.J. Kinetic analysis of biomarkers in a cohort of US patients with Ebola virus disease. Clin. Infect. Dis. 2016;63:460–467. doi: 10.1093/cid/ciw334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson A.J., Martin D.S., Maddox V., Rattenbury S., Bland D., Bhagani S., Cropley I., Hopkins S., Mepham S., Rodger A. Thromboelastography in the management of coagulopathy associated with Ebola virus disease. Clin. Infect. Dis. 2016;62:610–612. doi: 10.1093/cid/civ977. [DOI] [PubMed] [Google Scholar]
- 25.Kobinger G.P., Pesenti A., Langer M., Kabia S., Brogiato G., Amone J., Castilletti C. Relationship between viremia and specific organ damage in Ebola patients: A cohort study. Clin. Infect. Dis. 2018;66:36–44. doi: 10.1093/cid/cix704. [DOI] [PubMed] [Google Scholar]
- 26.Fisher-Hoch S.P., Platt G.S., Lloyd G., Simpson D.I., Neild G.H., Barrett A.J. Haematological and biochemical monitoring of Ebola infection in rhesus monkeys: Implications for patient management. Lancet. 1983;2:1055–1058. doi: 10.1016/S0140-6736(83)91041-3. [DOI] [PubMed] [Google Scholar]
- 27.Ryabchikova E.I., Kolesnikova L.V., Luchko S.V. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 1999;179:S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- 28.Geisbert T.W., Hensley L.E., Larsen T., Young H.A., Reed D.S., Geisbert J.B., Scott D.P., Kagan E., Jahrling P.B., Davis K.J. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: Evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 2003;63:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed D.S., Lackemeyer M.G., Garza N.L., Sullivan L.J., Nichols D.K. Aerosol exposure to Zaire ebolavirus in three nonhuman primate species: Differences in disease course and clinical pathology. Microbes Infect. 2011;13:930–936. doi: 10.1016/j.micinf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Smither S.J., Nelson M., Eastaugh L., Nunez A., Salguero F.J., Lever M.S. Experimental respiratory infection of marmosets (Callithrix jacchus) with Ebola virus Kikwit. J. Infect. Dis. 2015;212:S336–S345. doi: 10.1093/infdis/jiv371. [DOI] [PubMed] [Google Scholar]
- 31.Hensley L.E., Stevens E.L., Yan S.B., Geisbert J.B., Macias W.L., Larsen T., Daddario-DiCaprio K.M., Cassell G.H., Jahrling P.B., Geisbert T.W. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J. Infect. Dis. 2007;196:S390–S399. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 32.Geisbert T.W., Hensley L.E., Jahrling P.B., Larsen T., Geisbert J.B., Paragas J., Young H.A., Fredeking T.M., Rote W.E., Vlasuk G.P. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: A study in rhesus monkeys. Lancet. 2003;362:1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 33.Poliquin P.G., Biondi M., Ranadheera C., Hagan M., Bello A., Racine T., Allan M., Funk D., Hansen G., Hancock B.J., et al. Delivering prolonged intensive care to a non-human primate: A high fidelity animal model of critical illness. Sci. Rep. 2017;7:1204. doi: 10.1038/s41598-017-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]