Abstract
Background
Atopics have a lower risk for malignancies, and IgE targeted to tumors is superior to IgG in fighting cancer. Whether IgE-mediated innate or adaptive immune surveillance can confer protection against tumors remains unclear.
Objective
We aimed to investigate the effects of active and passive immunotherapy to the tumor-associated antigen HER-2 in three murine models differing in Epsilon-B-cell-receptor expression affecting the levels of expressed IgE.
Methods
We compared the levels of several serum specific anti-HER-2 antibodies (IgE, IgG1, IgG2a, IgG2b, IgA) and the survival rates in low-IgE ΔM1M2 mice lacking the transmembrane/cytoplasmic domain of Epsilon-B-cell-receptors expressing reduced IgE levels, high-IgE KN1 mice expressing chimeric Epsilon-Gamma1-B-cell receptors with 4-6-fold elevated serum IgE levels, and wild type (WT) BALB/c. Prior engrafting mice with D2F2/E2 mammary tumors overexpressing HER-2, mice were vaccinated with HER-2 or vehicle control PBS using the Th2-adjuvant Al(OH)3 (active immunotherapy), or treated with the murine anti-HER-2 IgG1 antibody 4D5 (passive immunotherapy).
Results
Overall, among the three strains of mice, HER-2 vaccination induced significantly higher levels of HER-2 specific IgE and IgG1 in high-IgE KN1, while low-IgE ΔM1M2 mice had higher IgG2a levels. HER-2 vaccination and passive immunotherapy prolonged the survival in tumor-grafted WT and low-IgE ΔM1M2 strains compared with treatment controls; active vaccination provided the highest benefit. Notably, untreated high-IgE KN1 mice displayed the longest survival of all strains, which could not be further extended by active or passive immunotherapy.
Conclusion
Active and passive immunotherapies prolong survival in wild type and low-IgE ΔM1M2 mice engrafted with mammary tumors. High-IgE KN1 mice have an innate survival benefit following tumor challenge.
Keywords: AllergoOncology, Cancer vaccine, IgE, HER-2, Onco-immunology
Abbreviations: ADCC, Antibody-dependent Cell-mediated Cytotoxicity; ADCP, Antibody-dependent Cellular Phagocytosis; BCR, B-Cell Receptor; HER-2, Human Epidermal Growth Factor Receptor-2, ErbB-2; IgA, Immunoglobulin A; IgE, Immunoglobulin E; IgG, Immunoglobulin G; TAA, Tumor-Associated Antigen; WT, wild type
Background
Amongst the clinically-applied monoclonal antibodies targeting tumor-associated antigens (TAA), especially trastuzumab (Herceptin®, Roche) is indispensable in the treatment of metastatic breast cancer and other cancer entities overexpressing the human epidermal growth factor-2, HER-2.1 This humanized IgG1 antibody leads to improved overall response rates and better progression-free as well as overall survival.2
Trastuzumab is a derivative of the mouse monoclonal antibody 4D5, an IgG1 antibody clone targeting human HER-2.3 Because of its favorable binding affinity and tumor growth inhibition, the complementarity-determining regions of 4D5 were inserted into the backbone of a human IgG1 antibody with increased affinity to HER-2 as a positive side-effect.4 All antibodies currently FDA-approved are of the IgG isotype,5 although nature has equipped the human immune system with five different immunoglobulin classes.6
IgE antibodies have gained attention in the past for their potential protective role in cancer.7, 8 IgE antibodies purified from pancreatic cancer patients were capable of mediating antibody-dependent cell-mediated cytotoxicity (ADCC) against pancreatic cancer cells in vitro.9 In vitro, higher levels of ADCC could be triggered by IgE antibodies, compared to their IgG counterparts.10, 11 This points towards a superior function of IgE in ADCC, compared to IgG, which is known to likely induce antibody-dependent cellular phagocytosis (ADCP).12, 13, 14, 15, 16, 17, 18, 19 Large epidemiological studies demonstrated inverse correlations between elevated IgE levels in atopics and malignant diseases, including colorectal, pancreatic, and gynecological cancers as well as gliomas, and childhood leukemia.16, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Moreover, recent epidemiological studies displayed an association of very low IgE levels (termed “IgE-deficient”) with higher rates of malignancies,31 independently of other risk factors, such as concomitant common variable immunodeficiency.32
IgE could thus play a considerable role in natural tumor immune surveillance, and potentially can be exploited in anticancer immunotherapy according to numerous studies, which support the AllergoOncology concept.7, 8, 33, 34, 35, 36, 37, 38
However, it remains unclear whether serum IgE levels and IgE-mediated innate or adaptive immune surveillance can confer protection against tumors,33 or vice versa, low IgE levels are in favor of tumor development.31, 32 Therefore, we investigated the impact of IgE against cancer at low-, normal- or high-IgE conditions in a mouse model. In order to address these aspects, we compared the levels of specific IgE and several other serum specific anti-HER-2 antibodies (IgG1, IgG2a, IgG2b, IgA) along with the survival rates between three different mouse strains engrafted with HER-2 overexpressing tumors, differing in their Epsilon-B-cell receptor expression and IgE expression levels: i) low-IgE ΔM1M2 mice lacking the transmembrane/cytoplasmic domain of the ε-B-cell receptor (BCR),39 ii) high-IgE KN1 mice expressing chimeric ε-γ1-BCRs with a 4 -to 6- fold elevated mean serum IgE level,40 compared to iii) wild type (WT) BALB/c mice with “normal” IgE levels.
Materials and methods
Cell lines, recombinant proteins and monoclonal antibodies
D2F2/E2 is a mouse mammary carcinoma cell line derived from D2F2 cells transfected with human HER-2. This cell line was established41 and kindly provided by Prof. Wei-Zen Wei (Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan, USA). D2F2/E2 cells were grown in a humidified atmosphere of 5% CO2 at 37 °C in IMDM medium supplemented with 10% fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 μg/mL) and 1 mg/ml geneticin (G418). Recombinant human HER-2 (rHER-2) was produced as previously described42, 43 in Lec-1 cells (a kind gift of Prof. Daniel J. Leahy, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA). Lec-1 cells were kept in DMEM/F12, supplemented with 5% FCS, 100 nM methotrexate and 10 μg/mL gentamicin sulfate. Trastuzumab (Herceptin®), a humanized IgG1 monoclonal anti-ErbB-2 (HER-2) antibody, was obtained from Roche (Basel, Switzerland). Rituximab (MabThera®), a chimeric IgG1 anti-CD20 monoclonal antibody, was employed as isotype control in ELISAs. Clone 4D5, the murine IgG1 precursor of trastuzumab was kindly provided by Genentech (South San Francisco, California, USA).
Mouse strains
Mice with different degrees of IgE-responses to foreign antigens were employed in this study. BALB/c mice served as wild type animals (WT). ΔM1M2 mice, which lack the transmembrane and cytoplasmic domain of IgE39 served as the IgE-reduced (IgE low) strain. KN1 mice, which express chimeric ε-γ1 B-cell receptors40 served as the IgE-overexpressing (high-IgE) model. Both transgenic mouse strains are of BALB/c background.
All mice were kept on the basis of authorization of the Animal Ethics Committee of the Medical University according to the Austrian, European Union and Federation for Laboratory Animal Science Associations (FELASA) guidelines for animal care and protection (GZ: BMWF-66.009/0086-C/GT/2007).
Immunization scheme and tumor graft trial
In order to achieve specific immunity to HER-2, mice were vaccinated subcutaneously four times at 2-week intervals with 50 μg of rHER-2 adjuvanted with aluminum hydroxide solution (Al(OH)3, Alum, Alu-Gel-S®, Cat-No.: 12261.01,Serva, Heidelberg, Germany). Control groups received phosphate buffered saline (PBS). Before the immunization trial and after each round of immunization serum samples were taken to monitor the immune response (Fig. 1, PIS … Pre-Immune Serum, MIS 1–4 … Mouse Immune Serum 1–4). For overview on mouse groups, strains and treatments see Table 1.
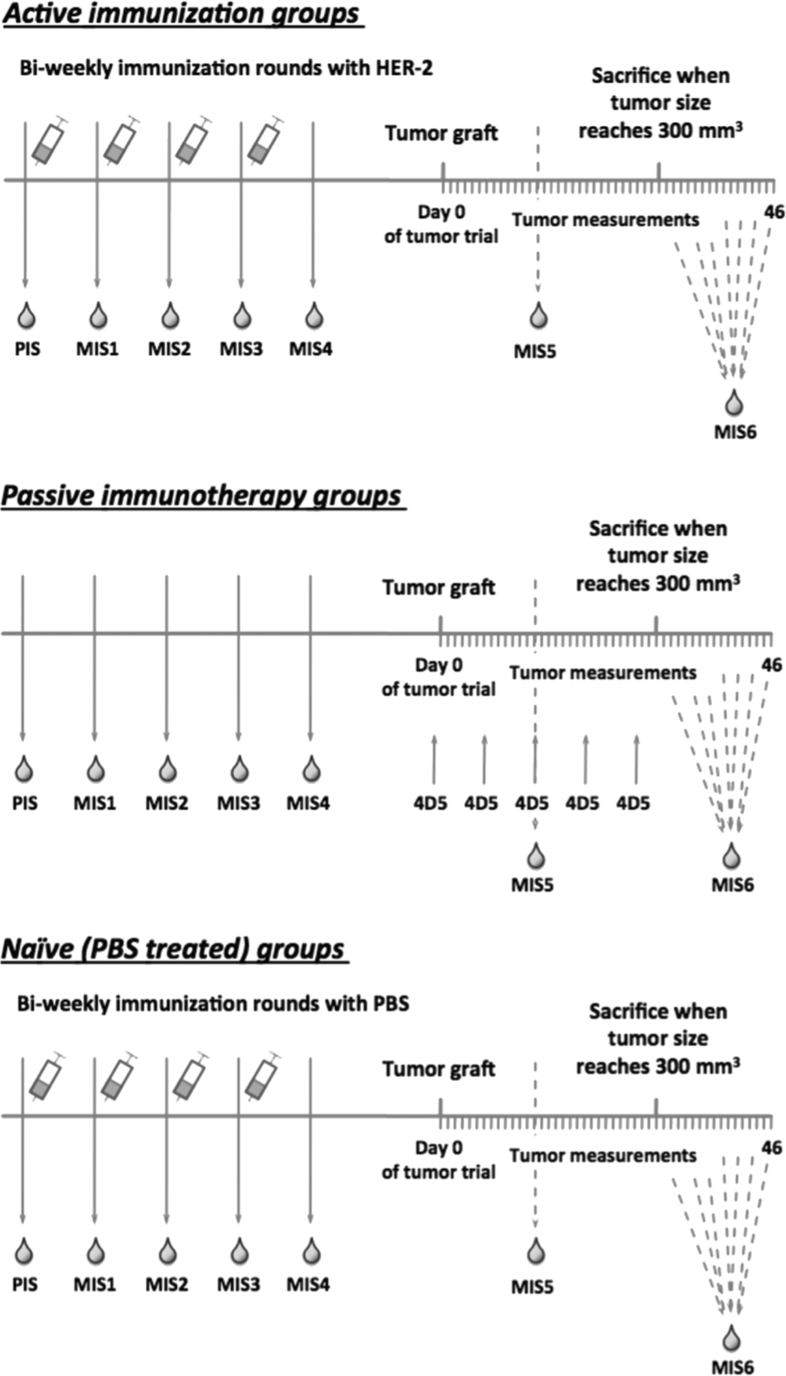
Fig. 1.
Schematic overview of immunization plan and tumor graft trial. Mice were immunized 4 times with rHER-2 or PBS + Al(OH)3 in bi-weekly intervals (top panel, bottom panel). Mouse sera were taken to monitor the immune response (PIS = PreImmuneSerum, MIS 1-4 = Mouse Immune Serum). Subsequently mice were grafted 2x106 HER-2 overexpressing D2F2/E2 cells s.c.; tumor growth was measured daily by caliper measurement. Passive immunotherapy groups received the murine anti-HER-2 antibody 4D5 i.p. 1 day prior to tumor grafting and every 7 days after tumor transplantation (middle panel). MIS5 was taken at day 14 of the tumor trial. Mice were sacrificed when tumor volume reached 300mm3. MIS6 was taken prior to sacrifice (at individual time points).
Table 1.
Overview on treatment groups.
| Active Immunotherapy | Passive Immunotherapy | Control | |
|---|---|---|---|
| WT BALBc (normal IgE levels) | rHER-2 | 4D5 | PBS |
| ΔM1M2 (low IgE levels) | rHER-2 | 4D5 | PBS |
| KN1 (high IgE levels) | rHER-2 | 4D5 | PBS |
On day 1 of tumor challenge experiments, immunized mice were grafted subcutaneously into their left flanks with 2 × 106 HER-2 overexpressing D2F2/E2 mouse mammary carcinoma cells, resuspended in 100 μl of IMDM (without supplements). Tumor size was measured daily by caliper measurement and tumor volume was calculated according to formula V (mm3) = d2 (mm2) x D (mm)/2, where d stands for the smallest and D for the largest diameter of the tumor. When tumors reached a volume of 300 mm3, mice had to be sacrificed and tumors were taken for histologic evaluation of HER-2 expression.
In order to treat one group with passive anti-HER-2 immunotherapy, respective mice received 100 μg 4D5 antibody intraperitoneally (i.p.) 1 day before the tumor graft and every 7 days after tumor transplantation. MIS5 (Mouse Immune Serum) was taken at day 14 of the tumor trial. Mice were sacrificed when tumor volume reached 300 mm3 MIS6 was taken prior to sacrifice (at individual time points; see Fig. 1).
Enzyme linked immunosorbent assay (ELISA)
Immune responses in mice were evaluated by ELISA. The tumor-associated antigen rHER-2 was coated on 96-well microtiter plates (Immuno Maxi-Sorp™, Nunc, Cat-No: M9410-1CS, Roskild, Denmark) at a concentration of 1 μg/ml. Unspecific binding was blocked with 1% dried milk powder (DMP) in TRIS-buffered saline, 0.05% Tween20 (TBST). Sera of treated mice, diluted in TBST/0.1% DMP, were allowed to adhere overnight on 4 °C. Bound murine immunoglobulins were detected with rat anti-mouse IgM (Cat-No: 553405), IgG1 (Cat-No: 553440), IgG2a (Cat-No: 553387), IgG2b (Cat-No: 553392), IgA (Cat-No: 556960) and IgE (Cat-No: 553416) antibodies (Pharmingen™, BD Biosciences, Franklin Lakes, New Jersey, USA), followed by detection of bound antibodies with a horseradish-peroxidase labeled goat anti-rat IgG antibody (Amersham ECL, GE Healthcare Europe GmbH; Cat-No: NA935; diluted 1:3000 in TBST + 0.1% DMP, Buckinghamshire, United Kingdom). For detection, 3,3′,5,5′-tetramethylbenzidine (TMB, BD OptEIA TMB Solution, BD Biosciences; Cat-No: 555214; 100μl/well) was added and the optical density (OD) was measured at 450 nm with 620 nm as reference wavelength with a multiwell plate reader (Infinite M200 PRO, Tecan Group AG, Maennedorf, Switzerland). For standard dilution curves, the following purified mouse immunoglobulins from Southern Biotechnologies were used: mouse IgM clone 11E10 (Cat-No.: 0101-01), mouse IgG1 clone 15H6 (Cat-No.: 0102-01), mouse IgG2a clone HOPC (Cat-No.: 0103-01), mouse IgG2b clone A-1 (Cat-No.: 0104-01), mouse IgA clone S107 (Cat-No.: 0106-01) and mouse IgE clone 15.3 (Cat-No.: 0114-01).
Data handling and statistics
Sera of animals were tested in duplicates for the presence of HER-2 specific antibodies. Levels at MIS4 and MIS6 were analyzed by means of one way-ANOVA followed by Tukey's multiple comparison's test. Statistical analysis of survival experiments was performed by plotting Kaplan-Meier curves of animal groups were and analyzed applying Log-rank (Mantel-Cox) Test.
In all statistical calculations of this study, significance was accepted at p < 0.05 (*), p < 0.01 (**), p < 0.001 (***) and p < 0.0001 (****). Statistical analyses were performed with GraphPad Prism (Version 7.0b).
Results
High-IgE expressing mice respond to HER-2 immunization by enhancing anti- HER-2 IgE and IgG1, while low-IgE ΔM1M2 mice develop higher IgG2a levels.
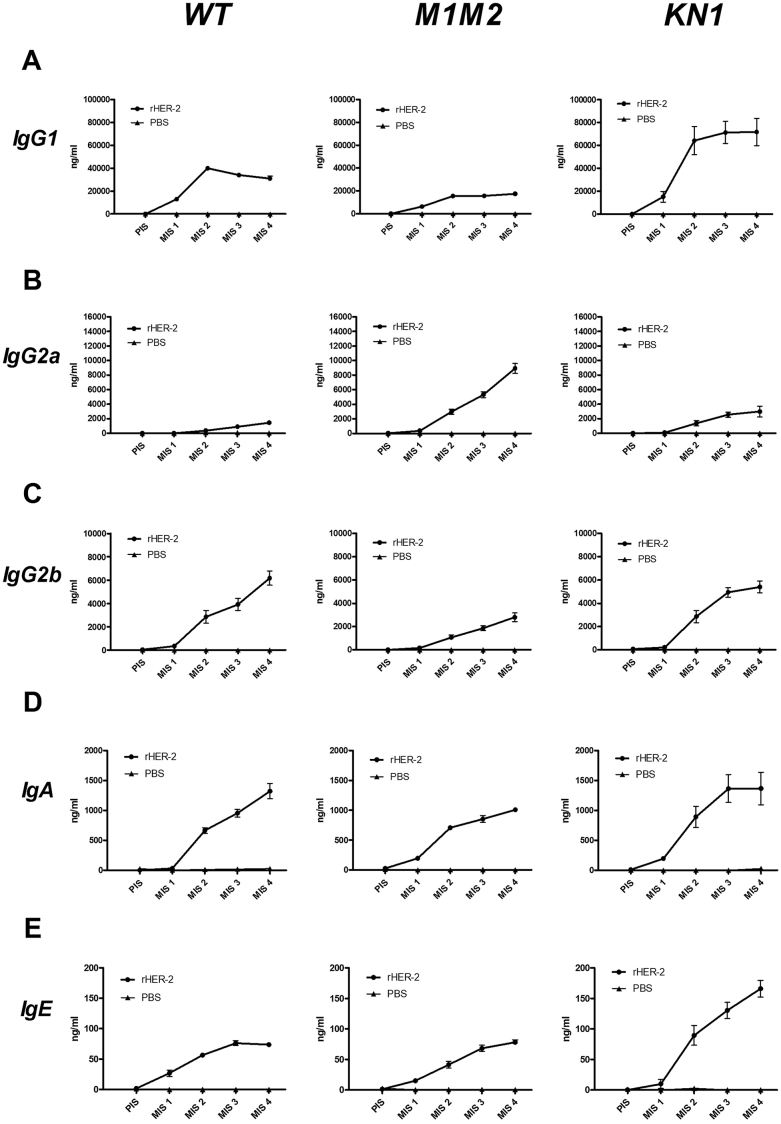
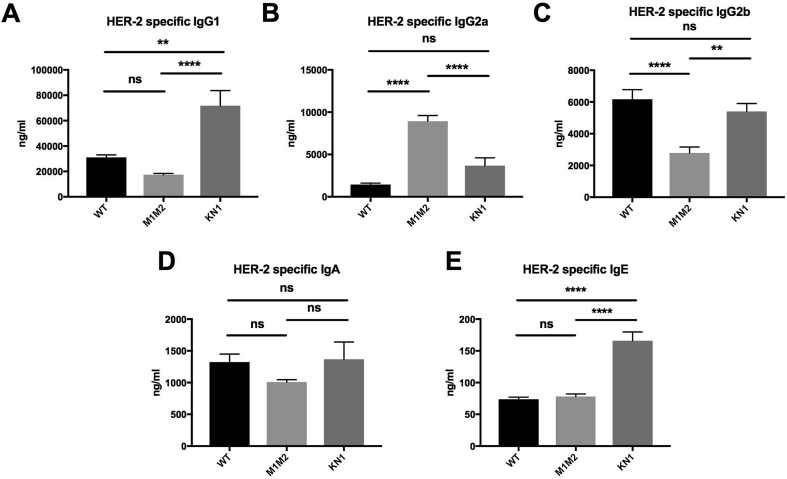
In mice of all three strains immunoglobulins could be specifically induced by the HER-2 vaccine (Fig. 2). IgG1 was by far the most prominent immunoglobulin isotype response in all three strains (Fig. 2A). After 4 immunizations (MIS4, Fig. 3), WT BALB/c mice displayed significantly less HER-2 specific IgG1 than high-IgE expressing KN1 (p < 0.0001, Fig. 3A), and more than low-IgE expressing ΔM1M2 (n.s., p = 0.346) mice. ΔM1M2 mice developed significantly higher levels of anti-HER-2 IgG2a (Fig. 2B) than either WT BALB/c (p < 0.0001, Fig. 3B) or KN1 (p < 0.0001, Fig. 3B). Both WT BALB/c and KN1 mice (Fig. 2C) displayed comparable (p = 0.5633), but significantly higher IgG2b levels than ΔM1M2 mice (p < 0.0001 for WT BALB/c; p = 0.0023 for KN1; Fig. 3C). For HER-2 specific IgA, the serum levels did not significantly differ between the three stains (WT BALB/c: ΔM1M2: p = 0.388; WT vs KN1: p = 0.9819; KN1: ΔM1M2: p = 0.2725; Fig. 2, Fig. 3D). Notably, after 4 rounds of immunization, HER-2 specific IgE levels were highest in the KN1 animals (p < 0.0001) but did not differ between normal-IgE WT BALB/c vs low-IgE ΔM1M2 mice (p = 0.8993; Fig. 2, Fig. 3E). The induction of HER-2 specific IgE occurred more slowly in ΔM1M2 mice (Fig. 2E).
Fig. 2.
Antibody responses of different mouse strains.A: HER-2 specific IgG1 antibody levels are displayed during the course of the immunization trial. Rectangle symbols depict the groups immunized with rHER-2, triangle symbols represent the groups receiving PBS. Serum was taken prior to immunization (PIS) and after each immunization round (MIS 1–4). Left panel depicts WT mice, middle panel ΔM1M2 mice and right panel KN1. Displayed are mean immunoglobulin levels in ng/ml ± SEM. B: HER-2 specific IgG2a antibody levels. C: HER-2 specific IgG2b. D: HER-2 specific IgA. E: HER-2 specific IgE.
Fig. 3.
Comparison of antibody responses in different mouse strains after 4 rounds of immunizations.A: HER-2 specific IgG1 levels in WT, ΔM1M2 and KN1 mice. B: HER-2 specific IgG2a antibody levels. C: HER-2 specific IgG2b. D: HER-2 specific IgA. E: HER-2 specific IgE. Displayed are mean values in ng/ml ± SEM. ns … not significant, * … p < 0.05, ** … p < 0.01, *** … p < 0.001, **** … p < 0.0001.
Together, these findings suggest that high-IgE expressing mice develop a prominent IgE-enhanced response following HER-2 vaccination.
Active and passive immunotherapy do not further prolong the survival rates in high-IgE mice
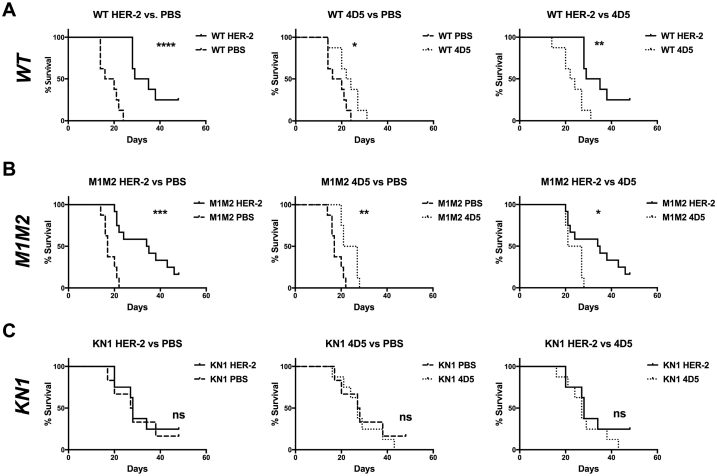
Following tumor challenge, WT HER-2-vaccinated mice had a significant survival benefit over PBS-treated control mice (Fig. 4A, left p < 0.0001), which exceeded the effect of passive immunotherapy with the HER-2 specific monoclonal IgG1 antibody 4D5 (Fig. 4A, middle, p = 0.0428). 4D5 treatment, however, provided a significant survival benefit compared to PBS sham treatments. (Fig. 4A, right, p = 0.0013).
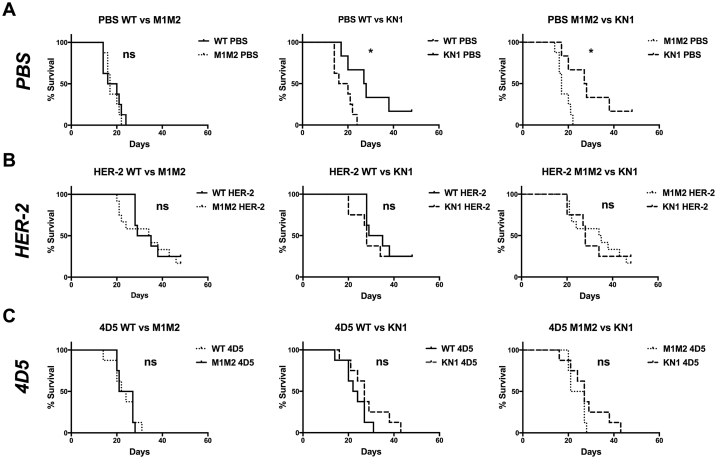
Fig. 4.
Survival curves of different treatment groups after tumor challenge.A: Survival curves of wild type animals distributed by different treatment groups. Left panel: HER-2 immunized animals compared to naive (PBS treated) mice, middle: mice treated with the anti-HER-2 antibody 4D5 compared to naive mice, right: comparison of the active (HER-2 immunized) and passive (4D5 treated) immunotherapy groups. B: Survival curves of different treatment groups in the ΔM1M2 mouse strain. Left panel: HER-2 treated mice compared to naive, middle: 4D5 treated animals compared to naive, right: HER-2 immunized mice compared to 4D5 treated. C: Survival curves of differently treated mice in the KN1 strain. Left: HER-2 treatment vs. naive, middle: 4D5 treatment vs. naive, right: HER-2 immunization vs. 4D5. Full lines: naive groups, dashed lines: HER-2 immunized groups, dotted lines: 4D5 treated groups; ns … not significant, * … p < 0.05, ** … p < 0.01, *** … p < 0.001, **** … p < 0.0001.
In low-IgE ΔM1M2 mice, the HER-2 vaccine (p = 0.0001) and 4D5 (p = 0.0081) prolonged survival compared to PBS (Fig. 4B left and middle). Again, survival of the HER-2 vaccinated mice was more pronounced than in mice treated with 4D5 (Fig. 4B right: HER-2 vs. 4D5 treated, p = 0.0251).
In the high-IgE KN1 group, neither HER-2 immunization (Fig. 4C left, p = 0.1557) nor 4D5 mediated any additional survival benefits compared to naïve animals (Fig. 4C middle, p = 0.8475).
Notably, untreated high-IgE KN1 mice displayed significantly longer survival after tumor grafting than both WT and ΔM1M2 mice (Fig. 5A, middle and right).
Fig. 5.
Survival curves of different mouse strains after tumor challenge.A: Survival curves of naive (PBS treated) animals distributed by different mouse strains. Left panel: wild type (WT) animals compared to ΔM1M2 mice, middle: WT animals compared to mice of the KN1 strain, right: comparison of ΔM1M2 and KN1 strains. B: Survival curves of HER-2 immunized animals distributed by different mouse strains. Left panel: WT mice compared to ΔM1M2, middle: WT animals compared to KN1, right: ΔM1M2 mice compared to KN1. C: Survival curves of 4D5 treated animals distributed by different mouse strains. Left: WT vs. ΔM1M2, middle: WT vs. KN1, right: ΔM1M2 vs. KN1. Full lines: WT mice, dashed lines: KN1 mice, dotted lines: ΔM1M2 mice; ns … not significant, * … p < 0.05, ** … p < 0.01.
Discussion
Promising approaches employ alternate immunoglobulin classes, such as IgA or IgE, to the commonly used IgG for cancer immunotherapy.10, 19, 44, 45, 46 A trastuzumab-like IgE with the same variable region and differing only in the constant region, mediated higher levels of ADCC in a side-by-side comparison with the corresponding IgG1 in vitro.10 These functional studies in parallel with data from epidemiological meta-analyses describing a negative correlation of specific and total IgE levels in allergics and atopics with the occurrence of specific cancers suggest that elevated levels of IgE antibody responses such as in allergy and atopy, may offer protection from cancer initiation.23, 24, 25, 26, 27, 28, 29, 30, 31, 32 In accordance with these observations, recent retrospective studies demonstrate that vice versa low levels of total IgE in 4488 patients of the 2005–2006 US National Health and Nutrition Examination Survey (NHANES) termed “IgE-deficient”, correlated with a higher frequency of occurrence of cancer, compared with normal, high, or very high IgE levels.31 In another study, the authors showed that in fact 2.7% of 2339 allergics visiting an outpatient allergy clinic correlated with significantly higher rates of diagnosis of any type of cancer during the observation period of 2010–2015 (33% vs 8.7%; odds ratio 5.51, 95% confidence interval 3.07e9.88).32
In the present study, we mimic the human setting of high, atopic IgE levels, versus low IgE levels employing three different mouse strains differing in their ε-B-cell receptor expression. Our findings confirm that IgE antibodies have a protective effect, in a model of HER-2 positive mammary cancer, even in a non-antigen specific manner.
In high-IgE KN1 mice, higher expression of the ε-BCR and of IgE seem to play a decisive role in significantly enhancing tumor-free survival time independent of active or passive immunotherapy. In WT and low-IgE ΔM1M2 mice, both passive and active immunotherapy significantly prolonged mouse survival, compared to sham treatments. These findings suggest the merit of anticancer immunotherapies targeting the tumor-associated antigen HER-2. Notably, high-IgE mice survived significantly longer than normal- and low-IgE mice following mammary tumor challenge when no immunotherapy was made. In the high-IgE KN1 mice, mimicking the atopic state in humans (Fig. 5A), the “innate” effects of high IgE could not be further increased by specific anti-HER-2 vaccination, or by passive anti-HER-2 IgG1 antibody treatment using 4D5. This is in favor of previous work demonstrating an innate “adjuvant function” of IgE in cooperation with FcεRI expressed by cytotoxic effector cells.38 In concordance, a gene expression signature incorporating the high affinity IgE receptor FcεRI was shown to correlate with improved prognosis in lung adenocarcinoma.47 These findings mirrored numerous epidemiologic studies highlighting an inverse correlation of high total or allergen-specific IgE in atopic and allergic patients, with the reduced risk of developing different cancers including those of the breast.
When mice were treated with passive or active immunotherapy, the advantage of innate IgE in KN1 was lost and all strains had a similar survival benefit (Fig. 5B and C). Based on these data we propose that low-IgE patients might have the greatest advantage of immunotherapies, while atopic patients already naturally exploit innate IgE-mediated mechanisms against malignancies.
Our study has several limitations: first of all, the sample size for each group is small, however, this is an exploratory pilot study addressing a novel aspect in cancer immunology, which may represent the basis for further research studies into this field. Second, in the absence of a recombinant mouse anti HER-2 IgE antibody of the same specificity as 4D5, a side-by-side comparison of IgE versus IgG istotypes could not be done to compare the potential of IgG versus IgE-based immunotherapies. Furthermore, adoptive transfer experiments of sera from high-IgE to low-IgE mice could have further elucidated the role of IgE in immunosurveillance and defense of cancer. Finally, the mouse immune system does not fully mirror the human setting and generally such experiments would only constitute an impression of the human immune system setting. For instance, murine dendritic cells do not express FcεRI, which hinders conclusions on the IgE-mediated antigen presentation capacity of the used transgenic mouse models.8
Conclusion
This study highlights: i) that anticancer vaccines targeting HER-2 provide a significant survival benefit and delay disease progression in an immunocompetent syngeneic mouse model of cancer, ii) that vaccination was superior to passive antibody therapy with the anti-HER-2 IgG1 antibody 4D5 in WT and low-IgE expressing mice, and iii) the protective effects of elevated natural IgE levels after tumor challenge compared with normal- or low-IgE expressing mice who did not undergo any intervention. IgE therefore has a natural surveillance function against malignant cells, together with its cytotoxic cellular effector arm. Epidemiologic studies indicate that atopics with elevated IgE may benefit from its innate immune surveillance and functions. We propose that in non-atopic patients, the benefits of IgE can be exploited and improved by targeting recombinant IgE antibodies to cancer antigens. While low-IgE conditions pose a higher risk for progressive cancer, these individuals may benefit from anticancer immunotherapies. Based on our study and in line with the AllergoOncology concept, we propose that IgE should be reinvented to target cancer antigens.7, 8, 13, 35, 48
Conflict of interest
Sophia N. Karagiannis is scientific founder of IGEM Therapeutics, a UK-based Immuno-Oncology company. The other authors declare no conflict of interest.
References
- 1.De Mattos-Arruda L., Cortes J. Advances in first-line treatment for patients with HER-2+ metastatic breast cancer. Oncol. 2012;17(5):631–644. doi: 10.1634/theoncologist.2011-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris C.A., Ward R.L., Dobbins T.A., Drew A.K., Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22(6):1308–1317. doi: 10.1093/annonc/mdq593. [DOI] [PubMed] [Google Scholar]
- 3.Fendly B.M., Winget M., Hudziak R.M., Lipari M.T., Napier M.A., Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990;50(5):1550–1558. [PubMed] [Google Scholar]
- 4.Carter P., Presta L., Gorman C.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89(10):4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinch M.S. An overview of FDA-approved biologics medicines. Drug Discov Today. 2015;20(4):393–398. doi: 10.1016/j.drudis.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Flajnik M.F. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2(9):688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 7.Jensen-Jarolim E, Bax HJ, Bianchini R. AllergoOncology: Opposite outcomes of immune tolerance in allergy and cancer. Allergy. 2017 doi: 10.1111/all.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen-Jarolim E, Bax HJ, Bianchini R. AllergoOncology - the impact of allergy in oncology: EAACI position paper. Allergy. 2017;72(6):866–887. doi: 10.1111/all.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu S.L., Pierre J., Smith-Norowitz T.A. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin Exp Immunol. 2008;153(3):401–409. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karagiannis P., Singer J., Hunt J. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol Immunother. 2009;58(6):915–930. doi: 10.1007/s00262-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillner E., Plum M., Blank S., Miehe M., Singer J., Braren I. Recombinant IgE antibody engineering to target EGFR. Cancer Immunol Immunother. 2012;61(9):1565–1573. doi: 10.1007/s00262-012-1287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephs D.H., Bax H.J., Dodev T. Anti-folate receptor-alpha IgE but not IgG recruits macrophages to attack tumors via tnfalpha/MCP-1 signaling. Cancer Res. 2017;77(5):1127–1141. doi: 10.1158/0008-5472.CAN-16-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crescioli S., Chiaruttini G., Mele S. Engineering and stable production of recombinant IgE for cancer immunotherapy and AllergoOncology. J Allergy Clin Immunol. 2018;141(4) doi: 10.1016/j.jaci.2017.12.986. 1519-1523 e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould H.J., Mackay G.A., Karagiannis S.N. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29(11):3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Josephs D.H., Nakamura M., Bax H.J. An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE. Allergy. 2018;73(12):2328–2341. doi: 10.1111/all.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josephs D.H., Spicer J.F., Corrigan C.J., Gould H.J., Karagiannis S.N. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy. 2013;43(10):1110–1123. doi: 10.1111/cea.12178. [DOI] [PubMed] [Google Scholar]
- 17.Karagiannis S.N., Bracher M.G., Beavil R.L. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57(2):247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karagiannis S.N., Bracher M.G., Hunt J. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179(5):2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 19.Karagiannis S.N., Josephs D.H., Karagiannis P. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother. 2012;61(9):1547–1564. doi: 10.1007/s00262-011-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner M.C., Chen Y., Krewski D., Ghadirian P., Thun M.J., Calle E.E. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162(3):212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 21.Turner M.C., Chen Y., Krewski D., Ghadirian P. An overview of the association between allergy and cancer. Int J Cancer. 2006;118(12):3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 22.Turner M.C. Epidemiology: allergy history, IgE, and cancer. Cancer Immunol Immunother. 2012;61(9):1493–1510. doi: 10.1007/s00262-011-1180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs E.J., Gapstur S.M., Newton C.C., Turner M.C., Campbell P.T. Cancer Epidemiol Biomarkers Prev; 2013. Hay Fever and Asthma as Markers of Atopic Immune Response and Risk of Colorectal Cancer in Three Large Cohort Studies. [DOI] [PubMed] [Google Scholar]
- 24.Wulaningsih W., Holmberg L., Garmo H. Investigating the association between allergen-specific immunoglobulin E, cancer risk and survival. OncoImmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1154250. e1154250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disney-Hogg L., Cornish A.J., Sud A. Impact of atopy on risk of glioma: a Mendelian randomisation study. BMC Med. 2018;16(1):42. doi: 10.1186/s12916-018-1027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amirian E.S., Zhou R., Wrensch M.R. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the glioma international case-control study. Cancer Epidemiol Biomark Prev. 2016;25(2):282–290. doi: 10.1158/1055-9965.EPI-15-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartzbaum J., Ding B., Johannesen T.B. Association between prediagnostic IgE levels and risk of glioma. J Natl Cancer Inst. 2012;104(16):1251–1259. doi: 10.1093/jnci/djs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C., Zhu Q.X. Allergy is associated with reduced risk of glioma: a meta-analysis. Allergol Immunopathol (Madr) 2017;45(6):553–559. doi: 10.1016/j.aller.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H., Cai W., Su S., Zhi D., Lu J., Liu S. Allergic conditions reduce the risk of glioma: a meta-analysis based on 128,936 subjects. Tumour Biol. 2014;35(4):3875–3880. doi: 10.1007/s13277-013-1514-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen C., Xu T., Chen J. Allergy and risk of glioma: a meta-analysis. Eur J Neurol. 2011;18(3):387–395. doi: 10.1111/j.1468-1331.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferastraoaru D., Rosenstreich D. IgE deficiency and prior diagnosis of malignancy: results of the 2005-2006 national Health and nutrition examination Survey. Ann Allergy Asthma Immunol. 2018;121(5):613–618. doi: 10.1016/j.anai.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Ferastraoaru D., Gross R., Rosenstreich D. Increased malignancy incidence in IgE deficient patients not due to concomitant Common Variable Immunodeficiency. Ann Allergy Asthma Immunol. 2017;119(3):267–273. doi: 10.1016/j.anai.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Jensen-Jarolim E., Achatz G., Turner M.C. Allergo Oncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63(10):1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen-Jarolim E., Pawelec G. The nascent field of AllergoOncology. Cancer Immunol Immunother. 2012;61(9):1355–1357. doi: 10.1007/s00262-012-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen-Jarolim E., Turner M.C., Karagiannis S.N. AllergoOncology: IgE- and IgG4-mediated immune mechanisms linking allergy with cancer and their translational implications. J Allergy Clin Immunol. 2017;140(4):982–984. doi: 10.1016/j.jaci.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Jensen-Jarolim E., Singer J. Why could passive Immunoglobulin E antibody therapy be safe in clinical oncology? Clin Exp Allergy. 2011;41(10):1337–1340. doi: 10.1111/j.1365-2222.2011.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer J., Jensen-Jarolim E. IgE-based immunotherapy of cancer: challenges and chances. Allergy. 2014;69(2):137–149. doi: 10.1111/all.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigro E.A., Brini A.T., Yenagi V.A. Cutting edge: IgE plays an active role in tumor immunosurveillance in mice. J Immunol. 2016;197(7):2583–2588. doi: 10.4049/jimmunol.1601026. [DOI] [PubMed] [Google Scholar]
- 39.Achatz G., Nitschke L., Lamers M.C. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276(5311):409–411. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 40.Achatz-Straussberger G., Zaborsky N., Konigsberger S. Migration of antibody secreting cells towards CXCL12 depends on the isotype that forms the BCR. Eur J Immunol. 2008;38(11):3167–3177. doi: 10.1002/eji.200838456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei W.Z., Shi W.P., Galy A. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81(5):748–754. doi: 10.1002/(sici)1097-0215(19990531)81:5<748::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Cho H.S., Mason K., Ramyar K.X. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 43.Singer J., Manzano-Szalai K., Fazekas J. Proof of concept study with an HER-2 mimotope anticancer vaccine deduced from a novel AAV-mimotope library platform. Oncoimmunology. 2016;5(7) doi: 10.1080/2162402X.2016.1171446. e1171446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouwendal G.J., van der Lee M.M., Meyer S. A comparison of anti-HER2 IgA and IgG1 in vivo efficacy is facilitated by high N-glycan sialylation of the IgA. mAbs. 2016;8(1):74–86. doi: 10.1080/19420862.2015.1102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer S., Nederend M., Jansen J.H. Improved in vivo anti-tumor effects of IgA-Her2 antibodies through half-life extension and serum exposure enhancement by FcRn targeting. mAbs. 2016;8(1):87–98. doi: 10.1080/19420862.2015.1106658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goritzer K., Maresch D., Altmann F., Obinger C., Strasser R. Exploring site-specific N-glycosylation of HEK293 and plant-produced human IgA isotypes. J Proteome Res. 2017;16(7):2560–2570. doi: 10.1021/acs.jproteome.7b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ly D., Zhu C.Q., Cabanero M., Tsao M.S., Zhang L. Role for high-affinity IgE receptor in prognosis of lung adenocarcinoma patients. Cancer Immunol Res. 2017;5(9):821–829. doi: 10.1158/2326-6066.CIR-16-0392. [DOI] [PubMed] [Google Scholar]
- 48.Daniels T.R., Leuchter R.K., Quintero R. Targeting HER2/neu with a fully human IgE to harness the allergic reaction against cancer cells. Cancer Immunol Immunother. 2012;61(7):991–1003. doi: 10.1007/s00262-011-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]