Key Points
Question
What is the relative association of management strategies for adult patients with moderate to severe acute respiratory distress syndrome with mortality and barotrauma?
Findings
In this systematic review and network meta-analysis of 25 randomized clinical trials including 7743 patients, venovenous extracorporeal membrane oxygenation and prone positioning were associated with significantly lower 28-day mortality compared with lung protective ventilation alone. Moreover, venovenous extracorporeal membrane oxygenation was the highest-ranked intervention associated with a reduction in 28-day mortality among the 9 interventions evaluated.
Meaning
These findings support the use of prone positioning in patients with moderate to severe acute respiratory distress syndrome and venovenous extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome.
This systematic review and network meta-analysis compares and ranks different therapeutic strategies to identify the best intervention associated with a reduction in mortality in adult patients with moderate to severe acute respiratory distress syndrome (ARDS).
Abstract
Importance
A number of interventions are available to manage patients with moderate to severe acute respiratory distress syndrome (ARDS). However, the associations of currently available ventilatory strategies and adjunctive therapies with mortality are uncertain.
Objectives
To compare and rank different therapeutic strategies to identify the best intervention associated with a reduction in mortality in adult patients with moderate to severe ARDS.
Data Sources
An electronic search of MEDLINE, MEDLINE In-Process/ePubs Ahead of Print, Embase, Cochrane Controlled Clinical Trial Register (Central), PubMed, and CINAHL was conducted, from database inception to May 29, 2019.
Study Selection
Randomized clinical trials of interventions for adults with moderate to severe ARDS that used lung protective ventilation. No language restrictions were applied.
Data Extraction and Synthesis
Data were independently extracted by 2 reviewers and synthesized with Bayesian random-effects network meta-analyses.
Main Outcomes and Measures
The primary outcome was 28-day mortality. Barotrauma was a secondary outcome.
Results
Among 25 randomized clinical trials evaluating 9 interventions, 2686 of 7743 patients (34.6%) died within 28 days. Compared with lung protective ventilation alone, prone positioning and venovenous extracorporeal membrane oxygenation were associated with significantly lower 28-day mortality (prone positioning: risk ratio, 0.69; 95% credible interval, 0.48-0.99; low quality of evidence; venovenous extracorporeal membrane oxygenation: risk ratio, 0.60; 95% credible interval, 0.38-0.93; moderate quality of evidence). These 2 interventions had the highest ranking probabilities, although they were not significantly different from each other. Among 18 trials reporting on barotrauma, 448 of 6258 patients (7.2%) experienced this secondary outcome. No intervention was superior to any other in reducing barotrauma, and each represented low to very low quality of evidence.
Conclusions and Relevance
This network meta-analysis supports the use of prone positioning and venovenous extracorporeal membrane oxygenation in addition to lung protective ventilation in patients with ARDS. Moreover, venovenous extracorporeal membrane oxygenation may be considered as an early strategy for adults with severe ARDS receiving lung protective ventilation.
Introduction
Acute respiratory distress syndrome (ARDS) is a lethal condition whereby the lung is injured by direct (eg, pneumonia or aspiration) or indirect (eg, extrapulmonary sepsis) insults. Clinically, patients with ARDS develop severe hypoxemia and/or hypercapnia, and most die of sepsis or multiorgan failure rather than from refractory respiratory failure. Acute respiratory distress is an important public health problem. A global epidemiologic study1 reported that 10.4% of total intensive care unit admissions and 23.4% of all patients who were intubated had ARDS, with an associated hospital mortality of 40%.
Since the first description of ARDS in 1967,2 a number of approaches to its management have been evaluated and used clinically.3,4 Despite more than 50 years of research, to our knowledge, none of the treatments currently available are aimed directly at the pathophysiological mechanism resulting in acute respiratory failure (ie, increased alveolar capillary permeability); current approaches are mainly supportive. Perhaps most important has been the recognition that although mechanical ventilation is critical for the survival of patients with ARDS, it can also be injurious (ie, ventilator-induced lung injury).5 Indeed, lung protective ventilation (LPV), using low tidal volumes and airway pressures to mitigate ventilator-induced lung injury, is considered the mainstay of management in patients with ARDS.6
In 2017, clinical practice guidelines on mechanical ventilation in adult patients with ARDS were published for 6 individual interventions, as follows: (1) LPV, (2) higher positive end-expiratory pressure (PEEP), (3) lung recruitment maneuvers (RMs), (4) high-frequency oscillatory ventilation (HFOV), (5) prone positioning, and (6) venovenous extracorporeal membrane oxygenation (VV ECMO).6 However, for clinicians, choosing between potentially efficacious treatments can be challenging if the treatments have not been directly compared in clinical trials (eg, prone positioning vs VV ECMO). Moreover, 4 randomized clinical trials (RCTs)—the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART),7 the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial,8 the Esophageal Pressure-Guided Ventilation 2 (EPVent2) trial,9 and the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial10—have been published since the guidelines were completed. Therefore, we conducted a systematic review and network meta-analysis to compare different therapeutic strategies simultaneously to identify the best strategy associated with a reduction in mortality and to rank those therapeutic modalities for adult patients with moderate to severe ARDS.
Methods
Eligibility Criteria, Literature Search, and Study Selection
We followed the steps outlined by the Cochrane Collaboration11 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.12 We included RCTs or quasi-RCTs enrolling adult patients (aged ≥18 years) with moderate to severe ARDS who received mechanical ventilation in the intensive care unit.13 For studies using the American-European Consensus Conference to diagnose ARDS, we checked Pao2-to-fraction of inspired oxygen (Fio2) ratio of inclusion criteria or the mean and distribution of Pao2/Fio2 ratio in each trial to determine the severity of ARDS among enrolled patients. We included interventions available for moderate to severe ARDS, either alone or in combination with LPV or another intervention. We defined LPV as a mechanical ventilation using low tidal volume of 4 to 8 mL/kg of predicted body weight. The interventions that we considered a priori were LPV, open lung strategies (ie, RM or PEEP), neuromuscular blockade (NMBA), inhaled nitric oxide (INO), HFOV, prone positioning, and VV ECMO. Participants in the comparator group could also have received 1 cointervention (eg, NMBA), as described above. Our primary outcome was 28-day mortality. If not reported explicitly, we identified or calculated 28-day mortality from Kaplan-Meier curves (using Digitaliser v10.9 [Engauge]) or from the closest reported time point, assuming constant mortality rate over time. Our prespecified secondary outcome was barotrauma at any time point. Inclusion and exclusion criteria are summarized in eAppendix 1 in the Supplement.
We performed an electronic search of MEDLINE, MEDLINE In-Process/ePub Ahead of Print, Embase, Cochrane Controlled Clinical Trial Register (Central) (via the Ovid search interface), PubMed (via the National Library of Medicine and excluding Medline records), and CINAHL (via EbscoHost) from database inception to May 29, 2019, using a sensitive search strategy (eAppendix 2 in the Supplement). We used controlled vocabulary terms (when available), text words, and keywords. No language restrictions were applied. We screened the reference lists of key articles for additional potentially relevant articles.
Two reviewers (H.A. and K.U.) independently identified and assessed potentially eligible studies for inclusion in the review, and any disagreement and discrepancies were resolved by discussion with and adjudication by a third reviewer (E.F.). Cohen κ was reported for agreement between the 2 reviewers.14 Data extraction and risk-of-bias assessment were also performed independently in duplicate by 2 authors (H.A. and K.U.).
Data Extraction and Risk-of-Bias Assessment
A standardized, piloted data collection form designed for this systematic review was developed for data extraction. Risk of bias for each eligible study was determined using the Cochrane risk-of-bias tool.11 The certainty of evidence for the network meta-analysis was assessed and determined using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) tool for network meta-analysis.15 The risk of bias was graded as low, high, or unclear on the basis of each study’s randomization, allocation concealment, blinding of outcome assessment, losses to follow-up, treatment of withdrawals, and selective reporting. For performance and detection bias domains, we judged that, because mortality is objective, it was unlikely to be influenced by lack of blinding as long as a strict protocol for both groups was provided. To determine imprecision, a sample size required to detect a 30% relative risk reduction (optimal information size) was calculated for each comparison for each outcome based on a total event rate in the control group.16,17,18 Funnel plots were used to assess publication bias for each arm of the comparison, and further statistical analysis of funnel plot asymmetry was planned if there were more than 10 trials in each arm.11 Assessment of heterogeneity, consistency, and intransitivity are described in eAppendix 3 in the Supplement.
Statistical Analysis
Standard pairwise meta-analysis methods with random-effect models were used to analyze interventions of eligible RCTs directly, where forest plots were constructed with subsequent calculation of risk ratios and 95% CIs for effect size. The I2 statistic was used to assess statistical heterogeneity in pairwise meta-analysis (eAppendix 3 in the Supplement). For network meta-analysis, we calculated effect sizes by determining risk ratios and 95% credible intervals (CrI) by the Bayesian hierarchical random-effects model, using Markov chain Monte Carlo simulation with noninformative prior distributions.19,20,21,22 For multiarm trials, correction of the treatment effects between arms was taken into account.23 Generalized linear models with a log-link function were applied for the analysis, with 4 chains and 2 000 000 iterated simulations, discarding the initial 1 500 000 iterations as burn-in. Potential scale reduction factor derived from the Brooks-Gelman-Rubin diagnostic was used for assessment of model convergence.24 Model fit was assessed by residual deviance, leverage, and the deviance information criterion.25 Consistency (ie, between-trial differences in the underlying treatment effects between comparisons) was assessed by the node-splitting method (ie, exploring differences between the treatment effects estimated by direct evidence and treatment effects estimated using indirect evidence), and transitivity (ie, the assumption that all treatments are equally likely candidates for the patients in the network) between comparisons was assessed by inspection of differences in potential effect modifiers (eAppendix 3 in the Supplement).
A rank statistic was determined and represented in a rankogram to illustrate the probability that a chosen treatment of all eligible interventions to be investigated was associated with the best, second best, and so on reduction in mortality.26 We also used the surface under cumulative ranking (SUCRA) curve to provide a numerical ranking of the association of all treatments with reduction in mortality, from 0 (certain to be the worst) to 100 (certain to be the best).26
Sensitivity analyses were conducted by excluding trials with high risk of bias and trials without a description of cointerventions. Model fit excluding small-sized trials was assessed as a sensitivity analysis. The other sensitivity analysis was to use Poisson models in the network meta-analysis for the primary outcome to adjust for different follow-up periods.27 Preplanned subgroup analysis was not conducted for the primary and secondary outcomes to assess the association of the distribution of outcome modifiers, including age and ARDS severity (ie, Pao2/Fio2 ratio), because network metaregression with treatment by covariate interactions showed none of the interventions included in our study were affected by age and ARDS severity at the study level (eAppendix 4 and eFigure 1 in the Supplement).
All statistical significance testing was 2-sided, and P < .05 was considered statistically significant. RevMan version 5.1 (The Nordic Cochrane Centre) was used to generate funnel plots and the risk-of-bias tables. Statistical analyses were conducted using R version 3.5.1 (The R Foundation) with R packages gemtc, coda, pcnetmeta, and rjags and using Just Another Giggs Samples version 4.3.0 (JAGS).
Results
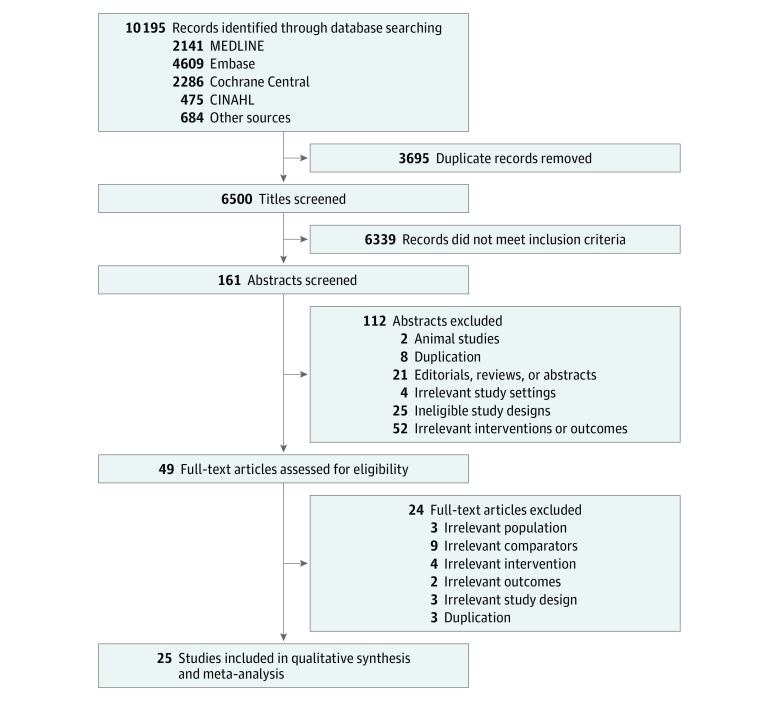
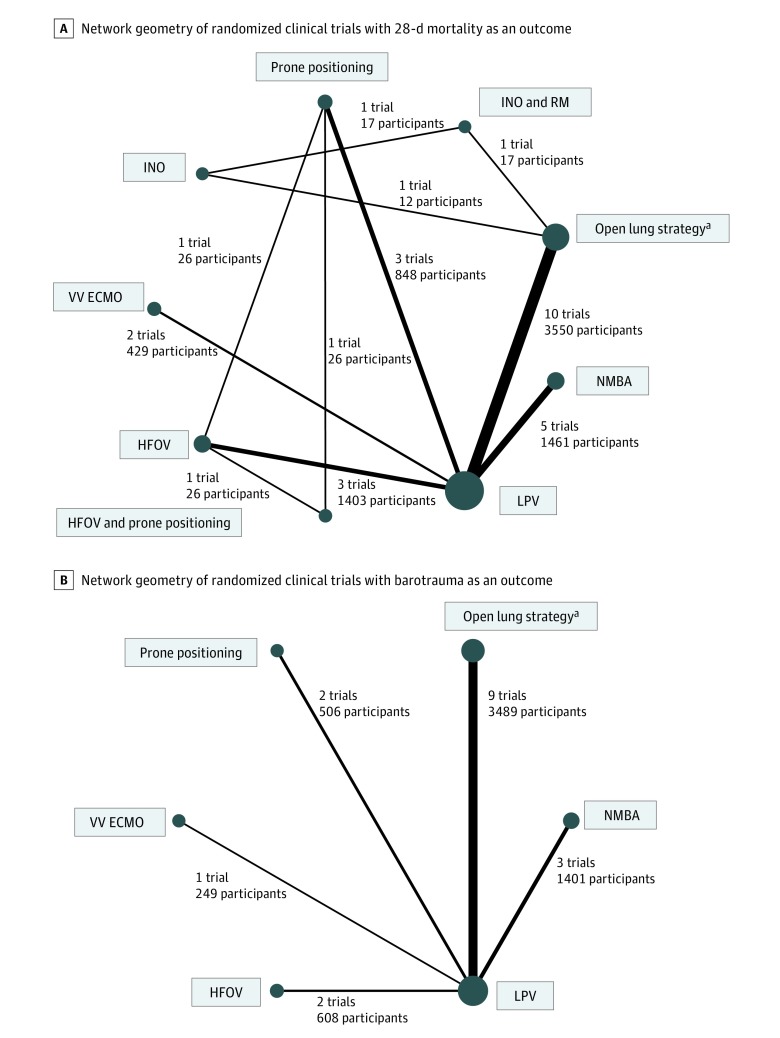
We identified 10 195 records in our electronic search (Figure 1). After screening by title and abstract, we obtained full-text articles for 49 citations that were potentially eligible for inclusion. We included 25 studies (7753 participants; range, 20-1010 participants) in this review (Table 1),7,8,9,10,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 while 24 studies did not meet our inclusion criteria (eTable 1 in the Supplement). There was near-perfect agreement on study inclusion between the 2 reviewers (κ = 0.97). Overall, 9 interventions were investigated (Figure 2) (eFigure 2 and eFigure 3 in the Supplement): LPV (23 trials),7,8,9,10,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 an open lung strategy using RM and/or higher PEEP (10 trials),7,9,39,40,41,42,43,44,45,46 NMBA using a 48-hour infusion of cisatracurium (5 trials),10,35,36,37,38 INO (1 trial),47 INO with RM (1 trial),47 HFOV (3 trials),28,29,30 HFOV with prone positioning (1 trial),48 prone positioning (3 trials),32,33,34 and VV ECMO (2 trials).8,31
Figure 1. PRISMA Flow Diagram.
Table 1. Summary of Identified 25 Studies.
| Source | Study Period | Study Sites (Country) | No. of Patients | Inclusion Criteria of Pao2/Fio2 in AECC Definition or ARDS Severity in Berlin Definition | Protocolized Vt Setting | Experimental/Control Age, Mean (SD), y | Experimental/Control Pao2/Fio2 Ratio at Enrollment, Mean (SD) | Primary Outcome | Experimental/Control, 28-d Mortality, % | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| HFOV | ||||||||||
| Ferguson et al,28 2013 | 2007-2012 | 39 Centers (Canada, the United States, Saudi Arabia, Chile, and India) | 548 | Pao2/Fio2 ≤ 200 | 6 mL/kg PBW | 55 (16)/54 (16) | 121 (46)/114 (38) | Hospital mortality | 40.4/28.6 | Unclear |
| Young et al,29 2013 | 2007-2012 | 29 Centers (United Kingdom) | 795 | Pao2/Fio2 ≤ 200 | 6-8 mL/kg PBW | 55 (19)/56 (16) | 113 (37)/113 (38) | 30-d Mortality | 41.7/41.1 | Low |
| Mohamed and Mohamed,30 2016 | 2007-2011 | Not specified (Saudi Arabia) | 60 | Pao2/Fio2 ≤ 200 | 4-6 mL/kg PBW | 46 (10)/44 (12) | Not reported | 30-d Mortality | 53.3/56.7 | High |
| VV ECMO | ||||||||||
| Peek et al,31 2009 | 2001-2006 | 68 Hospitals (United Kingdom) | 180 | Severea | 4-8 mL/kg PBW | 40 (13)/40 (13) | 76 (30)/75 (36) | 6-mo Mortality | 22.2/46.7 | Unclear |
| Combes et al,8 2018 | 2011-2017 | 23 Centers (France) | 249 | Pao2/Fio2 < 200 | 6 mL/kg of PBW | 52 (14)/54 (13) | 73 (30)/72 (24) | 60-d Mortality | 25.8/36.8 | Unclear |
| Prone Positioning | ||||||||||
| Fernandez et al,32 2008 | 2003-2004 | 17 Hospitals (Spain) | 40 | Pao2/Fio2 ≤ 200 | 6-8 mL/kg PBW | 54 (18)/55 (15) | 153 (59)/158 (84) | 60-d Mortality | 19.0/26.3 | Unclear |
| Taccone et al,33 2009 | 2004-2008 | 25 Centers (Italy and Spain) | 342 | Pao2/Fio2 ≤ 200 | ≤8 mL/kg PBW | Not available | Not available | 28-d Mortality | 31.0/32.8 | Unclear |
| Guérin et al,34 2013 | 2008-2011 | 27 Centers (France and Spain) | 466 | Pao2/Fio2 < 150 | 6 mL/kg PBW | 58 (16)/60 (16) | 100 (30)/100 (20) | 28-d Mortality | 16.0/32.8 | Low |
| NMBA | ||||||||||
| Gainnier et al,35 2004 | 2000-2001 | 4 Centers (France) | 56 | Pao2/Fio2 < 150 | 6-8 mL/kg PBW | 60 (18)/62 (15) | 130 (34)/119 (31) | Pao2/FIO2 during 120 h after randomization | 35.7/60.7 | Low |
| Forel et al,36 2006 | 2002-2003 | 3 Centers (France) | 36 | Pao2/Fio2 ≤ 200 | 4-8 mL/kg PBW | 52 (16)/61 (18) | Not available | Pulmonary and systemic inflammatory response | 27.8/55.6 | Low |
| Papazian et al,37 2010 | 2006-2008 | 20 Centers (France) | 339 | Pao2/Fio2 < 150 | 6-8 mL/kg PBW | 58 (16)/58 (15) | 106 (36)/115 (41) | 90-d Mortality | 23.7/33.3 | Low |
| Guervilly et al,38 2017 | 2012-2014 | 2 Centers (France) | 24 | Moderate | 6 mL/kg PBW | 72 (63-79)/60 (52-75)b | 158 (131-185)/150 (121-187)b | Change in transpulmonary pressure | 38.5/27.3 | Low |
| Moss et al,10 2019 | 2016-2018 | 48 Centers (United States) | 1006 | Pao2/Fio2 < 150 | 6 mL/kg PBW | 56.6 (14.7)/55.1 (15.9) | 98.7 (27.9)/99.5 (27.9) | 90-d Mortality | 36.7/37.0 | Low |
| Open Lung Strategy Using RM and/or Higher PEEP | ||||||||||
| Brower et al,39 2004 | 1999-2002 | 23 Centers (United States) | 549 | Allc | 6 mL/kg PBW | 54 (17)/49 (17) | 151 (67)/165 (77) | Hospital mortality | 24.2/28.7c | High |
| Meade et al,40 2008 | 2000-2006 | 30 Centers (Canada, Australia, and Saudi Arabia) | 983 | Pao2/Fio2 ≤ 250c | 6 mL/kg PBW with allowances for 4-8 mL | 55 (17)/57 (17) | 145 (48)/145 (49) | Hospital mortality | Low | |
| Mercat et al,41 2008 | 2002-2005 | 37 Centers (France) | 767 | Allc | 6 mL/kg PBW | 60 (16)/60 (15) | 144 (58)/143 (57) | 28-d Mortality | Low | |
| Talmor et al,42 2008 | 2004-2007 | 1 Center (United States) | 61 | Alld | 6 mL/kg PBW | 55 (16)/51 (23) | 147 (56)/145 (57) | Pao2/FIO2 72 h after randomization | 16.7/38.7 | Low |
| Huh et al,43 2009 | 2004-2006 | 1 Center (Korea) | 57 | Pao2/Fio2 ≤ 200 | 6 mL/kg PBW with allowances up to 8 mL | 55 (4)/62 (2) | 115 (9)/111 (6) | Pao2/FIO2 | 40.0/33.3 | Unclear |
| Xi et al,44 2010 | 2003-2006 | 14 Centers (China) | 110 | Pao2/Fio2 ≤ 200 | 6-8 mL/kg PBW | 62 (16)/66 (15) | 94 (69-150)/120 (88-140)b | ICU mortality | 29.1/43.6 | High |
| Hodgson et al,45 2011 | 2008-2009 | 1 Center (Australia) | 20 | Pao2/Fio2 ≤ 200 | ≤6 mL/kg PBW | 60 (5)/58 (4) | 155 (8)/149 (12) | IL-6 level | 50.0/20.0 | Low |
| Kacmarek et al,46 2016 | 2007-2013 | 20 Centers (Spain, Brazil, South Korea, Peru, Chile, and United States) | 200 | Pao2/Fio2 ≤ 200 | 4-8 mL/kg PBW | 52 (15)/53 (15) | 121 (37)/114 (33) | 60-d Mortality | 22.2/26.7 | Low |
| Cavalcanti et al,7 2017 | 2011-2017 | 120 Centers (Brazil, Argentina, Colombia, Italy, Poland, Portugal, Malaysia, Spain, and Uruguay) | 1010 | Moderate to severe | 4-6 mL/kg PBW | 51 (17)/51 (17) | 120 (44)/117 (42) | 28-d Mortality | 55.3/49.3 | Low |
| Beitler et al,9 2019 | 2012-2017 | 14 Centers (United States and Canada) | 200 | Moderate to severe | 4-8 mL/kg PBW | 58 (47-66)/57.5 (43-69)b | 95 (73-129)/90 (69-123)b | Ranked composite incorporating death and days free of mechanical ventilation among survivors | 30.6/32.4 | Low |
| INO and RM | ||||||||||
| Park et al,47 2003 | Not stated | 1 Center (Korea) | 23 | Pao2/Fio2 ≤ 200 | 6 mL/kg PBW | 52 (17)/50 (20)/59 (13)e | 140 (27)/162 (19)/105 (14)e | Not specified | 36.4/33.3/66.7e | High |
| HFOV and Prone Positioning | ||||||||||
| Papazian et al,48 2005 | Not stated | 1 Center (France) | 39 | Pao2/Fio2 < 150 | 6 mL/kg PBW | 55 (15)/51 (12)/51 (9)e | 106 (31)/101 (22)/103 (41)e | Not specified | 30.8/23.1/38.5e | Unclear |
Abbreviations: AECC, American-European Consensus Conference; ARDS, acute respiratory distress syndrome; HFOV, high-frequency oscillatory ventilation; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IL-6, interleukin 6; INO, inhaled nitric oxide; NMBA, neuromuscular blockade; PBW, predicted body weight; PEEP, positive end-expiratory pressure; RM, recruitment maneuver; Vt, tidal volume; VV ECMO, venovenous extracorporeal membrane oxygenation.
Severe but potentially reversible respiratory failure, Murray score 3.0 or higher, or uncompensated hypercapnia with pH less than 7.20.
Median (interquartile range) reported.
A subsequent systematic review of individual patient-level data, including Brower et al,39 Meade et al,40 Mercat et al,41 reported the total number of events and patients who were diagnosed as having ARDS with Pao2/Fio2 less than 200. These data were extracted and used for further analyses in this study.
Nearly all enrolled patients had moderate or severe ARDS.
Study included 3 arms.
Figure 2. Network Geometry and Ranking Probabilities for the Association of Interventions With Outcomes.
Network geometry shows the interventions as nodes and the direct comparisons as connections between the nodes. Larger node size indicates a larger total number of participants in arms including that intervention, and thicker connection indicates a larger number of trials investigating that comparison. HFOV indicates high-frequency oscillatory ventilation; INO, inhaled nitric oxide; LPV, lung protective ventilation; NMBA, neuromuscular blockade; RM, recruitment maneuver; and VV ECMO, venovenous extracorporeal membrane oxygenation.
aOpen lung strategy using RM and/or higher positive end-expiratory pressure.
There was high risk of bias for 1 or more key domains in 4 studies (eFigures 4-6 in the Supplement).30,39,44,47 All trials specified standardized protocols for the implementation of each intervention and ventilatory management; thus, blinding domains were judged low for our primary outcome. As a result, 14 of 25 studies (56%) had a low risk of bias across all domains.7,9,10,29,34,35,36,37,38,40,41,42,45,46
Among the 25 studies, overall 28-day mortality was 34.6% (2686 of 7753 patients). Overall, LPV was the most frequently investigated intervention, whereas 3 interventions were investigated by only 1 trial (Figure 2). Compared with LPV alone, prone positioning (risk ratio, 0.69; 95% CrI, 0.48-0.98; low quality of evidence) and VV ECMO (risk ratio, 0.60; 95% CrI, 0.38-0.93; moderate quality of evidence) were associated with significantly lower 28-day mortality (Table 2) (eTable 2, eFigure 2, and eFigure 7 in the Supplement). Prone positioning prevented 124 more deaths per 1000 patients compared with LPV alone, and VV ECMO prevented 161 more deaths per 1000 patients compared with LPV alone (Table 2). The results of comparisons between all possible pairs of interventions with quality of evidence are summarized in eTable 3 in the Supplement. In a sensitivity analysis restricted to studies without a high risk of bias (12 studies; 4213 patients) and studies with a description of cointerventions (17 studies; 7196 patients), prone positioning and VV ECMO remained significantly associated with lower 28-day mortality (eTable 4 and eTable 5 in the Supplement). A sensitivity analysis using Poisson models showed that VV ECMO and prone positioning were significantly associated with reduced mortality (eTable 6 in the Supplement). Exclusion of small-sized trials did not change model fit in the network meta-analysis (eTables 7-9 in the Supplement).
Table 2. Summary of Findings for 28-Day Mortality.
| Comparison | No. | Network Risk Ratio (95% CrI) | Anticipated Absolute Effecta | Quality of Evidence | ||
|---|---|---|---|---|---|---|
| Patients | Trials | With Intervention per 1000 | Difference (95% CrI) | |||
| LPV | NA | NA | 1 [Reference] | 401 | NA | NA |
| VV ECMO | 429 | 2 | 0.60 (0.38 to 0.93) | 240 | −161 (−249 to −28) | Moderate |
| HFOV | 1403 | 3 | 1.12 (0.83 to 1.54) | 449 | 48 (−68 to 200) | Low |
| HFOV and prone positioning | NA | Indirect evidence | 0.53 (0.12 to 1.60) | 212 | −188 (−353 to 241) | Very low |
| NMBA | 956 | 5 | 0.79 (0.57 to 1.02) | 318 | −83 (−173 to 8) | High |
| Open lung strategyb | 3452 | 10 | 0.96 (0.77 to 1.14) | 385 | −16 (−92 to 56) | Low |
| INO and RM | NA | Indirect evidence | 0.86 (0.22 to 3.83) | 345 | −56 (−313 to 599) | Very low |
| Prone positioning | 848 | 3 | 0.69 (0.48 to 0.98) | 277 | −124 (−208 to −8) | Low |
| INO | NA | Indirect evidence | 1.48 (0.42 to 6.08) | 593 | 192 (−233 to 599) | Very low |
| VV ECMO | NA | NA | 1 [Reference] | 240 | NA | NA |
| HFOV | NA | Indirect evidence | 1.88 (1.12 to 3.24) | 451 | 211 (29 to 538) | Low |
| HFOV and prone positioning | NA | Indirect evidence | 0.87 (0.19 to 2.90) | 209 | −31 (−194 to 456) | Very low |
| NMBA | NA | Indirect evidence | 1.30 (0.77 to 2.14) | 312 | 72 (−55 to 274) | Very low |
| Open lung strategyb | NA | Indirect evidence | 1.59 (0.99 to 2.56) | 382 | 142 (−2 to 374) | Very low |
| INO and RM | NA | Indirect evidence | 1.43 (0.34 to 6.60) | 343 | 103 (−158 to 640) | Very low |
| Prone positioning | NA | Indirect evidence | 1.15 (0.66 to 2.01) | 276 | 36 (−82 to 242) | Very low |
| INO | NA | Indirect evidence | 2.48 (0.67 to 10.90) | 595 | 355 (−79 to 760)b | Very low |
| HFOV | NA | NA | 1 [Reference] | 435 | NA | NA |
| HFOV and prone positioning | 26 | 1 | 0.47 (0.11 to 1.44) | 204 | −230 (−387 to 191) | Low |
| NMBA | NA | Indirect evidence | 0.70 (0.48 to 1.02) | 304 | −130 (−226 to 9) | Moderate |
| Open lung strategyb | NA | Indirect evidence | 0.85 (0.58 to 1.19) | 370 | −65 (−183 to 83) | Low |
| INO and RM | NA | Indirect evidence | 0.76 (0.18 to 3.50) | 331 | −104 (−357 to 565) | Very low |
| Prone positioning | 26 | 1 | 0.61 (0.39 to 0.95) | 265 | −170 (−265 to −22) | Moderate |
| INO | NA | Indirect evidence | 1.32 (0.35 to 5.51) | 574 | 139 (−283 to 565) | Very low |
| HFOV and prone positioning | NA | NA | 1 [Reference] | 231 | NA | NA |
| NMBA | NA | Indirect evidence | 1.48 (0.45 to 6.63) | 342 | 111 (−127 to 769) | Very low |
| Open lung strategyb | NA | Indirect evidence | 1.80 (0.56 to 8.01) | 416 | 184 (−102 to 769) | Very low |
| INO and RM | NA | Indirect evidence | 1.67 (0.26 to 13.36) | 386 | 155 (−171 to 769) | Very low |
| Prone positioning | 26 | 1 | 1.31 (0.41 to 5.83) | 303 | 72 (−136 to 769) | Low |
| INO | NA | Indirect evidence | 2.93 (0.49 to 21.97) | 677 | 446 (−118 to 769) | Very low |
| NMBA | NA | NA | 1 [Reference] | 314 | NA | NA |
| Open lung strategyb | NA | Indirect evidence | 1.22 (0.90 to 1.72) | 383 | 69 (−31 to 226) | Very low |
| INO and RM | NA | Indirect evidence | 1.10 (0.28 to 5.04) | 345 | 31 (−226 to 686) | Very low |
| Prone positioning | NA | Indirect evidence | 0.88 (0.56 to 1.40) | 276 | 38 (−138 to 126) | Very low |
| INO | NA | Indirect evidence | 1.91 (0.51 to 8.23) | 600 | 286 (−154 to 686) | Very low |
| Open lung strategyb | NA | NA | 1 [Reference] | 403 | NA | NA |
| INO and RM | 17 | 1 | 0.90 (0.24 to 3.91) | 363 | −40 (−306 to 597) | Very low |
| Prone positioning | NA | Indirect evidence | 0.72 (0.48 to 1.11) | 290 | −113 (−210 to 34) | Very low |
| INO | 12 | 1 | 1.55 (0.44 to 6.28) | 625 | 222 (−226 to 597) | Very low |
| INO and RM | NA | NA | 1 [Reference] | 364 | NA | NA |
| Prone positioning | NA | Indirect evidence | 0.80 (0.18 to 3.22) | 291 | −73 (−299 to 636) | Very low |
| INO | 17 | 1 | 1.74 (0.51 to 5.94) | 633 | 269 (−128 to 636) | Very low |
| Prone positioning | NA | NA | 1 [Reference] | 242 | NA | NA |
| INO | NA | Indirect evidence | 2.15 (0.59 to 8.99) | 520 | 278 (−99 to 758) | Very low |
Abbreviations: CrI, credible interval; HFOV, high-frequency oscillatory ventilation; INO, inhaled nitric oxide; LPV, lung protective ventilation; NA, not applicable; NMBA, neuromuscular blockade; RM, recruitment maneuver; VV ECMO, venovenous extracorporeal membrane oxygenation.
To compute anticipated absolute effect, risk ratio is less than or equal to 1 divided by event rate in the reference group (ie, 1/average control risk).
Open lung strategy using RM and/or higher positive end-expiratory pressure.
The incidence of barotrauma was 7.2% (448 of 6253 patients) from 17 trials evaluating 6 interventions (Figure 2). There were no significant differences between interventions in the risk of barotrauma, with variable quality of evidence (Table 3) (eTable 10, eTable 11, eFigure 3, and eFigure 7 in the Supplement).
Table 3. Summary of Findings for Barotrauma.
| Comparison | No. | Network Risk Ratio (95% CrI) | Anticipated Absolute Effecta | Quality of Evidence | ||
|---|---|---|---|---|---|---|
| Patients | Trials | With Intervention per 1000 | Difference (95% CrI) | |||
| LPV | NA | NA | 1 [Reference] | 68 | NA | NA |
| VV ECMO | 249 | 1 | 1.19 (0.24 to 5.88) | 81 | 13 (−52 to 332) | Moderate |
| HFOV | 608 | 2 | 1.69 (0.55 to 7.14) | 115 | 47 (−31 to 417) | Low |
| NMBA | 896 | 3 | 0.47 (0.18 to 1.03) | 32 | −36 (−56 to 2) | Low |
| Open lung strategyb | 3391 | 9 | 1.11 (0.54 to 1.84) | 75 | 7 (−31 to 57) | Low |
| Prone positioning | 506 | 2 | 0.78 (0.19 to 2.32) | 53 | −15 (−55 to 90) | Low |
| VV ECMO | NA | NA | 1 [Reference] | 145 | NA | NA |
| HFOV | NA | Indirect evidence | 1.35 (0.21 to 8.47) | 196 | 51 (−115 to 855) | Very low |
| NMBA | NA | Indirect evidence | 0.39 (0.07 to 1.86) | 56 | −89 (−135 to 125) | Very low |
| Open lung strategyb | NA | Indirect evidence | 0.94 (0.18 to 3.67) | 136 | −7 (−119 to 387) | Very low |
| Prone positioning | NA | Indirect evidence | 0.65 (0.08 to 3.55) | 94 | −51 (−133 to 370) | Very low |
| HFOV | NA | NA | 1 [Reference] | 134 | NA | NA |
| NMBA | NA | Indirect evidence | 0.29 (0.05 to 0.92) | 39 | −95 (−128 to −11) | Very low |
| Open lung strategyb | NA | Indirect evidence | 0.69 (0.14 to 1.88) | 92 | −42 (−115 to 118) | Very low |
| Prone positioning | NA | Indirect evidence | 0.48 (0.06 to 1.89) | 64 | −70 (−126 to 119) | Very low |
| NMBA | NA | NA | 1 [Reference] | 25 | NA | NA |
| Open lung strategyb | NA | Indirect evidence | 2.36 (0.79 to 6.91) | 59 | 34 (−5 to 148) | Very low |
| Prone positioning | NA | Indirect evidence | 1.66 (0.34 to 7.03) | 41 | 16 (−17 to 151) | Very low |
| Open lung strategyb | NA | NA | 1 [Reference] | 59 | NA | NA |
| Prone positioning | NA | Indirect evidence | 0.70 (0.17 to 2.61) | 41 | −18 (−49 to 95) | Very low |
Abbreviations: CrI, credible interval; HFOV, high-frequency oscillatory ventilation; LPV, lung protective ventilation; NA, not applicable; NMBA, neuromuscular blockade; VV ECMO, venovenous extracorporeal membrane oxygenation.
To compute anticipated absolute effect, risk ratio is less than or equal to 1 divided by event rate in the reference group (ie, 1/average control risk).
Open lung strategy using recruitment maneuver and/or higher positive end-expiratory pressure.
For mortality, both VV ECMO and prone positioning were ranked highly on the basis of SUCRA, with VV ECMO being highest (0.82). However, the ranking probability for VV ECMO did not differ significantly from prone positioning (eFigure 8 in the Supplement). For barotrauma, NMBA had the highest SUCRA (0.93), although no intervention was significantly different from any other in reducing barotrauma in studies reporting this outcome (eFigure 8 in the Supplement).
There were 36 direct or indirect comparisons for the primary outcome among 25 studies (eTable 3 in the Supplement). Only 7 comparisons were not affected by moderate or high heterogeneity (eTable 3 in the Supplement). Comparisons of LPV, HFOV, and open lung strategy using RM and/or higher PEEP in an arm with any intervention in the other arm met the optimal information size for imprecision. Node splitting found no significant inconsistency in 3 comparisons (HFOV vs LPV, prone positioning vs LPV, and prone positioning vs HFOV) (eTable 12 in the Supplement). Intransitivity was not found based on network metaregression with treatment by covariate interactions to measure potential effect modifiers. Based on these findings in the primary outcome among all 36 comparisons, the quality of evidence for network effects estimates was judged as high in 1 comparison, moderate in 4, low in 6, and very low in 25 (eTable 3 in the Supplement).
No comparison for barotrauma had a high risk of bias or met the optimal information size in imprecision (eTable 11 in the Supplement). All comparisons, except VV ECMO vs LPV, showed moderate to high heterogeneity. Because there was no closed loop of interventions for barotrauma, the assumption of consistency in any comparison cannot be violated. Intransitivity was not found. The quality of evidence for network effects estimates in barotrauma was judged as moderate in 1 comparison, low in 4, and very low in 10 (eTable 11 in the Supplement).
Discussion
Our systematic review and network meta-analysis of 25 RCTs, which included 7753 patients and 9 interventions, found that prone positioning was associated with significantly lower 28-day mortality in patients with moderate ARDS compared with LPV alone. In patients with severe ARDS, VV ECMO was associated with significantly lower 28-day mortality. Furthermore, these interventions were highly ranked, with VV ECMO being the highest, although there were no significant differences in ranking probabilities between these interventions. No intervention was superior to any other in reducing barotrauma.
While the use of LPV remains the mainstay of supportive care in patients with ARDS,6 LPV alone may be insufficient to maintain adequate gas exchange or prevent ventilator-induced lung injury, and adjunctive interventions may be required. While many of these adjunctive interventions have been evaluated in RCTs or meta-analyses, to our knowledge, there are limited data comparing their relative efficacy with each other. Wang et al49 assessed 26 ventilatory strategies in their network meta-analysis for ARDS but divided the interventions into complex groupings, making clinical interpretation difficult. Moreover, they made comparisons with a strategy of higher tidal volumes that has little clinical relevance in the current management of patients with ARDS.49 In contrast, we decided to focus on a limited number of interventions that are commonly used in patients with moderate to severe ARDS1 rather than include all possibilities to provide the most relevant guidance for clinicians at the bedside.
Our results are consistent with the strong recommendations from the American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine clinical practice guidelines6 based on conventional (pairwise) meta-analyses. Specifically, our study supports the use of prone positioning (ie, significant reduction in mortality and high ranking) as well as the strong recommendation against the routine use of HFOV (ie, increased mortality and low ranking). The conditional recommendations for RMs or higher PEEP did not include the results of the ART7 or the EPVent2 trial.9 Our pooled results did not find an association of RMs or higher PEEP with mortality. Importantly, the guideline could not make a recommendation on the use of VV ECMO in patients with severe ARDS owing to insufficient data. Our study supports the earlier consideration of VV ECMO along with LPV in patients with severe ARDS, consistent with the results of the EOLIA trial,8 the post hoc Bayesian reanalysis of that trial,50 and the updated meta-analysis of VV ECMO studies.51 The results for INO, which were not included in the guideline, are consistent with the 2016 meta-analysis52 that showed an increased risk for renal failure with no significant mortality benefit for INO. Finally, although the most recent meta-analysis of NMBA, to our knowledge, showed a reduction in mortality,53 our study results are consistent with the results of the ROSE trial,10 in which NMBA did not improve mortality of patients with moderate to severe ARDS.
By including the ART,7 EPVent2,9 EOLIA,8 and ROSE trials10 in our network meta-analysis, our findings could inform future updates of the clinical practice guidelines and decision-making process at the bedside. Given the limited clinical interpretation of SUCRA (relative efficacy assessment on eligible interventions),26 our findings demonstrate that prone positioning and VV ECMO should be considered early, with implementation of these interventions influenced by individual patient characteristics or available clinical resources. Our preplanned metaregression confirmed that ARDS severity (ie, aggregated Pao2/Fio2 ratio at study level) did not affect the findings of these analyses. However, VV ECMO should be restricted to severe ARDS, since the 2 included RCTs of VV ECMO only recruited patients with severe ARDS. Moreover, in addition to the statistical heterogeneity in severity among eligible studies, considerations such as the invasiveness, availability of resources, and need for clinical experience may also limit the use of VV ECMO to the patients with the most severe ARDS in high-volume centers.54 Therefore, prone positioning should be considered as a first-line approach for patients with moderate to severe ARDS in addition to LPV. Timely transfer to an ECMO-capable center and VV ECMO should be considered for patients with severe ARDS who have contraindications or who fail prone positioning, similar to the EOLIA trial.8 The strategy of HFOV combined with prone positioning was associated with a nonsignificant reduction in mortality but with a high SUCRA; however, inferences about its clinical efficacy and relevance remain uncertain owing to limitations from a single study with very small sample size.48 The lower rankings of the other 5 interventions (NMBA, HFOV, INO, INO with RM, and open lung strategy using RM and/or higher PEEP) suggests against their routine use.
Limitations
This study has several limitations. First, the network meta-analysis depends on the assumption that population and intervention characteristics were largely similar across the included studies (ie, transitivity). Thus, an analysis including trials applying different thresholds of Pao2/Fio2 ratio (ie, severity of ARDS) for the patient recruitment is subject to bias. For example, the EOLIA trial only enrolled patients with severe ARDS. Furthermore, this assumption may also be violated by the difference in the period of those trials performed. We confirmed that there was no effect modification of Pao2/Fio2 ratio on treatment by covariate interaction by network metaregressions with Pao2/Fio2 ratio (eFigure 1 in the Supplement). However, the most robust method for evaluating the potential for effect modification by Pao2/Fio2 ratio would be with a meta-analysis of individual patient data. Second, some studies allowed or recommended cointerventions in some settings for both experimental and control groups (eg, prone positioning for certain Pao2/Fio2 ratios in a study of NMBA) but not in all participants. However, many studies did not explicitly describe the use of cointerventions in the protocol or results of their published articles. We conducted a sensitivity analysis excluding studies without a description of cointerventions, which was robust for the main analysis (eTable 5 and eTable 13 in the Supplement). Moreover, we attempted to address this limitation only by downrating the quality in GRADE assessment. Future trials are strongly encouraged to provide a detailed description of cointerventions used or to protocolize their implementation within the trial. Third, we only evaluated short-term mortality. However, we believe that this approach is theoretically justified because most interventions studied have the main rationale of reducing ventilator-induced lung injury and more immediate death. Future studies should include an evaluation of long-term mortality and functional outcomes. Fourth, the adverse effects of treatments were limited because of the nature of different interventions. We only assessed barotrauma, and there were differences in definition of barotrauma in each study. Fifth, although intervention from smaller studies (eg, INO, HFOV with prone positioning) can lead to imprecision of estimated effects (ie, wider CrIs), we confirmed that excluding such small-sized studies did not change overall model fitting of the network meta-analysis and the other main findings (eTable 7-9 in the Supplement). Sixth, we could not specify 28-day mortality in 5 of 25 trials (20%) and computed mortality assuming constant risk of death during the study period. An alternative way to address this issue was to use Poisson models in the network meta-analysis and use follow-up period as offset in the model. Using this approach, we found no differences between Poisson models and our primary analyses (eTable 6 in the Supplement). Seventh, we did not account for covariates (ie, age and Pao2/Fio2 ratio) in the final models of network meta-analysis because we did not find treatment by covariate interaction from network metaregressions with these covariates. However, these findings were based on study-level data instead of individual patient data, so aggregation bias still remains possible. Eighth, a meta-analysis with individual patient data may be desirable to further separate the effectiveness of each intervention and explore outcomes in important patient subgroups. Despite these limitations, the use of network meta-analysis enabled us to compare clinically relevant management strategies in patients with ARDS. Ninth, we used robust methods as recommended by the Cochrane Collaboration, PRISMA, and GRADE for network meta-analysis.11,12,15
Conclusions
This network meta-analysis supports the use of prone positioning and VV ECMO in addition to LPV in patients with moderate to severe ARDS and severe ARDS, respectively. In addition, our results are consistent with recent data suggesting that VV ECMO may be considered as an early strategy for adults with severe ARDS.
eAppendix 1. Summary of Inclusion and Exclusion Criteria
eAppendix 2. Updated Search Strategy in MEDLINE, Embase, Cochrane, PubMed, and CINAHL
eAppendix 3. Assessment of Heterogeneity, Consistency, and Intransitivity
eAppendix 4. Network Metaregression With Treatment by Covariate Interactions to Measure the Impact of Age and ARDS Severity
eFigure 1. Results of Network Metaregression for Age (a) and ARDS Severity (b)
eFigure 2. Forest Plots of Direct, Indirect, and Pooled Comparisons for Primary Outcome
eFigure 3. Forest Plots of Direct, Indirect, and Pooled Comparisons for Secondary Outcome
eFigure 4. Risk-of-Bias Graph for All Eligible Studies
eFigure 5. Risk-of-Bias Summary for All Eligible Studies
eFigure 6. Funnel Plots for Primary (a) and (b) Secondary Outcomes
eFigure 7. Gelman Plots for Model Convergence With 4 Chains and 2 000 000 Iterated Simulations, Discarding the Initial 1 500 000 Iterations as Burn-in on Primary (a) and (b) Secondary Outcomes
eFigure 8. Ranking Probabilities for the Association of Interventions With Outcomes
eTable 1. Detailed Explanation for Excluded Studies With Full-Text Assessment
eTable 2. Comparison of the Included Interventions in Risk Ratio (95% CrI) for 28-Day Mortality
eTable 3. Network Meta-analysis for 28-Day Mortality and Quality of Evidence Assessment
eTable 4. Results of Sensitivity Analysis for 28-Day Mortality
eTable 5. Results of Preliminary Analysis Excluding Studies Without Description of Cointerventions
eTable 6. Results of Analysis Using Poisson Models for the Primary Outcome Adjusting for Different Timing of the Measurement
eTable 7. Potential Scale Reduction Factor From Brooks-Gelman-Rubin Diagnostic for Model Convergence on Primary (a) and (b) Secondary Outcomes
eTable 8. Assessment of Model Fits for Primary (a) and (b) Secondary Outcomes
eTable 9. Results of Assessment on Small Sample Size Effects
eTable 10. Comparison of the Included Interventions in Risk Ratio (95% CrI) for Barotrauma
eTable 11. Network Meta-analysis for Barotrauma and Quality of Evidence Assessment
eTable 12. Results of Node-Splitting Models
eTable 13. Summary of Cointerventions in Included Studies
eReferences
References
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):-. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319-323. doi: 10.1016/S0140-6736(67)90168-7 [DOI] [PubMed] [Google Scholar]
- 3.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698-710. doi: 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]
- 4.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562-572. doi: 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126-2136. doi: 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
- 6.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 7.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. ; Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators . Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335-1345. doi: 10.1001/jama.2017.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet . Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965-1975. doi: 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 9.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. ; EPVent-2 Study Group . Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846-857. doi: 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss M, Huang DT, Brower RG, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network . Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. doi: 10.1056/NEJMoa1901686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 http://handbook-5-1.cochrane.org/. Accessed June 27, 2019.
- 12.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 13.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213-220. doi: 10.1037/h0026256 [DOI] [PubMed] [Google Scholar]
- 15.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6: rating the quality of evidence: imprecision. J Clin Epidemiol. 2011;64(12):1283-1293. doi: 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Brignardello-Petersen R, Murad MH, Walter SD, et al. ; GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60-67. doi: 10.1016/j.jclinepi.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2002;21(11):1601-1623. doi: 10.1002/sim.1189 [DOI] [PubMed] [Google Scholar]
- 20.van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80-93. doi: 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897-900. doi: 10.1136/bmj.331.7521.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285-299. doi: 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- 23.Franchini AJ, Dias S, Ades AE, Jansen JP, Welton NJ. Accounting for correlation in network meta-analysis with multi-arm trials. Res Synth Methods. 2012;3(2):142-160. doi: 10.1002/jrsm.1049 [DOI] [PubMed] [Google Scholar]
- 24.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434-455. doi: 10.1080/10618600.1998.10474787 [DOI] [Google Scholar]
- 25.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64(4):583-639. doi: 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 26.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 27.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607-617. doi: 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson ND, Cook DJ, Guyatt GH, et al. ; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group . High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795-805. doi: 10.1056/NEJMoa1215554 [DOI] [PubMed] [Google Scholar]
- 29.Young D, Lamb SE, Shah S, et al. ; OSCAR Study Group . High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806-813. doi: 10.1056/NEJMoa1215716 [DOI] [PubMed] [Google Scholar]
- 30.Mohamed SAR, Mohamed NN. Efficacy and adverse events of early high-frequency oscillatory ventilation in adult burn patients with acute respiratory distress syndrome. Egypt J Anaesth. 2016;32(3):421-429. doi: 10.1016/j.egja.2016.01.001 [DOI] [Google Scholar]
- 31.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration . Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363. doi: 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 32.Fernandez R, Trenchs X, Klamburg J, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008;34(8):1487-1491. doi: 10.1007/s00134-008-1119-3 [DOI] [PubMed] [Google Scholar]
- 33.Taccone P, Pesenti A, Latini R, et al. ; Prone-Supine II Study Group . Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977-1984. doi: 10.1001/jama.2009.1614 [DOI] [PubMed] [Google Scholar]
- 34.Guérin C, Reignier J, Richard J-C, et al. ; PROSEVA Study Group . Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159-2168. doi: 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 35.Gainnier M, Roch A, Forel J-M, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32(1):113-119. doi: 10.1097/01.CCM.0000104114.72614.BC [DOI] [PubMed] [Google Scholar]
- 36.Forel J-M, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34(11):2749-2757. doi: 10.1097/01.CCM.0000239435.87433.0D [DOI] [PubMed] [Google Scholar]
- 37.Papazian L, Forel J-M, Gacouin A, et al. ; ACURASYS Study Investigators . Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107-1116. doi: 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 38.Guervilly C, Bisbal M, Forel JM, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(3):408-418. doi: 10.1007/s00134-016-4653-4 [DOI] [PubMed] [Google Scholar]
- 39.Brower RG, Lanken PN, MacIntyre N, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327-336. doi: 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 40.Meade MO, Cook DJ, Guyatt GH, et al. ; Lung Open Ventilation Study Investigators . Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637-645. doi: 10.1001/jama.299.6.637 [DOI] [PubMed] [Google Scholar]
- 41.Mercat A, Richard JC, Vielle B, et al. ; Expiratory Pressure (Express) Study Group . Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646-655. doi: 10.1001/jama.299.6.646 [DOI] [PubMed] [Google Scholar]
- 42.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095-2104. doi: 10.1056/NEJMoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh JW, Jung H, Choi HS, Hong S-B, Lim C-M, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22. doi: 10.1186/cc7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xi XM, Jiang L, Zhu B; RM group . Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl). 2010;123(21):3100-3105. [PubMed] [Google Scholar]
- 45.Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133. doi: 10.1186/cc10249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kacmarek RM, Villar J, Sulemanji D, et al. ; Open Lung Approach Network . Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med. 2016;44(1):32-42. doi: 10.1097/CCM.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 47.Park KJ, Lee YJ, Oh YJ, Lee KS, Sheen SS, Hwang SC. Combined effects of inhaled nitric oxide and a recruitment maneuver in patients with acute respiratory distress syndrome. Yonsei Med J. 2003;44(2):219-226. doi: 10.3349/ymj.2003.44.2.219 [DOI] [PubMed] [Google Scholar]
- 48.Papazian L, Gainnier M, Marin V, et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(10):2162-2171. doi: 10.1097/01.CCM.0000181298.05474.2B [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Wang X, Chi C, et al. Lung ventilation strategies for acute respiratory distress syndrome: a systematic review and network meta-analysis. Sci Rep. 2016;6:22855. doi: 10.1038/srep22855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251-2259. doi: 10.1001/jama.2018.14276 [DOI] [PubMed] [Google Scholar]
- 51.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(2):163-172. doi: 10.1016/S2213-2600(18)30452-1 [DOI] [PubMed] [Google Scholar]
- 52.Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;(6):CD002787. doi: 10.1002/14651858.CD002787.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17(2):R43. doi: 10.1186/cc12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality: analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894-901. doi: 10.1164/rccm.201409-1634OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Summary of Inclusion and Exclusion Criteria
eAppendix 2. Updated Search Strategy in MEDLINE, Embase, Cochrane, PubMed, and CINAHL
eAppendix 3. Assessment of Heterogeneity, Consistency, and Intransitivity
eAppendix 4. Network Metaregression With Treatment by Covariate Interactions to Measure the Impact of Age and ARDS Severity
eFigure 1. Results of Network Metaregression for Age (a) and ARDS Severity (b)
eFigure 2. Forest Plots of Direct, Indirect, and Pooled Comparisons for Primary Outcome
eFigure 3. Forest Plots of Direct, Indirect, and Pooled Comparisons for Secondary Outcome
eFigure 4. Risk-of-Bias Graph for All Eligible Studies
eFigure 5. Risk-of-Bias Summary for All Eligible Studies
eFigure 6. Funnel Plots for Primary (a) and (b) Secondary Outcomes
eFigure 7. Gelman Plots for Model Convergence With 4 Chains and 2 000 000 Iterated Simulations, Discarding the Initial 1 500 000 Iterations as Burn-in on Primary (a) and (b) Secondary Outcomes
eFigure 8. Ranking Probabilities for the Association of Interventions With Outcomes
eTable 1. Detailed Explanation for Excluded Studies With Full-Text Assessment
eTable 2. Comparison of the Included Interventions in Risk Ratio (95% CrI) for 28-Day Mortality
eTable 3. Network Meta-analysis for 28-Day Mortality and Quality of Evidence Assessment
eTable 4. Results of Sensitivity Analysis for 28-Day Mortality
eTable 5. Results of Preliminary Analysis Excluding Studies Without Description of Cointerventions
eTable 6. Results of Analysis Using Poisson Models for the Primary Outcome Adjusting for Different Timing of the Measurement
eTable 7. Potential Scale Reduction Factor From Brooks-Gelman-Rubin Diagnostic for Model Convergence on Primary (a) and (b) Secondary Outcomes
eTable 8. Assessment of Model Fits for Primary (a) and (b) Secondary Outcomes
eTable 9. Results of Assessment on Small Sample Size Effects
eTable 10. Comparison of the Included Interventions in Risk Ratio (95% CrI) for Barotrauma
eTable 11. Network Meta-analysis for Barotrauma and Quality of Evidence Assessment
eTable 12. Results of Node-Splitting Models
eTable 13. Summary of Cointerventions in Included Studies
eReferences