Abstract
The circRNA circAGFG1 is reported to be important in triple‐negative breast cancer progression. However, the mechanism of circAGFG1 in non‐small‐cell lung cancer (NSCLC) remains unknown. In this study, expression of circAGFG1 was determined by real‐time PCR in 20 pairs of NSCLC tissues and adjacent tissues. Next, functional experiments with circAGFG1 were performed in vitro to evaluate the role of circAGFG1 in tumor metastasis and growth. Meanwhile, a dual luciferase reporter assay, RNA pull‐down and RNA immunoprecipitation experiments were used to explore the interaction between circAGFG1 and miR‐203. Our results revealed that expression levels of circAGFG1 and miR‐203 are upregulated in non‐small‐cell lung cancer tissues. CircAGFG1 enhances NSCLC cell proliferation, invasion, migration and epithelial‐mesenchymal transition in vitro. Mechanistic analyses indicated that circAGFG1 acts as a sponge for miR‐203 to repress the effect of miR‐203 on its target, ZNF281. In conclusion, our study suggests that circAGFG1 promotes NSCLC growth and metastasis though a circAGFG1/miR‐203/ZNF281 axis and may represent a novel therapeutic target.
Keywords: circAGFG1, miR‐203, non‐small‐cell lung cancer, ZNF281
Introduction
There has recently been markedly increased morbidity and mortality reported in patients suffering from lung cancer,1 primarily due to non‐small‐cell lung cancer (NSCLC). It is no exaggeration to say that lung cancer is becoming one of the highest mortality cancers and is leading to a significant burden on society.2
CircRNAs, a group of newly discovered endogenous non‐coding RNAs, have 3′‐poly A tails and a continuous covalently closed loop without a 5′‐cap structure.3 Many circRNAs have been identified in mammalian tissues and cells by using advanced high throughput sequencing techniques and bioinformatics analysis. When circRNA was first discovered in mammalian cells, it was considered a byproduct of incorrectly spliced RNA. Currently, circRNA is recognized as a crucial regulator of various biological processes.4 CircRNAs are involved in the pathological processes of diseases, including cancers.5 Recent studies have reported that circular RNAs (circRNAs) play an important role in the viability of NSCLC.6, 7, 8 For example, circABCB reportedly promotes proliferation and migration of NSCLC.9 Furthermore, circFGFR3 enhances tumor progression in NSCLC.10 Yang et al.11 demonstrated that circAGFG1 promotes triple negative breast cancer progression through regulating CCNE1 expression. However, the role of circAGFG1 in NSCLC remains obscure.
MicroRNAs (miRNAs), known as small non‐coding RNAs, have a characteristic size of 18–25 nucleotides. MiRNAs primarily act as post‐transcriptional regulators of target mRNAs.12, 13 Increased oncogenic miRNA expression in cancer inhibits tumor suppressive genes, whereas decreased tumor suppressive miRNAs promote the expression of oncogenes. Some studies have demonstrated that miRNAs are involved in tumorigenesis and progression of various cancers, including lung cancer.14, 15, 16 Nevertheless, the upstream regulators of miRNAs are poorly understood. Recently, it has been hypothesized that competing endogenous RNAs (ceRNAs), such as lncRNAs, mRNAs and pseudogenes, could combine with and modulate each other via competitively binding to microRNA response elements (MREs).17 A number of studies have reported that circRNAs are capable of serving as ceRNAs to prevent miRNAs from accessing their target genes.18, 19 However, the biological role of most circRNAs with respect to the underlying mechanisms and pathogenesis of NSCLC remain largely uncertain.
Here, we demonstrated for the first time that circAGFG1 plays a critical role in the viability of NSCLC. We investigated the clinical significance of circAGFG1 expression in NSCLC tissue samples and further explored its function and underlying molecular mechanism in the development and progression of NSCLC.
Methods
NSCLC tissue samples
A total of 20 NSCLC tissue samples and paired adjacent noncancerous non‐small‐cell lung cancer tissue samples were obtained from patients who underwent surgery from September 2018 to December 2018 at the first affiliated hospital of Nanjing Medical University. Each sample was snap‐frozen and stored in liquid nitrogen prior to RNA extraction. Patients were informed regarding sample collection and signed the informed consent forms. Collection and use of tissue samples were approved by the Ethical Review Committee of the first affiliated hospital of Nanjing Medical University.
Cell culture
NSCLC cell lines, A549 and H1299, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in DMEM high‐glucose medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) at 37 °C in a humidified environment with 5% CO2.
Total RNA extraction and qRT‐PCR
Total RNA was extracted and reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara, Dalian, China) according to the manufacturer's instructions. QRT‐PCR was performed using a Bio‐Rad CFX96 system (Bio‐Rad, CA, USA) with TB Green Premix Ex Taq (Takara, Dalian, China). Internal reference genes for quantification of circRNA and mRNA was GAPDH and for miRNA was U6. Relative gene expression was calculated using the 2−ΔΔCt method. The PCR specific primers used in this study are listed in Table 1.
Table 1.
Primers used for qPCR
| Gene | Forward 5′‐3′ | Reverse 5′‐3′ |
|---|---|---|
| CircAGFG1 | CCAGTTGTAGGTCGTTCTCAAG | GGATTTAATCCTCGCCTGCATG |
| ZNF281 | GCCAGCCTTGGGACAATAATG | CTTGCACGTTGAGTTTGGGT |
| MiR‐203 | GGGGTGAAATGTTTAGGAC | CAGTGCGTGTCGTGGAGT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
RNAi and transfection
CircAGFG1‐siRNA, two ZNF281‐siRNAs and negative control (NC) siRNAs plasmids were designed, synthesized and purchased from GenePharma, China by using the pPG‐miR‐eGFP‐Blasticidin vector with has‐miR‐203 (pPG‐miR‐203) or hsa‐miR‐203 inhibitors (pPG‐anti‐miR‐203) or NC (pPG‐miR‐NC). The SiRNA sequences were as follows: 5′‐ATGCAGGCGAGGATTAAAT‐3′ for si‐circAGFG111 and 5′‐CCAGAATCTCAGGGAATCA‐3′ for si‐ZNF281‐1.20 All siRNA sequences were synthesized and purchased form GenePharma, China. The pcDNA3.1‐circAGFG1 construct and empty vector were designed, synthesized and purchased from Sangon Biotech, China. Cells were cultured in six‐well plates to 50–70% confluency followed by transfection with siRNAs or plasmids using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer's instructions. Cells were collected for subsequent experiments 48 hours after transfection.
Transwell invasion assay
Cell invasion assays were performed using 24‐well transwell plates (Corning, USA). There were approximately 2 × 105 cells in the upper chamber in serum‐free medium with each condition run in triplicate. Medium containing 10% fetal bovine serum (300 μL) acted as chemoattractant in the lower chamber. Cells above the Matrigel layer were removed using a cotton swab. Cells below the membrane were then fixed in 4% formaldehyde and stained with 0.1% crystal violet for 10 minutes. Finally, five randomly chosen fields were quantified for each well.
Dual‐luciferase reporter assay
A549 and H1299 cells were cotransfected with 150 ng empty pmiR‐GLO‐NC or pmiR‐GLO‐circAGFG1‐wt or with pmiR‐GLO‐circAGFG1‐mut, pmiR‐GLO‐ZNF281‐wt or pmiR‐GLO‐ZNF281‐mut (Sangon Biotech, China) and with 2 ng of internal control pRL‐TK (Promega, USA). A549 and H1299 cells were also cotransfected with pPG‐miR‐203 or pPG‐miR‐NC. A dual‐luciferase reporter assay kit (Promega, USA) was used to assess luciferase activity according to the manufacturer's instructions. Relative luciferase activity was normalized to Renilla luciferase activity.
RNA binding protein immunoprecipitation assay
RNA immunoprecipitation (RIP) assays were performed using the EZ‐Magna RIP RNA‐Binding Protein Immunoprecipitation Kit (Millipore, USA) according to the manufacturer's instructions. A549 cells were lysed in complete RIP lysis buffer, and then RIP lysis buffer staining magnetic beads conjugated to human anti‐Ago2 antibody (Proteintech, China) were added to cleared lysates. Normal mouse IgG (Beyotime, China) served as the NC, and SNRNP70 (Millipore, USA) was the positive control. Coprecipitated RNAs were extracted using TRIzol reagent (TakaRa, China) and subsequently subjected to qRT‐PCR.
Biotin‐labelled miRNA pull‐down assay
Biotinylated miR‐203, biotinylated miR‐203‐mut and biotinylated NC (GenePharma, China) were transfected into H1299 cells and harvested 48 hours later. Cell lysates were incubated with M‐280 streptavidin magnetic beads (Invitrogen, USA) coated with RNase‐free bovine serum albumin (Sigma‐Aldrich) and yeast tRNA (Sigma‐Aldrich) to prevent nonspecific binding of RNA and protein complexes. The beads were incubated at 4 °C for 3 hours. Next, beads were washed three times in ice cold lysis buffer and once in high salt buffer. Bound RNAs were isolated by using TRIzol for subsequent analysis.
Western blotting
Western blotting was performed as previously described.21 Cells were lysed using RIPA lysis buffer (Solarbio, China) supplemented with protease inhibitors (Roche Applied Science, Switzerland). Primary antibodies used were as follows: anti‐E‐cadherin (1:500, Abcam, UK), anti‐Vimentin (1:500, Abcam, UK), ZNF281 (1:500, Abcam, UK) and GAPDH (1:5000, Proteintech, China). Horseradish peroxidase‐conjugated goat anti‐rabbit or goat anti‐mouse IgG secondary antibodies (1: 1000, Beyotime, China) were used. All experiments were performed at least three times.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5. A paired‐sample t‐test was used to analyse significant differences between tumor tissue samples and adjacent nontumor tissue samples. Independent‐samples t‐test was used to analyse differences between two groups. For multiple comparisons, two‐way analysis of variance (ANOVA) was performed followed by Tukey's post hoc test. All P‐values were two‐sided, and *P < 0.05, **P < 0.01 and ***P < 0.001 were deemed statistically significant.
Results
CircAGFG1 is significantly overexpressed in non‐small‐cell lung cancer and represses the proliferation and migration of A549 and H1299 cells
To assess the expression of circAGFG1 in non‐small‐cell lung cancer, 20 pairs of NSCLC tissue samples and paired adjacent normal tissue samples were analysed by qRT‐PCR. As shown in Figure 1a, expression of circAGFG1 was significantly upregulated in NSCLC tissue samples compared to paired adjacent normal tissue samples. To further assess the role of circAGFG1 in NSCLC, we silenced the expression of circAGFG1 in A549 and H1299 cells by using si‐circAGFG1 (Fig 1b,d). CCK‐8 assay results showed that proliferation was inhibited in A549 cells transfected with si‐circAGFG1 (Fig 1c). Proliferation of H1299 cells was also decreased in response to transfection of si‐circAGFG1 (Fig 1e). Next, we assessed migration of NSCLC by evaluating transwell invasion, demonstrating that migration of A549 and H1299 cells was decreased after transfection of si‐circAGFG1 (Fig 1f). Quantitative analysis of transwell invasion assays indicated that the significant differences between si‐NC and si‐circAGFG1 groups was markedly significant. These findings revealed that circAGFG1 increases proliferation and migration of NSCLC cells.
Figure 1.
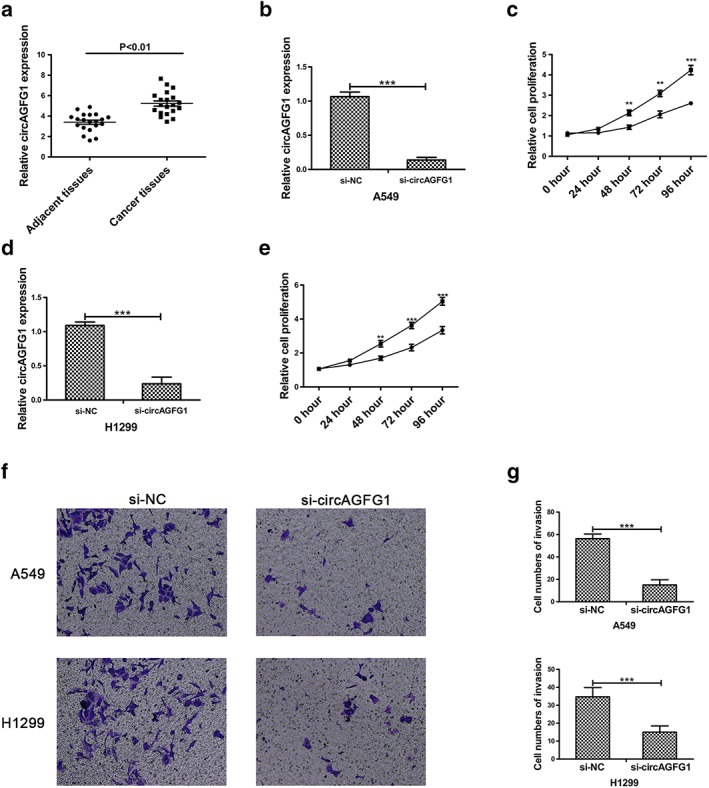
CircAGFG1 is upregulated in NSCLC tissue and promotes proliferation and migration of NSCLC cells. (a) Relative expression of circAGFG1 in non‐small‐cell lung cancer tissues and corresponding adjacent tissues was determined by qRT‐PCR (n = 20). (b) The expression of circAGFG1 was detected by qRT‐PCR in A549 cell lines transfected with si‐circAGFG1. (c) The relative cell proliferation of A549 cells transfected with si‐NC or si‐circAGFG1 was determined by using CCK‐8 assays.  a549/si‐NC,
a549/si‐NC,  a549/si‐circAGFG1. (d) The expression of circAGFG1 was detected by qRT‐PCR in H1299 cell lines transfected with si‐NC or si‐circAGFG1. (e) Relative cell proliferation of H1299 cells transfected with si‐NC or si‐circAGFG1 was determined by using CCK‐8 assays.
a549/si‐circAGFG1. (d) The expression of circAGFG1 was detected by qRT‐PCR in H1299 cell lines transfected with si‐NC or si‐circAGFG1. (e) Relative cell proliferation of H1299 cells transfected with si‐NC or si‐circAGFG1 was determined by using CCK‐8 assays.  H1299/si‐NC,
H1299/si‐NC,  H1299/si‐AGFG1. (f) Transwell invasion assays in A549 and H1299 cell lines transfected with si‐NC or si‐circAGFG1 were used to determine migration of A549 and H1299 cells. (g) Quantitative analysis of transwell invasion assays. All data are presented as mean ± s.d., and all experiments were performed at least three times. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
H1299/si‐AGFG1. (f) Transwell invasion assays in A549 and H1299 cell lines transfected with si‐NC or si‐circAGFG1 were used to determine migration of A549 and H1299 cells. (g) Quantitative analysis of transwell invasion assays. All data are presented as mean ± s.d., and all experiments were performed at least three times. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
MiR‐203 acts as a target of circAGFG1
To investigate the underlying mechanism between miR‐203 and circAGFG1, circAGFG1 was overexpressed by transfection of plasmids containing pcDNA‐circAGFG1 in H1299 and A549 cells (Fig 1a). A recent study demonstrated that circRNAs interact competitively with the same miRNA responsive elements to regulate protein expression via a molecular sponge or ceRNA.17 We hypothesized that circAGFG1 acts as a molecular sponge to modulate miRNA expression in the cytoplasm. To predict potential miRNAs modulated by circAGFG1, online databases (Circinteractome, https://circinteractome.nia.nih.gov) were used to identify potential sites. We uncovered potential complementary sequences between circAGFG1 and miR‐203 (Fig 2b). Importantly, miR‐203 was reported to be markedly downregulated, resulting in inhibited cell proliferation, invasion, and migration of NSCLC.22 Therefore, we performed qPCR to measure expression levels of miR‐203 in A549 and H1299 cells transfected with si‐circAGFG1 and pcDNA‐circAGFG1. Results showed that expression of miR‐203 was increased in A549 and H1299 in response to si‐circAGFG1 transfection and decreased in A549 and H1299 in response to pcDNA‐circAGFG1 transfection (Fig 2c,d).
Figure 2.
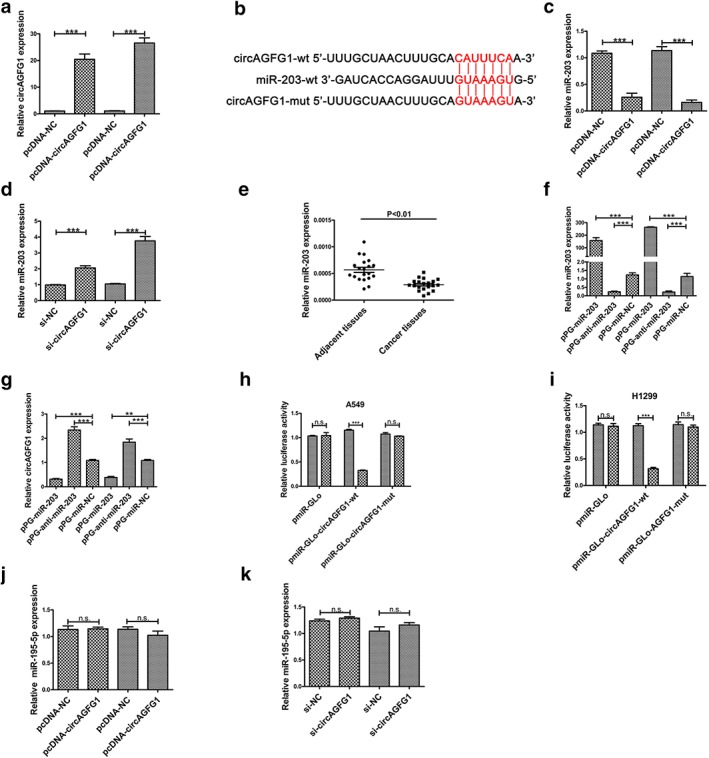
MiR‐203 is a target of circAGFG1. (a) Relative expression of circAGFG1 was upregulated in response to transfection of pcDNA‐circAGFG1 in A549 and H1299 cells.  A549,
A549,  H1299. (b) Alignment of miR‐203 with the potential binding site of wild type circAGFG1 and circAGFG1 mutated at the potential binding site. (c) Relative expression of miR‐203 was detected by qRT‐PCR in A549 and H1299 cells transfected with pcDNA‐NC or pcDNA‐circAGFG1.
H1299. (b) Alignment of miR‐203 with the potential binding site of wild type circAGFG1 and circAGFG1 mutated at the potential binding site. (c) Relative expression of miR‐203 was detected by qRT‐PCR in A549 and H1299 cells transfected with pcDNA‐NC or pcDNA‐circAGFG1.  A549,
A549,  H1299. (d) Relative expression of miR‐203 was detected by qRT‐PCR in A549 and H1299 cells transfected with si‐NC or si‐circAGFG1.
H1299. (d) Relative expression of miR‐203 was detected by qRT‐PCR in A549 and H1299 cells transfected with si‐NC or si‐circAGFG1.  A549,
A549,  H1299. (e) Relative expression of miR‐203 in non‐small‐cell (adenocarcinoma) lung cancer tissues and corresponding adjacent tissue was determined by qRT‐PCR (n = 20). (f) The relative expression of miR‐203 was silenced and upregulated by transfection of pPG‐anti‐miR‐203 and pPG‐miR‐203, respectively, in A549 and H1299 cells. (g) Relative expression of circAGFG1 was detected by qRT‐PCR in A549 and H1299 cells transfected with pPG‐miR‐NC, pPG‐anti‐miR‐203 and pPG‐miR‐203.
H1299. (e) Relative expression of miR‐203 in non‐small‐cell (adenocarcinoma) lung cancer tissues and corresponding adjacent tissue was determined by qRT‐PCR (n = 20). (f) The relative expression of miR‐203 was silenced and upregulated by transfection of pPG‐anti‐miR‐203 and pPG‐miR‐203, respectively, in A549 and H1299 cells. (g) Relative expression of circAGFG1 was detected by qRT‐PCR in A549 and H1299 cells transfected with pPG‐miR‐NC, pPG‐anti‐miR‐203 and pPG‐miR‐203.  A549,
A549,  H1299. (h) and (i) Luciferase reporter activity was determined in A549 and H1299 cells cotransfected with pPG‐miR‐203 (or empty vector as a control) and the luciferase empty vector (pmiR‐GLo) or with the vector containing the wild type circAGFG1 (pmiR‐GLo‐circAGFG1‐wt) or mutant transcripts (pmiR‐GLo‐circAGFG1‐mut). The relative ratio of firefly luciferase activity to Renilla luciferase activity is presented. (j) Relative expression of miR‐195‐5p was detected by qRT‐PCR in A549 and H1299 cells transfected with pcDNA‐NC or pcDNA‐circAGFG1.
H1299. (h) and (i) Luciferase reporter activity was determined in A549 and H1299 cells cotransfected with pPG‐miR‐203 (or empty vector as a control) and the luciferase empty vector (pmiR‐GLo) or with the vector containing the wild type circAGFG1 (pmiR‐GLo‐circAGFG1‐wt) or mutant transcripts (pmiR‐GLo‐circAGFG1‐mut). The relative ratio of firefly luciferase activity to Renilla luciferase activity is presented. (j) Relative expression of miR‐195‐5p was detected by qRT‐PCR in A549 and H1299 cells transfected with pcDNA‐NC or pcDNA‐circAGFG1.  pPG‐miR‐NC,
pPG‐miR‐NC,  pPG‐miR‐203 (k) Relative expression of miR‐195‐5p was detected by qRT‐PCR in A549 and H1299 cells transfected with si‐NC or si‐circAGFG1. All data are presented as the mean ± s.d., and all experiments were performed at least three times.
pPG‐miR‐203 (k) Relative expression of miR‐195‐5p was detected by qRT‐PCR in A549 and H1299 cells transfected with si‐NC or si‐circAGFG1. All data are presented as the mean ± s.d., and all experiments were performed at least three times.  A549,
A549,  H1299. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant
H1299. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant
Next, expression levels of miR‐203 were detected by qRT‐PCR in 20 pairs of NSCLC tissue samples, and the results confirmed its downregulation in NSCLC tissue samples. To further investigate whether miR‐203 is capable of downregulating circAGFG1, we transfected A549 and H1299 cells with pPG‐miR‐NC, pPG‐miR‐203 and pPG‐anti‐miR‐203 individually. qRT‐PCR results revealed that the expression level of miR‐203 in both A549 and H1299 cells was decreased in response to transfection with pPG‐anti‐miR‐203 and increased in response to pPG‐miR‐203 transfection (Fig 2f). Next, qRT‐PCR results showed that pPG‐anti‐miR‐203 transfection markedly increased circAGFG1 expression, while pPG‐miR‐203 transfection significantly increased circAGFG1 expression (Fig 2g).
To further investigate whether the potential identified binding site is functional, a dual‐luciferase reporter assay in A549 and H1299 cells was performed. Luciferase activity was decreased in A549 and H1299 cells cotransfected with pPG‐miR‐203 + pmiR‐Glo‐circAGFG1‐wt but not in cells cotransfected with pPG‐miR‐203 + pmiR‐Glo or pPG‐miR‐203 + pmiR‐Glo‐circAGFG1‐mut (Fig 3c,d). These results indicate that the binding site of circAGFG1 and miR‐203 is essential for their reciprocity.
Figure 3.
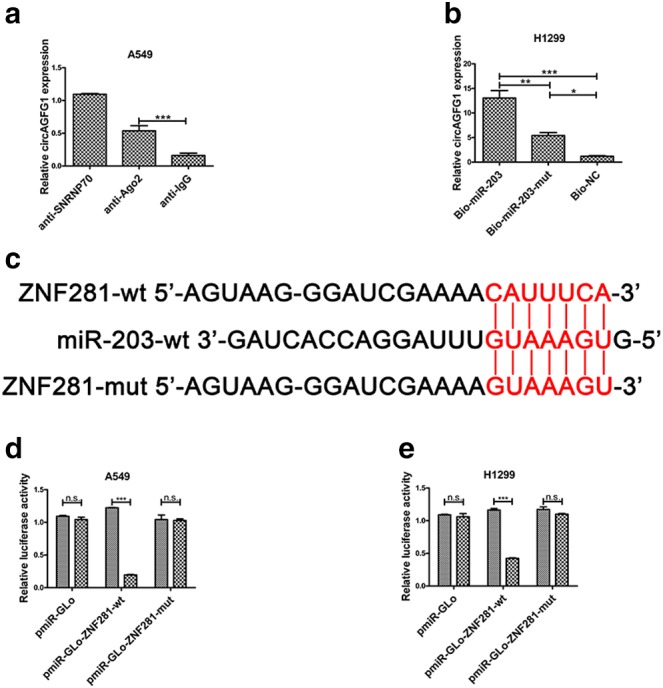
ZNF281 is a target of miR‐203. (a) Amount of circAGFG1 bound to SNRNP70 (positive control), Ago2 or IgG (negative control) detected by qPCR after RIP in A549 cells. (b) H1299 cells were transfected with biotinylated NC (Bio‐NC), biotinylated wild type miR‐203 (BiomiR‐203) or biotinylated mutant miR‐203 (Bio‐miR‐203‐mut), and biotin‐based miRNA pull‐down assays were performed 48 h after transfection. CircAGFG1 levels were analysed by qPCR. (c) Alignment of miR‐203 with the potential binding site of wild type ZNF281 and ZNF281 mutated at the potential binding site. (d) and (e) Luciferase reporter activity was measured in A549 and H1299 cells cotransfected with pPG‐miR‐203 (or empty vector as a control) and the luciferase empty vector (pmiR‐GLo) or with the vector containing wild type ZNF281 (pmiR‐GLo‐ZNF281‐wt) or mutant transcripts (pmiR‐GLo‐ZNF281‐mut). The relative ratio of firefly luciferase activity to Renilla luciferase activity is presented. All data are presented as mean ± s.d., and all experiments were performed at least three times.  pPG‐miR‐NC,
pPG‐miR‐NC,  pPG‐miR‐203. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
pPG‐miR‐203. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
A recent study reported that circAGFG1 acts as a sponger of miR‐195‐5p to promote triple negative breast cancer progression by regulating cyclin E1 expression.11 To confirm whether circAGFG1 regulates NSCLC progression by sponging miR‐195‐5p, pcDNA‐NC or pcDNA‐circAGFG1 was transfected into A549 and H1299 cells. qRT‐PCR results showed that miR‐195‐5p expression was unchanged (Fig 2j). Moreover, si‐NC or si‐circAGFG1 was also transfected into A549 and H1299 cells to investigate whether circAGFG1 regulates NSCLC progression by sponging miR‐195‐5p. As shown in Figure 2k, there was no change in the expression of miR‐195‐5p in A549 or H1299 cells transfected with si‐NC or si‐circAGFG1. These results confirm that circAGFG1 does not regulate expression of miR‐195‐5p. CircAGFG1 did not act as a sponge of miR‐195‐5p to promote EMT and metastasis in non‐small‐cell lung cancer.
MiR‐203 modulates ZNF281 by combining with the binding site of ZNF281
Ago, a core component of the RNA‐induced silencing complex (RISC), binds to miRNA to silence miRNA‐targeted genes.23 RNA was isolated from RISC by using an Ago2 antibody in RIP assays, and results revealed that circAGFG1 was markedly enriched in Ago2‐containing beads in A549 cells (Fig 3a). Moreover, miRNA pull‐down assays were used to further determine the binding affinity between circAGFG1 and miR‐203 by transfecting H1299 cells with biotinylated miR‐203, biotinylated miR‐203‐mut or biotinylated NC. The results showed that circAGFG1 was pulled down by miR‐203 (Fig 3b). These results also suggest that circAGFG1 binds directly to miR‐203.
To verify the target protein of miR‐203, an online database (TargetScan, http://www.targetscan.org/vert_72/) was used to predict potential targets of miR‐203. As shown in Figure 3c, the same binding site on ZNF281 targeted by circAGFG1 was matched to the miR‐203 sequence. To further confirm whether this potential binding site is functional, wild type and mutated luciferase reporters of ZNF281 were constructed to perform dual luciferase reporter assays in A549 and H1299 cells. We observed that luciferase activity was reduced in A549 and H1299 cells transfected with pmiR‐Glo‐ZNF281‐wt but not in cells transfected with pmiR‐Glo‐ZNF281‐mut. These results demonstrate that miR‐203 interacts with the 3’UTR of ZNF281 in A549 and H1299 cells.
CircAGFG1 regulates ZNF281 via competing with miR‐203 in NSCLC cells
We measured mRNA expression of ZNF281 in NSCLC in response to different treatments to explore whether circAGFG1 is capable of regulating ZNF281 by competing with miR‐203 in NSCLC cell lines. First, we observed that mRNA expression of ZNF281 was reduced in A549 and H1299 cells transfected with pPG‐miR‐203 and increased in cells transfected with pPG‐anti‐miR‐203 (Fig 4a,b). Moreover, we observed that mRNA expression of ZNF281 was reduced in A549 and H1299 transfected with si‐circAGFG1, while cotransfection with pPG‐anti‐miR‐203 reversed these effects (Fig 4c,d). Of note, mRNA expression of ZNF‐281 was increased in A549 and H1299 cells transfected with pcDNA‐circAGFG1, and pPG‐miR‐203 reversed these effects (Fig 4e,f). These results reveal that circAGFG1 regulates ZNF281 via competing with miR‐203 in NSCLC cells.
Figure 4.
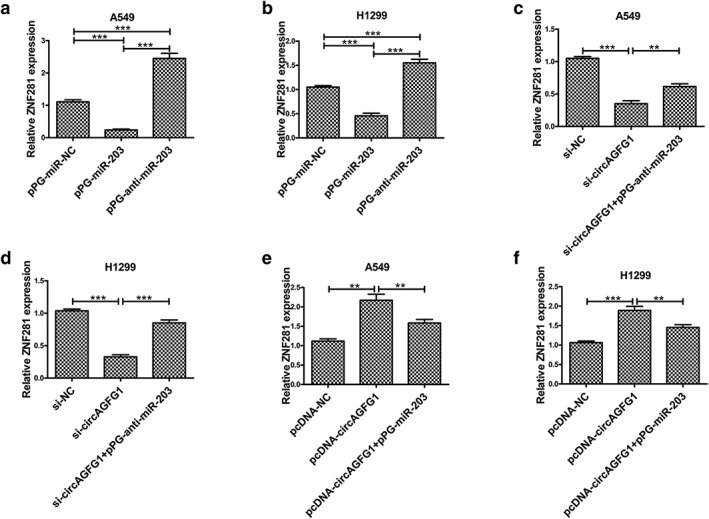
ZNF281 is regulated by miR‐203 and circAGFG1. (a) Relative ZNF281 expression was determined by qRT‐PCR in A549 cells transfected with pPG‐miR‐NC, pPG‐anti‐miR‐203 and pPG‐miR‐203. (b) Relative ZNF281 expression was determined by qRT‐PCR in H1299 cells transfected with pPG‐miR‐NC, pPG‐anti‐miR‐203 and pPG‐miR‐203. (c) Relative ZNF281 expression was measured by qRT‐PCR in A549 cells transfected with si‐NC, si‐circAGFG1 and si‐circAGFG1 + pPG‐anti‐miR‐203. (d) Relative ZNF281 expression was measured by qRT‐PCR in H1299 cells transfected with si‐NC, si‐circAGFG1 and si‐circAGFG1 + pPG‐anti‐miR‐203. (e) Relative ZNF281 expression was detected by qRT‐PCR in A549 cells transfected with pcDNA‐NC, pcDNA‐circAGFG1 and pcDNA‐circAGFG1 + pPG‐anti‐miR‐203. (f) Relative ZNF281 expression was detected by qRT‐PCR in H1299 cells transfected with pcDNA‐NC, pcDNA‐circAGFG1 and pcDNA‐circAGFG1 + pPG‐anti‐miR‐203. All data are presented as mean ± s.d., and all experiments were performed at least three times. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
Silencing of ZNF281 suppresses proliferation and invasion of NSCLC cells
Previous studies have demonstrated that ZNF281 is significantly involved in cancer.20, 24 However, the role of ZNF281 in NSCLC remains unknown. To determine the role of ZNF281 in NSCLC cells, we constructed si‐ZNF281 and si‐NC constructs. Results showed that ZNF281 mRNA expression was markedly silenced in A549 and H1299 cells in response to si‐ZNF281 transfection (Fig. 5a,b). Then, protein expression was evaluated in ZNF281 knockdown A549 and H1299 cells by using Western blotting. We discovered that E‐cadherin expression was increased, and Vimentin expression was decreased in si‐ZNF281 transfected A549 and H1299 cells (Fig 5c). In addition, transwell invasion assays were used to assess invasion of A549 and H1299 cells transfected with si‐ZNF281, and the results illustrated that invasion of A549 and H1299 was reduced in response to knock down of ZNF281 (Fig 5d). Quantitative analysis showed that si‐NC and si‐ZNF281 were significantly different from each other (Fig 5e). Colony formation experiments showed that proliferation of A549 and H1299 cells was reduced in response to transfection with si‐ZNF281 (Fig 5f). According to these data, ZNF281 enhances invasion and proliferation of NSCLC cells.
Figure 5.
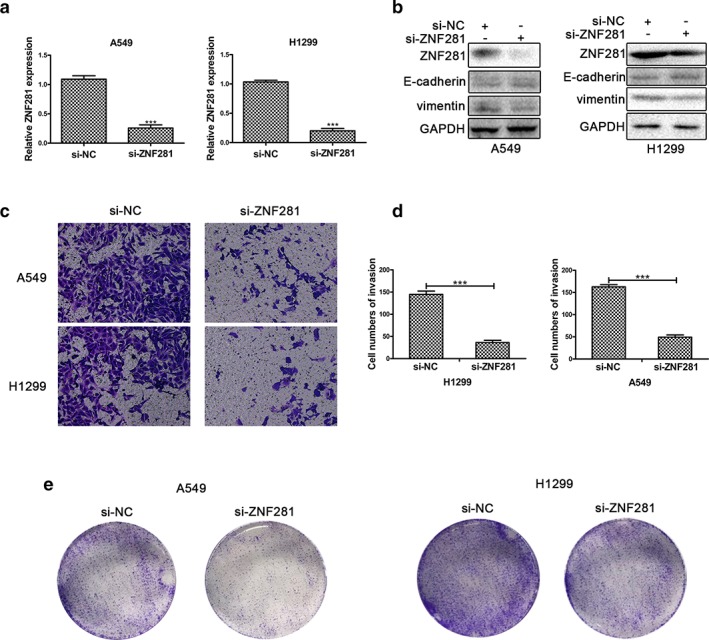
Silencing of ZNF281 inhibits A549 and H1299 cell proliferation and invasion. (a) Relative mRNA expression of ZNF281 was detected by qRT‐PCR in A549 and H1299 cells transfected with si‐NC or si‐ZNF281. (b) The protein expression of ZNF281, E‐cadherin, Vimentin and GAPDH was detected by Western blotting in A549 and H1299 cells transfected with si‐NC or si‐circAGFG1. (c) Transwell invasion assays in A549 and H1299 cell lines transfected with si‐NC or si‐ZNF281 were used to determine migration of A549 and H1299 cell lines. (d) Quantitative analysis of transwell invasion assays. (e) The colonizing ability of A549 and H1299 cells transfected with si‐NC or si‐ZNF281 was determined by using colony formation assays. All data are presented as mean ± s.d., and all experiments were performed at least three times. *P < 0.05, ** < 0.01, *** < 0.001; n.s: not statistically significant.
Discussion
In recent years, increasing circRNAs have been identified in mammalian cells by using advanced sequencing technology. CircRNAs possess powerful regulatory functions affecting all varieties of biological processes and are potential diagnostic markers and therapeutic targets for cancer.25 Recent studies reported that some circRNAs function as tumor suppressors or oncogenes in gastric cancer, bladder cancer, pancreatic cancer, hepatocellular carcinoma and other types of cancer. However, only a few circRNAs have been adequately investigated, and their biological function remains poorly understood.
Here, we demonstrated for the first time that circAGFG1 plays a crucial role in the development and progression of NSCLC. We observed that expression of circAGFG1 is upregulated in NSCLC tissues compared to adjacent noncancerous tissues. Subsequently, we showed that silencing of circAGFG1 inhibits proliferation and migration of NSCLC cell lines. These results indicate that circAGFG1 may act as an oncogene in the pathological progression of NSCLC.
The ceRNA hypothesis suggests that circRNAs, lncRNAs, mRNAs and pseudogenes communicate with and regulate the expression of one other by completely combining with shared miRNAs response elements (MREs), which establishes a new theory and mechanism of post‐transcriptional regulatory networks.17 In our study, the MRE of miR‐203 was identified to be contained within circAGFG1 via bioinformatics analyses. Expression of miR‐203 was decreased in NSCLC tissue samples compared to adjacent nontumor tissue samples. Importantly, we also found that increased expression of circAGFG1 decreases the expression of miR‐203, whereas decreased expression of circAGFG1 increases expression of miR‐203. Therefore, these results suggest that circAGFG1 might act as an oncogene by sponging miR‐203 in NSCLC. Further, dual‐luciferase assays, RNA‐binding protein immunoprecipitation assays and RNA pull‐down assays confirmed that circAGFG1 directly interacts with miR‐203. Another study reported that miR‐203 inhibits cell proliferation, migration and invasion of NSCLC.22 Our findings suggest that circAGFG1 may serve as a ceRNA for miR‐203 in NSCLC.
CircRNA regulates the expression of miRNA target genes via serving as a ceRNA. We found that ZNF281 is one potential target of miR‐203 using miR‐code and TargetScan. Further, we observed that mRNA expression of ZNF281 was upregulated in NSCLC tissue samples and downregulated in paired adjacent nontumor samples. ZNF281, a transcription factor, contains four Krüppel‐type zinc‐finger domains.26 Newly discovered findings indicate that ZNF‐281 could act as an oncogene to promote pathological progression of cancers, such as pancreatic cancer, colorectal cancer and glioma.20, 27, 28 Our studies confirm the notion that knockdown of ZNF281 inhibits EMT, migration and proliferation of NSCLC cells. Importantly, a dual‐luciferase reporter assay verified that miR‐203 directly interacts with the 3′‐untranslated region of ZNF281. In addition, silencing of miR‐203 led to increased expression of ZNF281, whereas upregulation of miR‐203 had the opposite effect. To confirm the correlation between circAGFG1 and ZNF281, we demonstrated that upregulation of circAFGF1 promotes mRNA and protein expression of ZNF281, while silencing of circAGFG1 reduces mRNA and protein expression of ZNF281. Furthermore, miR‐203 mimics or inhibitors partially inhibited these effects, which supports our hypothesis that circAGFG1 serves as a ceRNA to enhance ZNF281‐mediated migration and proliferation of NSCLC through sponging miR‐203.
In conclusion, our findings suggest for the first time that circAGFG1 regulates ZNF281 expression via acting as a decoy for miR‐203, resulting in tumorigenesis and development of NSCLC. Our results also imply that circAGFG1 is potentially a novel diagnostic, prognostic and therapeutic target for NSCLC. The regulatory network of the circAGFG1/miR‐203/ZNF281 axis provides an improved understanding of the potential mechanism of pathogenesis and progression in NSCLC.
Acknowledgments
We are grateful to colleagues in our laboratory.
References
- 1. Shash E, Peccatori FA, Azim HA. Optimizing the use of epidermal growth factor receptor inhibitors in advanced non‐small‐lung cancer (NSCLC). J Thorac Dis 2011; 3 (1): 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim JS, Ibaseta A, Fischer MM et al Intratumoural heterogeneity generated by Notch signalling promotes small‐cell lung cancer. Nature 2017; 545 (7654): 360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geng Y, Jiang J, Wu C. Function and clinical significance of circRNAs in solid tumors. J Hematol Oncol 2018; 11 (1): 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol Ther 2018; 187: 31–44. [DOI] [PubMed] [Google Scholar]
- 5. He J, Xie Q, Xu H, Li J, Li Y. Circular RNAs and cancer. Cancer Lett 2017; 396: 138–44. [DOI] [PubMed] [Google Scholar]
- 6. Yan Y, Zhang R, Zhang X, Zhang A, Zhang Y, Bu X. RNA‐Seq profiling of circular RNAs and potential function of hsa_circ_0002360 in human lung adenocarcinom. Am J Transl Res 2019; 11 (1): 160–75. [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L, Nan A, Zhang N et al Circular RNA 100146 functions as an oncogene through direct binding to miR‐361‐3p and miR‐615‐5p in non‐small cell lung cancer. Mol Cancer 2019; 18 (1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. An J, Shi H, Zhang N, Song S. Elevation of circular RNA circ_0003645 forecasts unfavorable prognosis and facilitates cell progression via miR‐1179/TMEM14A pathway in non‐small cell lung cancer. Biochem Biophys Res Commun 2019; 511: 921–5. [DOI] [PubMed] [Google Scholar]
- 9. Tian X, Zhang L, Jiao Y, Chen J, Shan Y, Yang W. CircABCB10 promotes nonsmall cell lung cancer cell proliferation and migration by regulating the miR‐1252/FOXR2 axis. J Cell Biochem 2019; 120 (3): 3765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu BQ, Zhang PF, Xiong D et al CircRNA fibroblast growth factor receptor 3 promotes tumor progression in non‐small cell lung cancer by regulating Galectin‐1‐AKT/ERK1/2 signaling. J Cell Physiol 2019; 234 (7): 11256–64. [DOI] [PubMed] [Google Scholar]
- 11. Yang R, Xing L, Zheng X, Sun Y, Wang X, Chen J. The circRNA circAGFG1 acts as a sponge of miR‐195‐5p to promote triple‐negative breast cancer progression through regulating CCNE1 expression. Mol Cancer 2019; 18 (1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9 (10): 775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 2011; 11 (12): 849–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hashemi ZS, Khalili S, Forouzandeh Moghadam M, Sadroddiny E. Lung cancer and miRNAs: A possible remedy for anti‐metastatic, therapeutic and diagnostic applications. Expert Rev Respir Med 2017; 11 (2): 147–57. [DOI] [PubMed] [Google Scholar]
- 15. Florczuk M, Szpechcinski A, Chorostowska‐Wynimko J. miRNAs as biomarkers and therapeutic targets in non‐small cell lung cancer: Current perspectives. Target Oncol 2017; 12 (2): 179–200. [DOI] [PubMed] [Google Scholar]
- 16. Switlik WZ, Szemraj J. Circulating miRNAs as non‐invasive biomarkers for non‐small cell lung cancer diagnosis, prognosis and prediction of treatment response. Postepy Hig Med Dosw 2017; 71 (0): 649–62. [DOI] [PubMed] [Google Scholar]
- 17. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011; 146 (3): 353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong Y, Mao J, Wu D et al Circ‐ZEB1.33 promotes the proliferation of human HCC by sponging miR‐200a‐3p and upregulating CDK6. Cancer Cell Int 2018; 18: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Hu J, Li L et al Upregulated circular RNA circ_0016760 indicates unfavorable prognosis in NSCLC and promotes cell progression through miR‐1287/GAGE1 axis. Biochem Biophys Res Commun 2018; 503 (3): 2089–94. [DOI] [PubMed] [Google Scholar]
- 20. Qian Y, Li J, Xia S. ZNF281 promotes growth and invasion of pancreatic cancer cells by activating Wnt/beta‐catenin signaling. Dig Dis Sci 2017; 62 (8): 2011–20. [DOI] [PubMed] [Google Scholar]
- 21. Ma MZ, Chu BF, Zhang Y et al Long non‐coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA‐218‐5p. Cell Death Dis 2015; 6: e1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chi Y, Jin Q, Liu X et al miR‐203 inhibits cell proliferation, invasion, and migration of non‐small‐cell lung cancer by downregulating RGS17. Cancer Sci 2017; 108 (12): 2366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karginov FV, Conaco C, Xuan Z et al A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci USA 2007; 104 (49): 19291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hahn S, Hermeking H. ZNF281/ZBP‐99: A new player in epithelial‐mesenchymal transition, stemness, and cancer. J Mol Med (Berl) 2014; 92 (6): 571–81. [DOI] [PubMed] [Google Scholar]
- 25. Zhu Q, Lu G, Luo Z et al CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR‐1324/FZD5/Wnt/beta‐catenin axis. Biochem Biophys Res Commun 2018; 497 (2): 626–32. [DOI] [PubMed] [Google Scholar]
- 26. Law DJ, Du M, Law GL, Merchant JL. ZBP‐99 defines a conserved family of transcription factors and regulates ornithine decarboxylase gene expression. Biochem Biophys Res Commun 1999; 262 (1): 113–20. [DOI] [PubMed] [Google Scholar]
- 27. Li XT, Li JC, Feng M, Zhou YX, Du ZW. Novel lncRNA‐ZNF281 regulates cell growth, stemness and invasion of glioma stem‐like U251s cells. Neoplasma 2019; 66 (1): 118–27. [DOI] [PubMed] [Google Scholar]
- 28. Zhu Y, Zhou Q, Zhu G et al GSK‐3beta phosphorylation‐dependent degradation of ZNF281 by beta‐TrCP2 suppresses colorectal cancer progression. Oncotarget 2017; 8 (51): 88599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]


