Abstract
Background
Lower thoracic esophageal cancer (LTEC) with celiac node metastasis and upper thoracic esophageal cancer (UTEC) with supraclavicular node metastasis were previously categorized as M1a diseases. Our study aimed to investigate whether the clinical significance of supraclavicular and celiac lymph node metastasis should be reevaluated in thoracic esophageal cancer.
Methods
A total of 6178 patients with thoracic esophageal cancer were identified from the Surveillance, Epidemiology, and End Results (SEER) database during 2004–2015. Treatment strategies and outcomes (OS, overall survival; CSS, cancer‐specific survival) of patients with different nodal status were reviewed. The Cox proportional hazards regression model was applied to evaluate the prognostic factors. Statistical analyses were performed in all subgroups.
Results
Multivariate analysis identified supraclavicular node metastasis but not celiac node metastasis as an independent predictor of both OS and CSS in LTEC. However, metastasis to supraclavicular or celiac nodes was not an independent predictor of OS and CSS in UTEC. Surgery was not associated with increased OS and CSS for UTEC with celiac or supraclavicular node metastasis but was favored as a predictor of better OS and CSS for LTEC with celiac or supraclavicular node metastasis. Radiotherapy benefited OS and CSS in LTEC involving celiac or supraclavicular nodes and in UTEC involving celiac nodes, while only OS benefited from radiotherapy in UTEC involving supraclavicular nodes.
Conclusions
These results provide preliminary evidence that the clinical significance of supraclavicular and celiac lymph node metastasis should be reevaluated in thoracic esophageal cancer with different prognostic information according to the primary sites.
Keywords: Esophageal cancer, lymph node metastasis, prognosis, treatment
Introduction
Esophageal cancer is ranked as one of the most common digestive system cancers worldwide1, 2 and as the seventh most frequent cause of cancer‐related death for males in the United States.3Surgical resection has been the mainstay of therapy for localized operable esophageal cancer.4However, the therapeutic strategies for patients with advanced‐stage esophageal cancer remain controversial, especially for those involving nonregional lymph nodes.5, 6, 7, 8
The latest eighth edition of the Union for International Cancer Control–American Joint Committee on Cancer (UICC–AJCC) TNM staging system for esophageal cancer and the Eleventh edition of the Japanese Classification of Esophageal Cancer by the Japan Esophageal Society (JES) are the two major classifications widely accepted for staging of esophageal cancer.9, 10, 11, 12 However, these two major staging systems have not reached a consensus on the prognostic significance of regional and nonregional lymph node involvement in thoracic esophageal cancer, or more specifically on the implication of supraclavicular node metastasis. Recently, many Japanese surgeons have proposed that the supraclavicular nodes should be classified as regional ones for upper thoracic esophageal cancer (UTEC)5, 8, 13 which was supported by some Asian and Western surgeons.14, 15 In fact, involvement of celiac lymph nodes (nodal station 20) was previously classified as metastatic (M1a) disease for lower thoracic esophageal cancer (LTEC) in the sixth edition of the AJCC staging system;5 similarly, involvement of the supraclavicular lymph nodes (nodal station 1) was designated as M1a disease for UTEC.7, 16 However, in later editions of the AJCC staging system, celiac lymph nodes were redefined as regional ones for LTEC, whereas supraclavicular lymph nodes remained as nonregional ones for UTEC.16, 17, 18, 19 Subsequently, celiac node involvement was reclassified as nodal (N) disease, while supraclavicular node involvement remained as metastatic (M1) disease. Therefore, it is important to ask if supraclavicular and celiac node metastasis can be classified into the M and N staging parameters respectively, which definitely affects the treatment for esophageal cancer.
In the present study, we reevaluated the prognostic significance of supraclavicular and celiac lymph node metastasis and assessed the treatment strategies for patients with either metastasis using the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Patients and population cohort
The National Cancer Institute’s SEER database was utilized for the present study with information of cancer patients.20 Data for patients with esophageal cancer diagnosed between 2004 and 2015 were extracted from the SEER database with eligibility criteria as follows: (i) age older than 18 years and histologically confirmed cancer arising from the esophagus (ICD‐O‐3 codes: 8000–8576, 8940–8950, 8980–8981); (ii) primary tumor located in the upper or lower thoracic esophagus;21 (iii) survival time ≥ 3 months. The Collaborative Stage Data Collection System codes at DX (Distant) and Lymph Nodes were used to query cases with celiac, supraclavicular or regional lymph node metastasis. All eligible patients were staged according to the sixth edition of the AJCC staging manual.22
Statistical analysis
The primary outcomes were overall survival (OS) and cancer‐specific survival (CSS). OS was defined as the interval between the diagnosis of cancer and death or last follow‐up. CSS was measured from the date of initial treatment to death from esophageal cancer. Prognostic factors were compared by the log‐rank test and Kaplan‐Meier methods. The chi‐squared test was used to compare categorical variables and Student’s t‐test was employed for continuous data. Multivariate analyses were performed by Cox regression. Hazard ratios (HR) with 95% confidence intervals (CI) were used to quantify the strength of the association between predictors and survival. All statistical analyses were performed using R software version 3.4.4 (Institute for Statistics and Mathematics, Vienna, Austria). A two‐tailed P‐value <0.05 was considered statistically significant.
Results
General information
The entire cohort consisted of 6178 patients with thoracic esophageal cancer, including 5803 patients with LTEC and 375 patients with UTEC. Table 1 lists the demographic parameters of all patients. Among patients with LTEC, there were 492 patients (8.5%) with supraclavicular lymph node involvement and 588 patients (10.1%) with celiac lymph node involvement. Among patients with UTEC, there were 25 patients (6.7%) with supraclavicular lymph node involvement and 27 patients with celiac lymph node involvement (7.2%).
Table 1.
Characteristics of patients in the entire cohort
| Clinicopathological characteristics Stratified by Site | Lower | Upper | P | |
|---|---|---|---|---|
| Number of patients | 5803 | 375 | ||
| Nodal status (%) | Regional node metastasis | 4723 (81.4) | 323 (86.1) | 0.067 |
| Supraclavicular node (station 1) metastasis | 492 (8.5) | 25 (6.7) | ||
| Celiac node (station 20) metastasis | 588 (10.1) | 27 (7.2) | ||
| Age at diagnosis (mean [SD]) | 64.83 (11.10) | 65.35 (10.73) | 0.377 | |
| Sex (%) | Female | 816 (14.1) | 119 (31.7) | <0.001 |
| Male | 4987 (85.9) | 256 (68.3) | ||
| CHSDA region (%) | East | 2224 (38.3) | 163 (43.5) | 0.167 |
| NP | 743 (12.8) | 39 (10.4) | ||
| PC | 2594 (44.7) | 161 (42.9) | ||
| SW | 242 (4.2) | 12 (3.2) | ||
| Marital status (%) | Married (including common law) | 3850 (66.3) | 183 (48.8) | <0.001 |
| Separated/widowed/divorced | 1159 (20.0) | 117 (31.2) | ||
| Single (never married) | 794 (13.7) | 75 (20.0) | ||
| Histology type (%) | Adenocarcinoma | 4372 (75.3) | 32 (8.5) | <0.001 |
| Squamous cell carcinoma | 816 (14.1) | 328 (87.5) | ||
| Others | 615 (10.6) | 15 (4.0) | ||
| T stage (%) | T1 | 819 (14.1) | 58 (15.5) | <0.001 |
| T2 | 805 (13.9) | 50 (13.3) | ||
| T3 | 3544 (61.1) | 169 (45.1) | ||
| T4 | 635 (10.9) | 98 (26.1) | ||
| N stage (%) | N0 | 2263 (39.0) | 116 (30.9) | 0.002 |
| N1 | 3540 (61.0) | 259 (69.1) | ||
| Tumor size (%) | ≤5 cm | 2562 (44.1) | 169 (45.1) | 0.809 |
| >5 cm | 1603 (27.6) | 106 (28.3) | ||
| Unknown | 1638 (28.2) | 100 (26.7) | ||
| Surgery (%) | No | 2949 (50.8) | 325 (86.7) | <0.001 |
| Yes | 2854 (49.2) | 50 (13.3) | ||
| Surgery type (%) | Esophagectomy | 2827 (48.7) | 44 (11.7) | <0.001 |
| Endoscopic treatment | 27 (0.5) | 6 (1.6) | ||
| Radiotherapy (%) | No | 1311 (22.6) | 69 (18.4) | 0.068 |
| Yes | 4492 (77.4) | 306 (81.6) | ||
| Chemotherapy (%) | No/unknown | 944 (16.3) | 75 (20.0) | 0.069 |
| Yes | 4859 (83.7) | 300 (80.0) | ||
| Race (%) | White | 5293 (91.2) | 257 (68.5) | <0.001 |
| Black | 277 (4.8) | 72 (19.2) | ||
| Others | 233 (4.0) | 46 (12.3) | ||
| Grade (%) | G1 | 240 (4.1) | 22 (5.9) | 0.002 |
| G2 | 2230 (38.4) | 175 (46.7) | ||
| G3 | 3227 (55.6) | 173 (46.1) | ||
| G4 | 106 (1.8) | 5 (1.3) | ||
| Diagnosed year (%) | 2004–2009 | 2446 (42.2) | 133 (35.5) | 0.013 |
| 2010–2015 | 3357 (57.8) | 242 (64.5) |
CHSDA, Contract Health Service Delivery Areas; NP, Northern Plains; PC, Pacific Coast; SW, South West; SD, standard deviation.
Status of lymph node metastases and survival rates
As shown in Figure 1a,b, the prognostic impact of different nodal status on UTEC was compared. In this subgroup, there was no significant difference in OS (P = 0.56; Fig 1a) and CSS (P = 0.36; Fig 1b) between UTEC with regional node metastasis and those with supraclavicular or celiac node metastasis. In contrast, Figure 1c,d presents the prognoses of LTEC stratified by nodal status. In this subgroup, significantly worse OS (P < 0.0001; Fig 1c) and CSS (P < 0.0001; Fig 1d) were observed in LTEC with supraclavicular node metastasis compared to those with regional or celiac node metastasis.
Figure 1.
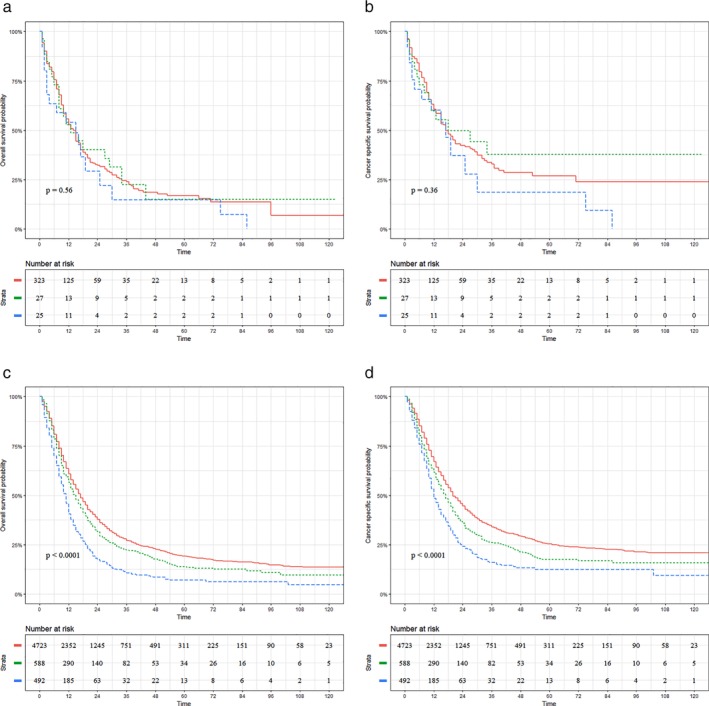
Prognostic impact of different nodal status on patients with thoracic esophageal cancer. (a) Overall Survival (OS) for upper thoracic esophageal cancer (UTEC) involving regional nodes (UR), supraclavicular nodes (U1) or celiac nodes (U20). (b) Cancer‐specific survival (CSS) for UTEC involving UR, U1 or U20 nodes. ( ) Group=UR, (
) Group=UR, ( ) Group=U20, and (
) Group=U20, and ( ) Group=U1. (c) OS for lower thoracic esophageal cancer (LTEC) involving regional nodes (LR), supraclavicular nodes (L1) or celiac nodes (L20). (d) CSS for LTEC involving LR, L1 or L20 nodes. (
) Group=U1. (c) OS for lower thoracic esophageal cancer (LTEC) involving regional nodes (LR), supraclavicular nodes (L1) or celiac nodes (L20). (d) CSS for LTEC involving LR, L1 or L20 nodes. ( ) Group=LR, (
) Group=LR, ( ) Group=L20, and (
) Group=L20, and ( ) Group=L1.
) Group=L1.
Univariate and multivariate analysis of prognostic factors
As for the OS and CSS of LTEC, univariate analysis identified 13 significant risk factors, including nodal status (supraclavicular and celiac), surgery, chemotherapy and radiotherapy (Table S1).
In the multivariate analysis, supraclavicular node metastasis was shown to be a significant independent predictor of both OS (HR, 1.19; 95% CI: 1.06–1.34; P = 0.004) and CSS (HR, 1.17; 95% CI: 1.02–1.33; P = 0.02) for LTEC (Table S2). In addition, surgery (OS, HR: 0.47; 95% CI: 0.43–0.5; P < 0.001; CSS, HR: 0.45; 95% CI: 0.42–0.49; P < 0.001), chemotherapy (OS, HR: 0.58; 95% CI: 0.52–0.63; P < 0.001; CSS, HR: 0.58; 95% CI: 0.52–0.65; P < 0.001) and radiotherapy (OS, HR: 0.81; 95% CI: 0.74–0.88; P < 0.001; CSS, HR: 0.81; 95% CI: 0.74–0.9; P < 0.001) were independent predictors of prognoses.
For patients with UTEC, eight significant risk factors were identified in univariate analysis for OS and CSS (Table S3). Notably, neither supraclavicular nor celiac nodal location was an independent predictor of OS and CSS for UTEC.
In the multivariate analysis, surgery (OS, HR: 0.63; 95% CI: 0.42–0.95; P = 0.026; CSS, HR: 0.6; 95% CI: 0.37–0.96; P = 0.034) and chemotherapy (OS, HR: 0.37;95% CI: 0.26–0.52; P < 0.001; CSS, HR: 0.33; 95% CI: 0.21–0.51; P < 0.001) were significant independent predictors of both OS and CSS for UTEC (Table S4).
Nodal status and therapeutic role of treatment
Figures 2 and 3 exhibit the therapeutic role of surgery in patients with UTEC (Fig 2a,d) and LTEC (Fig 3a,d).Surgery was not associated with increased OS (surgery vs. nonsurgery: 50.00% vs. 10.38%; P = 0.11; Fig 2a) or CSS (surgery vs. nonsurgery: 100.00% vs. 25.11%; P = 0.11; Fig 2b) for UTEC with celiac node metastasis. Similar results are displayed in Figure 2c,d which demonstrate that surgery did not improve the OS (surgery vs. nonsurgery: 0.00% vs. 12.20%; P = 0.635) or CSS (surgery vs. nonsurgery: 0.00% vs. 15.30%; P = 0.513) for UTEC with supraclavicular node metastasis. In contrast, significantly better OS (surgery vs. nonsurgery: 25.40% vs. 5.80%; P < 0.001) and CSS (surgery vs. nonsurgery: 31.40% vs. 7.90%; P < 0.001) were observed in LTEC with celiac node metastasis, as shown in Figure 3a,b. In addition, surgery was also associated with increased OS (surgery vs. nonsurgery: 27.00% vs. 6.10%; P < 0.001; Fig 3c) and CSS (surgery vs. nonsurgery: 42.70% vs. 9.10%; P < 0.11; Fig 3d) for LTEC with supraclavicular node metastasis.
Figure 2.
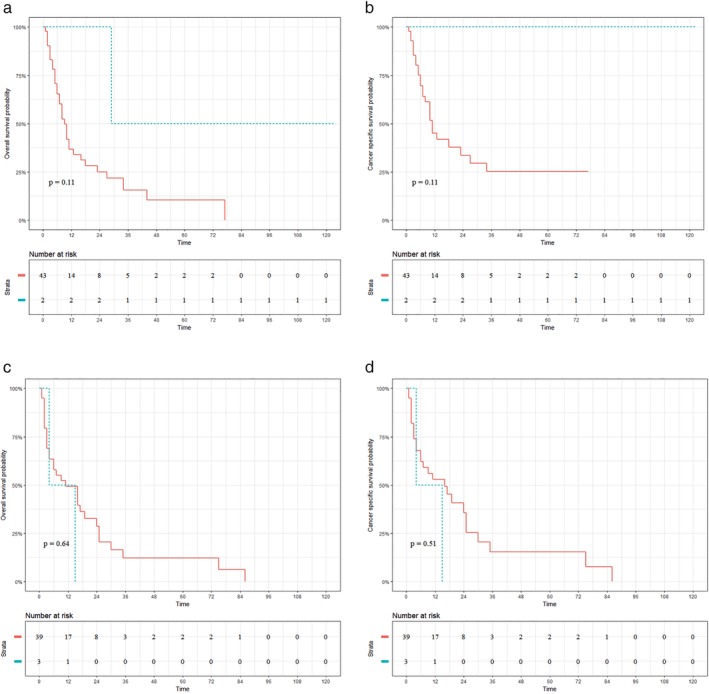
Prognostic impact of surgery on patients with upper thoracic esophageal cancer (UTEC). (a, b) Overall survival (OS) and cancer‐specific survival (CSS) for UTEC with celiac node metastasis. (c, d) OS and CSS for UTEC with supraclavicular node metastasis. ( ) surgery=No, and (
) surgery=No, and ( ) surgery=Yes.
) surgery=Yes.
Figure 3.
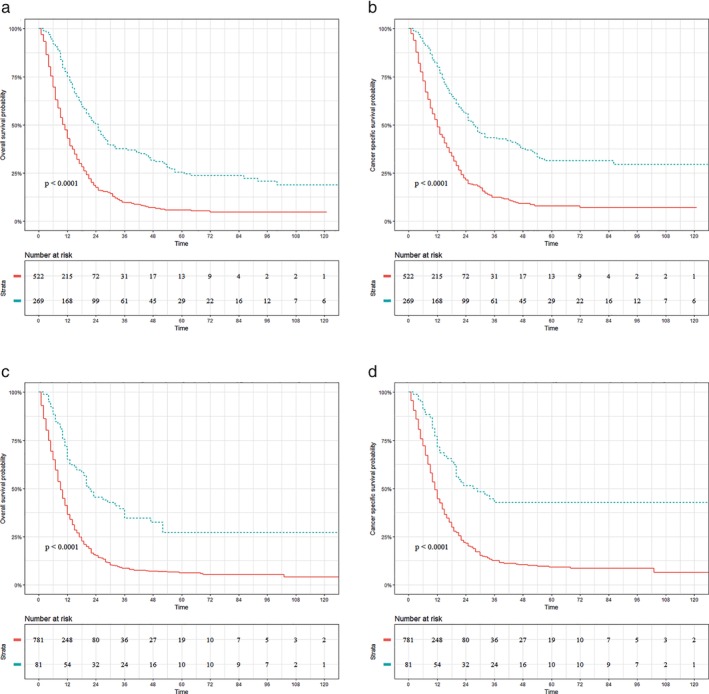
Prognostic impact of surgery on patients with lower thoracic esophageal cancer (LTEC). (a, b) Overall survival (OS) and cancer‐specific survival (CSS) for LTEC with celiac node metastasis. (c, d) OS and CSS for LTEC with supraclavicular node metastasis. ( ) surgery=No, and (
) surgery=No, and ( ) surgery=Yes.
) surgery=Yes.
Figures 4 and 5 present the prognostic impact of radiotherapy on patients with UTEC (Fig 4a,d) and LTEC (Fig 5a,d), respectively. Significantly better OS (radiotherapy vs. nonradiotherapy: 17.20% vs. 0.00%; P < 0.001; Fig 4a) and CSS (radiotherapy vs. nonradiotherapy: 15.60% vs. 0.00%; P < 0.001; Fig 4b) were observed in patients with celiac node metastasis who underwent radiotherapy. Interestingly, there was a significant difference in OS (radiotherapy vs. nonradiotherapy: 15.90% vs. 0.00%; P = 0.012; Fig 4c) but not in CSS (radiotherapy vs. nonradiotherapy: 17.70% vs. 0.00%; P = 0.075; Fig 4d) between radiotherapy and nonradiotherapy in UTEC with supraclavicular node metastasis. Meanwhile, radiotherapy was associated with improved OS (radiotherapy vs. nonradiotherapy: 14.50% vs. 4.20%; P < 0.001; Fig 5a) and CSS (radiotherapy vs. nonradiotherapy: 18.90% vs. 5.50%; P < 0.001; Fig 5b) for LTEC with celiac node metastasis. Similar results were also observed in LTEC with supraclavicular node metastasis (OS: radiotherapy vs. nonradiotherapy: 11.40% vs. 5.00%; P < 0.001; Fig 5c; CSS: radiotherapy vs. nonradiotherapy: 14.90% vs. 11.90%; P = 0.004; Fig 5d).
Figure 4.
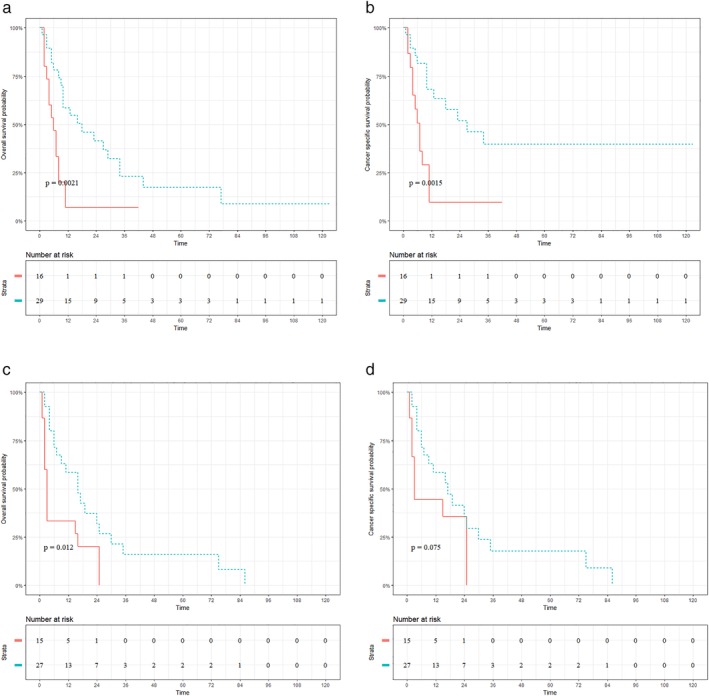
Prognostic impact of radiotherapy on patients with upper thoracic esophageal cancer. (a, b) Overall survival (OS) and cancer‐specific survival (CSS) for UTEC with celiac node metastasis. (c, d) OS and CSS for UTEC with supraclavicular node metastasis. ( ) radiotherapy=No, and (
) radiotherapy=No, and ( ) radiotherapy=Yes.
) radiotherapy=Yes.
Figure 5.
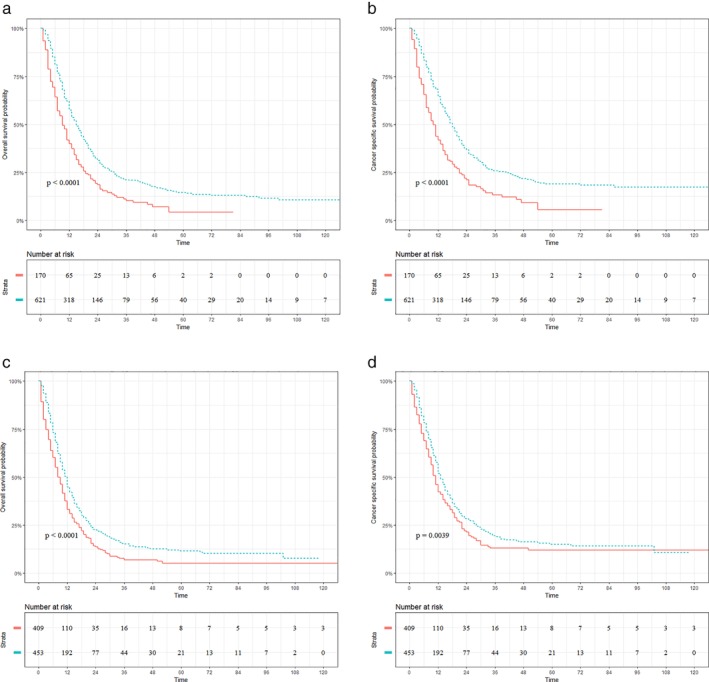
Prognostic impact of radiotherapy on patients with lower thoracic esophageal cancer (LTEC). (a, b) OS and CSS for LTEC with celiac node metastasis. (c, d) OS and CSS for LTEC with supraclavicular node metastasis. ( ) radiotherapy=No, and (
) radiotherapy=No, and ( ) radiotherapy=Yes.
) radiotherapy=Yes.
Discussion
Despite progress in esophageal cancer staging systems, the sixth edition of the AJCC staging system has continued to be the most widely used in China. However, the prognostic impact and therapeutic strategies for esophageal cancer with supraclavicular node metastasis remain controversial. As mentioned above, the implication of supraclavicular node metastasis in the AJCC and JES systems is very different. The AJCC TNM classification defines lymph nodes located in the defined area as “regional lymph nodes” regardless of the tumor location. Metastasis to lymph nodes other than regional lymph nodes, especially supraclavicular nodes, is categorized as M1.23 In the latest JES classification, regional nodes are subgrouped into groups 1 to 4 in five different patterns according to the main tumor location.24 In particular, supraclavicular nodes are classified as group 3 nodes for LTEC.24 Because group 3 nodes are the most distant regional nodes, proposals for their selective dissection have been reported.25, 26, 27 On the other hand, supraclavicular nodes are classified as group 2 for UTEC,24 the dissection of which has been widely accepted in Japan.9Our study revealed that the prognostic impact of supraclavicular node metastasis in UTEC was similar to that of regional or celiac node metastasis, which validates the subgrouping of regional nodes in the Japanese classification. Our study also coincides with a multi‐institutional study in which the prognostic impact of supraclavicular nodes was similar to that of regional nodes for thoracic esophageal cancer.8 Meanwhile, we showed that LTEC involving supraclavicular nodes resulted in significantly worse prognoses compared with those involving previously designated regional or celiac nodes, which supports the reclassification of regional nodes.
Although the outcomes of LTEC with celiac node metastasis were relatively worse than those with regional node metastasis, our data revealed no statistically significance between them. Moreover, celiac node metastasis was not an independent predictor of worse OS and CSS in either UTEC or LTEC. Our findings justify the reclassification of celiac node metastasis into the N parameter in seventh and eighth editions of the AJCC staging system which is consistent with other studies.16, 18, 28
Several studies7, 19, 29 have reported that treatment on esophageal cancer with supraclavicular node metastasis has been mainly palliative but not curative. However, other studies have demonstrated that surgery can benefit the prognosis of thoracic esophageal cancer with supraclavicular node metastasis.5, 6, 18, 28 In addition, up to 20% of patients with supraclavicular node metastasis showed reasonable survival after chemoradiation and surgical resection.14 From our data, surgery, chemotherapy and radiation were predictors of prolonged OS and CSS in both UTEC and LTEC, consistent with previous studies.8, 14 Meanwhile, our subgroup analysis indicated both surgery and radiotherapy were associated with better outcomes in LTEC with supraclavicular or celiac node metastasis. Our findings were also supported to some extent by studies which demonstrated that postoperative radiotherapy could not only improve the survival of stage II–III esophageal cancer irrespective of the tumor location, but also control local‐regional LTEC with supraclavicular nodeinvolvement.2, 30 Given that surgery could not improve prognoses of UTEC with either supraclavicular or celiac node metastasis, radiotherapy nonetheless exhibited its value in improving prognoses of UTEC. Our data thus indicate different therapeutic strategies should be considered according to the primary location of esophageal cancer.
We must acknowledge several limitations of this study. First, potential biases were inevitable because of the retrospective nature of our study. Although some advanced statistical methods were applied to balance the covariates among the arms, there were still some latent biases. Another potential criticism of the present study is that most patients included with UTEC had squamous cell carcinoma, whereas the majority of patients with LTEC had adenocarcinoma. The different main histology types might affect the therapeutic roles of surgery and radiotherapy. Also, compared with those without surgical resection, fewer UTEC patients with celiac node or supraclavicular node metastasis received surgery, which might affect the statistical power of our results. Furthermore, in the present study, patients diagnosed with upper esophageal cancer were more likely to have T4 stage and were less likely to receive surgery, which means this entity might be treated with less curative intent. However, according to the NCCN guideline, even for those with inoperable disease, surgical resection can also be considered for select patients.31 Moreover, the primary aim of this study was to evaluate the prognostic significance of supraclavicular and celiac lymph node metastasis. The large sample size of our study (especially those diagnosed with esophageal cancer located in the lower third) made our conclusion convincible. As a final comment, the SEER database could only provide a small number of thoracic esophageal cancer patients with supraclavicular node metastasis who underwent surgery. Large‐scale prospective randomized trials are needed to further validate our findings and better define the regional lymph nodes linked to tumor location.
In summary, our results provide preliminary evidence that the clinical significance of supraclavicular and celiac lymph node metastases should be reevaluated in thoracic esophageal cancer utilizing different prognostic information according to the primary sites.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Table S1. Univariate analysis of overall survival and cancer‐specific survival in patients with lower thoracic esophageal cancer.
Table S2. Cox proportional hazards regression model for overall survival and cancer‐specific survival in patients with lower thoracic esophageal cancer.
Table S3. Univariate analysis of overall survival and cancer‐specific survival in patients with upper thoracic esophageal cancer.
Table S4. Cox proportional hazards regression model for overall survival and cancer‐specific survival in patients with upper thoracic esophageal cancer.
Acknowledgments
This study was supported by Jiangsu Provincial Commission of Health and Family Planning (grant H201521), the Natural Science Foundation of Jiangsu Province (grant BK20161224), Suzhou Key Discipline for Medicine (SZXK201803) and the Science and Technology Research Foundation of Suzhou Municipality (SYS2018063). Donglai Chen, Junmiao Wen and Ting Zhao were responsible for the concept, data and for writing the original draft of the manuscript.
Jiayan Chen, Yuhuan Zhao and Di Liu carried out a formal analysis and validation. Wenjia Wang was responsible for the Methodology. Xinyan Xu and Chang Chen reviewed and edited the manuscript. Min Fan and Yongbing Chen were responsible for supervision, funding acquisition and also assisted in reviewing and editing the manuscript. The authors also wish to thank International Science Editing (http://www.internationalscienceediting.com) for editing the manuscript.
Contributor Information
Min Fan, Email: fanmin_fuscc@aliyun.com.
Yongbing Chen, Email: chentongt@sina.com.
References
- 1. Wu H, Liu C, Xu M, Guo M, Xu S, Xie M. Prognostic value of the number of negative lymph nodes in esophageal carcinoma without lymphatic metastasis. Thorac Cancer 2018; 9 (9): 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu J, Cai XW, Liu Q, Li HX, Cheng Y, Fu XL. Characteristics of the local recurrence pattern after curative resection and values in target region delineation in postoperative radiotherapy for lower thoracic esophageal squamous cell cancer. Thorac Cancer 2017; 8 (6): 630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67 (1): 7–30. [DOI] [PubMed] [Google Scholar]
- 4. Lertbutsayanukul C, Tharavej C, Klaikeaw N, Prayongrat A, Lowanitchai C, Sriuranpong V. High dose radiation with chemotherapy followed by salvage esophagectomy among patients with locally advanced esophageal squamous cell carcinoma. Thorac Cancer 2017; 8 (3): 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamasaki M, Miyata H, Miyazaki Y et al Evaluation of the nodal status in the 7th edition of the UICC‐TNM classification for esophageal squamous cell carcinoma: Proposed modifications for improved survival stratification : Impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol 2014; 21 (9): 2850–6. [DOI] [PubMed] [Google Scholar]
- 6. Schomas DA, Miller RC, Quevedo JF. Treatment for M1a cancer of the esophagus may not be largely palliative. J Thorac Oncol 2010; 5 (2): 284; author reply): 284–5. [DOI] [PubMed] [Google Scholar]
- 7. Trovo M, Bradley J, El Naqa I et al Esophageal carcinoma with celiac nodal metastases; curative or palliative. J Thorac Oncol 2008; 3 (7): 751–5. [DOI] [PubMed] [Google Scholar]
- 8. Tachimori Y, Ozawa S, Numasaki H et al Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi‐institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg 2014; 148 (4): 1224–9. [DOI] [PubMed] [Google Scholar]
- 9. Udagawa H, Ueno M. Comparison of two major staging systems of esophageal cancer‐toward more practical common scale for tumor staging. Ann Transl Med 2018; 6 (4): 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang D, Zheng Y, Wang Z et al Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery. Eur J Surg Oncol 2017; 43 (10): 1949–55. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Zheng Y, Wang Z, Zheng Q, Huang Q, Liu S. Nodal skip metastasis in esophageal squamous cell carcinoma patients undergoing three‐field lymphadenectomy. Ann Thorac Surg 2017; 104 (4): 1187–93. [DOI] [PubMed] [Google Scholar]
- 12. Huang W, Huang Y, Sun J et al Atlas of the thoracic lymph nodal delineation and recommendations for lymph nodal CTV of esophageal squamous cell cancer in radiation therapy from China. Radiother Oncol 2015; 116 (1): 100–6. [DOI] [PubMed] [Google Scholar]
- 13. Udagawa H, Ueno M, Shinohara H et al The importance of grouping of lymph node stations and rationale of three‐field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012; 106 (6): 742–7. [DOI] [PubMed] [Google Scholar]
- 14. Tong DK, Kwong DL, Law S, Wong KH, Wong J. Cervical nodal metastasis from intrathoracic esophageal squamous cell carcinoma is not necessarily an incurable disease. J Gastrointest Surg 2008; 12 (10): 1638–45 discussion 1645. [DOI] [PubMed] [Google Scholar]
- 15. Altorki NK, Skinner DB. Occult cervical nodal metastasis in esophageal cancer: Preliminary results of three‐field lymphadenectomy. J Thorac Cardiovasc Surg 1997; 113 (3): 540–4. [DOI] [PubMed] [Google Scholar]
- 16. Hofstetter W, Correa AM, Bekele N et al Proposed modification of nodal status in AJCC esophageal cancer staging system. Ann Thorac Surg 2007; 84 (2): 365–73 discussion 374–365. [DOI] [PubMed] [Google Scholar]
- 17. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: An eighth edition staging primer. J Thorac Oncol 2017; 12 (1): 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu WH, Hsu PK, Hsieh CC, Huang CS, Wu YC. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J Gastrointest Surg 2009; 13 (11): 1913–20. [DOI] [PubMed] [Google Scholar]
- 19. Christie NA, Rice TW, DeCamp MM et al M1A/M1B esophageal carcinoma: Clinical relevance. J Thorac Cardiovasc Surg 1999; 118 (5): 900–6. [DOI] [PubMed] [Google Scholar]
- 20. Surveillance, Epidemiology and End Results Program . [Cited 5 May 2018.] Available from URL: http//seercancergov/about. 2018.
- 21. SEER Program Coding and Staging Manual . 2018. Coding guidelines for esophagus C150‐C155, C158‐C159, 2018. [Cited 31 May 2018]. Available from URL: https://seercancergov/tools/codingmanuals/Accessed.
- 22. Greene FL, Page DL, Fleming ID et al AJCC Cancer Staging Manual. Springer New York, New York: 2002. [Google Scholar]
- 23. Japan Esophageal Society . Japanese classification of esophageal cancer, 11th Edition: Part II and III. Esophagus 2017; 14 (1): 37–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Japan Esophageal Society . Japanese classification of esophageal cancer, 11th Edition: Part I. Esophagus 2017; 14 (1): 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyata H, Yano M, Doki Y et al A prospective trial for avoiding cervical lymph node dissection for thoracic esophageal cancers, based on intra‐operative genetic diagnosis of micrometastasis in recurrent laryngeal nerve chain nodes. J Surg Oncol 2006; 93 (6): 477–84. [DOI] [PubMed] [Google Scholar]
- 26. Shimada Y, Sato F, Maeda M et al Validity of intraoperative pathological diagnosis of paratracheal lymph node as a strategy for selection of patients for cervical lymph node dissection during esophagectomy. Dis Esophagus 2003; 16 (3): 246–51. [DOI] [PubMed] [Google Scholar]
- 27. Nagatani S, Shimada Y, Kondo M et al A strategy for determining which thoracic esophageal cancer patients should undergo cervical lymph node dissection. Ann Thorac Surg 2005; 80 (5): 1881–6. [DOI] [PubMed] [Google Scholar]
- 28. Lee PC, Port JL, Paul S, Stiles BM, Altorki NK. Predictors of long‐term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg 2009; 88 (1): 186–92 discussion 192‐3. [DOI] [PubMed] [Google Scholar]
- 29. Liu J, Liu Q, Wang Y, Xia Z, Zhao G. Nodal skip metastasis is associated with a relatively poor prognosis in thoracic esophageal squamous cell carcinoma. Eur J Surg Oncol 2016; 42 (8): 1202–5. [DOI] [PubMed] [Google Scholar]
- 30. Zou BW, Pang J, Liu YM et al Postoperative chemoradiotherapy improves survival in patients with stage II‐III esophageal squamous cell carcinoma: An analysis of clinical outcomes. Thorac Cancer 2016; 7 (5): 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meerten EV, CV R, Tesselaar ME et al Definitive concurrent chemoradiation (CRT) with weekly paclitaxel and carboplatin for patients (pts) with irresectable esophageal cancer: A phase II study. J Clin Oncol 2010; 28 (15_suppl): e14508–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis of overall survival and cancer‐specific survival in patients with lower thoracic esophageal cancer.
Table S2. Cox proportional hazards regression model for overall survival and cancer‐specific survival in patients with lower thoracic esophageal cancer.
Table S3. Univariate analysis of overall survival and cancer‐specific survival in patients with upper thoracic esophageal cancer.
Table S4. Cox proportional hazards regression model for overall survival and cancer‐specific survival in patients with upper thoracic esophageal cancer.


