Abstract
It is well established that obesity increases the incidence and worsens the prognosis of women’s cancer. For breast cancer, women with obesity exhibit more than a twofold increase in the odds of being diagnosed with cancer, with a greater risk of advanced stage at diagnosis, and ≤40% greater risk of recurrence and death than their normal-weight counterparts. These findings are similar in gynecologic cancers, where women who are obese with a body mass index (BMI) >40 kg/m2 have up to six times greater risk of developing endometrial cancer and a 9.2% increase in mortality with every 10% increase in BMI. Likewise, patients with obesity exhibit a twofold higher risk of premenopausal ovarian cancer, and patients who are obese with advanced stage ovarian cancer have shown a shorter time to recurrence and poorer overall survival. Obesity is accompanied by changes in expression of adipose factors that act on local tissues and systemically. Once obesity was recognized as a factor in cancer incidence and progression, the adipose cytokine (adipokine) leptin became the focus of intense investigation as a putative link, with nearly 3000 publications on the topic. Leptin has been shown to increase cell proliferation, inhibit apoptosis, promote angiogenesis, and increase therapeutic resistance. These characteristics are associated with a subset of cells in both liquid and solid tumors known as cancer stem cells (CSCs), or tumor initiating cells. We will review the literature discussing leptin’s role in breast and gynecologic cancer, focusing on its role in CSCs, and consider goals for targeting future therapy in this arena to disrupt tumor initiation and progression in women’s cancer.
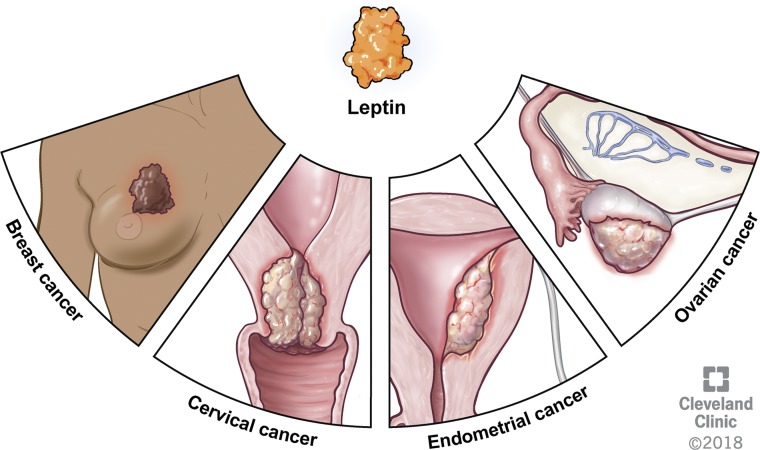
The adipokine leptin promotes breast and gynecologic cancers by engaging cancer stem cells to induce agiogenesis, proliferation, self-renewal, cancer progression, and chemotherapy resistance.
Obesity is characterized as excessive body fat, with a body mass index (BMI) >30 kg/m2. Obesity is a known risk factor for the initiation and progression of many types of cancer, including breast, endometrium, colon, and pancreatic cancer (1). Obesity is now a major epidemic, with 36.5% of adults in the United States categorized as obese and a global doubling of obesity rates from 6.4% to 12% over the past 30 years (2, 3). The incidence and mortality of many cancers have been steadily rising, with main contributory factors including obesity (4–6). In breast cancer, women with obesity are two times more likely to develop cancer, with 40% increased risk of cancer recurrence and death from disease (7). In endometrial cancer, women with obesity have a sixfold increased risk of developing malignancy. Reproductive-age women with obesity are twice as likely to develop ovarian cancer, with increased rates of cancer recurrence and death from disease (8, 9).
Although the correlation between obesity and cancer is accepted, the potential causal relationship between obesity and cancer continues to be an area of intense research investigation. Obesity is characterized by excess adipose tissue in the predominant fat storage depot, which drives the dysregulation of complex metabolic and endocrine activities. One of the major adipose tissue–secreted hormones is leptin, shown to correlate with the level of adiposity and BMI in women (10–12). Increasing evidence indicates that increased levels of leptin are associated with tumorigenic actions, such as inflammation, angiogenesis, chemoresistance, and cancer cell self-renewal (1, 13). Many of these activities are also known to be indicative of cancer stem cell (CSC) behavior, a population of cancer cells with tumor initiating and metastatic activity (14). This potential link between leptin and cancer incidence and progression has led to a broad exploration into its role in CSC biology. Over the past 5 years, a large body of literature has emerged on the role of leptin not only in breast cancer but also in its role in gynecologic cancers including endometrial, ovarian, and cervical malignancies (Fig. 1). Although leptin’s link to CSCs is well studied in breast cancer, leptin-mediated CSC maintenance in gynecologic malignancies is still a developing area of research that warrants further investigation. Here we review the current literature on leptin’s role in CSCs in breast and gynecologic cancers.
Figure 1.
Leptin has been found to play a role in breast, cervical, endometrial, and ovarian malignancies, with upregulation of leptin and LEPR in associated tissue samples. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2018. All Rights Reserved.
Potential Mechanisms of Leptin-Mediated CSC Maintenance
Leptin is an adipokine, or fat-secreted cytokine, discovered by Zhang et al. (15) in 1994. It is expressed and secreted primarily by adipose tissue (10). Low levels are also expressed in skeletal muscle, placenta, and the brain (15). Produced by the obesity (Ob) gene, this 16-kD hormone influences food intake and body weight via regulation of satiety and energy metabolism through activity in the arcuate nuclei neurons of the hypothalamus (16, 17). Multiple studies have also demonstrated that leptin influences fetal development, reproduction, hematopoiesis, wound healing, immune response, angiogenesis, and proliferation (17–25).
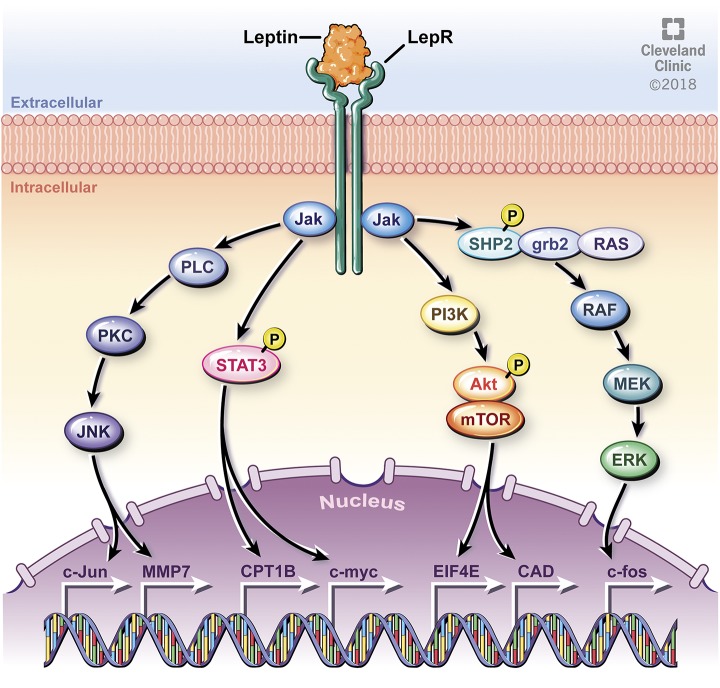
These physiologic activities are mediated through binding to the leptin receptor (LEPR), a class I cytokine receptor. There are six isoforms of LEPR (LEPRa through LEPRf), all differing in the length of their intracellular domain, with all isoforms except LEPRe containing a transmembrane region (26–29). LEPRb has an extended intracellular domain with typical cytokine receptor features (17). When leptin binds to the extracellular region of the LEPRb isoform of the LEPR, multiple downstream signaling pathways are activated (Fig. 2).
Figure 2.
Leptin binds to the LEPR and mediates multiple downstream pathways. The JAK/STAT pathway is mediated via homo-oligomerization of JAK2, with subsequent phosphorylation and activation of STAT transcription factors, such as CPT1B and c-myc. JAK also phosphorylates Tyr985, leading to phosphorylation of SHP2 and downstream activation of ERK and associated gene expression, including the oncogene c-fos. JAK also leads to activation of the PI3K pathway, whereby AKT is phosphorylated and mTOR is subsequently activated, leading to expression of EIF4E and CAD. JNK is another pathway activated by JAK, leading to downstream expression of genes including c-Jun (oncogene) and MMP7. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2018. All Rights Reserved.
Leptin binding to LEPRb activates Janus kinase 2 (JAK2), leading to recruitment and activation of signaling transducer and activator of transcription 3 (STAT3), ERK, PI3K/AKT, and JNK. The JAK2/STAT3 pathway is well studied, with binding of leptin to LEPRb causing homo-oligomerization and subsequent binding of JAK2 to form a complex (30, 31). This pathway leads to phosphorylation of tyrosines on the intracellular domain of LEPRb that provide a docking site for STAT3, STAT5, and SHP-2 (30, 32, 33). The ERK pathway is initiated by JAK2 phosphorylation of Tyr985, leading to phosphorylation of SHP-2, with subsequent activation downstream of ERK (33). JNK and ERK pathways lead to activation of multiple targets, including nuclear factor kappa B, a transcription factor essential in regulation of proinflammatory cytokines including TNF-α and IL-1. The PI3K pathway is mediated via IRS, which leads to phosphorylation of AKT and subsequent activation of mammalian target of rapamycin complex 1 (mTORC1), affecting downstream targets such as S6 kinase 1; S6 kinase 2; eukaryotic initiation factor 4 (EIF4); and carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) (17, 34–40). Phosphorylated STATs can form heterodimers or homodimers and translocate to the nucleus to induce specific gene transcription (13).
The leptin-activated pathways can lead to the expression of multiple target genes. For example, the JAK2/STAT3 pathway can induce expression of carnitine palmitoyltransferase 1B (CPT1B), a critical enzyme for fatty acid beta oxidation (41). JAK2/STAT3 can also induce expression of c-myc, a known oncogene (42). Likewise, ERK pathway activation leads to induction in expression of c-fos, whereas JNK pathway activation leads to induction of expression of c-jun (43). C-fos and c-Jun have been implicated as important proto-oncogenes serving as potential negative prognostic factors in gynecologic cancers, such as endometrial carcinoma (44–46). Similarly, the AKT/mTORC1/S6 kinase pathway is upregulated in breast cancer, with amplification of S6 kinase 1 and S6 kinase 2 associated with poor prognosis (29, 30). Downstream gene targets can vary, but they include carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, EIF4, and CAD, leading to cell growth and angiogenesis (35–39).
Leptin Regulation in Women’s Cancer
Leptin became an early focus for cancer investigation once the connection with obesity was established (47). Leptin has been shown to be associated with tumor progression via increased cell proliferation, attenuated apoptosis, enhanced angiogenesis, and metastasis, as well as chemotherapy and radiotherapy resistance. These activities are also associated with CSC behaviors, and so it has been proposed that leptin may promote different cancers via CSC enrichment (13, 48). Leptin’s role in CSC augmentation has been extensively studied in breast cancer cells, with leptin and its signaling pathways demonstrating increased stemness, chemoresistance, and poorer survival through CSC activity. These studies and preliminary investigation in this area of research in gynecologic malignancies are discussed.
Breast cancer
The leading type of cancer among women is breast cancer, with an estimated 252,710 new cases in 2017, representing 15% of all new cancer cases and 6.8% of all cancer deaths in the United States (49). There are several molecular subtypes of breast cancer, which include human epidermal growth factor receptor 2 (HER2) positive; luminal A, which is estrogen receptor (ER) positive and progesterone receptor (PR) positive; luminal B, which is ER and HER2 positive and PR negative; and triple negative breast cancer (TNBC), which is negative for ER, PR, and HER2 (50). Of these subtypes, TNBC, or basallike breast tumors, are more common in patients who are younger, African American, and overweight or obese (51). The link between obesity and breast cancer in both postmenopausal and premenopausal women is known, with an increase in incidence of disease and poorer prognosis in patients with high BMI (7, 8, 52). Other indicators of obesity correlate with breast cancer mortality, such as increased waist-to-hip ratio and enlarged waist circumference, which increase the risk of breast cancer mortality up to threefold (53, 54). Given this association between obesity and poorer outcomes in breast cancer, adipose tissue and adipocyte-related factors have been of research interest, with leptin being a primary focus.
Leptin and LEPR are elevated in breast cancer as compared with nonmalignant mammary tissue. Increased expression of leptin is associated with cancer progression, increased metastases, and poorer prognosis in patients with breast cancer (48, 55, 56). Increased serum leptin and elevated leptin mRNA levels within tumor tissue have also been associated with poor prognosis in patients with breast cancer (56). Initially it was reported that increased expression of LEPR was demonstrated predominantly in ER-positive breast cancer types, both α and β isoforms (ERα and ERβ), with the determination that leptin more specifically promotes cell proliferation in estrogen-sensitive tissue (57, 58). This was an expected finding given that adipose tissue produces elevated circulating estrogens in postmenopausal women. However, LEPR has also been found to be highly expressed in TNBC or ER-negative/PR- negative/HER2-negative breast cancers, with LEPR expressed in >90% and leptin in >80% of this breast cancer type (17, 59), indicating that leptin and LEPR may have a role in mammary tissue that is independent of hormonal effects.
Multiple leptin-mediated pathways have been described in breast cancer cells. Activation of proangiogenesis pathways via expression of vascular endothelial growth factor (VEGF) and VEGF receptor is mediated by leptin, which leads to increased breast cancer growth and tumor vasculature support (60). Lipsey et al. (61) demonstrated that inhibition of leptin signaling in TNBC via leptin antagonists bound to nanoparticles interrupted leptin-induced S phase progression of the cell cycle. Leptin has also demonstrated increased cell survival by regulation of Bcl-2 proteins with associated evasion of apoptosis in TNBC (62). Leptin has been linked to induction of telomerase activity along the STAT3 pathway in MCF-7 breast cancer cells, leading to increased cell proliferation (63).
Leptin’s role in CSC augmentation has been well studied in breast cancer. One of the first studies to indicate that leptin can promote CSC enrichment came from orthotopic tumor transplants of MMTV-Wnt-1 tumors into obese leptin-deficient (ob/ob) and obese leptin receptor–deficient (db/db) mice. Zheng et al. (64) found that tumors transplanted into leptin-deficient mice had fewer CSCs compared with tumors derived from wild type or LEPR-deficient mice. Using in vivo limiting dilution assays, Zheng et al. (64) showed that the cancer cells from wild type mice had a tumor-initiating frequency of 1 in 2000 cells, which decreased to 1 in 30,000 cells when tumors were derived from leptin-deficient mice. In subsequent studies, LEPR was determined to be expressed in mammary CSC and necessary for their maintenance. Moreover, short hairpin RNA silencing of the LEPR led to attenuation in expression of CSC transcription factors NANOG, SOX2, and OCT4. LEPR-silenced cells also exhibited significantly lower cell proliferation, self-renewal, and tumor growth in vivo (65). More recently, Thiagarajan et al. (66) further studied leptin effects on CSCs in TNBC and found that leptin promotes CSC survival via STAT3 phosphorylation. Furthermore, LEPR was sufficient to transform non-CSCs into a stem cell state with induction of NANOG, SOX2, and OCT4 expression in a STAT3-dependent manner. Collectively, these studies indicate the necessity of leptin and LEPR expression in cancer stem cell maintenance in vitro and in vivo, as discovered in breast cancer cells.
In a recent study by Wang et al. (41), the aforementioned JAK2/STAT3 pathway was studied in relation to breast CSCs and chemoresistance in breast cancer. They found that inhibition of the JAK2/STAT3 pathway leads to inhibition of breast CSC actions, including self-renewal and expression of genes involved in fatty acid beta oxidation, such as CPT1B. Importantly, leptin derived from mammary adipocytes was sufficient to induce CPT1B expression and fatty acid oxidation in breast CSCs. The findings indicate that this pathway can promote stemness and chemoresistance. Leptin inhibition attenuated fatty acid oxidation in mouse mammary tumors and subsequent chemosensitization of the CSCs (41). Bowers et al. (48) studied obesity correlated with TNBC and its association with CSC activity by using in vitro and in vivo models. In vitro, leptin increased sphere formation, cell viability, and cell migration and invasion in E-Wnt cells, a murine TNBC cell line. CSC-related gene expression was also increased, including SOX2 and AKT3. These effects were reduced with silencing of LEPR. Liu et al. (43) demonstrated that silencing a breast cancer stem cell gene, hematological and neurologic expressed 1-like (HN1L), decreases the population of breast CSCs, limits tumor initiation, inhibits cancer progression, and chemosensitizes previously docetaxel-resistant TNBC tumors. This gene operates via activation of the LEPR-STAT3 pathway, with HN1L acting as a transcription regulator in TNBC. Clinically, HN1L is significantly correlated with shorter overall and disease-free survival in patients with TNBC (67). Additionally, Lipsey et al. (61) and Guo et al. (68) illustrated a complex leptin-mediated crosstalk between Notch, a family of transmembrane proteins acting as receptors in adjacent cell membranes, and IL-1. It was called Notch, IL-1, and leptin crosstalk outcome (NILCO). They demonstrated that leptin induces Notch activation in ER-positive and TNBC cells in vitro and in vivo, with associated cell proliferation and migration. Taken together, these studies indicate the crucial role of CSCs in breast cancer initiation and progression, with the potential of leptin pathways leading to future areas of therapeutic management.
Endometrial cancer
Endometrial cancer, a type of uterine cancer, remains the most common gynecologic malignancy in the United States, with >60,000 new cases and >11,000 deaths estimated for 2018 (69). Obesity is a known risk factor for developing endometrial cancer, with risk up to six times greater in women with BMI >40 kg/m2 (70, 71). Obesity is also associated with increased recurrence risk of endometrial cancer, with women with BMI >30 kg/m2 incurring a higher relapse probability (72). Furthermore, in women with metabolic syndrome components (increased waist circumference, blood pressure, and blood glucose), endometrial cancer mortality is elevated (54).
Leptin and LEPR are known to be expressed in endometrial cells (73). Leptin has been found to stimulate proliferation, migration, and invasion in the endometrium (74, 75). This effect is seen in benign diseases; for example, serum leptin levels and peritoneal fluid levels are elevated in women with endometriosis (76). Given leptin’s role in inflammation and angiogenesis, increased development of endometriosis with elevated leptin levels is not unexpected.
Leptin is also associated with endometrial malignancy, with elevated serum leptin levels strongly correlating with a positive risk of endometrial cancer in a nested case-control study of postmenopausal women (77). Several studies indicate leptin’s involvement in malignant cell proliferation and progression via JAK/STAT, ERK, and PI3K pathways (44–46, 78, 79). Sharma et al. (79) found that when endometrial cancer cell lines were treated with leptin, cell proliferation increased via activation of STAT3 and ERK2 signaling pathways via JAK/STAT activation. Additionally, leptin increased invasion of endometrial cancer cells, which was inhibited by a JAK/STAT inhibitor (AG490) and PI3K inhibitor (LY294002). Leptin has been found to regulate angiogenic activity in a dose-dependent manner in endometrial carcinoma, with a large increase in VEGF in cancer vs benign cells, with leptin-mediated signaling pathways again including JAK2, PI3K, and mTOR (73).
Lipsey et al. (61) discussed the discovery of NILCO as a potential stimulus for leptin-induced tumorigenic activity in endometrial cancer. Daley-Brown et al. (80, 81) found that NILCO expression was greater in type II than in type I endometrial cancer. Type I is the common, hormonally linked form of endometrial cancer, whereas type II endometrial cancer is the more aggressive, non–hormonally linked form. Interestingly, when assessing endometrial cancer tissue via immunohistochemical analysis, Western blot, and real-time PCR, Lipsey et al. (61) found that NILCO was more highly expressed in type II endometrial cancer. With these studies indicating leptin’s involvement in more aggressive tumor types, including leptin-mediated angiogenic and proliferative activity, leptin’s role in endometrial CSC maintenance seems likely, but this link between leptin and CSC in endometrial cancer cannot yet be deduced and warrants further investigation.
As previously discussed, multiple studies link leptin to the activation of the mTOR pathway (Fig. 2). Metformin has been investigated as a therapeutic agent given its inhibition of this pathway and thus indirect inhibition of downstream leptin signaling. Metformin directly activates AMP-activated protein kinase, which then phosphorylates tuberous sclerosis 2 protein and subsequently inhibits mTOR signaling, leading to reduction in cell proliferation. Metformin also indirectly affects cell growth by increasing insulin sensitivity, causing increased intracellular glucose uptake and decreased insulin levels peripherally (82). Metformin also has been found to cause leptin sensitivity via upregulation of LEPR expression (83). In a prospective trial by Soliman et al. (82), patients with newly diagnosed endometrial cancer underwent pretreatment blood draws and endometrial biopsies, then were treated for ≥7 days with metformin, and subsequently underwent posttreatment blood draw and definitive surgery. After treatment, serum IGF-1, omentin, insulin, C-peptide, and leptin levels were significantly lower. Posttreatment tissue analysis indicated decreased phosphorylated AKT, phosphorylated S6rp, and phosphorylated p44/42MAPK. A systematic review of the literature analyzing 19 different studies supports metformin as a potential adjuvant therapeutic agent in endometrial cancer, with metformin found to reverse atypical endometrial hyperplasia to normal endometrium, decrease cell proliferation from 51.9% to 34.5%, and increase overall survival in metformin users with endometrial cancer (84). Currently, a Gynecologic Oncology Group clinical trial is in progress, randomly assigning patients with advanced or recurrent endometrial cancer undergoing standard chemotherapy with or without metformin as adjuvant therapy (85).
Ovarian cancer
Ovarian cancer is the most fatal gynecologic malignancy in the United States, with only a 46% survival at 5 years after diagnosis (69). Typically, advanced disease is treated with cytoreductive surgery and platinum-based chemotherapy or radiotherapy or a combination of these two modalities (86). In ovarian cancer, although ≤85% of patients will enter remission with standard treatment of debulking surgery and platinum/taxane chemotherapy, cancer will recur in most of these patients (87). For those who do enter remission, the progression to platinum-resistant disease is pervasive (88). The prognosis is particularly poor in those with platinum-resistant disease, with response rates <20% for subsequent lines of chemotherapy and continued decrease in disease-free intervals with each subsequent therapy (89). Given the poor prognosis in those with platinum-resistant disease, identification of pathways of chemoresistance and subsequent chemosensitive therapies are on the forefront of cancer treatment (90).
Self-renewing populations of CSCs are associated with both tumor recurrence and chemoresistance in multiple tumor types, including ovarian cancer (78, 91–93). Although leptin is known to induce cell proliferation, invasion, metastasis, and recurrence, the connection between leptin and CSCs in ovarian cancer is a developing hypothesis. Kumar et al. (94) assessed LEPR signaling in ovarian cancer cell lines and found that increased leptin led to increased cell count, potentially interpreted as proliferation, and decreased apoptosis, mediated via the JAK2/STAT3 pathway. This finding correlated clinically with associated decreased patient survival with an elevation of both leptin and LEPR. Ghasemi et al. (95) recently discovered a link between matrix metalloproteinase-7 (MMP7) and leptin-mediated cell invasion in multiple ovarian cancer cell lines (SKOV3, OVCAR3). Ovarian cancer cells were exposed to leptin, with activation of leptin signaling pathways ERK, JNK, and p38 MAP kinases assessed via immunoblotting. Small interfering RNA and ERK and JNK inhibitors were used to validate findings. Results indicated leptin-induced MMP7 expression through a receptor-dependent effect on the ERK and JNK pathways. Silencing of LEPR and inhibition of ERK and JNK reversed these effects. Gelatin zymography indicated that MMP7 gene silencing reduced leptin-induced activation. Thus, leptin does appear to play a role in cell invasion in ovarian cancer cells.
In a study looking at granulosa cancer cell lines (COV434, KGN) as compared with benign granulosa cells (HGrC1), Fiedor and Gregoraszczuk (96) sought to evaluate LEPR expression in estrogen-responsive ovarian tissue. Superactive human leptin antagonist (SHLA), Lan1, and Lan2 are LEPR antagonists, and these inhibitors were used to directly block leptin and analyze subsequent effects on cell proliferation, LEPR expression, and cell cycle protein expression. In ovarian cancer cells, LEPR gene expression was 50% higher than in the benign comparison cell line. Lan1 and Lan2 inhibitors decreased LEPR expression in cancer cells (COV434). Lan2 specifically reversed leptin-stimulated proliferation in these same ovarian cancer cells COV434. All three LEPR antagonists (SHLA, Lan1, and Lan2) inhibited leptin-stimulated proliferation in another ovarian cancer cell line (KGN), suggesting that LEPR antagonists such as SHLA, Lan1, and Lan2 may inhibit LEPR at different specificities in certain cell lines. In a study assessing OB3, a synthetic peptide, as compared with leptin, leptin was found to induce proliferation in ovarian cancer cells, whereas OB3 blocked this effect with coapplication. Leptin and OB3 both activated the PI3K and JAK/STAT3 pathways; however, these signaling pathways and subsequent expression of ERα-responsive genes were blocked when OB3 was coapplied to cancer cells, with subsequent reduction in cancer cell proliferation (97). Taken together, LEPR antagonists and peptides such as OB3 that compete with LEPR binding may show promise as a therapeutic option for ovarian cancer.
Although leptin has been shown to have tumorigenic activity in ovarian cancer, serum leptin levels have not been shown to be a reliable screening biomarker for patients with ovarian cancer. In fact, most research has found lower levels of leptin in patients with ovarian cancer compared with controls (98–100). For example, Mor et al. (99) used a panel of four serum protein markers that included leptin, prolactin, osteoponin, and IGF-2 to increase detection of ovarian cancer. Leptin was decreased in patients with ovarian cancer and was not found to be useful as a biomarker in isolation. When the four analytes were assessed together, however, the panel indicated utility in screening; sensitivity, specificity, and positive predictive value were all 95%, and the negative predictive value was 94%. Lane et al. (101) assessed six inflammation-regulating factors in the ascites of women with epithelial ovarian cancer and benign controls and found that there was no significant difference in the median ascites level of leptin in malignant and benign samples.
When considering leptin as a biomarker for prognosis, however, studies show potential. In the aforementioned study by Lane et al. (101) assessing leptin in ascites, in which leptin was not useful in diagnosis of disease, ascites leptin level was found to be a strong predictor of clinical resistance to first-line therapy when used in combination with serum CA-125. Kato et al. (67) assessed 70 patients with ovarian cancer and found progression-free and overall survival rates were significantly lower in patients who are overweight, with a worse overall survival rate seen in patients expressing higher leptin levels. Serum and ascites leptin levels were higher in patients who are overweight with associated poorer prognosis, with elevated LEPR expression in ascites and metastases as compared with primary tumor site. Leptin exposure led to increased cancer cell migration through known leptin-mediated pathways (JAK/STAT3, PI3K). Diaz et al. (102) compared leptin with another adipokine, adiponectin, in women with epithelial ovarian cancer and found that women with low leptin/adiponectin ratios had a statistically longer disease-specific survival compared with those with high leptin/adiponectin ratios, with disease-specific survival of 57 vs 37 months. Uddin et al. (103) investigated leptin and LEPR in tissue samples from patients with epithelial ovarian cancer and found that LEPR overexpression was significantly associated with worse progression-free survival. Thus, although leptin assessment may not assist in screening for ovarian malignancy, leptin’s role in CSC-mediated actions such as invasion, recurrence, and chemoresistance is clearly developing. Direct evidence of leptin’s role in ovarian CSC maintenance is needed, because it could play a key role in developing chemosensitization therapies for patients with otherwise incurable disease.
Cervical cancer
Cervical cancer is a common malignancy among women worldwide, ranked as the third most common cancer among women and the second most common cause of cancer-related death. In developing nations, it is often the most common cause of cancer-related death among women (104). Fortunately, because of the use of screening techniques and management of human papillomavirus (HPV), rates of new cervical cancer cases in the United States have decreased >50% in the past 30 years (105). Although HPV plays a key role in cervical cancer, there remain necessary stimuli to provoke and maintain the downstream oncogenic transformation of cervical cells.
Data regarding leptin levels and cervical cancer status are controversial. Lebrecht et al. (106) performed a study assessing VEGF and leptin serum levels in >100 patients with cervical cancer and pre–cervical cancer as compared with healthy controls and found that although elevated VEGF was correlated with cancer, precancer, and tumor stage, serum leptin levels were not significantly different between groups. However, Yuan et al. (42) found that leptin was significantly higher in HeLa cervical cancer cells in a dose-dependent manner, with upregulation of c-myc mRNA and protein. Yuan et al. (42) then assessed tumors in 80 patients with cervical cancer via immunohistochemical staining and found that leptin was significantly correlated with higher grade of cervical carcinoma and that increased expression of leptin led to an increased expression of c-myc and bcl-2 genes; C-myc is an established oncogene, and bcl-2 acts as an antiapoptotic gene. Increased leptin also resulted in cancer cell proliferation, whereas silencing of leptin indicated inhibition of proliferation in cervical cancer cells through decreased expression of c-myc and bcl-2. Thus, leptin appears to play a role in cervical cancer proliferation and progression, although further studies are needed.
Cervical cancer stem cells are an emerging area of research. In their analysis of the TGF-β1/ERK1/2 pathway, Wu et al. (107) assessed the associated expression of CK17 and its effects on CSCs in cervical cancer. TGF-β1–treated cervical cancer cells underwent epithelial mesenchymal transition with downregulation of E-cadherin, upregulation of fibronectin and vimentin expression, and increased oncospheres and side population cells on flow cytometry. Knockdown of CK17, a possible cervical cancer CSC marker, decreased colony formation and decreased the proportion of side population cells on flow cytometry, whereas CK17 overexpression increased lymph node metastases in vivo. Whereas TGF-β1 seems to induce CK17 expression, inhibiting ERK1/2, on the other hand, decreased CK17. Targeting the CK17 signaling pathway, and therefore associated CSC actions such as stemness and metastatic disease, may be an area for future therapies in patients with progressive, metastatic cervical cancer. As discussed earlier, ERK1/2 is a known downstream signaling pathway of leptin; however, more investigations into the direct connections between leptin and CSC maintenance are needed.
Vulvar and vaginal cancers
Vulvar and vaginal cancers are uncommon gynecologic malignancies, with an estimated 6020 new cases of vulvar cancer with an associated 1150 deaths from disease, and an estimated 5170 cases of vaginal cancer with associated 1330 deaths from disease in the last year (69, 108). These cancers are more common in older women, with most women diagnosed in the sixth decade of life. They are also more common in women with a history of HPV infection or genital warts. In a large study by the National Institutes of Health and the American Association of Retired Persons, >200,000 women were followed for nearly 14 years, and of 170 diagnosed invasive vulvar cancers, obesity was identified as a significant risk factor, with a hazard ratio of 1.62 with BMI >30 kg/m2 (109). Although the disease is rare, the National Cancer Institute has found that new cases have been rising on average 0.6% each year over the last 10 years, with death rates increasing an average of 1.2% each year (69).
With the development of knowledge about CSC’s role in progressive disease, Napoletano et al. (110) sought to review CSC involvement in vulvar cancer by assessing paraffin-embedded tissue specimens derived from 43 patients with vulvar cancer for expression of specific CSC markers: CD133, CD24, ABCG2, and the regulatory T-cell marker FOXP3. They found that CD133 expression was associated with a younger age at diagnosis, lymph node metastasis, and larger tumor diameter. Thus, increased CSC marker expression was associated with young age at diagnosis and more aggressive disease, although overall survival and progression-free survival were not significantly different.
Given the rarity of this disease, studies assessing obesity-related pathogenesis in vulvar and vaginal cancer have been limited. In a study in Vienna, patients with squamous cell cancer of the vulva were compared with healthy controls, with serum leptin levels elevated as compared with controls. However, this was a small sample population, and leptin level did not show a significant association with tumor grade, histology, or lymph node metastases, and there was no significant difference in disease-free or overall survival between groups (111). In a case report analyzing the immunohistochemical profile of a patient with squamous cell cancer of the clitoris, leptin antibody was found to have no tissue tumor uptake (112).
Studies are lacking in regard to the best treatment options for these patients. Research specifically exploring the role of leptin in CSC augmentation in vulvar cancer has not yet been adequately performed. Given that obesity is a risk factor for vulvar cancer and given the emerging data on increased CSC expression in younger women with more aggressive disease, further research is warranted to best elucidate the potential connections between adipokines such as leptin expression in CSCs in vulvar and vaginal cancer (96, 97).
Translational Relevance
Using our knowledge on how leptin activates the signaling pathways that lead to CSC stimulation and maintenance, we can pursue new therapeutic methods. Because LEPR expression is needed for maintenance of CSC in culture and LEPR silencing leads to lack of tumor formation in mice, inhibition of the LEPR directly may be a useful therapeutic target for all cancer types associated with leptin (66). Alternatively, signaling pathways further downstream could be inhibited. For example, initial investigation into STAT3 inhibition led to reversed obesity-induced mammary hyperplasia and reduced cell migration and invasion in an in vivo model (113). STAT3 is being assessed in a clinical trial of advanced cancer, including breast cancer, whereby an oral formulation of STAT3 inhibitor will be given to patients and safety and efficacy will be determined (114). Similarly, drawing on studies performed by Sharma et al. (79) in endometrial cancer (whereby increased leptin led to increased cell proliferation and invasion via JAK/STAT, with reversal with subsequent inhibition of this pathway), therapies targeting the JAK/STAT pathway could be used for endometrial cancers as well. For example, a preliminary open-label phase 1b study of multiple cancer types, including endometrial cancer, is testing a JAK inhibitor in combination with another therapy to assess for safety of therapy (115). Notch signaling pathway inhibitors are also an area of interest, with a phase 1 trial performed in advanced solid tumors, focusing on recurrent endometrial cancer (48, 116). In ovarian cancer, leptin inhibition with compounds such as SHLA and Lan2 may prove to be a useful targeted therapy for granulosa cell ovarian cancer (96). As seen in cervical cancer, the signaling pathway TGF-β1/ERK1/2 that led to expression of CK17 with increased CSC actions could be further pursued (107), with most clinical studies in this pathway being pursued for head and neck cancers thus far (117). mTOR inhibitors are also an area of interest, and patients with advanced or recurrent gynecologic malignancies that have failed initial treatment may be candidates for an inhibitor of the known PI3k/AKT/mTOR pathway (118). Accordingly, a current clinical trial is assessing metformin as adjuvant therapy for advanced and recurrent endometrial cancer (85). Given the evolving research on leptin-mediated CSC actions in breast and gynecologic cancer, additional preclinical and clinical trials must be pursued to impede cancer progression and reduce therapeutic failure in women’s cancer.
Acknowledgments
We thank Elliott Richards, Emily Esakov, and Chad Braley for critical reading of this article.
Financial Support: Research in the Reizes laboratory is supported by National Institutes of Health Grants CA191263 and P30 CA043703, institutional funds from the Cleveland Clinic Foundation, and VeloSano Bike to Cure. Dr. Reizes holds the Laura J. Fogarty Endowed Chair for Uterine Cancer Research. Dr. Crean-Tate is a gynecologic oncology fellow at Cleveland Clinic.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CAD
carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase
- CPT1B
carnitine palmitoyltransferase 1B
- CSC
cancer stem cell
- EIF4
eukaryotic initiation factor 4
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- HPV
human papillomavirus
- JAK2
Janus kinase 2
- LEPR
leptin receptor
- MMP7
matrix metalloproteinase-7
- mTORC1
mammalian target of rapamycin complex 1
- NILCO
Notch, IL-1, and leptin crosstalk outcome
- PR
progesterone receptor
- SHLA
superactive human leptin antagonist
- STAT3
signaling transducer and activator of transcription 3
- TNBC
triple negative breast cancer
- VEGF
vascular endothelial growth factor
References
- 1. Tchio Mantho CI, Harbuzariu A, Gonzalez-Perez RR. Histone deacetylases, microRNA and leptin crosstalk in pancreatic cancer. World J Clin Oncol. 2017;8(3):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Adult obesity facts. 2018. Available at: www.cdc.gov/obesity/data/adult.html. Accessed March 2018.
- 3. Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) . National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colditz GA, Sellers TA, Trapido E. Epidemiology: identifying the causes and preventability of cancer? Nat Rev Cancer. 2006;6(1):75–83. [DOI] [PubMed] [Google Scholar]
- 5. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 6. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. [DOI] [PubMed] [Google Scholar]
- 7. Jiralerspong S, Goodwin PJ. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J Clin Oncol. 2016;34(35):4203–4216. [DOI] [PubMed] [Google Scholar]
- 8. Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13(2):85–92. [DOI] [PubMed] [Google Scholar]
- 9. Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55(1):3–23. [DOI] [PubMed] [Google Scholar]
- 10. Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59(3):359–371. [DOI] [PubMed] [Google Scholar]
- 11. Wulaningsih W, Holmberg L, Ng T, Rohrmann S, Van Hemelrijck M. Serum leptin, C-reactive protein, and cancer mortality in the NHANES III. Cancer Med. 2016;5(1):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89(3):980S–984S. [DOI] [PubMed] [Google Scholar]
- 13. Candelaria PV, Rampoldi A, Harbuzariu A, Gonzalez-Perez RR. Leptin signaling and cancer chemoresistance: Perspectives. World J Clin Oncol. 2017;8(2):106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 16.Connor ERO, Saygin C. Obesity, adipokines, and gynecologic cancer. In: Berger NKA, Lu K, eds. Focus on Gynecologic Malignancies. Cleveland, OH: Springer International Publishing; 2017:73–102.
- 17. Thiagarajan PS, Reizes O. Mouse models to study leptin in breast cancer stem cells. In: Berger NA, ed. Murine Models, Energy Balance, and Cancer. Cleveland, OH: Springer International Publishing; 2015:127–143. [Google Scholar]
- 18. Andò S, Barone I, Giordano C, Bonofiglio D, Catalano S. The multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol. 2014;4:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andò S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011;8(5):263–275. [DOI] [PubMed] [Google Scholar]
- 20. Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98(11):6390–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Blasio MJ, Lanham SA, Blache D, Oreffo ROC, Fowden AL, Forhead AJ. Sex- and bone-specific responses in bone structure to exogenous leptin and leptin receptor antagonism in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R781–R790. [DOI] [PubMed] [Google Scholar]
- 22. Hoggard N, Haggarty P, Thomas L, Lea RG. Leptin expression in placental and fetal tissues: does leptin have a functional role? Biochem Soc Trans. 2001;29(Pt 2):57–63. [DOI] [PubMed] [Google Scholar]
- 23. Jéquier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967(1):379–388. [DOI] [PubMed] [Google Scholar]
- 24. Lee M, Lee E, Jin SH, Ahn S, Kim SO, Kim J, Choi D, Lim KM, Lee ST, Noh M. Leptin regulates the pro-inflammatory response in human epidermal keratinocytes. Arch Dermatol Res. 2018;310(4):351–362. [DOI] [PubMed] [Google Scholar]
- 25. Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194(1):6–11. [DOI] [PubMed] [Google Scholar]
- 26. Allison MB, Myers MG Jr. 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223(1):T25–T35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56(2 Pt 2):s38–46; discussion s54–75. [DOI] [PubMed] [Google Scholar]
- 28. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. [DOI] [PubMed] [Google Scholar]
- 29. Villanueva EC, Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. 2008;32(suppl 7):S8–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin N, Wang D, Zhang H, Yi X, Sun X, Shi B, Wu H, Wu G, Wang X, Shang Y. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 2004;64(16):5870–5875. [DOI] [PubMed] [Google Scholar]
- 32. Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, Montanaro D, Maggiolini M, Panno ML, Andó S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279(19):19908–19915. [DOI] [PubMed] [Google Scholar]
- 33. Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis. 2007;22(4):401–409. [DOI] [PubMed] [Google Scholar]
- 34. Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26(2):364–371. [DOI] [PubMed] [Google Scholar]
- 35. Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–580. [DOI] [PubMed] [Google Scholar]
- 36. Karlsson E, Magić I, Bostner J, Dyrager C, Lysholm F, Hallbeck AL, Stål O, Lundström P. Revealing different roles of the mTOR-targets S6K1 and S6K2 in breast cancer by expression profiling and structural analysis. PLoS One. 2015;10(12):e0145013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laudański P, Kowalczuk O, Klasa-Mazurkiewicz D, Milczek T, Rysak-Luberowicz D, Garbowicz M, Baranowski W, Charkiewicz R, Szamatowicz J, Chyczewski L. Selective gene expression profiling of mTOR-associated tumor suppressor and oncogenes in ovarian cancer. Folia Histochem Cytobiol. 2011;49(2):317–324. [DOI] [PubMed] [Google Scholar]
- 38. Rad E, Murray JT, Tee AR. Oncogenic signalling through mechanistic target of rapamycin (mTOR): a driver of metabolic transformation and cancer progression. Cancers (Basel). 2018;10(1):E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanwar PS, Zhang L, Kaneko-Tarui T, Curley MD, Taketo MM, Rani P, Roberts DJ, Teixeira JM. Mammalian target of rapamycin is a therapeutic target for murine ovarian endometrioid adenocarcinomas with dysregulated Wnt/β-catenin and PTEN. PLoS One. 2011;6(6):e20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei M, Wang L, Zhong M. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37(1):91–101. [DOI] [PubMed] [Google Scholar]
- 41. Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27(1):136–150 e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan Y, Zhang J, Cai L, Ding C, Wang X, Chen H, Wang X, Yan J, Lu J. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol Rep. 2013;29(6):2291–2296. [DOI] [PubMed] [Google Scholar]
- 43. Liu Z, Uesaka T, Watanabe H, Kato N. High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int J Oncol. 2001;19(5):1009–1014. [DOI] [PubMed] [Google Scholar]
- 44. Bamberger AM, Milde-Langosch K, Rössing E, Goemann C, Löning T. Expression pattern of the AP-1 family in endometrial cancer: correlations with cell cycle regulators. J Cancer Res Clin Oncol. 2001;127(9):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin CY, Chao A, Wang TH, Hsueh S, Lee YS, Wu TI, Chao AS, Huang HJ, Chou HH, Chang TC, Lai CH. A dual tyrosine kinase inhibitor lapatinib suppresses overexpression of matrix metallopeptidase 1 (MMP1) in endometrial cancer. J Mol Med (Berl). 2014;92(9):969–981. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Zhang W, Li X, Li D, Zhang X, Yin Y, Deng X, Sheng X. Prognostic factors and genes associated with endometrial cancer based on gene expression profiling by bioinformatics analysis. Arch Gynecol Obstet. 2016;293(6):1287–1295. [DOI] [PubMed] [Google Scholar]
- 47. Park J, Scherer PE. Leptin and cancer: from cancer stem cells to metastasis. Endocr Relat Cancer. 2011;18(4):C25–C29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bowers LW, Rossi EL, McDonell SB, Doerstling SS, Khatib SA, Lineberger CG, Albright JE, Tang X, deGraffenried LA, Hursting SD. Leptin signaling mediates obesity-associated CSC enrichment and EMT in preclinical TNBC models. Mol Cancer Res. 2018;16(5):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National Cancer Institute Cancer stat facts: female breast cancer. National Cancer Institute 2018. Available at: seer.cancer.gov/statfacts/html/breast.html. Accessed 23 March 2018.
- 50. Cancer Treatment Centers of America Breast cancer molecular subtypes. 2018. Available at: www.cancercenter.com/breast-cancer/types/tab/molecular-subtypes/. Accessed 23 March 2018.
- 51. Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, Lee MM, Ambrosone CB, Caan BJ. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7(4):333–340. [DOI] [PubMed] [Google Scholar]
- 53. Borugian MJ, Sheps SB, Kim-Sing C, Olivotto IA, Van Patten C, Dunn BP, Coldman AJ, Potter JD, Gallagher RP, Hislop TG. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol. 2003;158(10):963–968. [DOI] [PubMed] [Google Scholar]
- 54. Gathirua-Mwangi WG, Song Y, Monahan PO, Champion VL, Zollinger TW. Associations of metabolic syndrome and C-reactive protein with mortality from total cancer, obesity-linked cancers and breast cancer among women in NHANES III. Int J Cancer. 2018;143(3):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–1453. [DOI] [PubMed] [Google Scholar]
- 56. Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118(6):1414–1419. [DOI] [PubMed] [Google Scholar]
- 57. Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293(1):622–628. [DOI] [PubMed] [Google Scholar]
- 58. Fusco R, Galgani M, Procaccini C, Franco R, Pirozzi G, Fucci L, Laccetti P, Matarese G. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-alpha in breast cancer: molecular basis for a novel therapeutic setting. Endocr Relat Cancer. 2010;17(2):373–382. [DOI] [PubMed] [Google Scholar]
- 59. Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105(4):956–964. [DOI] [PubMed] [Google Scholar]
- 60. Gonzalez-Perez RR, Lanier V, Newman G. Leptin’s Pro-Angiogenic Signature in Breast Cancer. Cancers (Basel). 2013;5(3):1140–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30(6):1499–1509. [PubMed] [Google Scholar]
- 63. Ren H, Zhao T, Wang X, Gao C, Wang J, Yu M, Hao J. Leptin upregulates telomerase activity and transcription of human telomerase reverse transcriptase in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2010;394(1):59–63. [DOI] [PubMed] [Google Scholar]
- 64. Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18(4):491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thiagarajan PS, Zheng Q, Bhagrath M, Mulkearns-Hubert EE, Myers MG, Lathia JD, Reizes O. STAT3 activation by leptin receptor is essential for TNBC stem cell maintenance. Endocr Relat Cancer. 2017;24(8):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kato S, Abarzua-Catalan L, Trigo C, Delpiano A, Sanhueza C, García K, Ibañez C, Hormazábal K, Diaz D, Brañes J, Castellón E, Bravo E, Owen G, Cuello MA. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget. 2015;6(25):21100–21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6(6):e21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA SEER cancer statistics review, 1975–2014. National Cancer Institute Available at: seer.cancer.gov/csr/1975_2014/. Accessed 23 March 2018.
- 70. Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008;98(9):1582–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers. 2014;29(1):e21–e29. [DOI] [PubMed] [Google Scholar]
- 72. Yang YF, Liao YY, Liu XL, Su SG, Li LZ, Peng NF. Prognostic factors of regression and relapse of complex atypical hyperplasia and well-differentiated endometrioid carcinoma with conservative treatment. Gynecol Oncol. 2015;139(3):419–423. [DOI] [PubMed] [Google Scholar]
- 73. Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123(12):2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dos Santos E, Duval F, Vialard F, Dieudonné MN. The roles of leptin and adiponectin at the fetal-maternal interface in humans. Horm Mol Biol Clin Investig. 2015;24(1):47–63. [DOI] [PubMed] [Google Scholar]
- 75. Ahn JH, Choi YS, Choi JH. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod. 2015;21(10):792–802. [DOI] [PubMed] [Google Scholar]
- 76. Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab. 2000;85(7):2483–2487. [DOI] [PubMed] [Google Scholar]
- 77. Luhn P, Dallal CM, Weiss JM, Black A, Huang WY, Lacey JV Jr, Hayes RB, Stanczyk FZ, Wentzensen N, Brinton LA. Circulating adipokine levels and endometrial cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kyo S, Maida Y, Inoue M. Stem cells in endometrium and endometrial cancer: accumulating evidence and unresolved questions. Cancer Lett. 2011;308(2):123–133. [DOI] [PubMed] [Google Scholar]
- 79. Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13(2):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Daley-Brown D, Oprea-Iles G, Vann KT, Lanier V, Lee R, Candelaria PV, Quarshie A, Pattillo R, Gonzalez-Perez RR. Type II endometrial cancer overexpresses NILCO: a preliminary evaluation. Dis Markers. 2017;2017:8248175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Daley-Brown D, Oprea-Ilies GM, Lee R, Pattillo R, Gonzalez-Perez RR. Molecular cues on obesity signals, tumor markers and endometrial cancer. Horm Mol Biol Clin Investig. 2015;21(1):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Soliman PT, Zhang Q, Broaddus RR, Westin SN, Iglesias D, Munsell MF, Schmandt R, Yates M, Ramondetta L, Lu KH. Prospective evaluation of the molecular effects of metformin on the endometrium in women with newly diagnosed endometrial cancer: a window of opportunity study. Gynecol Oncol. 2016;143(3):466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tang X, Li J, Xiang W, Cui Y, Xie B, Wang X, Xu Z, Gan L. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J Endocrinol. 2016;230(2):227–237. [DOI] [PubMed] [Google Scholar]
- 84. Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, Guerra ENS, Lofrano-Porto A. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017;147(1):167–180. [DOI] [PubMed] [Google Scholar]
- 85. US National Library of Medicine Paclitaxel and carboplatin with or without metformin hydrochloride in treating patients with stage III, IV, or recurrent endometrial cancer. 2018. Available at: clinicaltrials.gov/ct2/show/NCT02065687. Accessed 23 March 2018.
- 86. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R; Gynecologic Oncology Group . Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. [DOI] [PubMed] [Google Scholar]
- 87. Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17(12):1147–1158. [DOI] [PubMed] [Google Scholar]
- 88. Pujade-Lauraine E, Combe P. Recurrent ovarian cancer. Ann Oncol. 2016;27(suppl 1):i63–i65. [DOI] [PubMed] [Google Scholar]
- 89. Herzog TJ, Monk BJ. Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need? Gynecol Oncol Res Pract. 2017;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Monk BJ, Herzog TJ, Tewari KS. Evolution of chemosensitivity and resistance assays as predictors of clinical outcomes in epithelial ovarian cancer patients. Curr Pharm Des. 2016;22(30):4717–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 92. Wiechert A, Saygin C, Thiagarajan PS, Rao VS, Hale JS, Gupta N, Hitomi M, Nagaraj AB, DiFeo A, Lathia JD, Reizes O. Cisplatin induces stemness in ovarian cancer. Oncotarget. 2016;7(21):30511–30522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xiang D, Shigdar S, Bean AG, Bruce M, Yang W, Mathesh M, Wang T, Yin W, Tran PH, Al Shamaileh H, Barrero RA, Zhang PZ, Li Y, Kong L, Liu K, Zhou SF, Hou Y, He A, Duan W. Transforming doxorubicin into a cancer stem cell killer via EpCAM aptamer-mediated delivery. Theranostics. 2017;7(17):4071–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kumar J, Fang H, McCulloch DR, Crowley T, Ward AC. Leptin receptor signaling via Janus kinase 2/Signal transducer and activator of transcription 3 impacts on ovarian cancer cell phenotypes. Oncotarget. 2017;8(55):93530–93540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ghasemi A, Hashemy SI, Aghaei M, Panjehpour M. Leptin induces matrix metalloproteinase 7 expression to promote ovarian cancer cell invasion by activating ERK and JNK pathways. J Cell Biochem. 2018;119(2):2333–2344. [DOI] [PubMed] [Google Scholar]
- 96. Fiedor E, Gregoraszczuk EL. Superactive human leptin antagonist (SHLA), triple Lan1 and quadruple Lan2 leptin mutein as a promising treatment for human folliculoma. Cancer Chemother Pharmacol. 2017;80(4):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, Yang YSH, Chiu HC, Fu E, Davis PJ, Tang HY, Lin HY. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J Biomed Sci. 2017;24(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jin JH, Kim HJ, Kim CY, Kim YH, Ju W, Kim SC. Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet Gynecol Sci. 2016;59(4):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, Yue L, Bray-Ward P, Ward DC. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci USA. 2005;102(21):7677–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nick AM, Sood AK. The ROC ‘n’ role of the multiplex assay for early detection of ovarian cancer. Nat Clin Pract Oncol. 2008;5(10):568–569. [DOI] [PubMed] [Google Scholar]
- 101. Lane D, Matte I, Garde-Granger P, Laplante C, Carignan A, Rancourt C, Piché A. Inflammation-regulating factors in ascites as predictive biomarkers of drug resistance and progression-free survival in serous epithelial ovarian cancers. BMC Cancer. 2015;15(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129(2):353–357. [DOI] [PubMed] [Google Scholar]
- 103. Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, Al-Kuraya KS. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. 2009;8(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. National Institutes of Health. Cervical cancer. 2010. Available at: report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed 23 March 2018.
- 105. Practice bulletin no. 157: cervical cancer screening and prevention. Obstet Gynecol. 2016;127(1):e1–e20. [DOI] [PubMed] [Google Scholar]
- 106. Lebrecht A, Ludwig E, Huber A, Klein M, Schneeberger C, Tempfer C, Koelbl H, Hefler L. Serum vascular endothelial growth factor and serum leptin in patients with cervical cancer. Gynecol Oncol. 2002;85(1):32–35. [DOI] [PubMed] [Google Scholar]
- 107. Wu L, Han L, Zhou C, Wei W, Chen X, Yi H, Wu X, Bai X, Guo S, Yu Y, Liang L, Wang W. TGF-β1-induced CK17 enhances cancer stem cell-like properties rather than EMT in promoting cervical cancer metastasis via the ERK1/2-MZF1 signaling pathway. FEBS J. 2017;284(18):3000–3017. [DOI] [PubMed] [Google Scholar]
- 108. American Cancer Society Key statistics for vaginal cancer. 2018. Available at: www.cancer.org/cancer/vaginal-cancer/about/key-statistics.html. Accessed 23 March 2018.
- 109. Brinton LA, Thistle JE, Liao LM, Trabert B. Epidemiology of vulvar neoplasia in the NIH-AARP Study. Gynecol Oncol. 2017;145(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Napoletano C, Bellati F, Ruscito I, Pernice M, Zizzari IG, Caponnetto S, Tomao F, Frigerio L, Liberati M, Rughetti A, Caserta D, Panici PB, Nuti M. Immunological and clinical impact of cancer stem cells in vulvar cancer: role of CD133/CD24/ABCG2-Expressing cells. Anticancer Res. 2016;36(10):5109–5116. [DOI] [PubMed] [Google Scholar]
- 111. Lebrecht A, Hefler L, Schneeberger C, Koelbl H. Serum leptin in patients with vulvar cancer. Gynecol Oncol. 2001;83(1):164–165. [DOI] [PubMed] [Google Scholar]
- 112. Semczuk A, Skomra D, Jankiewicz K, Adamiak A, Korobowicz E, Rechberger T. The immunohistochemical profile of the primary and metastatic carcinoma of the clitoris: a case report. Arch Gynecol Obstet. 2005;273(3):187–191. [DOI] [PubMed] [Google Scholar]
- 113. Park JW, Zhao L, Willingham MC, Cheng SY. Inhibition of STAT3 signaling blocks obesity-induced mammary hyperplasia in a mouse model. Am J Cancer Res. 2017;7(3):727–739. [PMC free article] [PubMed] [Google Scholar]
- 114. US National Library of Medicine Oral STAT3 inhibitor, C188-9, in patients with advanced cancers. 2017. Available at: www.clinicaltrials.gov/ct2/show/NCT03195699?term=stat3+breast&rank=1. Accessed 18 April 2018.
- 115. US National Library of Medicine Pembrolizumab combined with itacitinib (INCB039110) and/or pembrolizumab combined with INCB050465 in advanced solid tumors. 2016. Available at: www.clinicaltrials.gov/ct2/show/NCT02646748?term=jak&cond=endometrial&rank=1. Accessed 18 April 2018.
- 116. US National Library of Medicine Gamma-secretase/notch signalling pathway inhibitor RO4929097 and temsirolimus in treating patients with advanced solid tumors. 2014. Available at: www.clinicaltrials.gov/ct2/show/NCT01198184?term=notch+signaling&rank=18. Accessed 18 April 2018.
- 117. US National Library of Medicine Phase II study of RAD001 head and neck cancer. 2017. Available at: www.clinicaltrials.gov/ct2/show/NCT01051791?term=erk&cond=cervical&rank=3. Accessed 18 April 2018.
- 118. Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol Oncol. 2014;133(2):375–381. [DOI] [PubMed] [Google Scholar]