Abstract
Objective
Insulin secretion (IS) declines with age, which increases the risk of impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM) in older adults. IS is regulated by the incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). Here we tested the hypotheses that incretin release is lower in older adults and that this decline is associated with β-cell dysfunction.
Research Design
A total of 40 young (25 ± 3 years) and 53 older (74 ± 7 years) lean nondiabetic subjects underwent a 2-hour oral glucose tolerance test (OGTT). Based on the OGTT, subjects were divided into three groups: young subjects with normal glucose tolerance (Y-NGT; n = 40), older subjects with normal glucose tolerance (O-NGT; n = 32), and older subjects with IGT (O-IGT; n = 21).
Main Outcome Measures
Plasma insulin, C-peptide, GLP-1, and GIP concentrations were measured every 15 to 30 minutes. We quantitated insulin sensitivity (Matsuda index) and insulin secretory rate (ISR) by deconvolution of C-peptide with the calculation of β-cell glucose sensitivity.
Results
Matsuda index, early phase ISR (0 to 30 minutes), and parameters of β-cell function were lower in O-IGT than in Y-NGT subjects but not in O-NGT subjects. GLP-1 concentrations were elevated in both older groups [GLP-1 area under the curve (AUC)0–120 was 2.8 ± 0.1 in Y-NGT, 3.8 ± 0.5 in O-NGT, and 3.7 ± 0.4 nmol/L∙120 minutes in O-IGT subjects; P < 0.05], whereas GIP secretion was higher in O-NGT than in Y-NGT subjects (GIP AUC0–120 was 4.7 ± 0.3 in Y-NGT, 6.0 ± 0.4 in O-NGT, and 4.8 ± 0.3 nmol/L∙120 minutes in O-IGT subjects; P < 0.05).
Conclusions
Aging is associated with an exaggerated GLP-1 secretory response. However, it was not sufficient to increase insulin first-phase release in O-IGT and overcome insulin resistance.
Aging is associated with an exaggerated GLP-1 secretory response. However, this response was not sufficient to increase insulin release and overcome insulin resistance in older subjects with IGT.
Aging is associated with major changes in glucose metabolism. Various studies indicate that increasing age is accompanied by impaired glucose tolerance (IGT). The 2-hour plasma glucose concentration during an oral glucose tolerance test (OGTT) rises on average 5.3 mg/dL per decade (1). The Baltimore Longitudinal Study of Aging showed a progressive decline in glucose tolerance from the third through the ninth decade of life (2). This decline in glucose tolerance with age also was evident in the National Health and Nutrition Examination Survey III, in agreement with recent estimates that approximately one-third of subjects aged ≥65 have diabetes (3). The cause for the high prevalence of glucose intolerance and type 2 diabetes mellitus (T2DM) in the older population is not clear. Yet many factors have been implicated, including changes in fat distribution (4), physical activity (5), muscle insulin sensitivity (6), and β-cell function (7).
A decrease in β-cell function with advancing age has been previously documented (8). For example, β-cell responsiveness during a frequent sampled intravenous glucose tolerance test is lower in older nondiabetic subjects than in younger subjects (9, 10). In addition, β-cell insulin response upon arginine stimulation also is impaired in older subjects (9, 10). Despite these data indicating that aging leads to decreases in β-cell function, the cause of this age-dependent functional decline is not known. Some studies have found that β-cell mass declines with age (11), although other studies have not reported such decline (12).
Incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) regulate β-cell function and mass (13). These peptides are secreted by the gut in response to nutrients, increasing insulin secretion rate (ISR) by β cells and reducing glucagon release (14). Although T2DM and adiposity are associated with altered incretin release and β-cell resistance to both GLP-1 and GIP (15, 16), it is unclear whether the decline in β-cell function seen in normal aging (i.e., in the absence of T2DM) also is associated with defects in incretin secretion.
The purpose of this study was to evaluate incretin hormone secretion in lean older subjects with either normal glucose tolerance (NGT) or IGT, compared with lean young subjects with NGT. We also examined whether possible age-related differences in incretin release are associated with changes in β-cell function. Because incretins increase β-cell function and mass (15), we hypothesized that aging leads to a reduced or impaired incretin release in response to glucose load and that this impairment would be associated with reduced β-cell function.
Methods
Subjects
We studied 40 young (18 to 30 years old) and 53 older (≥65 years old) nondiabetic, nonobese subjects. Each subject underwent a medical history, physical examination, screening laboratory tests, and a 75-g OGTT. All subjects were sedentary (not more than one session of exercise per week) and community-dwelling. Subjects were not obese (body mass index = 23 to 26 kg/m2) and did not have a family history (first-degree relative) of diabetes. Body weight was stable (±1 kg) for at least 3 months before enrollment. Subjects were not taking medication known to affect glucose metabolism. The study was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio, and all subjects gave written voluntary consent.
OGTT
Plasma glucose, insulin, and C-peptide concentrations were measured at baseline and every 15 minutes for 2 hours after the ingestion of 75 g glucose; GLP-1 and GIP were measured every 30 minutes in samples collected in prechilled test tubes containing aprotinin and EDTA. Based on the OGTT, subjects from the older group were subdivided into NGT or IGT groups. All subjects in the young group had NGT. The incremental area under the curve (AUC) for plasma glucose and insulin during the OGTT was calculated with the trapezoidal rule (17).
We calculated the homeostatic model assessment of insulin resistance (IR) index and the Matsuda index for insulin sensitivity, as previously described (18, 19). The primary stimulus for ISR is the increment in plasma glucose in the first minutes after the glucose load. Thus, we calculated the insulinogenic index as the incremental AUC for plasma insulin concentration (ΔI) divided by the incremental AUC for plasma glucose concentration (ΔG) from 0 to 30 minutes and the late insulin response as ΔI/ΔG from 30 to 120 minutes. The prehepatic ISR was calculated by plasma C-peptide deconvolution using MLAB (Civilized Software, Inc.; Silver Spring, MD) (20, 21). We also calculated the indexes of β-cell insulin secretion (IS), the rate sensitivity, that is, the IS in response to changes in glucose concentration, and the β-cell glucose sensitivity, defined as the slope of the dose response between ISR and glucose excursion, as previously described (22, 23). The disposition index during OGTT was calculated as ΔI/ΔG × Matsuda index from 0 to 30 and 0 to 120 minutes, respectively (21, 24). We also calculated the ratio of IS to IR, or IS/IR index, as ΔISR/ΔG × Matsuda index (21, 24).
Laboratory analyses
Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), glucose was measured with the oxidase method on a Beckman analyzer (Beckman Coulter, Inc., Brea, CA), and hemoglobin A1c was measured with a DCA 2000 analyzer (Bayer Corporation, Tarrytown, NY). GIP was measured by radioimmunoassay with a C-terminally directed antiserum code #867, raised against a synthetic peptide corresponding to the C-terminus of human GIP (University of Copenhagen, Copenhagen, Denmark), thus measuring “total” GIP (intact GIP + the primary metabolite GIP 3-42). Total GLP-1 (intact GLP-1 + the primary metabolite GLP-1 9-36 amide) was measured by radioimmunoassay according to standards of synthetic GLP-1 7-36 amide and antiserum code no. 89390, which is specific for the amidated C-terminus of GLP-1 (University of Copenhagen, Copenhagen, Denmark). Plasma concentrations of total cholesterol and triglyceride were measured enzymatically (Boehringer-Mannheim, Indianapolis, IN). Plasma high-density lipoprotein cholesterol was measured enzymatically on Hitachi 704 autoanalyzer (Boehringer-Mannheim) after precipitation of chylomicron and very low-density lipoprotein and low-density lipoprotein (LDL) cholesterol by phosphotungstic acid precipitation. LDL cholesterol was calculated from the Friedwald equation.
Statistical methods
All continuous data (mean ± standard error) and qualitative variables were expressed as percentages. The Kolmogorov-Smirnov test was performed to evaluate distribution of the variables. Comparison between groups (young vs old) was performed with t tests for quantitative variables with normal distribution and with Mann-Whitney U tests for those with nonnormal distribution. To compare more than two groups we used analysis of variance and Tukey’s test for normally distributed variables and the Kruskal-Wallis procedure for nonnormal data distribution. Correlations between continuous variables were carried out with Pearson correlations for variables with normal distribution and Spearman for those with nonnormal distribution.
Results
Subject characteristics
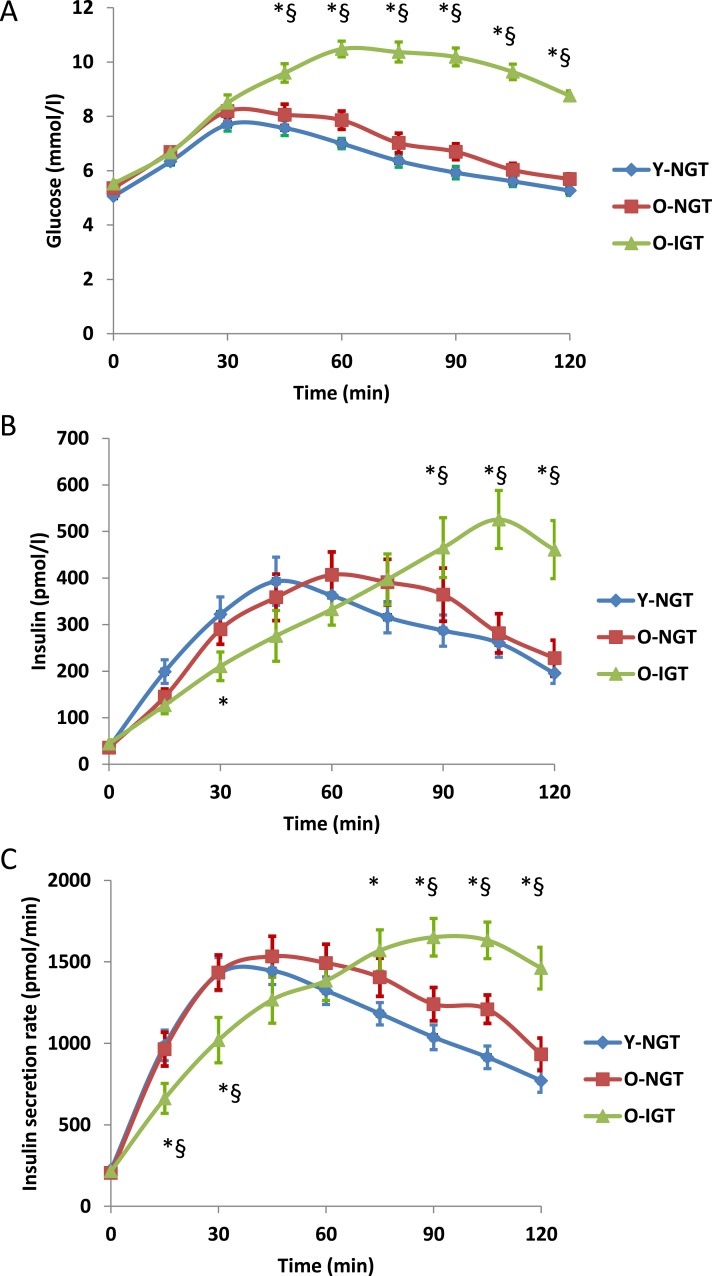
We studied 93 subjects subdivided into three groups according to age and degree of glucose tolerance: young subjects with NGT (Y-NGT; n = 40, mean age 25 years), older subjects with NGT (O-NGT; n = 32, mean age 72), and older subjects with IGT (O-IGT; n = 21, mean age 77). Anthropometric and metabolic characteristics of the subjects are shown in Table 1. Sex distribution and body mass index were not statistically different between the three groups. Total and LDL cholesterol concentrations were higher in older IGT compared with NGT subjects (both Y-NGT and O-NGT), whereas high-densitylipoprotein cholesterol and triglycerides were similar in all groups. Fasting plasma insulin was similar in the three groups, whereas the fasting plasma glucose was slightly but significantly higher in both older groups (O-NGT, O-IGT) than in the Y-NGT group (Table 1). The glucose AUC increased progressively from Y-NGT to O-NGT to O-IGT (Fig. 1A).
Table 1.
Baseline Subject Characteristics
| Y-NGT | O-NGT | O-IGT | P a | |
|---|---|---|---|---|
| No. | 40 | 32 | 21 | |
| Age, y | 25.4 ± 3.4 | 71.9 ± 7.2b | 76.6 ± 6.7b | <0.0001 |
| Sex, female/male | 26/14 | 14/19 | 11/9 | NS |
| BMI, kg/m2 | 23.8 ± 2.5 | 25.2 ± 2.9 | 23.8 ± 2.8 | 0.16 |
| Hemoglobin A1c, % | 5.1 ± 0.3 | 5.5 ± 0.3 | 5.6 ± 0.3b | <0.0001 |
| Insulin, pmol/L | 36.8 ± 4.9 | 35.9 ± 3.5 | 44.3 ± 7.8 | 0.46 |
| Glucose, mmol/L | 5.06 ± 0.55 | 5.36 ± 0.54b | 5.52 ± 0.46b | 0.001 |
| Total cholesterol, mmol/L | 3.89 ± 0.17 | 4.39 ± 0.23 | 4.40 ± 0.17b | 0.04 |
| HDL cholesterol, mmol/L | 1.43 ± 0.08 | 1.51 ± 0.09 | 1.54 ± 0.11 | 0.39 |
| LDL cholesterol, mmol/L | 1.98 ± 0.18 | 2.43 ± 0.20 | 2.41 ± 0.13b | 0.07 |
| Triglycerides, mmol/L | 1.07 ± 0.17 | 0.97 ± 0.18 | 1.05 ± 0.08c | 0.91 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; NS, not significant.
Old vs young.
P < 0.05 vs Y-NGT.
P < 0.05 vs O-NGT.
Figure 1.
(A) Glucose and (B) insulin concentrations and (C) ISRs on OGTT in Y-NGT, O-NGT, and O-IGT nonobese subjects. *P < 0.05 O-IGT vs Y-NGT. §P < 0.05 O-IGT vs O-NGT.
Indexes of insulin sensitivity
The homeostatic model assessment of IR, which represents primarily hepatic insulin sensitivity, was not significantly different between the three groups (Table 2). Peripheral insulin sensitivity, calculated with the Matsuda index, was similar among NGT subjects (i.e., O-NGT and Y-NGT) but was significantly lower in the O-IGT group (P < 0.05 vs Y-NGT) (Table 2). ΔG0–120 was significantly higher in the O-IGT group than in both Y-NGT and O-NGT groups. The O-IGT group displayed a significant increase in glucose concentrations, particularly from 30 to 120 minutes (Table 2, Fig. 1A).
Table 2.
OGTT Indexes of Insulin Sensitivity and β-Cell Function
| Y-NGT | O-NGT | O-IGT | P a | |
|---|---|---|---|---|
| Glucose excursions during OGTT | ||||
| ΔG0–30, mmol/L | 39.3 ± 3.0 | 41.2 ± 3.1 | 39.6 ± 4.0 | 0.74 |
| ΔG30–120, mmol/L | 129.5 ± 17.2 | 156.8 ± 19.0 | 387.0 ± 21.4b,c | 0.0001 |
| Insulin sensitivity | ||||
| HOMA-IR | 1.21 ± 0.18 | 1.26 ± 0.13 | 1.56 ± 0.27 | 0.17 |
| Matsuda index | 11.0 ± 1.4 | 8.1 ± 0.7 | 6.5 ± 0.7b | 0.009 |
| Insulin concentrations and secretion during OGTT | ||||
| ΔI0–30, nmol/L | 4.6 ± 0.6 | 3.6 ± 0.5 | 2.5 ± 0.5b | 0.03 |
| ΔI30–120, nmol/L | 24.9 ± 2.6 | 27.7 ± 3.6 | 31.0 ± 4.5b | 0.48 |
| ΔISR0–30, (nmol/min | 22.6 ± 2.2 | 22.6 ± 2.4 | 13.7 ± 2.2b,c | 0.02 |
| ΔISR30–120, nmol/min | 83.8 ± 5.2 | 101.6 ± 7.8 | 111.8 ± 8.4b | 0.02 |
| (ΔI/ΔG)0–30, pmol/mmol | 127 ± 20 | 102 ± 17 | 68 ± 16b | 0.02 |
| (ΔI/ΔG)30–120, pmol/mmol | 578 ± 479 | 415 ± 119 | 84 ± 12b,c | 0.002 |
| (ΔISR/ΔG)0–30, nmol/min/mmol/L | 0.710 ± 0.099 | 0.683 ± 0.140 | 0.380 ± 0.073b,c | 0.004 |
| (ΔISR/ΔG)30–120, nmol/min/mmol/L | 4.327 ± 4.429 | 1.480 ± 0.403 | 0.300 ± 0.025b,c | <0.0001 |
| β-Cell function | ||||
| Kd, pmol/m2/mM | 4097 ± 1011 | 3560 ± 1379 | 1919 ± 508 | 0.03 |
| Glucose sensitivity, pmol/min/m2/mM | 509 ± 55 | 479 ± 65 | 266 ± 28b,c | 0.04 |
| (ΔI/ΔG)0–30 × Matsuda | 1134 ± 176 | 752 ± 141 | 358 ± 74b,c | <0.0005 |
| (ΔI/ΔG)0–120 × Matsuda | 4026 ± 1864 | 1685 ± 351 | 462 ± 63b,c | <0.001 |
| (ΔISR/ΔG)0–30 × Matsuda | 7.9 ± 1.7 | 5.1 ± 1.1 | 2.3 ± 0.4b,c | <0.0002 |
| (ΔISR/ΔG)0–120 × Matsuda | 17.9 ± 5.9 | 7.4 ± 1.7 | 1.9 ± 0.3b,c | <0.0001 |
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; Kd, rate sensitivity.
Old vs young.
P < 0.05 vs Y-NGT.
P < 0.05 vs O-NGT.
Insulin response to a glucose load
Plasma glucose concentrations were similar in all subjects in the first 30 minutes of the OGTT (Fig. 1A); glucose concentrations of O-NGT overlapped those of Y-NGT, whereas in O-IGT glucose concentrations were higher (Fig. 1A). The early incremental plasma insulin response (both ΔI0–30 and ΔISR0–30) to the OGTT was reduced only in O-IGT vs Y-NGT (Fig. 1B and 1C), whereas IS from 30 to 120 minutes (ΔI30–120 and ΔISR30–120) was increased in the O-IGT group (Table 2). Because older subjects had a late response in IS, both ΔI0–120 and ΔISR0–120 were similar in the three groups (Table 2).
β-Cell function
We have calculated rate sensitivity (i.e., the IS in response to changes in glucose concentration) and the β-cell glucose sensitivity (i.e., the slope of the dose response between ISR and glucose excursion) as previously described. The β-cell glucose sensitivity was reduced only in the O-IGT group. Also, the disposition index and the IS/IR index were reduced only in the O-IGT group, whether calculated in the first 30 minutes or throughout the OGTT (Table 2).
Incretin secretion
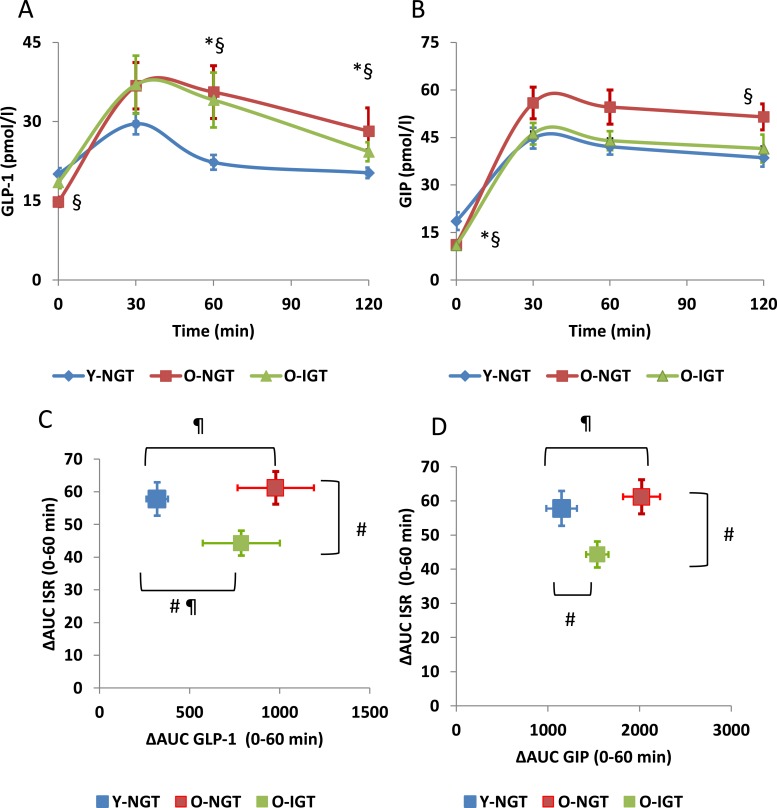
GLP-1 secretion was significantly higher in both older groups than in the Y-NGT group (GLP-1 AUC0–120 was 2.8 ± 0.1 in Y-NGT, 3.8 ± 0.5 in O-NGT, and 3.7 ± 0.4 nmol/L·120 minutes in O-IGT; P < 0.05). There was no difference in GLP-1 secretion between older groups (Fig. 2A). GIP secretion was significantly higher in the O-NGT group (AUC0–120 6.0 ± 0.4 nmol/L × 120 minutes) than in the Y-NGT and O-IGT groups (4.7 ± 0.3 and 4.8 ± 0.3 nmol/L × 120 minutes) (Fig. 2B). Even though the main effect of aging on β-cell function is most evident in the first 30 minutes of the OGTT, the GLP-1 AUC in the last 60 minutes of the curve remained significantly higher in O-NGT compared with Y-NGT subjects, suggesting a compensatory response of GLP-1 secretion in O-NGT. We analyzed the relationship between incretin secretion and insulin response by plotting the incremental incretin concentrations in the first 60 minutes vs the incremental ISR. We observed that O-IGT had higher GLP-1 secretion than Y-NGT but significantly lower ISR than both Y-NGT and O-NGT groups (Fig. 2C). O-NGT and Y-NGT had similar ISR response in the first hour, but GIP secretion was higher in O-NGT, perhaps indicating lower sensitivity to GIP in older subjects (Fig. 2D). On the other hand, O-IGT had lower GIP and ISR during the first 60 minutes of the OGTT compared with O-NGT (Fig. 2D).
Figure 2.
(A) GLP-1 and (B) GIP concentrations on OGTT in Y-NGT, O-NGT, and O-IGT nonobese subjects. *P < 0.05 O-IGT vs Y-NGT. §P < 0.05 O-NGT vs Y-NGT. P = ns O-IGT vs O-NGT. Comparison of incremental ISR between 0 and 60 minutes vs incremental (C) GLP-1 and (D) GIP concentrations. #P < 0.05 changes in ΔAUC ISR vs Y-NGT. ¶P < 0.05 changes in ΔAUC GLP-1 or GIP vs Y-NGT. ΔAUC, incremental area under the curve.
Discussion
We investigated the relationship between age-related changes in β-cell function with incretin secretion in response to oral glucose. The loss of first-phase IS (first-phase ISR) is one of the earliest abnormalities observed in glucose-intolerant people (25–27). Chen et al. (9) demonstrated that older subjects may lose first-phase ISR and have a delayed insulin response in the first hour after an oral glucose load. In this study, the first-phase insulin response (measured as ΔISR/ΔG and ΔI/ΔG during the first 30 minutes of OGTT; Table 2) was lower in O-IGT but not in O-NGT compared with Y-NGT subjects. Similarly, O-IGT subjects had a lower 0- to 120-minute β-cell response.
We investigated whether the impairment in insulin release seen in O-IGT subjects is caused by reduced incretin secretion. Against our prediction, we found that the incretin response was exaggerated in the older subjects (both O-NGT and O-IGT) compared with young. The higher GLP-1 response observed in both older groups might be interpreted as a physiological response to prevent loss of early-phase IS. Nonetheless, this exaggerated GLP-1 response was not sufficient to normalize first-phase IS in the O-IGT. Notably, β-cell sensitivity to glucose was altered only in older subjects with IGT but not in those with NGT. This suggests resistance of the β cells to the incretin effect such that the gut responds to the glucose load with an exaggerated GLP-1 release in an attempt to exert normal IS responses. A negative feedback relationship between insulin and GIP has been proposed to exist (28) but was never convincingly demonstrated, and the influence of IS on GLP-1 secretion is unclear.
A deleterious effect of aging per se on β-cell function has not been consistently observed. For example, previous studies that used the hyperglycemic clamp technique have shown little or no decrease in IS, both first and second phase, with aging (29–31). Our results are therefore in line with previous findings showing that when early IS is preserved, older subjects have NGT.
The exaggerated GLP-1 response shown in this study is in line with the study from Ranganath et al. (32). Yet others did not find any difference in GLP-1 concentration between younger healthy controls and older healthy controls and older subjects with T2DM (33). Unlike our study, these previous studies did not account for the level of IR (34) and did not exclude subjects with positive family history for T2DM, which is known to affect incretin responses (35). We found that the incretin response to the glucose load is higher in older subjects (NGT and IGT) than in young controls, but β-cell sensitivity to glucose is altered only in older subjects with IGT. This finding indicates a resistance of the β cell to the incretin effect, and therefore the gut may respond to the glucose load with an increased incretin release to obtain similar insulin responses, although the mechanism remains obscure. We propose that with aging the β cell becomes resistant to the incretin effect, thus needing an increased release of GLP-1 and GIP to stimulate adequate IS in response to the glucose load.
Conclusions
We conclude that the incretin response in older adults is not impaired but rather increased. The insulin response in the older NGT group is similar to that of the young NGT group, suggesting that resistance of the β cell to the incretin effect could contribute to the glucose intolerance seen with aging.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (R01-DK80157 and R01-DK089229) and the American Diabetes Association to N.M. This work also was supported by grants UL1TR000149 (CTSA), AG044271 (San Antonio Claude D. Pepper Older Americans Independence Center), and AG013319 (San Antonio Nathan Shock Center) and grants from the NovoNordisk Foundation (J.J.H.). A.G. has received research funds from the Italian Ministry of Research (MIUR) (Consiglio Nazionale delle Ricerche) for “Progetto Premiale” and “Ageing Project.”
Author Contributions: J.d.J.G.-G. and R.L. performed the studies. J.d.J.G.-G., A.G., J.J.H., and N.M. analyzed the data and wrote the manuscript. J.J.H. and R.A.D. contributed to revising and reviewing the manuscript. N.M. is the guarantor of this work and takes full responsibility for the integrity of information and concepts presented in the manuscript.
Disclosure Summary: J.d.J.G.-G. is a speaker for Novo-Nordisk, Sanofi Aventis, AstraZeneca, Boheringer Ingelheim, and Janssen. A.G. is a consultant for Eli-Lilly, Menarini, Gilead, Inventiva, and Sanofi. R.A.D. is a member of the Advisory Board of Takeda, Bristol Myers Squibb, Janssen, Boehringer Ingelheim, Novo Nordisk, and Amylin; he is a member of the Speaker Bureau of Novo Nordisk, Amylin, BMS, and Janssen; and he has grant support from Takeda, Amylin, and BMS. The salaries of N.M. and R.A.D. are paid in part by the South Texas Veterans Healthcare System. J.J.H. is a member of advisory boards for MSD and NovoNordisk. The remaining author has nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide 1
- IGT
impaired glucose tolerance
- IR
insulin resistance
- IS
insulin secretion
- ISR
insulin secretory rate
- LDL
low-density lipoprotein
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- O-IGT
older subjects with impaired glucose tolerance
- O-NGT
older subjects with normal glucose tolerance
- T2DM
type 2 diabetes mellitus
- Y-NGT
young subjects with normal glucose tolerance
References
- 1. Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab. 2005;31:5S27–5S34. [DOI] [PubMed] [Google Scholar]
- 2. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R; Baltimore Longitudinal Study of Aging . The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–1484. [DOI] [PubMed] [Google Scholar]
- 3. Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CC, Glickman SG, Dengel DR, Brown MD, Supiano MA. Abdominal adiposity assessed by dual energy X-ray absorptiometry provides a sex-independent predictor of insulin sensitivity in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(7):872–877. [DOI] [PubMed] [Google Scholar]
- 5. Peterson MJ, Morey MC, Giuliani C, Pieper CF, Evenson KR, Mercer V, Visser M, Brach JS, Kritchevsky SB, Goodpaster BH, Rubin S, Satterfield S, Simonsick EM; Health ABC Study . Walking in old age and development of metabolic syndrome: the health, aging, and body composition study. Metab Syndr Relat Disord. 2010;8(4):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71(6):1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia GVFR, Freeman RV, Supiano MA, Smith MJ, Galecki AT, Halter JB. Glucose metabolism in older adults: a study including subjects more than 80 years of age. J Am Geriatr Soc. 1997;45(7):813–817. [DOI] [PubMed] [Google Scholar]
- 8. Weir GC. Islet-cell biology in 2015: understanding secretion, ageing and death in β cells. Nat Rev Endocrinol. 2016;12(2):72–74. [DOI] [PubMed] [Google Scholar]
- 9. Chen M, Bergman RN, Pacini G, Porte D Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60(1):13–20. [DOI] [PubMed] [Google Scholar]
- 10. Pacini G, Beccaro F, Valerio A, Nosadini R, Crepaldi G. Reduced beta-cell secretion and insulin hepatic extraction in healthy elderly subjects. J Am Geriatr Soc. 1990;38(12):1283–1289. [DOI] [PubMed] [Google Scholar]
- 11. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. [DOI] [PubMed] [Google Scholar]
- 12. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritzel R, Schulte M, Pørksen N, Nauck MS, Holst JJ, Juhl C, März W, Schmitz O, Schmiegel WH, Nauck MA. Glucagon-like peptide 1 increases secretory burst mass of pulsatile insulin secretion in patients with type 2 diabetes and impaired glucose tolerance. Diabetes. 2001;50(4):776–784. [DOI] [PubMed] [Google Scholar]
- 14. Holst JJVT, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136. [DOI] [PubMed] [Google Scholar]
- 15. Brubaker PL. Minireview: update on incretin biology: focus on glucagon-like peptide-1. Endocrinology. 2010;151(5):1984–1989. [DOI] [PubMed] [Google Scholar]
- 16. Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57(5):1340–1348. [DOI] [PubMed] [Google Scholar]
- 17. Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17(2):152–154. [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 19. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 20. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 21. DeFronzo RA, Tripathy D, Abdul-Ghani M, Musi N, Gastaldelli A. The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J Clin Endocrinol Metab. 2014;99(10):3774–3781. [DOI] [PubMed] [Google Scholar]
- 22. Gastaldelli A, Brodows RG, D’Alessio D. The effect of chronic twice daily exenatide treatment on β-cell function in new onset type 2 diabetes. Clin Endocrinol (Oxf). 2014;80(4):545–553. [DOI] [PubMed] [Google Scholar]
- 23. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio Metabolism Study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. [DOI] [PubMed] [Google Scholar]
- 25. Osei K, Gaillard T, Schuster DP. Pathogenetic mechanisms of impaired glucose tolerance and type II diabetes in African-Americans. The significance of insulin secretion, insulin sensitivity, and glucose effectiveness. Diabetes Care. 1997;20(3):396–404. [DOI] [PubMed] [Google Scholar]
- 26. Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48(11):2197–2203. [DOI] [PubMed] [Google Scholar]
- 27. Festa A, D’Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53(6):1549–1555. [DOI] [PubMed] [Google Scholar]
- 28. Creutzfeldt W, Talaulicar M, Ebert R, Willms B. Inhibition of gastric inhibitory polypeptide (GIP) release by insulin and glucose in juvenile diabetes. Diabetes. 1980;29(2):140–145. [DOI] [PubMed] [Google Scholar]
- 29. Elahi D, Muller DC, McAloon-Dyke M, Tobin JD, Andres R. The effect of age on insulin response and glucose utilization during four hyperglycemic plateaus. Exp Gerontol. 1993;28(4-5):393–409. [DOI] [PubMed] [Google Scholar]
- 30. Ahrén B, Pacini G. Age-related reduction in glucose elimination is accompanied by reduced glucose effectiveness and increased hepatic insulin extraction in man. J Clin Endocrinol Metab. 1998;83(9):3350–3356. [DOI] [PubMed] [Google Scholar]
- 31. Bourey RE, Kohrt WM, Kirwan JP, Staten MA, King DS, Holloszy JO. Relationship between glucose tolerance and glucose-stimulated insulin response in 65-year-olds. J Gerontol. 1993;48(4):M122–M127. [DOI] [PubMed] [Google Scholar]
- 32. Ranganath L, Sedgwick I, Morgan L, Wright J, Marks V. The ageing entero-insular axis. Diabetologia. 1998;41(11):1309–1313. [DOI] [PubMed] [Google Scholar]
- 33. Korosi J, McIntosh CH, Pederson RA, Demuth HU, Habener JF, Gingerich R, Egan JM, Elahi D, Meneilly GS. Effect of aging and diabetes on the enteroinsular axis. J Gerontol A Biol Sci Med Sci. 2001;56(9):M575–M579. [DOI] [PubMed] [Google Scholar]
- 34. Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ, Ahrén B; Northern Sweden Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) . Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24(9):1640–1645. [DOI] [PubMed] [Google Scholar]
- 35. Matikainen N, Bogl LH, Hakkarainen A, Lundbom J, Lundbom N, Kaprio J, Rissanen A, Holst JJ, Pietiläinen KH. GLP-1 responses are heritable and blunted in acquired obesity with high liver fat and insulin resistance. Diabetes Care. 2014;37(1):242–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.