Abstract
Entomopathogenic fungi (EPFs), Isaria fumosorosea and Beauveria bassiana, are efficient biological agents in the management of multiple arthropod pests. In this study, the effects of both EPF species on various life stages of Spodoptera litura (F.) (Lepidoptera: Noctuidae) and its natural enemy Rhynocoris marginatus (Fab.) (Hemiptera: Reduviidae) were determined under laboratory conditions. I. fumosorosea significantly (P < .05) reduced the growth rate of the third and fourth instar larvae of S. litura. For relative consumption rate (RCR), the maximum impact was recorded for I. fumosorosea, which reduced the RCR of the larvae. The larvae of S. litura treated with I. fumosorosea showed significantly lower efficiency of conversion of ingested food (ECI) and the larval mortality rate (58.0%) was also higher compared with B. bassiana (33.3%). Similarly, I. fumosorosea had a significant effect on the pupal formation of S. litura; however, no significant effect was found on adult emergence percentage. To determine the effect of EPF-infected prey on the adult predator, their handling time, predatory rate, consumption rate, and the survival rate were recorded. No significant effect of EPF species on the predation rate was found. Furthermore, no significant difference was found in the survival rate of predators fed on either EPF-infected prey or healthy larvae. The interaction of these EPFs with a reduviid predator suggested that both EPF species, especially I. fumosorosea, could be used together with the predator to boost the biological control of S. litura in commercial crops.
Keywords: Microbial control, field crop pest, integrated pest management, Beauveria bassiana, Isaria fumosorosea, reduviid predator
Introduction
Armyworm, Spodoptera litura (F.) (Lepidoptera: Noctuidae), is an economically important insect pest known to attack various agricultural crops and is widely distributed in the tropical and temperate zones of Asia, Australasia, and Pacific Islands.1 It is native to India and South-East Asia2 and is well established in Pakistan.3,4 It is reported to potentially cause 35% to 55% yield losses at the blossom and vegetative stages of the crops.5 In south Punjab, Pakistan, it causes damage to the economically important crops6 like cotton, tomatoes, tobacco, groundnut, soybean, lucerne, cabbage, sunflower, castor, cauliflower, onion, brinjal, and turnip.7-10 Recently, it has also been reported to feed on citrus cultivars in Sargodha region of Pakistan.10
Due to its economic importance and widely known losses to agricultural crops, insecticide application is considered the best method to manage S. litura.11 However, repeated applications and extensive use of insecticides have resulted in ecological imbalances such as toxic effects on natural enemies and humans.12 Development of insecticide resistance has also been reported for S. litura.13-16 Hence, it is important to explore eco-friendly (ecological and economical) insect pest management (IPM) strategies using natural enemies providing similar efficacy against S. litura. The use of microorganisms has achieved a prominent position among different options to control insect pests that cause considerable losses to agroecosystems.17-19 Use of entomopathogens is one of the management strategies against notorious insect pests. Different entomopathogens, such as Pseudomonas fluorescens (Trevisan) Migula, Metarhizium anisopliae (Metsch.) Sorokin, and S. litura nucleopolyhedrovirus (SpltNPV), have been used against different pests.19,20 Entomopathogenic fungi (EPFs), namely, Isaria fumosorosea and Beauveria bassiana, have been reported to effectively reduce the population of lepidopterous insect pests.21,22
Furthermore, the release of natural enemies including predators and parasitoids is another strategy to manage insect pests in an eco-friendly way. Previously reported biological control predators of S. litura are Platymeris laevicollis (Distant),23 Zelus renardii Kolenati,24 Rhynocoris marginatus (Fab.),25 Rhynocoris kumarii Ambrose and Livingstone, Rhynocoris fuscipes (Fab.), Pristhesancus plagipennis Walker (Reduviidae: Hemiptera), Acanthaspis pedestris (Stål), Catamiarus brevipennis (Serville), and Ectomocoris tibialis Distant26; all belong to the family Reduviidae. A number of insect species from different orders like Lepidoptera, Coleoptera, Hemiptera, Orthoptera, and Isoptera have been controlled by hunter reduviid predators (Reduviidae).23 These are commonly found in the agricultural fields and suppress the population of many important pests including Creontiades dilutus (Stål) (Miridae: Hemiptera), S. litura, Helicoverpa armigera (Hubner), Anomis flava F. (Noctuidae: Lepidoptera), Phenacoccus solenopsis (Tinsley) (Pseudococcidae: Hemiptera), Dysdercus cingulatus (Fab.) (Pyrrhocoridae: Hemiptera), and Aphis gossypii Glover (Aphididae: Hemiptera).27,28 R. marginatus (Heteroptera: Reduviidae), a polyphagous reduviid predator, feeds on more than 20 economically important insect pests29 and has paralytic potential using salivary gland extract against its host.30 Sahayaraj and Ravi31 reported a 66.6% reduction of S. litura population after releasing R. marginatus into groundnut field.
Few control strategies focus on limiting the use of insecticides because of their hazardous and non-target effects. Sometimes, insecticides are used synergistically at low doses with microbes to suppress pest populations.32 However, the non-target effect of these microbial insecticides should be determined before recommendation. Other alternate strategies involve the use of natural enemies entirely.
Due to lack of information, the possible combination of these natural enemies in several pest management programs has not yet been explored. No study has been reported on the reduviid predation against S. litura in Pakistan. This study focused on investigating the interaction of EPFs, B. bassiana and I. fumosorosea, with reduviid predators, especially R. marginatus (Reduviidae: Hemiptera), and their combined effect on S. litura. Hence, in this study, the effectiveness of EPFs was checked against various life stages of S. litura and the performance and survival of its predator, R. marginatus, was investigated.
Materials and Methods
To determine the effect of EPFs on S. litura and their non-target effect on reduviid predator R. marginatus, experiments were conducted in the Entomology Laboratory at College of Agriculture, University of Sargodha, Pakistan.
S. litura culture
Egg batches and larvae of S. litura were collected from the lucerne field nearby the University (32°07′42.9″N 72°41′27.2″E). The culture was maintained at 27°C ± 2°C temperature and 75% ± 5% relative humidity (RH) in the laboratory. Newly hatched larvae were provided with artificial diet till pupation. Artificial diet was prepared in accordance with Sorour et al.33 The adults were shifted into clean plastic cages (120 mm × 116 mm × 95 mm) covered with muslin cloth where they were fed on 10% sugar solution. The cotton wool strips (1 cm wide, 5-10 cm long) were kept in plastic cages as a suitable oviposition substrate to collect eggs. In the experiments, the F2 generation was used.
Entomopathogenic fungi
The commercial formulations of B. bassiana NCIM 1216 ATCC 26851 and I. fumosorosea IF-171201 (Agri Life, India) were used and tested at 1 × 108 cfu. Both EPF species were purchased from the Ali Akbar Group of Companies, Lahore, Pakistan. At the time of treatment, spore viability was determined by spraying 1 mL aliquots of suspension on potato dextrose agar (PDA) and incubated at 25°C. The viability of both fungi was more than 90%.
R. marginatus culture
The egg batches of R. marginatus were collected from the tobacco fields (32°07′38.8″N 72°40′33.6″E). The eggs were placed in clean glass Petri plates lined with a filter paper. The culture was maintained at 25°C ± 2°C and 70% ± 5% RH. R. marginatus was reared for second generation prior to the experiments. Newly hatched nymphs were provided with early instar larvae of S. litura and the mature nymphs and adults were provided the later instars of S. litura. About 2 larvae of hosts were provided to each predator nymph and adult on a daily basis. S. litura larvae were collected from the reared culture in the laboratory.
Effect of EPFs on eggs and larvae of S. litura
Freshly laid egg batches were collected and placed in separate Petri plates lined with a filter paper. The eggs were sprayed with EPFs (B. bassiana and I. fumosorosea) at 1 × 108 cfu using a hand sprayer (Taizhou Longshixiang Plastic, China). Distilled water was used in control treatment. Each treatment was replicated 3 times and 1 batch of the egg was considered as 1 replication. The total number of eggs was counted per batch before application. The average number of eggs was 130 per batch in each treatment. The eggs were kept in an incubator maintained at 25°C ± 1°C and 70% ± 5% RH. The color changes in eggs were observed daily. After 3, 5, and 7 days of treatment application, egg hatchability was recorded.
The efficacy of EPFs was also tested on the third and fourth instar larvae of S. litura. Each treatment was replicated 4 times and 5 larvae of each instar were tested in each treatment. The experiment for both eggs and larvae was repeated thrice. The topical bioassay was performed to test the efficacy of EPFs. About 1-µL drop of each treatment was applied on the thorax of each larva. The treated larvae were shifted into new Petri plates containing sunflower leaves. The leaves were collected from unsprayed field and brought into laboratory. Leaves were washed with water to remove contaminants and dried at room temperature. The leaves were cut into disk size (6 cm) of Petri dish and changed daily. The weights of larvae before and after 24, 72, and 120 hours of the application were recorded. The survival rates of larvae were also recorded at 24-hour interval for a total of 10 days. To determine the consumption rate, the leaves were weighed before and after 24 hours of application. Dead larvae due to the application of EPFs were separated into clean Petri plates and sealed with parafilm. The Petri plates were kept in an incubator at 26°C ± 2°C and 70% ± 5% RH. The pupal formation and adult emergence rate of S. litura were also recorded. The digestion, consumption, and use of the third and fourth instar larvae of S. litura after infection were calculated using the formulae described by Waldbauer34
where ΔB is the change in body weight of the insect (mg), BI is the initial larval weight, T is the duration of the feeding period (days), I is the dry weight of food (mg) consumed, and B is the insect dry weight gain (mg).
Effect of EPFs on the performance of R. marginatus predator
S. litura larvae were treated with EPFs for the reduviid R. marginatus predator. Freshly molted third and fourth instar larvae of S. litura were starved for 12 hours and then treated with either B. bassiana or I. fumosorosea on an artificial diet. Larvae for control treatment were fed on diet inoculated with distilled water. Larvae that consumed the entire diet within 24 hours were separated and transferred to clean Petri dishes containing fresh uncontaminated diet and reared under controlled conditions. Larvae that did not eat the diet were discarded. The microbial infection was identified based on their sluggish behavior, food consumption, and later the growth of conidia.
To record the effect of EPFs on the performance of the predator, newly emerged R. marginatus adults from the lab culture were selected that were reared on healthy S. litura larvae. Throughout their nymphal instar, they were provided the healthy S. litura larvae. However, the third and fourth nymphal instars and adults were starved for 12 hours and then EPF-infected third and fourth instar prey were provided separately to each category of R. marginatus. For the experimental treatment, 10 nymphs/adult predators (10 replicates/treatment) were provided 5 EPF-infected prey for each category separately. In the case of control treatment, healthy larvae were provided to the predators. Handling time (paralyzing plus sucking), predator rate (number of prey/predator/day), number of preys consumed, and the survival rate were recorded daily for 5 days. A digital video camera (DSC-WX60, 16.2 MP HD, China) was set over the experimental setup for 4 hours daily to estimate the handling time (paralyzing + sucking act). Food consumption of R. marginatus adults was calculated using the formula35
where FC is the food consumption, PWB is the prey weight before providing to predators, and PWA is the prey weight after feeding of predators.
To record the effect of EPF-infected prey on the developmental biology of R. marginatus, 10 pairs of newly emerged adults from nymphs were fed on healthy S. litura larvae throughout their lives, and then they were selected for further experiment. In total, 10 newly emerged nymphs were fed on EPF-treated prey and 10 were fed on healthy S. litura larvae that served as the control treatment. Data on the number of days required for the completion of each stage were recorded.
Data analysis
To check the significance of EPFs on the various stages of S. litura, 1-way analysis of variance (ANOVA) was performed. Mortality was analyzed using Kaplan-Meier survival analysis with a log-rank test. Similarly, 1-way ANOVA was also performed to check the effect of EPFs on the handling time, predatory rate, food consumption, and developmental biology of predators. Means were separated with the least significant difference (LSD) test at a probability level of 5%. All the analyses were performed using the Minitab 17.0 software.
Results
Effect of EPFs on eggs and larvae of S. litura
There was a significant effect of EPFs on the hatchability of S. litura after 3 (F = 48.7, P < .05), 5 (F = 10.6, P < .05), and 7 (F = 14.1, P < .05) days of exposure. After 7 days, egg hatchability was found lower (55.6%) by the application of I. fumosorosea followed by B. bassiana (71.1%) compared with the control treatment (92.9%) (Figure 1). A significant effect (P < .001) of EPFs was observed on all nutritional indices in both the third and fourth instar larvae of S. litura. The relative growth rates (RGRs) of the third (0.07 mg/mg/day) and fourth (0.13 mg/mg/day) instar larvae were affected more by I. fumosorosea compared with B. bassiana (0.20 mg/mg/day for the third instar and 0.26 mg/mg/day for the fourth instar). The values of the relative consumption rate (RCR) and efficiency of conversion of ingested food (ECI) indices were also significantly (P < .001) lower in the I. fumosorosea treatment (Table 1). Both microbial treatments had a similar effect on the third and fourth larval instars of S. litura for the different nutritional indices following a general trend: I. fumosorosea < B. bassiana < control. The survival rate of S. litura larvae was significantly (Kaplan-Meier log rank: df = 2, χ2 = 32.34, P < .001) affected after the application of EPFs. However, 33.3% of larvae survived after 10-day exposure of I. fumosorosea followed by 53.3% in the B. bassiana treatment (Figure 2).
Figure 1.
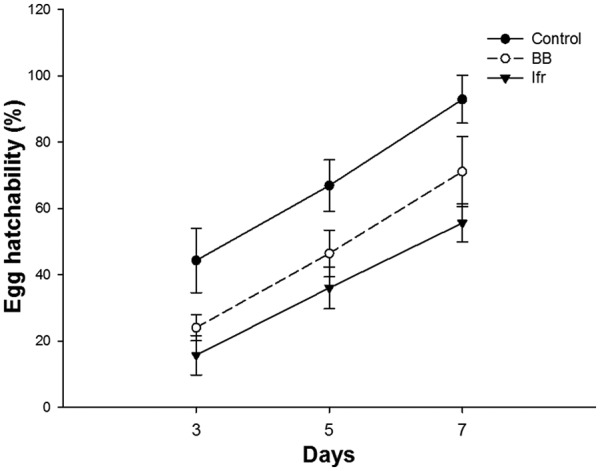
Percent (mean ± SE, n = 3) egg hatchability of Spodoptera litura after the application of entomopathogenic fungi. BB indicates Beauveria bassiana; Ifr, Isaria fumosorosea.
Table 1.
Different nutritional indices: relative growth rate (RGR), efficiency of conversion of ingested food (ECI), and relative consumption rate (RCR) of 2 larval instars of Spodoptera litura sprayed with Ifr and BB after exposure to sunflower leaf disks.
| Treatments | RGR (mg/mg/day) |
RCR (mg/mg/day) |
ECI (%) |
|||
|---|---|---|---|---|---|---|
| Third instar | Fourth instar | Third instar | Fourth instar | Third instar | Fourth instar | |
| Control | 0.32 ± 0.027a | 0.39 ± 0.022a | 0.65 ± 0.042a | 0.84 ± 0.139a | 71.9 ± 2.491a | 74.2 ± 3.541a |
| Ifr | 0.07 ± 0.011c | 0.13 ± 0.065c | 0.23 ± 0.026c | 0.27 ± 0.045b | 32.9 ± 1.701c | 38.6 ± 2.332c |
| BB | 0.20 ± 0.012b | 0.26 ± 0.022b | 0.35 ± 0.027b | 0.52 ± 0.077b | 49.7 ± 2.464b | 55.0 ± 3.181b |
| F-value | 45.9 | 10.2 | 42.6 | 8.94 | 75.9 | 33.8 |
| P-value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Abbreviations: BB, Beauveria bassiana; Ifr, Isaria fumosorosea.
All values are represented as means ± SE, n = 20.
Means sharing similar letters within the column are not significantly different at P > .05.
Figure 2.
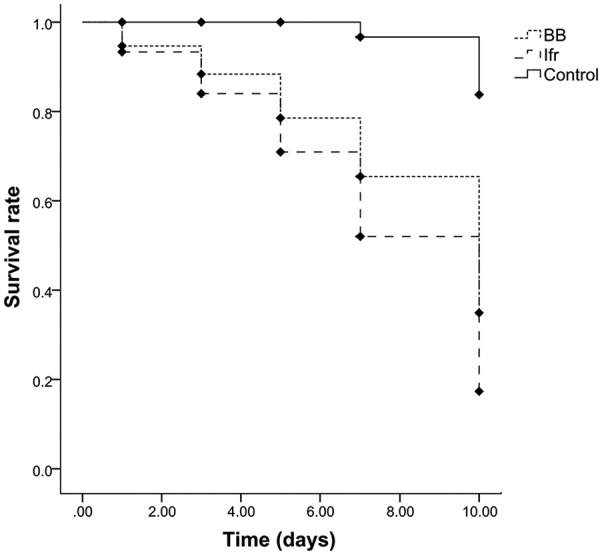
Effect of entomopathogenic fungi on survival rate (%) (mean ± SE, n = 20) of Spodoptera litura larvae. BB indicates Beauveria bassiana; Ifr, Isaria fumosorosea.
Entomopathogenic fungi had a significant effect (F = 7.00, P < .05) on pupal formation of S. litura. However, no significant (F = 0.44, P > .05) effect of EPFs was observed on adult emergence. I. fumosorosea affected the pupal formation and adult emergence of S. litura comparatively more than B. bassiana. In comparison with control, I. fumosorosea lowered 53.3% pupal formation and 14.4% adult emergence rate (Figure 3).
Figure 3.
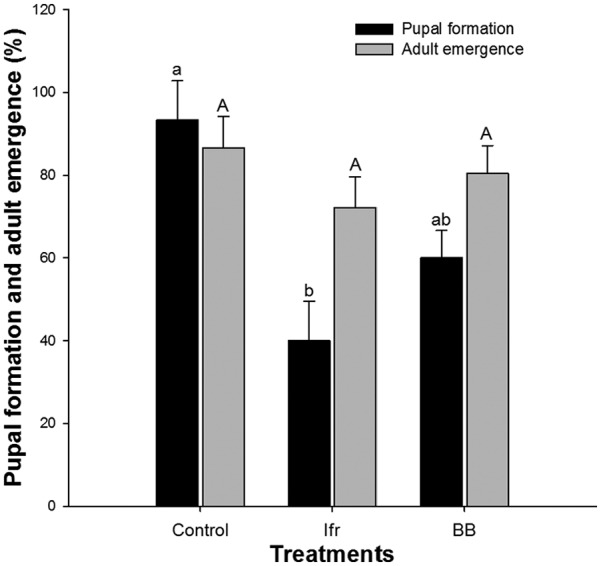
Effect (mean ± SE, n = 10) of entomopathogenic fungi on percent pupation and adult emergence of Spodoptera litura. Means sharing similar letters are not significantly different at P > .05. BB indicates Beauveria bassiana; Ifr, Isaria fumosorosea.
Effect of EPFs on R. marginatus
During predation, R. marginatus showed the sequential pattern of behavior such as locating, capturing, sucking, and paralyzing the prey. Food consumption, handling time, and predatory rate of adults of R. marginatus fed on EPF-infected S. litura prey are presented in Table 2. Food consumption of R. marginatus was not significantly (P > .05) affected by the application of EPFs. However, a significant (P < .001) difference in the handling time of R. marginatus on EPF-treated and untreated prey was found. The handling time was longer (172.2 minutes) on untreated prey compared with the EPF-treated one. Between the EPF treatments, the handling time of R. marginatus fed on I. fumosorosea-infected prey was longer. The R. marginatus consumed almost the same amount (P > .05) of S. litura larvae that were either untreated or EPF treated (Table 2).
Table 2.
Effect of EPF-infected Spodoptera litura larvae (third and fourth instars) on food consumption (FC), handling time (HT), and predatory rate (PR) of the Rhynocoris marginatus predator.
| Treatments | FC (mg) | HT (minutes) | PR (no. of prey/predators) |
|---|---|---|---|
| Control | 20.0 ± 1.457a | 172.4 ± 1.077a | 1.30 ± 0.133a |
| BB | 24.2 ± 1.218a | 72.4 ± 0.690c | 1.25 ± 0.133a |
| Ifr | 24.5 ± 2.299a | 81.5 ± 0.702b | 1.20 ± 0.101a |
| F-value | 2.05 | 7051.0 | 0.16 |
| P-value | .1483NS | <.001*** | .8563NS |
Abbreviations: BB, Beauveria bassiana; EPF, entomopathogenic fungus; Ifr, Isaria fumosorosea; NS, not significant.
All values are represented as means ± SE, n = 10.
Means sharing similar letters within the columns are not significantly different at P > .05.
***P < .001.
When B. bassiana-infected prey was fed to the third and fourth instar nymphs and adults of R. marginatus, the developmental period delayed compared with I. fumosorosea and untreated prey. Similarly, the total nymphal period was also affected significantly (F = 5.68, P < .05) fed on EPF-treated prey. The total nymphal period of R. marginatus was 61.0 days in the control treatment followed by 62.0 days in the I. fumosorosea and 64.3 days in the B. bassiana treatment. The adults lived 23.2 days fed on healthy S. litura larvae (control treatment). Total adult longevity of predator was 22.7 days fed on I. fumosorosea-treated prey followed by 26.9 days when they fed on B. bassiana-treated prey. No significant effect of EPFs was found on the survival rate of the third (F = 0.82, P > .05), fourth (F = 0.15, P > .05), and adult longevity (F = 2.21, P > .05) of R. marginatus (Table 3).
Table 3.
Effect of EPF-treated and untreated Spodoptera litura prey consumed by Rhynocoris marginatus nymphal stages and the total nymphal developmental period (days) and survival rate (%).
| Treatments | Developmental period (days) of Rhynocoris marginatus predator |
|||
|---|---|---|---|---|
| Third instar | Fourth instar | Adult longevity | Total nymphal period* | |
| Control | 11.0 ± 0.422b (100) | 12.4 ± 0.401b (95) | 23.2 ± 0.573b (95) | 61.0 ± 0.667b |
| BB | 13.1 ± 0.585a (85) | 14.4 ± 0.618a (90) | 26.9 ± 0.481a (80) | 64.3 ± 0.760a |
| Ifr | 12.2 ± 0.326ab (90) | 12.7 ± 0.495b (90) | 22.7 ± 0.701b (100) | 62.0 ± 0.699b |
| F-value | 5.30 | 4.43 | 15.0 | 5.68 |
| P-value | <.05 | <.05 | <.05 | <.05 |
Abbreviations: BB, Beauveria bassiana; EPF, entomopathogenic fungus; Ifr, Isaria fumosorosea; LSD, least significance difference.
Data include for the third to fifth nymphal instars, n = 10.
Mean values (±SEM) followed by different letters in a column are significantly different (LSD test, P < .05). Values for percent survival rate are presented in parentheses.
Discussion
As this study was based on EPFs and their effects, it was observed that EPFs significantly affected the egg hatchability of S. litura. The abnormal reduction in egg hatchability of S. litura treated with EPFs was in accordance with Leckie et al36 where lower egg hatchability rate and delayed development of Heliothis zea (Boddie) (Lepidoptera: Noctuidae) larvae fed on diet treated with B. bassiana were reported. Similarly, Malarvannan et al37observed complete arrest in fecundity of S. litura by the application of B. bassiana (2.4 × 107 spores/mL). Gindin et al38 observed an 80% to 82% reduction in the hatchability rate of EPF-treated red palm weevil adults, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae).
In this study, among both EPFs, I. fumosorosea proved to be more effective as it reduced the consumption rate and affected the relative growth of S. litura larvae compared with B. bassiana. Among larval instars, I. fumosorosea reduced the consumption rate of S. litura by 14.8% and had more effect in reducing the growth rate of small larvae (RGR: 46.1%) compared with large larval instars. Due to the application of I. fumosorosea, the values of RGR and RCR remained lower in both larval instars and, consequently, the conversion of ingested food remained low. In this investigation, I. fumosorosea-treated larvae showed a higher reduction in ECI which ranged from 32% to 38% with respect to the third and fourth instar larvae. In a similar study by Moorthi et al,21 a 46% reduction in ECI in S. litura larvae treated with I. fumosorosea was observed. This finding is suggestive of the altered digestive activity of S. litura after treatment with I. fumosorosea which could be used as a suitable biological control agent after testing it in the field. I. fumosorosea might have a direct effect on the metabolic process of S. litura as a significant decrease in food consumption (FC) and growth rate (GR) was observed (Table 1). It has been reported that entomopathogenic fungi (EPF) degrades the insect cuticle through the enzymes and enters hemocoel where they take on host nutrients and multiply in numbers.39
I. fumosorosea reduced the feeding indices and was effective in killing the S. litura larvae. The current results were well supported by Moorthi et al,21 Tefera and Pringle,40 and Asaff et al41 who observed more decline in food consumption of S. litura after I. fumosorosea application in comparison with B. bassiana and Paecilomyces variotii. A significant effect on the pupal formation of S. litura was observed after treatment with EPFs; however, no significant effect was recorded for the adult emergence rate. EPFs reduced the larval weight of S. litura during development, due to which the formation of shriveled pupa was observed.42 Many researchers have reported the effectiveness of I. fumosorosea against wide host range, especially lepidopterous insect pests.43-46
Before considering the application of these EPFs in field conditions either individually or in combination with other control strategies, such as the release of natural enemies (predators or parasitoids), it is essential to understand the interactions and compatibility of these EPFs with natural enemies. The current results showed that the provision of EPF-infected S. litura larvae to R. marginatus predator did not affect its food consumption. Furthermore, in the presence of EPFs, the predator took less time to handle the prey compared with untreated prey. This could be due to the sluggish behavior or slow movement of EPF-infected larvae that were easy for predators in locating, capturing, consuming, and digesting the larvae. These findings were well supported by Sahayaraj et al47 where no significant effect of EPF-infected larvae of S. litura on the performance of R. kumarii predator was reported. When the EPF-infected prey fed to different life stages of R. marginatus, the developmental period was prolonged but did not reduce the survival rate of nymphal and adult R. marginatus. Zhang et al48 have confirmed that Isaria cateniannulata fungus has no deleterious effects on the vitality and fertility of the predator Euseius nicholsi (Ehara & Lee) (Acari: Phytoselidae). Similarly, Scorsetti et al49 also reported that B. bassiana-infected Rhopalosiphum padi L. (Hemiptera: Aphididae) did not affect the development of the predator Eriopis connexa (Germar) (Coleoptera: Coccinellidae). The efficacy of these entomopathogens may vary under the field condition where the predator interacts with the pest and environment. However, Nalepa and Weir50 reported that even if the EPF invades the cuticle of coccinellid predators, there are no known deleterious impacts on the host.
The non-target effect of EPFs on the reduviid predator R. marginatus under laboratory conditions was mainly studied to assess the usefulness of the integration of natural predator and FPF. The findings demonstrated that there is a potential for combining R. marginatus with other entomopathogenic microbes, especially I. fumosorosea. Many researchers have encouraged the integration of commercial formulations of EPFs with other components for IPM.51-56 Reduviid as generalist predators, abundant in many agroecosystems and distributed worldwide, have been recommended for IPM programs23 and could be used together with I. fumosorosea.
Conclusions
The EPFs, I. fumosorosea and B. bassiana, proved to be effective in reducing the egg hatchability, food consumption, and growth rate of S. litura. However, among both treatments, I. fumosorosea significantly proved to affect the growth and development parameters in prey. Moreover, the integration of I. fumosorosea with the reduviid predator, R. marginatus, also yielded beneficial results as the predator easily handled and captured the EPF-infected prey due to its altered behavior. These findings allude to the consideration of I. fumosorosea as an effective eco-friendly mycoinsecticide against S. litura. However, limited numbers of studies are available on the interaction of microbes with natural enemies under field conditions. Such studies where more combinations of EPFs and natural enemies are exploited would be helpful in developing effective pest control programs.
Acknowledgments
We are thankful to two anonymous reviewers for useful comments on an earlier version of the manuscript.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SM and NA performed the experimental work and analysis in conducting this research study. MIU and MAf contributed in concept and designing of this study and also research supervision. MAr, NM, MR, SA, AA contributed equally in literature search, interpretation of results, manuscript drafting, improvement and critical reviewing of drafted manuscript. All authors approved and agreed for the submission of this article for publication.
ORCID iDs: Muhammad Irfan Ullah  https://orcid.org/0000-0002-2463-2665
https://orcid.org/0000-0002-2463-2665
Naunain Mehmood  https://orcid.org/0000-0001-7852-9113
https://orcid.org/0000-0001-7852-9113
Muhammad Riaz  https://orcid.org/0000-0002-5524-7735
https://orcid.org/0000-0002-5524-7735
References
- 1. Ahmad M, Sayyed AH, Saleem MA, Ahmad M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008;27:1367-1372. [Google Scholar]
- 2. Waterhouse DF, Norris KR. Biological Control: Pacific Prospects. Clayton, VIC, Australia: Inkata Press; 1987. [Google Scholar]
- 3. Shad SA, Sayyed AH, Saleem MA. Cross-resistance, mode of inheritance and stability of resistance to emamectin in Spodoptera litura (Lepidoptera: Noctuidae). Pest Manag Sci. 2010;66:839-846. [DOI] [PubMed] [Google Scholar]
- 4. Ahmad M, Gull S. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. Can Entomol. 2017;149:649-661. [Google Scholar]
- 5. Rao MS, Manimanjari D, Rao ACR, Swathi P, Maheswari M. Effect of climate change on Spodoptera litura Fab. on peanut: a life table approach. Crop Prot. 2014;66:98-106. [Google Scholar]
- 6. Ghaffar A, Attique M, Naveed M, Jan M. Host range and population dynamics of beet armyworm, Spodoptera exigua (Hubner) (Noctuidae: Lepidoptera) in cotton agroecosystem of Punjab [Pakistan]. Pak J Zool. 2002;34:209-213. [Google Scholar]
- 7. Ahmad M, Ghaffar A, Rafiq M. Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J Agric Biol. 2013;1:23-28. [Google Scholar]
- 8. Baskar K, Duraipandiyan V, Ignacimuthu S. Bioefficacy of the triterpenoid friedelin against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Pest Manag Sci. 2014;70:1877-1883. [Google Scholar]
- 9. Suresh U, Murugan K, Panneerselvam C, et al. Suaeda maritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodoptera litura. Physiol Mol Plant Pathol. 2018;101:225-235. [Google Scholar]
- 10. Ullah MI, Arshad M, Afzal M, et al. Incidence of Spodoptera litura (Lepidoptera: Noctuidae) and its feeding potential on various citrus (Sapindales: Rutaceae) cultivars in the Sargodha Region of Pakistan. Fla Entomol. 2016;99:192-196. [Google Scholar]
- 11. Liu Y, Li X, Zhou C, Liu F, Mu W. Toxicity of nine insecticides on four natural enemies of Spodoptera exigua. Sci Rep. 2016;6:39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kranthi K, Jadhav D, Kranthi S, Wanjari R, Ali S, Russell D. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 2002;21:449-460. [Google Scholar]
- 13. Armes NJ, Wightman JA, Jadhav DR, Ranga Rao GV. Status of insecticide resistance in Spodoptera litura in Andhra Pradesh, India. Pestic Sci. 1997;50:240-248. [Google Scholar]
- 14. Kranthi K, Jadhav D, Wanjari R, Ali SS, Russell D. Carbamate and organophosphate resistance in cotton pests in India, 1995 to 1999. Bull Entomol Res. 2001;91:37-46. [PubMed] [Google Scholar]
- 15. Ahmad M, Arif MI, Ahmad M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007;26:809-817. [Google Scholar]
- 16. Abbas N, Shad SA, Razaq M. Fitness cost, cross resistance and realized heritability of resistance to imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae). Pest Biochem Physiol. 2012;103:181-188. [Google Scholar]
- 17. Sabbour M, Sahab A. Efficacy of some microbial control agents against cabbage pests in Egypt. Pak J Biol Sci. 2005;8:1351-1356. [Google Scholar]
- 18. Hu QB, Ren SX, An XC, Qian MH. Insecticidal activity influence of destruxins on the pathogenicity of Paecilomyces javanicus against Spodoptera litura. J Appl Entomol. 2007;131:262-268. [Google Scholar]
- 19. Yang MM, Li ML, an Zhang Y, et al. Baculoviruses and insect pests control in China. Afr J Microbiol Res. 2012;6:214-218. [Google Scholar]
- 20. Bhanu Prakash GV, Padmaja V, Jami SK, Kirti PB. Expression of chitinase genes of Metarhizium anisopliae isolates in lepidopteran pests and on synthetic media. J Basic Microbiol. 2012;52:628-635. [DOI] [PubMed] [Google Scholar]
- 21. Moorthi VP, Balasubramanian C, Selvarani S, Radha A. Efficacy of sub lethal concentration of entomopathogenic fungi on the feeding and reproduction of Spodoptera litura. Springerplus. 2015;4:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandarilla-Pacheco FL, del Socorro Flores-González M, Morales-Ramos LH, Elías-Santos M, Galán-Wong LJ, Quintero-Zapata I. Effect of native Mexican isolates of Isaria fumosorosea (Wize) Brown & Smith on Spodoptera exigua (Hübner) and Helicoverpa zea (Boddie). Southwest Entomol. 2015;40:721-730. [Google Scholar]
- 23. Sahayaraj K. Reduviids and their merits in biological control. In Sahayaraj K. ed. Basic and Applied Aspects of Biopesticides. New York: Springer; 2014:195-214. [Google Scholar]
- 24. Petrakis P, Moulet P. First record of the nearctic Zelus renardii (Heteroptera, Reduviidae, Harpactocorinae) in Europe. Entomol Hell. 2011;20:75-81. [Google Scholar]
- 25. Ambrose D, Maran S. Polymorphic diversity in salivary and haemolymph proteins and digestive physiology of assassin bug Rhynocoris marginatus (Fab.) (Het., Reduviidae). J Appl Entomol. 2000;124:315-317. [Google Scholar]
- 26. Ambrose DP. A checklist of Indian assassin bugs (Insecta: Hemiptera: Reduviidae) with taxonomic status, distribution and diagnostic morphological characteristics. Zoos Print J. 2006;21:2388-2406. [Google Scholar]
- 27. Grundy P, Maelzer D. Assessment of Pristhesancus plagipennis (Walker) (Hemiptera: Reduviidae) as an augmented biological control in cotton and soybean crops. Aust J Entomol. 2000;39:305-309. [Google Scholar]
- 28. Sahayaraj K, Kalidas S, Tomson M. Stage preference and functional response of Rhynocoris longifrons (Stål) (Hemiptera: Reduviidae) on three hemipteran cotton pests. Braz Arch Biol Technol. 2012;55:733-740. [Google Scholar]
- 29. Sahayaraj K. Pest Control Mechanism of Rediviids. Jaipur, India: Oxford Book Company; 2007. [Google Scholar]
- 30. Maran PM. Chosen Reduviid Predators–Prey Interaction: Nutritional and Pheromonal Chemical Ecology (Insecta: Heteroptera: Reduviidae) [PhD thesis]. Chennai, India: Manonmaniam Sundaranar University; 2000. [Google Scholar]
- 31. Sahayaraj K, Ravi C. Evaluation of reduviid predators and plant products against chosen groundnut pests. Arch Phytopathol Plant Prot. 2007;40:281-290. [Google Scholar]
- 32. Guo W, Yan X, Zhao G, Han R. Increased efficacy of entomopathogenic nematode-insecticide combinations against Holotrichia oblita (Coleoptera: Scarabaeidae). J Econ Entomol. 2016;110:41-51. [DOI] [PubMed] [Google Scholar]
- 33. Sorour M, Khamiss O, El-Wahab A, El-Sheikh M, Abul-Ela S. An economically modified semi-synthetic diet for mass rearing the Egyptian cotton leaf worm Spodoptera littoralis Acad J Entomol. 2011;4:118-123. [Google Scholar]
- 34. Waldbauer G. The consumption and utilization of food by insects. Adv Insect Physiol. 1968;5:229-288. [Google Scholar]
- 35. Tuan SJ, Yeh CC, Atlihan R, Chi H, Tang LC. Demography and consumption of Spodoptera litura (Lepidoptera: Noctuidae) reared on cabbage and taro. J Econ Entomol. 2015;109:732-739. [DOI] [PubMed] [Google Scholar]
- 36. Leckie BM, Ownley BH, Pereira RM, Klingeman WE, Jones CJ, Gwinn KD. Mycelia and spent fermentation broth of Beauveria bassiana incorporated into synthetic diets affect mortality, growth and development of larval Helicoverpa zea (Lepidoptera: Noctuidae). Biocontrol Sci Techn. 2008;18:697-710. [Google Scholar]
- 37. Malarvannan S, Murali P, Shanthakumar S, Prabavathy V, Nair S. Laboratory evaluation of the entomopathogenic fungi, Beauveria bassiana against the tobacco caterpillar, Spodoptera litura Fabricius (Noctuidae: Lepidoptera). J. Biopesticides. 2010;3:126-131. [Google Scholar]
- 38. Gindin G, Levski S, Glazer I, Soroker V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006;34:370-379. [Google Scholar]
- 39. Rath S, Prasad B, Sinha B. Food utilization efficiency in fifth instar larvae of Antheraea mylitta (Lepidoptera: Saturniidae) infected with Nosema sp. and its effect on reproductive potential and silk production. J Invertebr Pathol. 2003;83:1-9. [DOI] [PubMed] [Google Scholar]
- 40. Tefera T, Pringle K. Effect of exposure method to Beauveria bassiana and conidia concentration on mortality, mycosis, and sporulation in cadavers of Chilo partellus (Lepidoptera: Pyralidae). J Invertebr Pathol. 2003;84:90-95. [DOI] [PubMed] [Google Scholar]
- 41. Asaff A, Cerda-Garcia-Rojas C, de la Torre M. Isolation of dipicolinic acid as an insecticidal toxin from Paecilomyces fumosoroseus. Appl Microbiol Biotechnol. 2005;68:542-547. [DOI] [PubMed] [Google Scholar]
- 42. Malarvannan S, Giridharan S, Sekar S, Prabavathy V, Sudha N. Bioefficacy of crude and fraction of Argemone mexicana against tobacco caterpillar, Spodoptera litura Fab.(Noctuidae: Lepidoptera). J Biopesticides. 2008;1:55-62. [Google Scholar]
- 43. Asi M, Bashir M, Muhammad A, et al. Potential of entomopathogenic fungi against larvae and eggs of Spodoptera litura (Lepidoptera: Noctuidae). Pak Entomol. 2012;34:151-156. [Google Scholar]
- 44. Hussein H, Zemek R, Habuštová S, Prenerová E, Adel MM. Laboratory evaluation of a new strain CCM 8367 of Isaria fumosorosea (syn. Paecilomyces fumosoroseus) on Spodoptera littoralis (Boisd.). Arch Phytopathol Plant Protect. 2013;46:1307-1319. [Google Scholar]
- 45. Han JH, Jin BR, Kim JJ, Lee SY. Virulence of entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus for the microbial control of Spodoptera exigua. Mycobiology. 2014;42:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimmermann G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Sci Techn. 2008;18:865-901. [Google Scholar]
- 47. Sahayaraj K, Subash N, Allingham RW, et al. Lethal and sublethal effects of three microbial biocontrol agents on Spodoptera litura and its natural predator Rhynocoris kumarii. Insects. 2018;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang XN, Jin DC, Zou X, Guo JJ, Qu JJ. Screening of highly virulent strain of Isaria cateniannulata against Tetranychus urticae and its effect to Euseius nicholsi. J Environ Entomol. 2014;36:372-380. [Google Scholar]
- 49. Scorsetti AC, Pelizza SA, Fogel MN, Vianna MF, Schneider MI. Interactions between the entomopathogenic fungus Beauveria bassiana and the neotropical predator Eriopis connexa (Coleoptera: Coccinellidae): implications in biological control of pest. J Plant Prot Res. 2017;57:389-395. [Google Scholar]
- 50. Nalepa CA, Weir A. Infection of Harmonia axyridis (Coleoptera: Coccinellidae) by Hesperomyces virescens (Ascomycetes: Laboulbeniales): role of mating status and aggregation behavior. J Invertebr Pathol. 2007;94:196-203. [DOI] [PubMed] [Google Scholar]
- 51. Hajek AE, Butler L. Predicting the host range of entomopathogenic fungi. In Follett PA, Duan JJ, eds. Nontarget Effects of Biological Control. New York: Springer; 2000:263-276. [Google Scholar]
- 52. Cottrell TE, Shapiro-Ilan DI. Susceptibility of a native and an exotic lady beetle (Coleoptera: Coccinellidae) to Beauveria bassiana. J Invertebr Pathol. 2003;84:137-144. [DOI] [PubMed] [Google Scholar]
- 53. Bayissa W, Ekesi S, Mohamed SA, et al. Interactions among vegetable-infesting aphids, the fungal pathogen Metarhizium anisopliae (Ascomycota: Hypocreales) and the predatory coccinellid Cheilomenes lunata (Coleoptera: Coccinellidae). Biocontrol Sci Techn. 2016;26:274-290. [Google Scholar]
- 54. Gonzalez F, Tkaczuk C, Dinu MM, et al. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J Pest Sci 2016;89:295-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jarrahi A, Safavi SA. Sublethal effects of Metarhizium anisopliae on life table parameters of Habrobracon hebetor parasitizing Helicoverpa armigera larvae at different time intervals. Biocontrol. 2016;61:167-175. [Google Scholar]
- 56. Jaber LR, Araj SE. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol Control. 2018;116:53-61. [Google Scholar]


