Abstract
Background:
Streptococcus pneumoniae is one of the primary cause of community-acquired pneumonia (CAP) worldwide. However, scant data are available on the prevalence of etiological organisms for CAP in adolescent and adult Indian population.
Objective:
We performed a systematic review and meta-analysis to determine the contribution of S. pneumoniae in the causation of CAP in Indian patients aged 12 years or above.
Methodology:
We performed a systematic search of both indexed and non-indexed publications using PubMed, databases of National Institute of Science Communication and Information Resources (NISCAIR), Annotated Bibliography of Indian Medicine (ABIM), Google Scholar, and hand search including cross-references using key terms ‘community acquired pneumonia AND India’. All studies, published between January 1990 and January 2017, that evaluated Indian patients aged above 12 years with a confirmed diagnosis of CAP were eligible for inclusion. Our search retrieved a total of 182 studies, of which only 17 and 12 qualified for inclusion in the systematic review of all etiological organisms, and meta-analysis of S. pneumonia, respectively.
Results:
A total of 1435 patients met the inclusion criteria. The pooled proportion of patients with S. pneumoniae infection was 19% (95% confidence interval [CI]: 12%-26%; I2 = 94.5% where I2 represents heterogeneity, P < .01). Other major etiological agents are Mycoplasma pneumoniae (15.5% [1.1%-35.5%]), Klebsiella pneumoniae (10.5% [1.6%-24.0%]), and Legionella pneumophila (7.3% [2.5%-23.8%]).
Conclusions:
Analysis found approximately a one-fifth proportion of adult Indian patients of CAP with S. pneumoniae infection, suggesting it as a leading organism for causing CAP compared with other etiological organisms.
Keywords: Community-acquired pneumonia, Streptococcus pneumoniae, bacterial pneumonia, pneumonia, aetiology
Introduction
Pneumococcal pneumonia comprises about two-thirds of all bacterial pneumonia and is the most common cause of morbidity in patients with community-acquired pneumonia (CAP).1 A recent global burden of disease report estimated that there were 291.8 million episodes of lower respiratory tract infection (LRTI) (95% uncertainty interval [UI] 276.3 million to 307.0 million) each year. More than one-third of these episodes (101.8 million) occurred in children less than 5 years of age.2 In a 2017 global burden of disease study, Streptococcus pneumoniae was the most commonly identified LRTI pathogen in all age groups, causing more than 1.5 million LRTI deaths. In particular, there were 0.7 deaths in patients above 70 years and 0.4 million fatalities among children less than 5 years of age. In India, CAP due to S. pneumoniae was responsible for 82 000 deaths among children less than 5 years of age.2
Empirical therapy for CAP starts with antibiotics, which include those that target S. pneumoniae; however, the misuse of antibiotics can lead to drug resistance. Consequently, empirical microbial treatment for CAP should be based on the knowledge of the causative pathogen to avoid treatment failure and the associated costs. Studies reveal a case-fatality rate ranging from 4% to 33% where there was an incorrect initial selection of antibiotics.3-5
There is some Indian literature on the microbiological aetiology of CAP among adults.6 Hence, this systematic review and meta-analysis were performed to analyse the proportion of CAP due to S. pneumoniae infection in Indian patients >12 years of age.
Methodology
This systematic review and meta-analysis were conducted in accordance with the Preferred Recording Items for Systematic Reviews and Meta-Analysis (PRISMA).
Eligibility criteria for studies
All studies, published between January 1990 and January 2017, that evaluated Indian patients aged above 12 years of age with a confirmed diagnosis of CAP were eligible for inclusion. Adolescence was taken to begin at 12 years of age.
Exclusion criteria for studies
All studies on CAP patients that were conducted outside India or/and not conducted in Indian population were excluded from the analysis. Studies were also excluded if they were conducted in Indian patient populations <12 years of age or where the full text was not available.
Measurements
The primary outcome of this study was the proportion of patients with CAP caused by S. pneumoniae. The secondary outcome was to determine the proportion of all other aetiological agents causing CAP. Sensitivity analysis was carried out based on the reporting quality of the included studies.
Search strategy
We performed a systematic search on PubMed, using the key terms ‘Community-Acquired Pneumonia AND India’, ‘Community-Acquired Pneumonia AND aetiology’, ‘Community-Acquired Pneumonia AND Diagnosis’, or ‘Community-Acquired Pneumonia AND Management’. The search was performed after applying constant filters based on these additional search criteria: Article Types – Randomized Clinical Trials, Meta-Analysis, Systematic Literature Reviews, Literature Reviews, Observational Studies; Language – English; Publication Date – 01/01/1990-08/01/2017; Species – Humans; Adult and Adolescent – 12+ years. Additional records were identified through other sources (the National Institute of Science Communication and Information Resources [NISCAIR], the Annotated Bibliography of Indian Medicine [ABIM], and Google Scholar) using the search terms: ‘Community-Acquired Pneumonia AND ‘India’. A hand search was also performed using the same key terms, based on cross-references and review of journals from the library. A medical librarian was not involved in designing or reviewing the research strategy.
Risk of bias
The risk of bias was avoided by assessing the quality of information from each study. The instrument used to assess the risk of bias analysis was the instrument developed by Joanna Briggs Institute for systematic reviews addressing questions of prevalence.7
Data extraction
Data was collected from all the primary studies using a structured sheet in Microsoft Excel. Any discrepancies arising while entering the data were sorted out by discussion among all the contributors. Two reviewers were involved in determining the risk of bias analysis and data extraction. Reviewers resolved any disagreements by discussion between themselves. Study characteristics extracted included authors details, year of publication, title of study, place of study, and type of study. Patient parameters included number of study participants and their mean age, gender, educational level, and marital status. CAP was classified by aetiology.
Statistical analysis
A meta-analysis of proportion for aetiological agents with corresponding 95% confidence interval (CI) for all included individual studies was performed. Also, meta-analysis using a random effects method (DerSimonian and Laird), with the assumption of a degree of heterogeneity (i)2 among the studies, was performed. The outcomes were presented as pooled estimates with 95% CI.8 The i2 test assessed variation in the outcome of all included studies with respect to the primary and secondary objectives. The meta-analyses were carried out using open software.9
Results
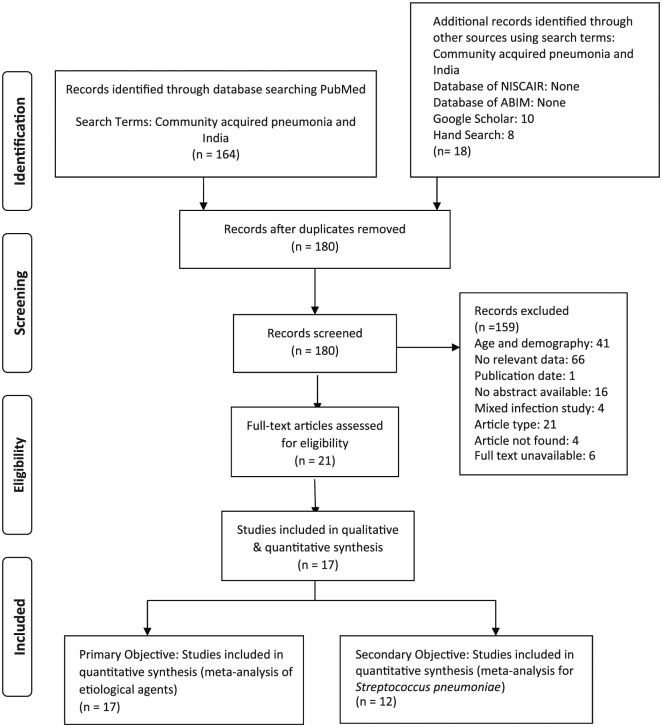
PubMed searches retrieved 164 studies and Google Scholar searches retrieved ten. Eight additional studies were retrieved via hand search. The ABIM and NISCAIR database searches did not retrieve any relevant study. The analysis identified 17 relevant studies (Figure 1). All 17 studies10-26 were considered for qualitative as well as the quantitative synthesis of aetiological agents. Ultimately, only 12 of 17 studies were included for S. pneumoniae meta-analysis,10,13,15,16,18-22,24-26 since the remaining five studies did not include S. pneumoniae among the aetiological agents in their analyses. Table 1 represents the characteristics of the studies included in the analysis.
Figure 1.
PRISMA flow diagram. ABIM indicates Annotated Bibliography of Indian Medicine; NISCAIR, National Institute of Science Communication and Information Resources.
Table 1.
Study characteristics table.
| First author | Year of publication | Study design | Number of patients | Risk factors/aetiology | Diagnostic test | Mean age (years) (full range) of population | Geographical location/type of hospital |
|---|---|---|---|---|---|---|---|
| Shah et al | 2010 | Prospective study | 150 | Streptococcus pneumonia, Klebsiella pneumonia, Mycoplasma pneumonia | Sputum gram stain and culture, blood culture | 53.68 years (15-80) | Northern India (Srinagar)/Tertiary Care Hospital |
| Dharmadhikari et al | 2013 | Observational study | 104 | S. pneumoniae, K. pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa | Sputum gram stain and culture, blood culture | Not available | Pune (India)/Tertiary Care Hospital |
| Mythri et al | 2013 | Cross-sectional observational clinical study | 100 | S. pneumoniae, Klebsiella spp., Pseudomonas spp. | Sputum gram stain and culture, blood culture | Not available | Bangalore (India)/Tertiary Care Hospital |
| Sreekanth and Reddy | 2015 | Prospective observational study | 50 | S. pneumoniae, K. pneumoniae, Enterobacter, Escherichia coli, P. aeruginosa, S. aureus | Bronchoscopy, sputum analysis and culture | Mean age not available (Minimum age, 18 years, but maximum age, not available) | Andhra Pradesh (India)/Tertiary Care Hospital |
| Bansal et al | 2004 | Prospective observational study | 70 | S. pneumoniae, K. pneumoniae, S. aureus, M. pneumoniae, E. coli, beta-haemolytic streptococci, other Gram-negative bacilli | Blood culture, sputum stain and culture, pleural fluid culture, ELISA | 52.77 years (17-93) | Himachal Pradesh/Tertiary Care Hospital |
| Dey et al | 1997 | Prospective observational study | 72 | S. pneumoniae, K. pneumoniae | Blood culture, sputum stain and culture | 50.6 years (18-80) | New Delhi/Tertiary Care Hospital |
| Dey et al | 2000 | Prospective observational study | 62 | M. pneumoniae | Blood culture, sputum analysis and culture, gelatin particle agglutination test, ELISA | 41.77 years (14-67) | New Delhi/Tertiary Care Hospital |
| Khadanga et al | 2014 | Observational study | Enrolled: 464; culture isolated: 149 | S. pneumoniae, K. pneumoniae, P. aeruginosa, S. aureus | Blood culture, sputum stain and culture | Not available | Madhya Pradesh and Odisha/Tertiary Care Hospital |
| Ravindranath et al | 2016 | Observational study | 150 | S. aureus | Blood Culture, sputum stain and culture | 55.71 years (<40 years and ⩾80) | Hyderabad/Tertiary Care Hospital |
| Jain et al | 2014 | Prospective observational study | 120 | S. pneumoniae, K. pneumoniae, S. aureus, Haemophilus influenza, other Gram-negative bacilli | Sputum analysis, blood culture | 52.36 years (15-85) | Gwalior/Tertiary Care Hospital |
| Kejriwal et al | 2015 | Prospective observational study | 60 | S. pneumoniae, K. pneumoniae, P. aeruginosa, E. coli, Acinetobacter, S. aureus | Sputum stain and culture, ELISA | Mean age not available (Minimum age, 14 years, but maximum age, not available) |
Mumbai/Tertiary Care Hospital |
| Shrikhande et al | 2015 | Prospective observational study | 50 | S. pneumoniae, K. pneumoniae, S. aureus, P. aeruginosa, E. coli | Sputum stain and culture, blood culture, pleural fluid | 49.34 years (Minimum age, 12 years, but maximum age, not available) | Rajasthan/Tertiary Care Hospital |
| Acharya et al | 2014 | Prospective observational study | 100 | S. pneumoniae, K. pneumoniae, P. aeruginosa | Sputum stain and culture | Mean age not available (14-70) | Mangalore, Coastal Karnataka/Tertiary Care Hospital |
| Menon et al | 2013 | Prospective observational study | 145 | K. pneumoniae, P. aeruginosa, alpha-haemolytic streptococci, E. coli, beta-haemolytic streptococci, Atypical coli | Sputum culture | Mean age not available (18-90) | Kerala/Secondary Care Hospital |
| Anbumani et al | 2010 | Observational study | 470 samples | Legionella pneumophila, other aetiological agents of bacterial pneumonia | Sputum stain and culture, blood culture, pleural fluid analysis, endotracheal aspirate | Mean age not available (24-76) | Tirupati/Tertiary Care Hospital |
| Angrup et al | 2016 | Observational study | 134 | L. pneumophila | Blood culture, urine analysis, respiratory tract fluids (throat swab, nasopharyngeal aspirates, endotracheal aspirates, bronchoalveolar lavage, sputum culture), PCR, ELISA | The study included paediatric and adult patients, but age not mentioned | New Delhi/Tertiary Care Hospital |
| Dorairaj et al | 2015 | Observational study | 107 | M. pneumoniae, Chlamydia pneumoniae | Sputum culture, urine antigen, serological diagnosis by ELISA | 44.42 years (18 to >65) | Chennai/Tertiary Care Hospital |
Abbreviations: ELISA enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.
Risk of bias analysis
The risk of bias assessment was delineated in the reporting of the following items (Supplementary Table 1): adequate sample size, appropriate recruitment, appropriate reporting of study subjects and of setting, reliable and objective measurement of outcome condition, statistical analysis, and accountability for confounding factors.
Primary outcome
The meta-analysis included 1435 patients. The patients’ ages ranged from 12 to 93 years, with a predominance of the male gender. Clinical diagnosis was made both by physical examination along with a chest X-ray. The microbiological assessment of sputum culture was made in all studies, while in 12 studies, both sputum and blood cultures were obtained (Table 1).
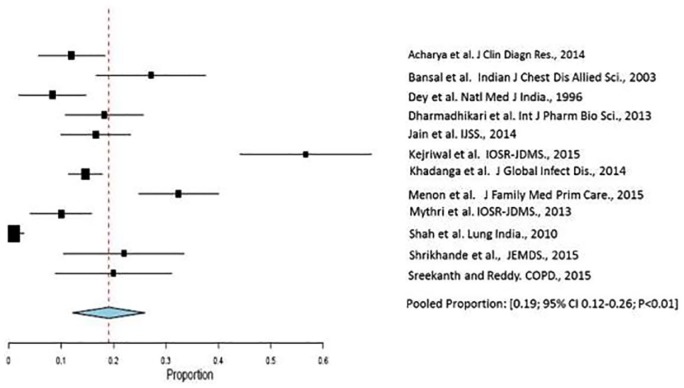
The pooled proportion of patients with S. pneumoniae infection was 19% (95% CI: 12%-26%; I2 = 94.5%; P < .01) (Tables 2 and 3, Figure 2). The degree of heterogeneity was significant.10,13,15,16,18-22,24-26
Table 2.
Proportion of Streptococcus pneumoniae infection in patients with community-acquired pneumonia.
| Study Reference | Proportion of prevalence of S. pneumoniae | CI lower | CI upper | Weight% |
|---|---|---|---|---|
| Acharya et al10 | 0.12 | 0.05 | 0.19 | 8.68 |
| Bansal et al13 | 0.27 | 0.15 | 0.39 | 7.73 |
| Dey et al15 | 0.08 | 0.02 | 0.15 | 8.68 |
| Dharmadhikari et al16 | 0.18 | 0.10 | 0.26 | 8.46 |
| Jain et al18 | 0.17 | 0.09 | 0.24 | 8.62 |
| Kejriwal et al19 | 0.57 | 0.38 | 0.76 | 7.16 |
| Khadanga et al20 | 0.15 | 0.11 | 0.18 | 9.18 |
| Menon et al21 | 0.32 | 0.23 | 0.42 | 8.41 |
| Mythri and Nataraju22 | 0.10 | 0.04 | 0.16 | 8.78 |
| Shah et al24 | 0.01 | −0.01 | 0.03 | 9.30 |
| Sreekanth and Reddy26 | 0.20 | 0.08 | 0.32 | 7.45 |
| Shrikhande et al25 | 0.22 | 0.09 | 0.35 | 7.55 |
Abbreviation: CAP, community-acquired pneumonia; CI, confidence interval.
Table 3.
Pooled aetiology data of studies.
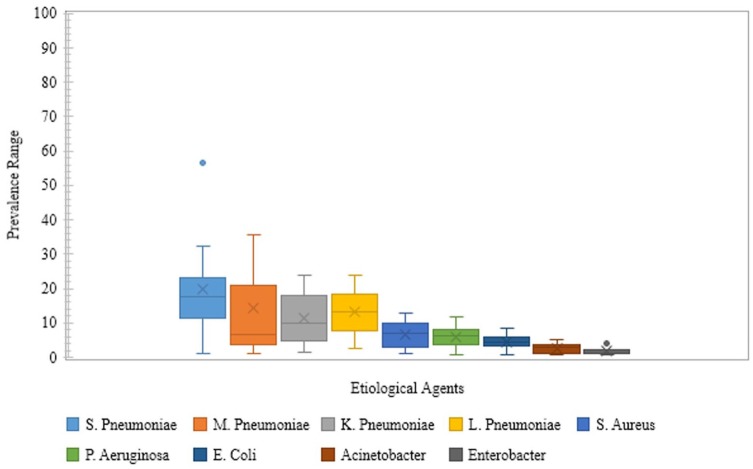
| Etiological agent (EA) | No. of subjects with EA | Total no. of subjects with confirmed culture report | Total no. of subjects with CAP | Pooled proportion (%) | Median (IQR) |
|---|---|---|---|---|---|
| S. pneumoniae | 257 | 699 | 1435 | 19.0 | 17.5 (11.7) |
| K. pneumonia | 151 | 699 | 1435 | 10.52 | 9.8 (13.1) |
| M. pneumoniae | 37 | 128 | 239 | 15.48 | 6.5 (17.2) |
| P. aeruginosa | 89 | 699 | 1435 | 6.20 | 6.3 (4.5) |
| S. aureus | 79 | 570 | 1468 | 5.38 | 7.0 (7.2) |
| Acinetobacter | 14 | 330 | 604 | 2.31 | 2.9 (2.3) |
| Enterobacter | 5 | 176 | 340 | 1.47 | 1.2 (1.1) |
| E. coli | 38 | 539 | 899 | 4.22 | 4.5 (2.8) |
| L. pneumophila | 44 | 604 | 604 | 7.28 | 13.2 (10.7) |
Abbreviations: CAP, community-acquired pneumonia; IQR: interquartile range.
Figure 2.
Forest plot displaying meta-analysis of proportion of Streptococcus pneumoniae infection in patients with community-acquired pneumonia. Binary random effects model was applied to get pooled proportion and 95% confidence interval (0.19; 95% CI 0.12–0.26; P < .01).
Secondary outcome
Other causative organisms of CAP in the Indian adolescent and adult population were Mycoplasma pneumoniae (1.1%-35.4%),13,14,17 Klebsiella pneumoniae (1.6%-24.0%),10,13,15,16,18-22,24-26 Legionella pneumophila (2.5%-23.8%),11,12 Staphylococcus aureus (1.0%-12.8%),10,13,15,16,18-22,24-26 Pseudomonas aeruginosa (0.83%-11.6%),10,13,15,16,18-22,24-26 Escherichia coli (0.83%-8.57%),10,13,16,18,19,21,22,24-26 Acinetobacter spp. (0.83%-5.0%),10,13,16,18,19,24,26 and Enterobacter spp. (0.83%-4.0%)10,13,18,26 (Supplementary Table 2, Figure 3).
Figure 3.
Prevalence range of etiological agents in community-acquired pneumonia in Indian setting. Blue and Grey dots are outliers; the cross (X) mark depicts the Mean.
Discussion
Our systematic review suggests that S. pneumoniae is responsible for 19% of CAP in Indian patients >12 years of age. In a recently published study, Para et al evaluated the microbial aetiology of CAP in adult patients in a tertiary care hospital from North India. They observed that S. pneumoniae was the most common micro-organism accounting for nearly 31% of cases. Other organisms identified were L. pneumophila (17.5%), influenza viruses (15.4%), and M. pneumoniae (7.2%), with 4% of patients having multiple etiologies.27 Kumar et al recently conducted a study from South India to assess CAP among children between 2 months and 16 years of age. They observed M. pneumoniae as the most common pathogen (20% cases), followed by respiratory syncytial virus isolated in about 11% cases.28
Historically, common laboratory tests for pneumonia have included leukocyte count, sputum Gram stain, two sets of blood cultures, and urine antigens. Although previous administration of antibiotics may contribute to false-negative cultures rates, culture positivity rate values for the microbiological diagnosis of S. pneumoniae CAP by sputum culture may be confounded by upper airway contamination leading to false-positive culture rates.29 Furthermore, while isolation of S. pneumoniae from the sputum may represent colonization and overestimate its role in CAP, the prevalance of S. pneumoniae as a cause of CAP is underestimated due to lack of sensitivity of isolation technique from the blood. The urinary antigen method may enhance the sensitivity to detect S. pneumoniae; however, only two studies included in the present review performed urinary antigen testing, which was to detect L. pneumophila.29 Our results suggest that approximately one-fifth of adult Indian CAP patients had S. pneumonia identified as an aetiological agent. The other predominant aetiological agents reported in adult Indian CAP patients were K. pneumoniae (10.5%) and M. pneumoniae (15.4%).
Our results are in corroboration with the studies conducted in other parts of the world. For example, a Spanish study conducted on 109 CAP patients, found S. pneumoniae to be responsible for 25% of all cases.30 In the United Kingdom, the Research Committee of the British Thoracic Society and the Public Health Laboratory Service conducted a study to determine the aetiology of CAP in adult British patients. S. pneumoniae was identified as the foremost aetiological agent.31 Similar results were reported in adult CAP patients in other studies.32,33 The results from the studies indicate the importance and contribution of S. pneumoniae in the burden of CAP across the globe over long periods.
CAP is a major cause of adult mortality across Asia.34 Similar mortality patterns exist in developed western countries as well; for example, CAP is the sixth leading cause of death in the USA.35 People in the older age group have increased mortality due to CAP as compared with the younger age group, with Chronic Obstructive Pulmonary Disease (COPD) being a common predisposing condition for CAP in the elderly.34,35
These deaths can be prevented by promoting vaccination against CAP in susceptible adults, the coverage of which is still far from being adequate in India.36 Apart from focusing on optimal use of antibiotics in this antibiotic-resistance era, vaccination against the common causative organism may be of substantial preventive benefit against adult CAP in India. Furthermore, it may also reduce the economic burden due to CAP.37
There are two types of pneumococcal vaccines currently used globally – conjugate vaccines that contain 10 (PCV-10) or 13 (PCV-13) pneumococcal serotypes, and the plain polysaccharide vaccine that contains 23 pneumococcal serotypes (PPV23).38 Polysaccharide pneumococcal vaccine (PPV) was first introduced in 1983.38 The dawn of the 21st century saw the introduction of pneumococcal conjugate vaccine (PCV): heptavalent followed by 10-valent and 13-valent.39 In contrast to the plain polysaccharide vaccine, the conjugate vaccines induce T-cell dependent immune response. With respect to elderly adults, CAPITA, a randomized, double-blind, placebo-controlled trial, involving nearly 85 000 adults sought to establish the safety and efficacy of PCV13. Vaccine-serotype-specific CAP (diagnosed by either blood culture or a serotype-specific urine antigen detection assay) occurred in 49 participants in the PCV13 group as compared with 90 in the placebo group (vaccine efficacy, 45.6%; 95.2% CI, 21.8-62.5).40
The strength of this meta-analysis is that the included studies represent each of the regions of India. In this respect, an important consideration was the heterogeneity of included Indian studies. The random effects model found a varied distribution pattern. Our qualitative analysis also revealed similar results, but the source of heterogeneity could not be identified among the studies. The sensitivity analysis on 11 studies after excluding the study with the largest sample size led to comparable observations, suggesting that pooling these studies despite the difference in methodology was reasonable for this meta-analysis.
Nonetheless, a major limitation of this analysis was that the inclusion of all the existing eligible participants having different comorbidities could not be guaranteed. The other limitation was the reliance on sputum culture to make the microbiological diagnosis, in spite of the fact that the 17 studies included in this systematic review and meta-analysis were done in tertiary care hospitals in the urban area. Although most reported diagnosis based on clinical signs and symptoms along with microbiological tests (sputum culture and blood culture), 6 of 17 reported diagnoses were based on sputum culture and leukocyte count.
In addition, with the criteria established for this analysis, studies on viral pneumonia, or a comparison of viral with bacterial aetiology, were not included. Also, unpublished data such as conference abstracts or papers presented at scientific symposia were not included in the current study. Furthermore, no Indian study included in this study had reported multiple or mixed infections.
Future research should focus on larger epidemiological studies to identify aetiological organisms of CAP. This will help to establish a precise estimate and reliable association between the aetiological agents and CAP.
Conclusions
This systematic review and meta-analysis identified the aetiological agents of bacterial CAP from published studies in the adolescent and adult Indian population, finding the predominant causes to be S. pneumonia (19%), M. pneumoniae (15.4%), and K. pneumoniae (10.5%).
Supplemental Material
Supplemental material, Supplemental for Streptococcus pneumoniae as a Cause of Community-Acquired Pneumonia in Indian Adolescents and Adults: A Systematic Review and Meta-Analysis by Canna J. Ghia, Raja Dhar, Parvaiz A Koul, Gautam Rambhad and Mark A Fletcher in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Pfizer.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CJG, GR, and MAF are employees of Pfizer, the manufacturer of pneumococcal conjugate vaccines. RD and PAK declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CJG, RD and PAK were involved in the design of the systemic review and meta-analysis, did the data analyses, and wrote the first draft of this manuscript with input from GR and MAF, who also advised on the statistical analyses and assisted with the figures. All authors contributed to refining their approved submitted manuscript.
ORCID iD: Canna J. Ghia  https://orcid.org/0000-0001-9839-3209
https://orcid.org/0000-0001-9839-3209
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults infectious diseases society of America. Clin Infect Dis. 2000;31:347–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troeger C. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis. 2017;17:1133–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. File TM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. [DOI] [PubMed] [Google Scholar]
- 5. Luna HI, Pankey G. The utility of blood culture in patients with community-acquired pneumonia. Ochsner J. 2001;3:85–93. [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar R, Ghia C, Waghela S, et al. Community-acquired pneumonia: bacteriological profile and microbiological investigations. J Assoc Physicians India. 2016;64:12–16. [PubMed] [Google Scholar]
- 7. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 9. Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:15. [Google Scholar]
- 10. Acharya VK, Padyana M. B U, R A Acharya PR, Juneja DJ. Microbiological profile and drug sensitivity pattern among community acquired pneumonia patients in tertiary care centre in Mangalore, coastal Karnataka, India. J Clin Diagn Res. 2014;8:MC04–MC06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anbumani S, Gururajkumar A, Chaudhury A. Isolation of Legionella pneumophila from clinical & environmental sources in a tertiary care hospital. Indian J Med Res. 2010;131:761–764. [PubMed] [Google Scholar]
- 12. Angrup A, Chaudhry R, Sharma S, et al. Application of real-time quantitative polymerase chain reaction assay to detect Legionella pneumophila in patients of community-acquired pneumonia in a tertiary care hospital. Indian J Med Microbiol. 2016;34:539–543. [DOI] [PubMed] [Google Scholar]
- 13. Bansal S, Kashyap S, Pal L, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 14. Dey A, Chaudhry R, Kumar P, et al. Mycoplasma pneumoniae and community-acquired pneumonia. Natl Med J India. 2000;13:66–70. [PubMed] [Google Scholar]
- 15. Dey A, Nagarkar K, Kumar V. Clinical presentation and predictors of outcome in adult patients with community-acquired pneumonia. Natl Med J India. 1996;10:169–172. [PubMed] [Google Scholar]
- 16. Dharmadhikari V, Joseph T, Kulkarni A. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Int J Pharm Bio Sci. 2013;4:695–702. [Google Scholar]
- 17. Dorairaj A, Kopula SS, Kumar K. Atypical pneumonia-screening in a tertiary care centre. J Clin Diagn Res. 2015;9:DC18–DC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain SK, Jain S, Trikha S. Study of clinical, radiological, and bacteriological profile of community-acquired pneumonia in hospitalized patients of Gajra Raja Medical College, Gwalior, Central India. Int J Sci Stud. 2014;2:96–100. [Google Scholar]
- 19. Kejriwal A, Shenoi A, Pusukuru R, et al. A clinical, bacteriological and radiological profile of community acquired pneumonia in Navi Mumbai, India. IOSR J Dental Med Sci. 2015;14:58–61. [Google Scholar]
- 20. Khadanga S, Karuna T, Thatoi PK, Behera SK. Changing bacteriological profile and mortality trends in community acquired pneumonia. J Glob Infect Dis. 2014;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menon RU, George AP, Menon UK. Etiology and anti-microbial sensitivity of organisms causing community acquired pneumonia: a single hospital study. J Family Med Prim Care. 2013;2:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mythri S, Nataraju HV. Bacteriological profile of community acquired pneumonia. IOSR J Dental Med Sci. 2013;12:16–19. [Google Scholar]
- 23. Ravindranath M, Raju C. Validity of pneumonia severity index/pneumonia outcome research trial and Curb-65 severity scoring systems in community acquired pneumonia in Indian setting. Indian J Chest Dis Allied Sci. 2016;3:338–344. [PubMed] [Google Scholar]
- 24. Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Lung India. 2010;27: 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shrikhande A, Khangarot S, Saxena A, et al. Clinical, bacteriological and radiological study of community acquired pneumonia. J Evol Med Dent Sci. 2015;4: 2112–2119. [Google Scholar]
- 26. Sreekanth A, Reddy SPK. Study of clinical presentations and treatment outcome of severe community acquired pneumonia in the department of pulmonology of a tertiary care hospital. COPD. 2015;10:20. [Google Scholar]
- 27. Para RA, Fomda BA, Jan RA, Shah S, Koul PA. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India. 2018;35:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar KJ, Ashok Chowdary KV, Usha HC, Kulkarni M, Manjunath VG. Etiology of community acquired pneumonia among children in India with special reference to atypical pathogens. Lung India. 2018;35:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37:1405–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruiz-Gonzalez A, Falguera M, Nogues A, Rubio-Caballero M. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? a microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am J Med. 1999;106:385–390. [DOI] [PubMed] [Google Scholar]
- 31. Research Committee of the British Thoracic Society the Public Health Laboratory Service. Community-acquired pneumonia in adults in British hospitals in 1982-1983: a survey of aetiology, mortality, prognostic factors and outcome: the British thoracic society and the public health laboratory service. Q J Med. 1987;62:195–220. [PubMed] [Google Scholar]
- 32. Lim WS, Macfarlane JT, Boswell TCJ, et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peto L, Nadjm B, Horby P, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. File TM., Jr. Streptococcus pneumoniae and community-acquired pneumonia: a cause for concern. Am J Med 2004;117:39s–50s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma BB, Singh V. Pneumonia bugs and determinants of their occurrence. Lung India. 2018;35:95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murdoch DR, Laing RTR, Mills GD, et al. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39: 3495–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tin Tin Htar M, Stuurman AL, Ferreira G, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS ONE. 2017;12:e0177985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scully IL, Swanson K, Green L, Jansen KU, Anderson AS. Anti-infective vaccination in the 21st century – new horizons for personal and public health. Curr Opin Microbiol. 2015;27:96–102. [DOI] [PubMed] [Google Scholar]
- 40. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372: 1114–1125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental for Streptococcus pneumoniae as a Cause of Community-Acquired Pneumonia in Indian Adolescents and Adults: A Systematic Review and Meta-Analysis by Canna J. Ghia, Raja Dhar, Parvaiz A Koul, Gautam Rambhad and Mark A Fletcher in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine