Abstract
Locally/regionally advanced melanoma confers a major challenge in terms of surgical and medical management. Surgical treatment carries the risks of surgical morbidities and potential complications that could be lasting. In addition, these patients continue to have a high risk of relapse and death despite the use of standard adjuvant therapy. Neoadjuvant therapy has the potential to significantly improve the clinical outcome of these patients, particularly in this era of newer and effective targeted and immunotherapeutic agents. Previous neoadjuvant studies tested chemotherapy with temozolomide where the clinical activity was limited. Biochemotherapy (BCT) was tested in two studies in the neoadjuvant setting and showed high tumor response rates; however, BCT was ultimately abandoned following its failure to demonstrate survival benefits in randomized trials of metastatic disease. Success of immunotherapy and targeted therapy in prolonging the lives of patients with metastatic melanoma generated considerable interest to investigate these novel strategies in the adjuvant and neoadjuvant settings. A number of neoadjuvant targeted and immunotherapy studies have been completed in melanoma to date and have yielded promising clinical activity. Given these encouraging results, a number of studies with other molecularly targeted and immunotherapeutic agents and their combinations are ongoing in the neoadjuvant setting; long-term outcome data are eagerly awaited. Such studies also provide access to biospecimens before and during therapy, allowing for the conduct of biomarker and mechanistic studies that may have a significant impact in guiding adjuvant therapy choices and drug development.
Keywords: anti-PD 1, cutaneous melanoma, immunotherapy, interferon, neoadjuvant, targeted therapy
Introduction
Melanoma is increasing in incidence at a rate that exceeds that of all other malignancies.1 In 2019, in the United States, it is estimated that 96,480 new cases of melanoma will be diagnosed and 7230 will die as a result of this disease.1 The American Joint Committee on Cancer (AJCC) divides cutaneous melanoma into four stages.2 Stage I melanoma has the best prognosis with surgical treatment alone and a 10-year survival rate of about 94–98%.2 The 5-year post-surgical relapse rate in patients with stages IIA, IIB, and IIC ranges from over 20% to over 50%, whereas for patients with stage III melanoma and clinically detectable regional lymph nodes with or without in-transit metastases, the reported 5-year relapse rate is 68–89%.3–5
Historically, patients with clinically palpable regional lymphatic metastases (stage IIIB-D, AJCC 8th edition) carried a risk of relapse and death that exceeds 70% at 5 years.2,5–8 Similarly, patients who develop local or regional recurrence after initial surgical management carry an even poorer prognosis.9–11 In the Melanoma Intergroup Surgical Trial, a local recurrence was associated with 5- and 10-year survival rates of 9–11% and 5%, respectively.10 Surgical excision of the primary, therapeutic lymphadenectomy, and adjuvant systemic therapy are the cornerstones of current management. Until recently, adjuvant therapy consisted of high-dose interferon alfa-2b (HDI), pegylated interferon alfa, or ipilimumab at 10 mg/kg.12–15 These adjuvant therapies provided improved survival benefit as compared with observation or placebo, however, high rates of toxicities were significant concerns.16,17 Recent studies have supported the adjuvant use of anti-PD1 monoclonal antibodies nivolumab and pembrolizumab as well as the combination of dabrafenib and trametinib (in patients with stage III BRAF-mutated melanoma).18–20 However, the relapse free survival for these patients is still poor, with median recurrence-free survival (RFS) of approximately 70–80% at year 1 and 50–60% at 3 years.18–20 There is a clear unmet need to improve locally/regionally advanced stage III melanoma outcomes, and early data have shown that neoadjuvant therapy may significantly improve disease response and patient survival.
Advantages of neoadjuvant therapy
Neoadjuvant therapy has improved the prognosis of patients with different types of solid tumors, including head and neck, breast, bladder, esophageal, and rectal cancer.21–24 Potential benefits that could be conferred through neoadjuvant therapy include reduction in tumor burden with improvements in surgical resectability, increased locoregional control rates, organ preservation, and improvement in overall survival (OS). Further, neoadjuvant therapy allows for the evaluation of pathologic responses in addition to clinical and radiologic responses. Neoadjuvant studies provide access to blood and tumor biospecimens before and during systemic therapy, supporting studies of immunologic and histologic correlates of tumor response. Such studies can allow for better understanding of the antitumor mechanisms of action and ultimately would enable more selective application of therapeutic agents to patients who are more likely to benefit. This would lead to improvement in the therapeutic index and the cost effectiveness of these agents.
A list of completed neoadjuvant studies in locally/regionally advanced melanoma is summarized in Table 1.
Table 1.
Completed neoadjuvant studies in locally/regionally advanced melanoma.
| Reference | Study | Number of patients | Design | Regimen | Findings |
|---|---|---|---|---|---|
| Neoadjuvant immunotherapy | |||||
| Moschos et al.30 | Moschos | 20 | Phase II single arm |
Interferon-α2b 20 MU/m2 i.v., 5 days a week for 4 wks before surgery (induction) Interferon-α2b 20 MU/m2 s.c., 3 days a week for 48 wks starting after surgery (maintenance) |
• Objective clinical response in 55% (11 patients) and pCR in 15% (three patients) • Significant increases in endotumoral CD11c+ and CD3+ cells • Upregulation of pSTAT1 and TAP2 and downregulation of pSTAT3 • Downregulation of phospho-ERK1/2 in tumor cells, not in lymphocytes |
| Tarhini et al.34,35 | Tarhini | 33 | Phase I/II single arm |
Ipi10 mg/kg i.v. every 3 wks × 2 doses, bracketing surgery | • Potentiation of type I CD4 and CD8 tumor specific T cells • Downregulation of MDSC • Increased infiltration of CD8+ TIL and T memory (CD45RO+) • An immune-related gene expression signature predicts clinical benefit |
| Tarhini et al.36 | Tarhini | 30 | Phase I/II single arm |
Ipi 3 or 10 mg/kg i.v. every 3 weeks × 4 doses bracketing definitive surgery, then every 12 wks × 4 doses. HDI (20 MU/m²/d i.v. × 5 days (d)/wks for 4 wks then 10 MU/m²/d s.c. every other day TIW for 48 wks) given concurrently | • Preoperative radiological response in 36% and pCR in 32% • Higher tumor T cell fraction in patients with pCR • Higher tumor T cell clonality associated with improved Relapse-free survival |
| Huang et al.37 | Huang | 30 | Phase I, single arm | Pembro 200 mg 1 dose followed by surgery after 3 weeks, then q3wks pembro for 1 year | • 30% complete or near-complete (<10% viable tumor) pathologic response • 2 year RFS of 63% and median OS of 93%. • Rapid accumulation of exhausted CD8 T cells in the tumor at 3 weeks and in blood at 1 week associated with pathologic and clinical responses. • A pretreatment immune signature associated with clinical benefit |
| Tarhini et al.38 | Tarhini | 20 | Phase I, single arm | Pembro 200 mg i.v./qq3wks × 2 doses followed by surgery, then q3wks × 1 year. HDI (20 MU/m2/d i.v. for 5 days/wks for 4 wks then 10 MU/m2/d s.c. every other day TIW for 48 wks) given concurrently | • Radiological preoperative response of 65% (WHO) • pCR of 35% (7/20 patients) |
| Blank et al.39 | OpACIN, Blank | 20 | Phase Ib | Arm A: Adjuvant i.v. ipi 3 mg/kg q3wks + i.v. nivo 1 mg/kg q3wks for 12 weeks Arm B: IV Ipi 3 mg/kg q3wks + i.v. nivo 1 mg/kg q3wks for 6 weeks bracketing surgery |
• Neoadjuvant ipi + nivo led to 3pCR, 4 near pCR (microscopic metastatic disease) and 1 PR • Grade 3–4 adverse events in 18/20 patients |
| Blank et al.40 | OpACIN-neo, Phase II | 90 | Phase II, III arms | Arm A: Ipi (3 mg/kg) + Nivo (1 mg/kg) q3wks for 6 wks before surgery Arm B: Ipi (1 mg/kg) + Nivo (3 mg/kg) q3wks for 6 weeks before surgery Arm C: Ipi (3 mg/kg) q3wks for 6 wks followed immediately by Nivo 3 mg/kg q2wks for 4 wks |
• Grade 3/4 adverse events: 40% in arm A, 20% in arm B, and 50% in arm C • Complete radiologic response rate: 7% in arm A, 10% in arm B, and 4% in arm C • pCR rate: 47% in arm A, 47% in arm B, and 23% in arm C |
| Amaria et al.41 | Amaria | 23 | Phase II | Arm A: Neoadjuvant Nivo 3mg/kg i.v. q2wks × 4 doses, followed by adjuvant Nivo 3 mg/kg IV q2wks × 13 doses Arm B: Neoadjuvant Nivo 1 mg/kg + Ipi 3 mg/kg q3wks × 3 doses, followed by adjuvant Nivo 3 mg/kg i.v. q2wks × 13 doses |
• Arm A: 25% pCR and 25% radiological response rate • Arm B: 45% pCR and 73% radiological response rate • Grade 3 adverse events: 8% in arm A versus 73% in arm B • Higher total tumor mutational burden associated with response • Responders had higher pre-existing T cell clonality |
| Neoadjuvant targeted therapy | |||||
| Menzies et al.42 | Menzies | 35 | Phase II, single arm | Dabrafenib + trametinib × 12 wks before surgery, followed by dabrafenib + trametinib × 40 wks | • 17/33 (52%) had pCR, 16% (48%) had RECIST CR and 16 (48%) had metabolic CR. |
| Amaria et al.43 | Combi-Neo, Amaria | 21 | Phase II, double arm | Arm A: 7 pts- surgery + SOC adjuvant therapy Arm B: 14 pts- Neoadjuvant dabrafenib + trametinib for 8 wks, adjuvant dabrafenib + trametinib for 44 wks |
• Median event free-survival 19.7 months (arm B) versus 2.9 months (arm A) • pCR rate of 58% and pathological partial response rate of 17% • Lower pERK concentration at baseline predictive of pCR. • Increased expression of TIM-3 and LAG-3 in baseline biopsies of non-pCR patients |
| Blankenstein et al.44 | Reductor trial, Blankenstein | 17 | Phase II | Dabrafenib + trametinib for 8 wks before surgery | • Complete R0 resections: 13 patients (93%) • Median RFS of 9 months in patients undergoing surgery after median follow-up time of 22 months • 6 pCR, 5pPR • 1-year and 2-year OS rate of 88% and 59% |
| Neoadjuvant oncolytic viral therapy | |||||
| Andtbacka et al.45 | Andtbacka | 150 | Phase II, double arm | Arm A: 6 cycles of neoadjuvant T-VEC followed by surgical resection Arm B: upfront surgical resection |
• pCR rate of 21% and OR rate (CR + PR) of 14.7% in arm A • 11 patients in arm A had progressive disease before surgery |
| Neoadjuvant biochemotherapy | |||||
| Buzaid et al.46 | Buzaid | 64 | Phase II single arm |
2–4 (3 weeks) cycles D1–4: Cisplatin 20 mg/m2 i.v.; vinblastine 1.5 mg/m2 i.v.; Interleukin-2 9 MIU/m2/day continuous i.v. D1 only: Dacarbazine 800 mg/m2 i.v. D1–5: Interferon-α2a 5 MU/m2 s.c. |
• High tumor response rates seen • BCT was abandoned with the failure to demonstrate survival benefits in randomized trials of metastatic disease |
| Gibbs et al.47 | Gibbs | 48 | Phase II single arm |
2 (3 weeks) cycles D1–4: Cisplatin 20 mg/m2 i.v.; vinblastine 1.6 mg/m2 i.v.; Interleukin-2 9 MIU/m2/day continuous i.v. D1 only: Dacarbazine 800 mg/m2 i.v. D1–5: Interferon-α2a 5 MU/m2 s.c. |
|
| Neoadjuvant chemotherapy | |||||
| Shah et al.48 | Shah | 19 | Phase II single arm |
2 (8 weeks) cycles Temozolomide 75 mg/m2/day × 6 weeks then 2 weeks off |
Limited clinical activity |
Ipi, ipilimumab; i.v., intravenous; LAG-3, lymphocyte-activation gene 3; MDSC, myeloid-derived suppressor cells; MU/m², million units/meter square; Nivo, nivolumab; OR, overall response; pCR, pathologic complete response; Pembro, pembrolizumab; pERK, phosphorylated extracellular signal-regulated kinases; PR, partial response; pSTAT1, phosphorylated signal transducer and activator of transcription 1; QxW, x times a week; qxwks, every x weeks; RFS, recurrence-free survival; s.c., subcutaneous; SOC, standard of care; TAP2, transporter 2, ATP binding cassette subfamily B member; TIL, tumor infiltrating lymphocytes; TIM 3, T cell immunoglobulin and mucin-domain containing-3; TIW, three times per week; T-VEC, talimogene laherparepvec; wks, weeks.
Neoadjuvant immunotherapy for locally/regionally advanced melanoma
Immunity in melanoma is important for disease control in the adjuvant, neoadjuvant, and advanced disease stages. Spontaneous regression of melanoma has been noted, supporting a role for host immunity, also indirectly supported by the presence of lymphoid infiltrates at primary melanoma associated with tumor regression.25 Host cellular immune response within the tumor has potential prognostic and predictive significance. T cell infiltrates are prognostic of disease outcome in primary melanoma,26 and in melanoma metastatic to regional lymph nodes.27–29 Furthermore, T cell infiltrates within regional nodal metastasis are associated with clinical benefit from neoadjuvant interferon-α therapy.27,30,31 These characteristics of melanoma have long supported the testing of systemic immunotherapy in its management, translating important findings into the clinic including therapy with interferon-α (adjuvant), interleukin (IL)-2, various tumor vaccination strategies, adoptive cell therapy, oncolytic viral therapy, and immune checkpoint inhibitors. When considering the operable versus advanced inoperable disease, the quality of the host immune response seems to differ. Considering that T helper type 1 (Th1)-type CD4+ antitumor T cell function appears necessary for the induction and maintenance of antitumor cytotoxic T lymphocyte (CTL) responses in vivo, and Th2- or Th3/Tr-type CD4+ T cell responses may subvert Th1-type cell mediated immunity providing a microenvironment favorable to disease progression, patients with melanoma have been noted to display strong tumor antigen specific Th2-type polarization. On the other hand, melanoma patients who were disease free following therapy demonstrate either weak mixed Th1-/Th2-type or strongly polarized Th1-type responses to the same epitopes.32 Therefore, factors of host immune tolerance that seem to impede advanced disease therapy, may be less pronounced in the high-risk operable setting, where the host may be more susceptible to immunologic interventions supporting a potential important therapeutic role for immunotherapy in this setting. Interestingly, preclinical studies have suggested improved efficacy with neoadjuvant immunotherapies in eradicating distant metastases following primary tumor resection in breast cancers in mice models as compared with adjuvant immunotherapy treatment.33
Neoadjuvant immunotherapy studies
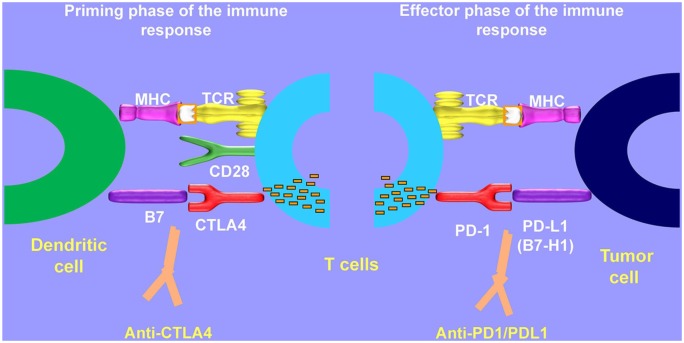
Checkpoint blockade-based immunotherapy has certainly been the most exciting advancement in cancer treatment. Checkpoint molecules such as PD-1, PD-L1, and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) block immunologic signals and prevent T cells from attacking the tumor cells. Therapeutic antibodies blocking CTLA-4 or PD-1 unleash an immune response against tumor cells by interfering with the T cell priming and effector phases, reactivating T cell proliferation and activity (Figure 1).49 Neoadjuvant immunotherapy for melanoma has been investigated extensively in the recent past following success in the metastatic settings, and several important findings have been demonstrated. Therapies studied to date include HDI, ipilimumab, the combination of HDI with ipilimumab or pembrolizumab as well as combination of ipilimumab and nivolumab. These studies have provided a model for later neoadjuvant immunotherapy studies in this disease.
Figure 1.
Mechanism of CTLA 4 and PD-1/PD-L1 inhibition.
Neoadjuvant HDI was tested in a study of melanoma patients with palpable regional lymph node metastases either presenting with clinical IIIB-C disease (AJCC 7th edition) or with recurrent regional lymphadenopathy.30 Twenty patients received standard intravenous (i.v.) HDI (20 million units/m2, 5 days per week) for 4 weeks followed by therapeutic lymphadenectomy. After recovery from surgery, maintenance subcutaneous HDI (10 million units/m2 3 times per week) was administered for 48 weeks. Objective clinical and radiologic responses were seen in 11 patients (55%) including three patients (15%) that had a complete pathologic response (pCR) on histologic assessment. At a median follow up of 18.5 months (range, 7–50 months), only 10 patients had no evidence of recurrent disease. By comparison, in treating advanced inoperable stage IV melanoma, response rates of less than 20% were reported, although a small number of patients were reported to have durable responses ranging from 26 to more than 30 months.50 Mechanistic studies revealed the ability of HDI to upregulate pSTAT1, while downregulating pSTAT3 and total STAT3 levels in both tumor cells and lymphocytes. Higher pSTAT1/pSTAT3 ratios in pretreated tumor cells were associated with longer OS (p = 0.032).51 Further, it was noted that responders to treatment had significantly greater increases in endotumoral CD11c+ and CD3+ cells and significantly greater decreases in endotumoral CD83+ cells compared with nonresponders.
Another study tested neoadjuvant ipilimumab with the goal to evaluate its safety and toxicity in locally/regionally advanced melanoma and define markers of activity in blood and in the tumor microenvironment.34,35 Ipilimumab was administered at 10 mg/kg IV every 3 weeks for two doses followed by surgery. Two additional doses of ipilimumab at 10 mg/kg were offered after recovery from surgery in the absence of previous dose-limiting toxicities. A total of 35 patients were enrolled: stage IIIB (3; N2b), stage IIIC (32; N2c, N3), stage IV (2). Worst toxicities included grade 3 diarrhea/colitis (5; 14%), hepatitis (2; 6%), rash (1; 3%), and elevated lipase (3; 9%). Median follow up was 18 months: among 33 evaluable patients, the preoperative radiologic assessment by positron emission tomography–computed tomography (PET-CT) scans 6–8 weeks after the initiation of ipilimumab revealed three patients (9%) with an objective response [two complete response (CR) and one partial response (PR)]. A total of 21 patients (64%) had stable disease and 8 patients (24%) had disease progression by PET-CT. Median RFS was 11 months (95% CI = 6.2–19.2). With respect to biomarker analysis, a greater decrease in the monocyte gate MDSC (Lin1-/HLA-DR-/CD33+/CD11b+) was associated with improved RFS (p = 0.03). In addition, lower baseline levels of circulating regulatory T cells (Tregs, CD4+CD25hi+CD39+) correlated with a better RFS (p = 0.04).52 Baseline IL-17 correlated with later development of grade 3 diarrhea/colitis. In tumor, there was a significant increase in CD8+ T cells after ipilimumab treatment (p = 0.02). Ipilimumab induced increased tumor infiltration by fully activated (CD69+) CD3+/CD4+ and CD3+/CD8+ T cells with evidence of induction/potentiation of memory T cells (CD45RO+). The change in Tregs observed within tumor showed an inverse relationship with clinical benefit while a greater decrease in tumor myeloid-derived suppressor cell (MDSC) subsets (Lin1-/HLA-DR-/CD33+/CD11b+) was associated with improved RFS at 1 year. Gene expression profiling performed on the tumor biopsies of 27 patients identified biologically relevant pathways enriched with immune related genes that are significantly associated with clinical outcome.53 The association of the immune related gene signature with clinical benefit was consistent when tested at baseline and on treatment and across multiple clinical endpoints tested.
Given the improvements in clinical outcome seen with HDI and ipilimumab as monotherapy in the neoadjuvant setting, it was hypothesized that the combination of neoadjuvant ipilimumab and interferon-α would lead to more durable antitumor responses based on synergistic antitumor immune mechanisms. Interferon α is known to mount a potent pro-inflammatory (Th1 polarized) immune response that can be suppressed by host immune suppressive elements including CTLA-4; the addition of a CTLA-4 blocker may release inhibitory influences on activated CD4+ and CD8+ effector T cells, leading to a more effective antitumor response. The results of a phase II trial testing neoadjuvant ipilimumab (3 or 10 mg/kg) and HDI in patients with locally/regionally advanced melanoma were published in 2018.36 A total of 30 patients were included in the trial and were randomized to receive ipilimumab 3 or 10 mg/kg every 3 weeks for two doses bracketing definitive surgery and HDI was given concurrently. Favorable outcomes were achieved at both doses of ipilimumab with an overall pCR rate of 32% and a preoperative radiological response rate of 36%. In terms of safety, toxicities were consistent with the known profiles of both ipilimumab and HDI, and a higher rate of grade 3/4 immune related adverse events was observed with ipilimumab at 10 mg/kg as compared with ipilimumab 3 mg/kg. Immunosequencing of T cell receptor β chains revealed that neoadjuvant therapy was associated with a significant increase in tumor and peripheral blood mononuclear cell (PBMC) clonality following treatment, and this correlated with improved clinical outcomes. Thus, the trial supported the use of the combination regimen in future studies with the lower doses of ipilimumab.
Efficacy and safety of neoadjuvant pembrolizumab monotherapy was assessed in patients with resectable stage IIIB/C or stage IV melanoma.37 A total of 27 patients received a single dose of 200 mg i.v. of pembrolizumab followed by curative-intent surgical resection 3 weeks later; adjuvant pembrolizumab therapy at the same dose was continued for up to 1 year. At the 3-week resection time point, 8 out of 27 patients (30%) had a complete (no residual tumor, n = 5) or near-complete (<10% viable tumor, n = 3) pathologic response and all 8 patients remained recurrence free at a median follow up of 25 months post-surgical intervention. Further, the disease-free survival rate and OS rate at 2 years was 63% and 93%, respectively. Treatment was shown to have acceptable toxicity with grade 3 adverse events noted in six patients and no grade 4 adverse events or delay in surgical management owing to toxicity. In addition, tumor infiltrating lymphocyte (TIL) infiltration after the single neoadjuvant dose was associated with both clinical and pathologic response. Patients with brisk TILs achieved 1-year RFS of 89% as compared with 27% with nonbrisk TILs, and all patients with pCR or near-pCR had brisk TILs at the time of surgical resection. Further, translational studies using paired patient samples revealed that there was robust increases in a subset of exhaustive CD8 T cells in the blood at day 7 and in the tumors at 3 weeks after initiation of neoadjuvant treatment and it correlated with pathologic and clinical responses. The investigators concluded that such rapidity and robustness in expansion of a subset of CD8 T cells in responders support the hypothesis that therapeutic benefit from PD-1 blockade is probably due to the presence of pre-existing T cell responses where previously primed T cells became exhausted and PD-1 blockade led to reinvigoration of exhausted-phenotype CD8 T cells.
The combination of neoadjuvant pembrolizumab at 200 mg and HDI for locally/regionally advanced melanoma has also been tested, and interim data of the phase I/II trial were presented recently.38 Out of the 20 evaluable patients, the radiological preoperative response rate was 65% and the pCR rate was 35%, demonstrating a promising clinical activity of the combination. The most common grade 3–4 toxicities were fatigue, elevation of serum creatine phosphokinase and decrease in phosphates, and most patients required dose a reduction or discontinuation of HDI. Longer follow-up results are currently underway to define the long-term benefits of this combination regimen and define the biomarkers of response and toxicity.
With the success of the combination of nivolumab and ipilimumab in advanced stages of disease,54 various studies are evaluating different protocols to optimize neoadjuvant combination therapy with ipilimumab and nivolumab. In the phase I OpACIN trial, 20 melanoma patients with palpable nodal disease (stage IIIB-C, AJCC 7th edition) received the combination of ipilimumab 3 mg/kg and nivolumab 1 mg/kg, either as four courses of postoperative adjuvant therapy alone or two courses of neoadjuvant therapy followed by two courses of postoperative adjuvant therapy.39 Neoadjuvant immunotherapy led to tumor response in 7/9 evaluable patients [3 pCR, 3 near pCR (⩽10% viable tumor cells) and 1 pathologic partial response (defined as ⩽50% viable tumor cells)]. However, grade 3–4 adverse events were observed in 90% of patients in each arm of the study leading to the premature discontinuation of the planned doses in the study. In addition, studies for biomarker assessment revealed that neoadjuvant immunotherapy treatment led to a greater expansion of tumor-resident T cell clones in the peripheral blood as compared with adjuvant treatment.
In follow up to the results of OpACIN trial, a reduced-dose regimen of the combination neoadjuvant ipilimumab and nivolumab was evaluated in the phase II trial OpACIN-neo trial.40 Patients with resectable stage III melanoma were randomized into three neoadjuvant arms (arm A: two doses of ipilimumab 3 mg/kg + nivolumab 1 mg/kg every 3 weeks; arm B: two doses of ipilimumab 1 mg/kg + nivolumab 3 mg/kg every 3 weeks; and arm C: two doses of ipilimumab 3 mg/kg every 3 weeks followed immediately by two doses of nivolumab 3 mg/kg every 2 weeks). All patients were scheduled to undergo complete lymph node dissection at week 6. Grade 3/4 toxicity was seen in 40%, 20%, and 50% of patients in arm A, B, and C, respectively. Arm C was closed early because of toxicity concerns. Further, it was noted that grade 3/4 toxicity of arm A (40%) was significantly lower as compared with the previous trial utilizing the same dosing combination (90%), after reduction in treatment courses from four to two. With respect to responses, among 30 patients in arms A and B and 26 patients in arm C, there was an 80% pathologic response rate with a 47% pCR observed in arm A, 77% pathologic response rate with 57% pCR observed in arm B and 65% pathologic response rate with 23% pCR observed in arm C. Two courses of ipilimumab 1 mg/kg and nivolumab 3 mg/kg (Arm B) was identified as the most optimal combination regimen in terms of safety while maintaining a high response rate.
Another phase II trial reported the results of neoadjuvant nivolumab with or without ipilimumab, followed by adjuvant nivolumab in the management of patients with high-risk, resectable clinical stage III or oligometastatic stage IV melanoma.41 A total of 23 patients were randomized in a 1:1 ratio to receive either nivolumab 3 mg/kg for four doses or combination of nivolumab 1 mg/kg and ipilimumab 3 mg/kg for three doses before definitive surgery. All patients who underwent surgery were further offered nivolumab 3 mg/kg for 13 doses every 2 weeks. Combined treatment with neoadjuvant nivolumab and ipilimumab achieved higher response rates [RECIST objective response rate (ORR) 73%, pCR 45%] as compared with neoadjuvant nivolumab alone treatment (ORR 25%, pCR 25%). While all patients in the combination cohort underwent definitive surgical resection, two patients receiving neoadjuvant nivolumab therapy were unable to undergo surgery due to development of synchronous metastatic disease as well as local progression. As expected, patients in the combination arm experienced greater toxicity as compared with the monotherapy arm (incidence of grade 3 treatment related adverse events was 73% in the combination arm versus 8% in the monotherapy arm), causing dose delays in 64% of the patients in the combination arm. Nevertheless, treatment with combined checkpoint blockade was found to be more clinically efficacious, supporting the rationale for further evaluation of combination immunotherapy agents in the neoadjuvant setting. Mechanistic studies performed on blood and tumor samples revealed several important insights. As previously observed in the metastatic setting,55 higher total mutational burden was associated with benefit from neoadjuvant immunotherapy treatment in this study. Further, immune profiling of tumor samples showed that the presence of higher CD8+ T cell infiltrate, tumor cell PD-L1 expression, and expression of various lymphoid markers including granzyme B, CD4, FoxP3, and PD-1 were associated with antitumor response. T cell receptor sequencing of tumor tissue revealed that responders to neoadjuvant therapy had higher clonality at baseline as well as greater expansion of the T cell repertoire following neoadjuvant therapy.
Neoadjuvant targeted therapy for locally/regionally advanced melanoma
Therapies targeting the products of driver oncogenic mutations in melanoma have led to important advances in disease control and the OS of patients with metastatic melanoma.56,57 A series of phase I, II, and III trials demonstrating efficacy successfully led to the regulatory approval of six mitogen-activated protein (MAP) kinase inhibitors (vemurafenib, dabrafenib, encorafenib, trametinib, cobimetinib, and binimetinib) for the management of advanced inoperable melanoma.58–62 Early case reports utilizing neoadjuvant vemurafenib and dabrafenib documented promising results in patients with operable stage III melanoma.63,64 Neoadjuvant dabrafenib and trametinib combination have been tested in a number of phase II trials.42–44 In a phase II trial, neoadjuvant dabrafenib and trametinib targeted therapy was administered to 35 patients with bulky stage IIIB/C BRAF V600E/K mutated melanoma.42 Standard dose of dabrafenib and trametinib was given for 12 weeks prior to therapeutic lymphadenectomy followed by 40 weeks as adjuvant therapy. At the time of surgical evaluation, 17 patients had achieved pCR (52%), and 16 had a residual CR (48%). No progression of disease was observed during the neoadjuvant therapy period. At a median follow-up period of 12.1 months (95% CI 8.8–14.8 months), recurrence of disease was observed in 12 patients (36%), with 4 patients having recurrence while undergoing adjuvant targeted therapy.
Similarly, a recently reported trial demonstrated safety and efficacy of neoadjuvant and adjuvant dabrafenib and trametinib combination therapy in comparison with standard of care (SOC) surgery in patients with surgically resectable stage III or oligometastatic stage IV BRAFV600E or BRAFV600K mutated melanoma.43 A total of 21 patients were randomized in a 1:2 ratio to either upfront surgery and consideration for standard adjuvant therapy (SOC group) or neoadjuvant dabrafenib plus trametinib therapy for 8 weeks followed by surgery and up to 44 weeks of adjuvant dabrafenib plus trametinib therapy. Patients who received neoadjuvant and adjuvant targeted therapy had a 60-fold reduced risk of relapse after surgery in comparison with patients who underwent SOC surgery (median event-free survival was 19.7 months versus 2.9 months, respectively, hazard ratio 0.016, p < 0.0001), and the trial was stopped early because it reached the primary objective of improved RFS with neoadjuvant treatment. In the group receiving neoadjuvant dabrafenib and trametinib, the overall radiographic response was seen in 11 (85%) of 13 evaluable patients (2 CR, 9 PR, and 2 stable disease), and all 13 patients achieved radiographic disease control. Among the 12 patients in the neoadjuvant therapy group who underwent surgery, 7 (58%) achieved pCR and 2 (17%) achieved a pathological partial response. Patients with a pCR had significantly longer distant metastatic-free survival. Despite limitations of the number of patients studied, the trial provided an important proof of concept to support the rationale for the use of neoadjuvant targeted therapy in patients with high-risk, surgically resectable melanoma with an inherited BRAF mutation. The data also showed a manageable toxicity profile of the combination regimen, similar to that reported in studies of patients with metastatic melanoma, with fever and chills being the most common adverse events. Biomarker studies performed in this trial provided valuable insights for understanding the determinants of tumor drug response. Molecular characterization revealed that patients achieving a pCR had significantly lower baseline pERK positivity of nonviable melanoma within the sampled tissue as compared with patients not achieving pCR. Interestingly, expression of TIM-3 (T cell immunoglobulin and mucin-domain containing-3) and LAG-3 (lymphocyte-activation gene 3) was significantly increased on T cells within baseline tumors that did not achieve a pCR compared with tumors with pCR. This may support the notion of adding checkpoint-inhibitor immunotherapy to neoadjuvant dabrafenib and trametinib targeted therapy in an effort to augment responses and delay relapse by overcoming checkpoint mediated T cell resistance mechanisms.
Neoadjuvant oncolytic viral therapy
Talimogene laherparepvec (T-VEC) is a genetically modified herpes simplex virus 1 that specifically infects and replicates in human tumor cells. It is the first oncolytic viral therapy approved by the FDA for the treatment of advanced melanoma based on the results of the phase III OPTiM trial.65 The clinical utility of single agent T-VEC was also explored in the neoadjuvant settings for patients with resectable stage IIIB/C and IVM1a melanoma in an effort to improve local control rate and reduce the incidence of distant metastasis.45 In this phase II trial, 150 patients were randomized to either immediate surgical resection or six doses of neoadjuvant T-VEC for up to 12 weeks, followed by surgical resection. Interim data released demonstrated promising clinical response of T-VEC with pCR rate of 21% in patients that underwent surgery, and no unexpected toxicities were noted. Negative margin resection rate was 56.1% in the T-VEC arm and 40.6% in the surgical resection alone arm. However, 11 patients in the T-VEC study arm showed disease progression before planned surgical resection. The primary analysis of RFS has not yet matured.
Neoadjuvant chemotherapy in melanoma
Neoadjuvant chemotherapy with temozolomide (TMZ) showed clinical activity that was not different from what is expected with metastatic melanoma and, therefore, did not support further development of this regimen. In a phase II study of neoadjuvant TMZ in patients with surgically resectable but locally/regionally advanced melanoma,48 TMZ was given orally at 75 mg/m2/day for 6 weeks of every 8-week cycle. A total of two cycles were given preoperatively. The study yielded a response rate of 16%, including 1 PR and 2 CR. Four patients had stable disease and 12 progressed before surgery.
Neoadjuvant biochemotherapy in melanoma
Neoadjuvant biochemotherapy (BCT) as tested in locally/regionally advanced but operable melanoma demonstrated high tumor response rates approaching 50%, including pCRs. However, BCT was eventually abandoned with the failure of this regimen to demonstrate OS benefits in randomized trials of metastatic melanoma when compared with chemotherapy alone. Patients with surgically resectable local/regional metastases of cutaneous melanoma (stage III, AJCC 7th edition; nodal, satellite/in-transit metastases, or local recurrence) were treated neoadjuvantly in a study testing the combination of cisplatin, vinblastine, dacarbazine, IL-2, and interferon-α2a.46 A total of 65 patients received 2–4 cycles of BCT preoperatively and postoperatively; 2 additional courses were provided to patients who experienced tumor response after preoperative courses. Overall, the partial response rate was 43.5% and pCR rate was 6.5%, with an ORR of 50%. Patients with pCR were noted to have a significantly lower tumor burden (p = 0.02).46 Another phase II study utilized a similar BCT regimen to the prior neoadjuvant study. A total of 48 patients were enrolled. Two cycles of BCT were administered prior to and after therapeutic lymphadenectomy. The ORR was 38.9%, including 13 (36.1%) PR and 1 (2.8%) CR. Four patients (11.1%) were reported to have pCR. After a median follow up of 31 months, 38 of the 48 patients (79.2%) were alive and 31 (64.6%) were free of melanoma recurrence.47
Neoadjuvant radiation therapy in combination with checkpoint blockade in melanoma
Traditionally, melanoma cells were considered resistant to radiation therapy, with its role largely limited to palliative settings in advanced melanoma or adjuvant therapy after surgical resection. However, increased understanding of the intricacies of the tumor immune system have unfolded mechanisms underlying the immunomodulatory effects of radiation therapy. Radiotherapy can act as an in situ vaccine stimulating a robust antitumor CD8+ T cell response by releasing endogenous adjuvants while upregulating and liberating tumor neoantigens.66–68 These immune-stimulatory effects of radiotherapy are enhanced by checkpoint blockade and occasionally lead to tumor responses outside the radiation field.69–71 Known as the abscopal effect, this phenomenon has been observed in different tumor types including melanoma.72 A number of clinical trials are currently investigating relationship between radiation therapy and immunotherapy in advanced melanoma.73,74 The results of these trials, so far, have been mixed in terms of efficacy, likely secondary to a heterogeneous tumor population and poor patient selection.67,74,75 They all have, however, demonstrated safety with a suggestion of synergism between the two modalities (radiotherapy and checkpoint blockade).67,74,75 A retrospective study by Doyen et al. showed encouraging clinical outcomes with concurrent multisite radiotherapy and immune checkpoint inhibitors with a 1 year PFS and OS rates of 46.2% and 58.4%, respectively.76 Important data from Twyman et al., although primarily preclinical, has shown that radiotherapy and dual-checkpoint blockade activate nonoverlapping immunologic mechanisms with checkpoint blockade promoting T cell expansion and radiotherapy broadening the T cell receptor diversity.67 No published data exist to evaluate the effect of neoadjuvant radiotherapy with checkpoint blockade in melanoma, but the rationale is clear: an intact tumor would provide a reservoir of neoantigens and adjuvants to activate/reinvigorate antitumor T cells. These activated cytotoxic T cells may then help control the primary tumor while also migrating to and eliminating any micrometastatic disease within draining nodes or at distant sites.77 If the tumor is removed prior to radiotherapy than the potential supply of neoantigens/adjuvants are also eliminated, and the likelihood of an abscopal response is reduced.78
Ongoing and planned neoadjuvant studies in melanoma
Several neoadjuvant studies in melanoma are either ongoing or are planned to be initiated in the near future. These include neoadjuvant targeted therapy with BRAF/MEK inhibitor combinations, neoadjuvant immunotherapy and their combinations. A list of ongoing clinical trials is given in Table 2.
Table 2.
Ongoing trials evaluating various neoadjuvant therapies for locally/regionally advanced melanoma.
| Study name, phase | ClinicalTrials.gov identifier | Neoadjuvant treatment | Study population | Outcomes |
|---|---|---|---|---|
| Neoadjuvant immunotherapy | ||||
| NeoPembroMel, phase II | NCT02306850 | Neoadjuvant pembro 200 mg for ⩾24 wks, then adjuvant pembro up to 2 years | 15 | Response rate, resectability rate |
| Phase II | NCT02519322 | Arm A: Nivo 3 mg/kg for q2wks for 4 doses, followed by surgery and adjuvant Nivo 3 mg/kg q2wks for 13 doses Arm B: Nivo 1 mg/kg + ipi 3 mg/kg q3wks for 3 doses followed by surgery and adjuvant Nivo 3 mg/kg q2wks for 13 doses Arm C: Nivo 480 mg + relatlimab 160 mg q4wks for 2 doses followed by surgery. Adjuvant Nivo 480 mg + relatlimab 160 mg q4wks for 10 doses |
53 | Response rate, survival, immunologic response |
| PRADO trial, phase II | NCT02977052 | 2 doses of Nivo 3 mg/kg + ipi 1 mg/kg q3wks for 6 weeks followed by resection of the index lymph node | 100–110 | Pathological response rate, relapse-free survival at 24 months |
| Neoadjuvant targeted therapy | ||||
| Phase II | NCT02036086 | Neoadjuvant Vemurafenib 960 mg BD oral + cobimetinib 60 mg QID for 8 weeks followed by adjuvant combination therapy for 1 year | 20 patients with BRAF V600 mutant melanoma with palpable lymph node metastasis | Resectability post neoadjuvant therapy, response rates, safety |
| NEO-VC, phase II | NCT02303951 | Neoadjuvant Vemurafenib 960 mg BD oral + cobimetinib 60 mg 21/7 oral | 110 patients with hardly resectable/unresectable stage III/IV melanoma with BRAF V600 mutation | Operability at 18 weeks |
| Combination of neoadjuvant immunotherapy and targeted therapy | ||||
| NeoTrio, phase II | NCT02858921 | 3 arms with neoadjuvant therapy for 12 wks and adjuvant therapy for 40 wks Arm A: Dabrafenib + trametinib for 2 wks, followed by 4 pembro doses until wk 12. Adjuvant pembro 3 weekly Arm B: Dabrafenib + trametinib + pembrolizumab for 12 wks bracketing surgery Arm C: Pembro alone bracketing surgery |
60 patients BRAF V600 stage III melanoma | Tumor response, safety, biomarker analysis |
| NeoACTIVATE, phase I |
NCT03554083 | Arm A: Neoadjuvant Atezo + cobimetinib, followed by adjuvant Atezo Arm B: Neoadjuvant Atezo + cobimetinib + Vemurafenib followed by adjuvant Atezo |
High-risk stage III BRAF (mutant) and BRAF (wildtype) melanoma | Response rate, safety, biomarker assessment |
| Combination of neoadjuvant immunotherapy and oncolytic therapy | ||||
| Neo-NivoHF10, phase II | NCT03259425 | Nivo 240 mg q2wks for 7 doses + 9 injections of HF10 for 12 wks, followed by surgery and adjuvant Nivo 480 mg q4wks for up to 1 year | Resectable Stage IIIB, IIIC, IVM1a Melanoma | Response rate, survival |
Atezo, atezolimumab; Ipi, ipilimumab; Nivo, nivolumab; qxwks, every x weeks; Pembro, pembrolizumab; TIW, three times per week; wks, weeks.
Combination immunotherapy studies utilizing checkpoint inhibitors have shown significant results in treating metastatic melanoma, primarily CTLA4 and PD1 blockade as well as other studies leading to ongoing randomized trials.79–83 A number of combination immunotherapy studies are ongoing based on nonredundant immune activation and T cell differentiation mechanisms.80 Studies of other novel immune checkpoint modulators targeting IDO-1, CD40, OX40, CD137, TIM3, and LAG-3 among others are also ongoing.84 This is in addition to a number of combination studies testing checkpoint inhibitors and other proinflammatory cytokines such as NKTR, NHS-IL12, recombinant human IL-12, and IL-15. More recently, these immunotherapeutic agents and combinations are being translated into the neoadjuvant setting as well. A randomized phase II trial (ClinicalTrials.gov identifier: NCT02519322) is evaluating neoadjuvant and adjuvant nivolumab with or without ipilimumab or anti-LAG-3 antibody relatlimab for the treatment of patients with resectable stage IIIB–IV melanoma. Another phase II neoadjuvant trial (ClinicalTrials.gov identifier: NCT03259425) is investigating safety and efficacy of the combination of nivolumab and intratumoral HF10 oncolytic viral therapy in resectable stage IIIB, IIIC, and IVM1a melanoma. Other combinations of checkpoint inhibitors may emerge depending on the clinical efficacy and low-toxicity data of new combinations.
In the phase II, PRADO extension study (ClinicalTrials.gov identifier: NCT02977052), the preferred combination regimen of OPACIN-neo trial (1 mg/kg + NIVO 3 mg/kg, 2 doses for 6 weeks) is being studied in around 100 patients to confirm its pathological response rate and toxicity.85 In addition, it addresses the relevance of undergoing therapeutic lymph node dissection and subsequent adjuvant therapy in patients who achieve pathological response in their index node after neoadjuvant therapy.
Triplet therapy with the combination of anti-PD1/PD-L1 inhibitors and BRAF and MEK inhibitors has shown encouraging clinical activity in early phase clinical trials for patients with BRAF-mutated advanced melanoma, and this approach represents a new potential strategy for improving clinical outcomes without significant increase in treatment-related toxicity.86 Various preclinical studies have confirmed that the treatment with BRAF and MEK inhibitors is associated with favorable effects on the tumor microenvironment, including increased tumor antigen expression, increased T cell infiltration at the site of tumor with improved T cell function, reduction in immunosuppressive cytokines, and upregulation of PD-L1 on tumor cells.87–89 In the clinical trial of neoadjuvant dabrafenib plus trametinib in patients with surgically resectable BRAF mutant melanoma, transcriptional profiling of baseline and early on-treatment tumor samples revealed strong upregulation of cytotoxic CD8+ T-cell genes in patients experiencing pCR, which was not seen in tumors from patients without a pCR.43 Thus, there is evidence to support the rationale of combinatorial trials using BRAF-MEK inhibitors with anti-PD-1 antibody inhibitors in the neoadjuvant settings. Several ongoing trials are evaluating the combination strategy, including the NeoTrio trial, which is evaluating sequential and concurrent combination of dabrafenib, trametinib, and pembrolizumab in patients with BRAF V600 mutant, stage IIIB-D resectable melanoma.90 Similarly, the NeoACTIVATE trial (ClinicalTrials.gov identifier: NCT03554083) is testing the combination of cobimetinib, vemurafenib and atezolizumab in patients with stage IIIB-D BRAF mutant melanoma. It is possible that the triplet combination therapy may represent a potential new SOC for patients with BRAF mutated melanoma, pending the outcome of ongoing phase III trials.
Selection of clinical trial endpoint in neoadjuvant studies
There has been increasing recognition among the oncology community to establish standard, quantifiable clinical trial endpoints to define the effectiveness of various therapeutic approaches in adjuvant and neoadjuvant settings in melanoma. In the adjuvant settings, OS was initially used as a primary endpoint for early trials, but the use of subsequent post-progression therapies in the metastatic settings potentially confounded the investigation of adjuvant therapies’ survival benefit. Thus, more recent adjuvant therapy trials have selected other markers of efficacy such as RFS. In breast cancer, use of pCR after neoadjuvant chemotherapy has been shown to act as an excellent prognostic marker that is significantly associated with durable disease control and favorable long-term survival outcomes.91–93 As a result, FDA has accepted the use of pCR as a primary endpoint in neoadjuvant breast cancer trials. It is hypothesized that in comparison with chemotherapy, use of pCR as a clinical endpoint with neoadjuvant immunotherapy trials is more likely to correlate with durable response and survival benefit in patients with melanoma. Recently, the study utilizing neoadjuvant ipilimumab and HDI combination therapy for patients with locally/regionally advanced melanoma showed that out of the 11 patients achieving pCR, 10 patients remained event free after a median follow-up interval of 32 months.36 With respect to targeted therapy, Amaria and colleagues noted similar observations on long-term follow up.43 It was reported that none of the patients who achieved pCR after undergoing treatment with neoadjuvant dabrafenib and trametinib developed distant metastatic disease while three (60%) out of five patients who did not achieve a pCR developed distant metastatic disease. Table 3 lists the likelihood of relapse in patients with pCR at the latest follow up in selected neoadjuvant trials with reported data. Finally, pooled analysis of six modern neoadjuvant clinical trials of immunotherapy and targeted therapy in melanoma, conducted across institutions participating in the International Neoadjuvant Melanoma Consortium showed that pCR correlated with improved RFS.94 In addition, pCR associated with immunotherapy studies were more durable than pCR associated with targeted therapy studies. Future evaluation of pCR as a primary endpoint in larger clinical trials may provide more robust evidence of the association of pCR with durable responses and survival and reinforce the use of pCR in clinical practice for neoadjuvant therapies in melanoma.
Table 3.
Comparison of relapse rate in patients with pCR and non-pCR in few selected trials of neoadjuvant therapy in locally/regionally advanced melanoma.
| Reference | Study | Time of surgical resection after neoadjuvant therapy initiation | pCR rate | Median follow-up time | Durability of pCR | Durability of non-pCR |
|---|---|---|---|---|---|---|
| Immunotherapy | ||||||
| Moschos et al.30 | Moschos, HDI | 4 weeks | 15% (3/20) | 18.5 months | Not given | Not given |
| Tarhini et al.34,35 | Tarhini, ipi mono | ⩾6 weeks | 0 | 42 months | Not applicable | 23/33 relapsed |
| Tarhini et al.36 | Tarhini, ipi + HDI | 6–8 weeks | 39% (11/28) | 32 months | 1/11 relapsed | 11/17 SD/PR/CR relapsed |
| Tarhini et al.38 | Tarhini, pembro + HDI | 6 weeks | 35% | 11 months | None relapsed | Not given |
| Blank et al.39 | OpAcin, blank, nivo + ipi | 6 weeks | 30% (3/10) | 25.6 months | None relapsed | 2/10 relapsed |
| Blank et al.40 | OpAcin neo, Arm A: Ipi (3 mg/kg) + Nivo (1 mg/kg) Arm B: Ipi (1 mg/kg) + Nivo (3 mg/kg) Arm C: Ipi (3 mg/kg) Q3W for 6 wks followed immediately by NIVO 3 mg/kg Q2W for 4 wks |
6 weeks | 47% in Arm A, 47% in Arm B, and 23% in arm C | 7.7 months | None relapsed | 9/21 relapsed |
| Amaria et al.41 | Amaria, nivo + ipi Arm A: Neoadjuvant Nivo 3 mg/kg i.v. q2wks × 4 doses, followed by adjuvant Nivo 3 mg/kg i.v. q2wks × 13 doses Arm B: Neoadjuvant Nivo 1 mg/kg + Ipi 3 mg/kg q3wks × 3 doses, followed by adjuvant Nivo 3 mg/kg i.v. q2wks × 13 doses |
8–9 weeks | Arm A- 25% pCR Arm B- 45% pCR |
15.6 months | None relapsed at 20.5 mon | 71% RFS at 16 mon |
| Targeted therapy | ||||||
| Menzies et al.42 | Menzies, dabrafenib + trametinib | 12 weeks | 17/33 (52%) had pCR | 12.1 months | 6 with pCR relapsed | 6 with non-pCR relapsed |
| Amaria et al.43 | Combi-Neo, Amaria, dabrafenib + trametinib | 8 weeks | 7/12 (pCR rate of 58%) | 18.6 months | 1 pt with pCR relapsed | 3 pts with non-pCR relapsed |
HDI, high-dose interferon-α2b; Ipi, ipilimumab; i.v. intravenous; Nivo, nivolumab; pCR, pathologic complete response; pembro, pembrolizumab; qxwks, every x weeks; QxW, x times a week.
Pathological assessment of resected melanoma specimens following neoadjuvant therapy
With pCR emerging as the primary endpoint in numerous neoadjuvant trials, it is critical to standardize approaches for pathologic tumor response assessment, in order to facilitate comparison of results across different clinical trials. The International Neoadjuvant Melanoma Consortium has prepared consensus guidelines for pathologic examination and reporting of surgical specimens from AJCC (8th edition) stage IIIB/C/D or oligometastatic stage IV melanoma patients treated with neoadjuvant anticheckpoint immunotherapy or targeted therapies.95 A criteria for quantifying the extent of pathologic response has been defined in the tumor beds including pCR (complete absence of residual viable tumor), major pathologic response/near-pCR (<10% of viable tumor), partial pathologic response (<50% of viable tumor), and pathologic non-response (>50% of viable tumor). Further, histopathologic patterns of specimens after neoadjuvant treatment have also been provided in these guidelines.
Conclusion
Neoadjuvant therapy would be ideally implemented in the management of melanoma in patients with locally advanced disease with the goal of allowing definitive surgical resection with minimum complications, reducing the risk of recurrence and prolonging melanoma-specific survival. Effective neoadjuvant therapy that may lead to a significant rate of pCRs may minimize the extent of surgery needed. In providing access to biospecimens before and after therapy, such studies are likely to provide notable mechanistic insights and biomarker findings that may have important and lasting impacts. In drug development, such studies may allow important insights into melanoma and its biological and immunologic response to the novel therapeutic regimens being tested.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Arjun Khunger, Zachary S. Buchwald, Mohammad K. Khan, and Keith A. Delman have no conflicts of interest to declare. Michael Lowe reports research funding from Vaccinex. Ahmad A. Tarhini declares consultancy roles with Bristol Myers Squibb, Merck, Novartis, Genentech-Roche, Array Biopharma, HUYA, Immunocore, and NewLink Genetics.
ORCID iDs: Michael Lowe  https://orcid.org/0000-0003-4853-7352
https://orcid.org/0000-0003-4853-7352
Ahmad A. Tarhini  https://orcid.org/0000-0002-3193-9702
https://orcid.org/0000-0002-3193-9702
Contributor Information
Arjun Khunger, Department of Hematology and Oncology, Cleveland Clinic Taussig Cancer Center, Cleveland, OH, USA.
Zachary S. Buchwald, Department of Radiation Oncology, Emory University School of Medicine, Atlanta, GA, USA
Michael Lowe, Department of Surgery, Emory University School of Medicine, Atlanta, GA, USA.
Mohammad K. Khan, Department of Radiation Oncology, Emory University School of Medicine, Atlanta, GA, USA
Keith A. Delman, Department of Surgery, Emory University School of Medicine, Atlanta, GA, USA
Ahmad A. Tarhini, Department of Hematology and Medical Oncology, Emory University School of Medicine, Winship Comprehensive Cancer Center, 1365 Clifton Rd Atlanta, GA 30322, USA.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67: 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manola J, Atkins M, Ibrahim J, et al. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern cooperative oncology group trials. J Clin Oncol 2000; 18: 3782–3793. [DOI] [PubMed] [Google Scholar]
- 4. Kirkwood J. Systemic cytotoxic and biologic therapy melanoma. In: Cancer principles and practice of oncology. 7th ed. Lippincott Williams & Wilkins, 1993, pp.1–16. [Google Scholar]
- 5. Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol 2010; 28: 3042–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American joint committee on cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19: 3635–3648. [DOI] [PubMed] [Google Scholar]
- 7. Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg 1981; 193: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American joint committee on cancer melanoma staging system. J Clin Oncol 2001; 19: 3622–3634. [DOI] [PubMed] [Google Scholar]
- 9. Balch CM, Urist MM, Karakousis CP, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann Surg 1993; 218: 262–267; discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol 2001; 8: 101–108. [DOI] [PubMed] [Google Scholar]
- 11. Karakousis CP, Balch CM, Urist MM, et al. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol 1996; 3: 446–452. [DOI] [PubMed] [Google Scholar]
- 12. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern cooperative oncology group trial EST 1684. J Clin Oncol 1996; 14: 7–17. [DOI] [PubMed] [Google Scholar]
- 13. Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 2000; 18: 2444–2458. [DOI] [PubMed] [Google Scholar]
- 14. Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol 2001; 19: 2370–2380. [DOI] [PubMed] [Google Scholar]
- 15. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016; 375: 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 2010; 102: 493–501. [DOI] [PubMed] [Google Scholar]
- 17. Oliver DE, Sondak VK, Strom T, et al. Interferon is associated with improved survival for node-positive cutaneous melanoma: a single-institution experience. Melanoma Manag 2018; 5: MMT02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–1835. [DOI] [PubMed] [Google Scholar]
- 19. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017; 377: 1813–1823. [DOI] [PubMed] [Google Scholar]
- 20. Eggermont AM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018; 378: 1789–1801. [DOI] [PubMed] [Google Scholar]
- 21. Estevez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res 2004; 10: 3249–3261. [DOI] [PubMed] [Google Scholar]
- 22. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859–866. [DOI] [PubMed] [Google Scholar]
- 23. Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; 359: 1727–1733. [DOI] [PubMed] [Google Scholar]
- 24. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol 1997; 15: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 25. Nathanson L. Spontaneous regression of malignant melanoma: a review of the literature on incidence, clinical features, and possible mechanisms. Natl Cancer lnst Monogr 1976; 44: 67–77. [PubMed] [Google Scholar]
- 26. Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 27. Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996; 74: 43–47. [PubMed] [Google Scholar]
- 28. Erdag G, Schaefer JT, Smolkin ME, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012; 72: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 2009; 106: 20429–20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol 2006; 24: 3164–3171. [DOI] [PubMed] [Google Scholar]
- 31. Hakansson A, Gustafsson B, Krysander L, et al. Tumour-infiltrating lymphocytes in metastatic malignant melanoma and response to interferon alpha treatment. Br J Cancer 1996; 74: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med 2002; 196: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016; 6: 1382–1399. [DOI] [PubMed] [Google Scholar]
- 34. Tarhini AA, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in locally/regionally advanced melanoma: clinical outcome and biomarker analysis. J Clin Oncol 2012: 76. [Google Scholar]
- 35. Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014; 9: e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tarhini A, Lin Y, Lin H, et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J Immunother Cancer 2018; 6: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019; 25: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarhini AA, Lin Y, Drabick JJ, et al. Neoadjuvant combination immunotherapy with pembrolizumab and high dose IFN-α2b in locally/regionally advanced melanoma. J Clin Oncol 2018; 36(Suppl. 5): 181. [Google Scholar]
- 39. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018; 24: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 40. Blank C, Rozeman E, Menzies A, et al. LBA42 OpACIN-neo: a multicenter phase II study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO). Ann Oncol 2018; 29(Suppl. 8): mdy424 052. [Google Scholar]
- 41. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018; 24: 1942. [DOI] [PubMed] [Google Scholar]
- 42. Menzies A, Gonzalez M, Guminski A, et al. 1220PDPhase 2 study of neoadjuvant dabrafenib+ trametinib (D+ T) for resectable stage IIIB/C BRAF V600 mutant melanoma. Ann Oncol 2017; 28(Suppl. 5). [Google Scholar]
- 43. Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol 2018; 19: 181–193. [DOI] [PubMed] [Google Scholar]
- 44. Blankenstein S, Rohaan M, Klop M, et al. High response rates after neoadjuvant cytoreductive treatment with BRAF/MEK inhibition of prior unresectable regionally advanced melanoma, REDUCTOR trial. Eur J Surg Oncol 2019; 45: e6. [DOI] [PubMed] [Google Scholar]
- 45. Andtbacka RHI, Dummer R, Gyorki DE, et al. Interim analysis of a randomized, open-label phase 2 study of talimogene laherparepvec (T-VEC) neoadjuvant treatment (neotx) plus surgery (surgx) vs surgx for resectable stage IIIB-IVM1a melanoma (MEL). J Clin Oncol 2018: 9508. [Google Scholar]
- 46. Buzaid AC, Colome M, Bedikian A, et al. Phase II study of neoadjuvant concurrent biochemotherapy in melanoma patients with local-regional metastases. Melanoma Res 1998; 8: 549–556. [DOI] [PubMed] [Google Scholar]
- 47. Gibbs P, Anderson C, Pearlman N, et al. A phase II study of neoadjuvant biochemotherapy for stage III melanoma. Cancer 2002; 94: 470–476. [DOI] [PubMed] [Google Scholar]
- 48. Shah GD, Socci ND, Gold JS, et al. Phase II trial of neoadjuvant temozolomide in resectable melanoma patients. Ann Oncol 2010; 21: 1718–1722. [DOI] [PubMed] [Google Scholar]
- 49. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018; 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirkwood JM, Ernstoff MS, Davis CA, et al. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Ann Intern Med 1985; 103: 32–36. [DOI] [PubMed] [Google Scholar]
- 51. Wang W, Edington HD, Rao UN, et al. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res 2007; 13: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 52. Retseck J, Nasr A, Lin Y, et al. Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma. J Transl Med 2018; 16: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tarhini AA, Lin Y, Lin HM, et al. Expression profiles of immune-related genes are associated with neoadjuvant ipilimumab clinical benefit. Oncoimmunology 2017; 6: e1231291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949. [DOI] [PubMed] [Google Scholar]
- 57. Jakob JA, Bassett RL, Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012; 118: 4014–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. [DOI] [PubMed] [Google Scholar]
- 60. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367: 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 62. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19: 603–615. [DOI] [PubMed] [Google Scholar]
- 63. Seremet T, Lienard D, Suppa M, et al. Successful (neo)adjuvant BRAF-targeted therapy in a patient with locally advanced BRAF V600E mutant melanoma. Melanoma Res 2015; 25: 180–183. [DOI] [PubMed] [Google Scholar]
- 64. Sondak VK, Khushalani NI. Adjuvant and neoadjuvant therapy in high-risk stage III cutaneous melanoma. Int J Radiat Oncol Biol Phys 2017; 98: 16–17. [DOI] [PubMed] [Google Scholar]
- 65. Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol 2010; 6: 941–949. [DOI] [PubMed] [Google Scholar]
- 66. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018; 24: 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Buchwald ZS, Wynne J, Nasti TH, et al. Radiation, immune checkpoint blockade and the abscopal effect: a critical review on timing, dose and fractionation. Front Oncol 2018; 8: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Buchwald ZS, Efstathiou JA. Immunotherapy and radiation - a new combined treatment approach for bladder cancer? Bladder Cancer 2015; 1: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014; 3: e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Escorcia FE, Postow MA, Barker CA. Radiotherapy and immune checkpoint blockade for melanoma: a promising combinatorial strategy in need of further investigation. Cancer J 2017; 23: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018; 36: 1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017; 23: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Doyen J, Picard A, Naghavi AO, et al. Clinical outcomes of metastatic melanoma treated with checkpoint inhibitors and multisite radiotherapy. JAMA Dermatol 2017; 153: 1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Demaria S, Formenti SC. Can abscopal effects of local radiotherapy be predicted by modeling T cell trafficking? J Immunother Cancer 2016; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018; 18: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol 2012; 30: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 2014; 312: 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014; 2: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005; 12: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012; 62: 309–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Reijers I, Rozeman EA, Menzies AM, et al. Personalized response-driven adjuvant therapy after combination ipilimumab and nivolumab in high-risk resectable stage III melanoma: PRADO trial. J Clin Oncol 2019; 37(Suppl. 15): TPS9605-TPS. [Google Scholar]
- 86. Ribas A, Hodi FS, Lawrence D, et al. 1216OKEYNOTE-022 update: phase 1 study of first-line pembrolizumab (pembro) plus dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma. Ann Oncol 2017; 28(Suppl. 5): mdx377.003–mdx377.003. [Google Scholar]
- 87. Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 89. Sanlorenzo M, Vujic I, Floris A, et al. BRAF and MEK inhibitors increase PD-1-positive melanoma cells leading to a potential lymphocyte-independent synergism with anti–PD-1 antibody. Clin Cancer Res 2018; 24: 3377–3385. [DOI] [PubMed] [Google Scholar]
- 90. Gonzalez M, Menzies AM, Saw R, et al. 1256TiPA phase II, randomised, open label study of neoadjuvant pembrolizumab with/without dabrafenib and trametinib (D+T) in BRAF V600 mutant resectable stage IIIB/C/D melanoma (NeoTrio Trial). Ann Oncol 2017; 28(Suppl. 5): mdx377.041–mdx377.041. [Google Scholar]
- 91. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–785. [DOI] [PubMed] [Google Scholar]
- 92. Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 93. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 94. Menzies AM, Rozeman EA, Amaria RN, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the international neoadjuvant melanoma consortium (INMC). J Clin Oncol 2019: 9503. [DOI] [PubMed] [Google Scholar]
- 95. Tetzlaff M, Messina J, Stein J, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 2018; 29: 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]