Abstract
Background:
This analysis aimed to evaluate the impact of rivaroxaban exposure and patient characteristics on efficacy and safety outcomes in patients with acute coronary syndrome (ACS) and to determine whether therapeutic drug monitoring might provide additional information regarding rivaroxaban dose, beyond what patient characteristics provide.
Methods:
A post hoc exposure–response analysis was conducted using data from the phase III ATLAS ACS 2 Thrombolysis in Myocardial Infarction (TIMI) 51 study, in which 15,526 randomized ACS patients received rivaroxaban (2.5 mg or 5 mg twice daily) or placebo for a mean of 13 months (maximum follow up: 31 months). A multivariate Cox model was used to correlate individual predicted rivaroxaban exposures and patient characteristics with time-to-event clinical outcomes.
Results:
For the incidence of myocardial infarction (MI), ischemic stroke, or nonhemorrhagic cardiovascular death, hazard ratios (HRs) for steady-state maximum plasma concentration (Cmax) in the 5th and 95th percentiles versus the median were statistically significant but close to 1 for both rivaroxaban doses. For TIMI major bleeding events, a statistically significant association was observed with Cmax [HR, 1.08; 95% CI, 1.06–1.11 (95th percentile versus median, 2.5 mg twice daily)], sex [HR, 0.56; 95% CI, 0.38–0.84 (female versus male)], and previous revascularization [HR, 0.62; 95% CI, 0.44–0.87 (no versus yes)].
Conclusions:
The shallow slopes of the exposure–response relationships and the lack of a clear therapeutic window render it unlikely that therapeutic drug monitoring in patients with ACS would provide additional information regarding rivaroxaban dose beyond that provided by patient characteristics.
Keywords: acute coronary syndromes, bleeding, exposure–response analysis, monitoring, population pharmacokinetics, rivaroxaban
Introduction
The oral direct factor Xa inhibitor rivaroxaban, co-administered with acetylsalicylic acid (ASA) alone or with ASA plus clopidogrel or ticlopidine, is licensed in the European Union (EU) for the prevention of recurrent atherothrombotic events in adult patients with acute coronary syndrome (ACS) and elevated cardiac biomarkers.1 Rivaroxaban has a predictable pharmacokinetic and pharmacodynamic profile,2 and has been developed for fixed-dose administration without routine coagulation or therapeutic drug monitoring.3
The approval of rivaroxaban for the treatment of patients with ACS was based on the results of the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with ACS-Thrombolysis in Myocardial Infarction (ATLAS ACS-TIMI) program. The phase II randomized, placebo-controlled, dose-finding ATLAS ACS-TIMI 46 trial (ClinicalTrials.gov identifier: NCT00402597) compared rivaroxaban (at total daily doses ranging from 5 to 20 mg) with placebo in patients receiving background antiplatelet therapy (aspirin alone or aspirin plus a thienopyridine).4 The two lowest doses of rivaroxaban [2.5 mg twice daily (BID) and 5 mg BID] demonstrated promising efficacy with lower bleeding rates than the higher doses and were selected for further evaluation in the subsequent phase III ATLAS ACS 2-TIMI 51 trial (ClinicalTrials.gov identifier: NCT00809965).5 The rivaroxaban 2.5 mg BID regimen showed similar efficacy to the 5 mg BID regimen with a lower incidence of bleeding, and consequently, authorization for use of rivaroxaban in treating patients with ACS was granted for the 2.5 mg BID regimen in the EU.1
The benefit–risk balance of rivaroxaban may be affected by both rivaroxaban exposure and patient characteristics. Older age and impaired renal function increase rivaroxaban exposure; this is not unexpected because approximately one-third of the rivaroxaban dose is eliminated unchanged by the kidneys,2,6 and renal function declines with advancing age.7 Furthermore, advancing age is a risk factor for ACS, along with other patient characteristics, such as a history of diabetes or myocardial infarction (MI).8 Given these considerations, it has been suggested that therapeutic drug monitoring (i.e. plasma concentration-based dose adjustment) may be useful to guide rivaroxaban dosing. To explore this possibility, we performed a post hoc exposure–response analysis using data from the ATLAS ACS 2-TIMI 51 trial population to evaluate the impact of predicted rivaroxaban exposures and patient characteristics on the occurrence of efficacy and safety outcomes in patients with ACS receiving rivaroxaban.
Methods and materials
Study design
The ATLAS ACS 2-TIMI 51 study was a double-blind, placebo-controlled, event-driven trial in which 15,526 patients with a recent ACS event were randomized to receive rivaroxaban 2.5 mg BID or 5 mg BID or placebo with a maximum follow up of 31 months (mean duration of treatment: 13.1 months).5,9 Study drugs were administered in addition to the standard of care, which included aspirin alone or aspirin plus a thienopyridine. A clinical events committee whose members were unaware of study-group assignments adjudicated all components of the key efficacy and safety outcomes. Study protocols and amendments were approved by independent ethics committees. All participants provided written informed consent prior to study enrollment. Full details of the methodology and ethical conduct of the study have been published previously.5,9
The efficacy outcomes evaluated in the current exposure–response analysis were a composite of MI, ischemic stroke, or nonhemorrhagic cardiovascular (CV) death, and a composite of MI, ischemic stroke, or death from all causes. TIMI major bleeding events (excluding bleeding associated with coronary artery bypass graft surgery) and clinically significant bleeding (a composite of first occurrence of any TIMI major bleeding, TIMI minor bleeding or bleeding requiring medical attention) were evaluated as safety outcomes. The exposure–response analysis included efficacy and safety events occurring from the first day of study-drug administration until 2 days after the last dose.
Patient characteristics
A list of patient characteristics (including potential risk factors for efficacy and safety outcomes) were a priori selected for inclusion in the exposure–response evaluation based on a review of the literature (e.g. GRACE10 and TIMI8,11 risk scores) and experience in the ATLAS ACS 2-TIMI 51 study.9 The variables were either categorical in nature or grouped categorically to aid clinical interpretation.
Rivaroxaban exposure predictions
Rivaroxaban plasma concentrations were not measured in the ATLAS ACS 2-TIMI 51 study. Therefore, rivaroxaban exposure metrics [steady-state area under the plasma concentration–time curve from time 0 to 24 h after dosing (AUC0–24), steady-state maximum plasma concentration (Cmax), and steady-state trough plasma concentration (Ctrough)] were predicted for each patient based on individual patient characteristics [age, weight, renal function measured as rate of creatinine clearance (CrCl) and sex] and rivaroxaban dose using an integrated population PK model, described elsewhere.12
Exposure predictions for exposure–efficacy analyses were made in patients who were randomized, received at least one dose of a study drug, and had available efficacy outcome data. For exposure–safety analyses, exposure predictions were made in patients who were randomized and received at least one dose of a study drug (the safety population of ATLAS ACS 2-TIMI 515,9). For patients randomized to the placebo group, rivaroxaban exposures were set to 0 with appropriate units. Relationships between exposure metrics and time-to-event clinical outcomes were explored graphically using Kaplan–Meier plots.
Regression analyses
Relationships between rivaroxaban exposure metrics, patient characteristics, and each of the efficacy and safety outcomes were quantified using the following methods. Initially, a univariate regression analysis was performed using AUC0–24, Cmax, or Ctrough as independent variables, assuming a linear relationship with the log-hazard of outcome events. The exposure metric with the lowest Akaike information criterion (AIC) value generated from the univariate analyses was then combined with the selected patient characteristics as independent variables for predicting the probability of the outcome events in a multivariate Cox proportional regression analysis, which resulted in the ‘full model’. The significance of each independent variable was generated from fitting the full model. Statistically nonsignificant variables (with associated p values >0.01 according to the likelihood ratio test) were each removed from the model, with the exception of the selected exposure metric, age, and CrCl calculated using the Cockcroft–Gault equation. The selected exposure metric, age, and CrCl were expected to have an impact on outcomes and were kept in the model as forced input variables, regardless of their statistical significance level. The derived model, which contained the forced input variables and the remaining statistically significant variables, was considered the final model for the evaluated response outcome.
The hazard ratios (HRs) of the variables in the final models were shown in forest plots, in which the hazard at a given value of a variable was compared with its corresponding reference value. The reference value was typically the most common value of the variable, with the exception of hospital region, for which Western Europe was set as the reference. The final models were used to simulate the probability of efficacy or safety events at 1 year versus exposure in a typical patient population (i.e. with individual patient characteristics set to reference values). The time interval of 1 year is close to 13.1 months, the mean duration of treatment in the ATLAS ACS 2-TIMI 51 study.9
Results
Patient characteristics
Table S1 shows the patient characteristics selected for evaluation in the models and the prevalence of those patient characteristics in the study population, including the rivaroxaban and placebo treatment groups (15,167 and 15,350 patients in the efficacy and safety populations, respectively). About one-quarter of the patients were female (25.3%), and 36.4% were ⩾65 years of age. The most common type of ACS was ST-segment elevation MI (50.3%), and 93.2% of patients were receiving dual antiplatelet therapy (aspirin plus a thienopyridine) at baseline.
Histories of heart failure, hypertension, diabetes mellitus, stroke/transient ischemic attack, MI, and previous revascularization were present in 11.1%, 67.8%, 31.9%, 2.6%, 27.1%, and 60.9% of patients in the efficacy population, respectively. In the safety population, the corresponding percentages were 10.9%, 67.5%, 32.0%, 2.6%, 27.0%, and 60.3%, respectively. Baseline CrCl was <50 ml/min in 6.9% and 7.0% of patients in the efficacy and safety populations, respectively. Gastrointestinal bleeding, smoking status, and alcohol use were evaluated only for the safety outcomes (Table S1).
Rivaroxaban exposure predictions and event rates
Rivaroxaban exposure data were predicted in 10,225 patients in the safety population (5115 and 5110 patients in the 2.5 mg BID and 5 mg BID groups, respectively) and 10,105 patients in the efficacy population (5055 and 5050 patients in the 2.5 mg BID and 5 mg BID groups, respectively; Table 1). The predicted exposure metrics were all highly correlated (>0.98) within a given individual. The observed event rates of efficacy and safety outcomes are shown in Table 2.
Table 1.
Summary of predicted rivaroxaban exposure in the efficacy and safety populations of the ATLAS ACS 2-TIMI 51 study.
| Rivaroxaban dose | Exposure measure | n | P05 | Median | P95 | Mean | CV (%) |
|---|---|---|---|---|---|---|---|
| Efficacy population | |||||||
| 2.5 mg BID | AUC0–24 (ug/l*h) | 5055 | 681.44 | 800.75 | 984.52 | 816.01 | 26.01 |
| Cmax (µg/l) | 5055 | 39.86 | 45.18 | 53.47 | 45.80 | 17.98 | |
| Ctrough (µg/l) | 5055 | 13.23 | 17.26 | 23.55 | 17.85 | 47.83 | |
| 5 mg BID | AUC0–24 (ug/l*h) | 5050 | 1294.98 | 1509.30 | 1853.47 | 1533.15 | 11.53 |
| Cmax (µg/l) | 5050 | 75.38 | 85.48 | 100.31 | 86.27 | 9.02 | |
| Ctrough (µg/l) | 5050 | 25.02 | 32.51 | 44.42 | 33.35 | 18.67 | |
| Safety population | |||||||
| 2.5 mg BID | AUC0–24 (ug/l*h) | 5115 | 681.44 | 800.88 | 985.21 | 816.44 | 25.97 |
| Cmax (µg/l) | 5115 | 39.87 | 45.19 | 53.47 | 45.83 | 17.95 | |
| Ctrough (µg/l) | 5115 | 13.23 | 17.26 | 23.60 | 17.86 | 47.74 | |
| 5 mg BID | AUC0–24 (ug/l*h) | 5110 | 1295.10 | 1509.45 | 1853.47 | 1533.54 | 11.52 |
| Cmax (µg/l) | 5110 | 75.38 | 85.50 | 100.31 | 86.29 | 9.01 | |
| Ctrough (µg/l) | 5110 | 25.03 | 32.51 | 44.42 | 33.36 | 18.65 | |
AUC0–24, steady-state area under the concentration–time curve from 0 to 24 h post dose; BID, twice daily; Cmax, steady-state maximum plasma concentration; Ctrough, steady-state trough plasma concentration; CV, coefficient of variation; n, number of subjects; P05, 5th percentile; P95, 95th percentile.
Table 2.
Observed event rates of efficacy and safety outcomes.
| Patients with event/total patients (%) |
||||
|---|---|---|---|---|
| Stratum | Placebo | 2.5 mg BID | 5 mg BID | |
| Efficacy outcomes | ||||
| MI, ischemic stroke, nonhemorrhagic CV death | Aspirin | 35/350 (10.0) | 24/343 (7.0) | 23/341 (6.7) |
| Aspirin plus thieno | 317/4712 (6.7) | 256/4712 (5.4) | 246/4709 (5.2) | |
| Combined | 352/5062 (7.0) | 280/5055 (5.5) | 269/5050 (5.3) | |
| MI, ischemic stroke, death from all causes | Aspirin | 35/350 (10.0) | 24/343 (7.0) | 23/341 (6.7) |
| Aspirin plus thieno | 326/4712 (6.9) | 263/4712 (5.6) | 256/4709 (5.4) | |
| Combined | 361/5062 (7.1) | 287/5055 (5.7) | 279/5050 (5.5) | |
| Safety outcomes | ||||
| TIMI major bleeding | Aspirin | 2/352 (0.6) | 2/343 (0.6) | 4/342 (1.2) |
| Aspirin plus thieno | 25/4773 (0.5) | 66/4772 (1.4) | 81/4768 (1.7) | |
| Combined | 27/5125 (0.5) | 68/5115 (1.3) | 85/5110 (1.7) | |
| Clinically significant bleeding | Aspirin | 11/352 (3.1) | 19/343 (5.5) | 23/342 (6.7) |
| Aspirin plus thieno | 316/4773 (6.6) | 567/4772 (11.9) | 725/4768 (15.2) | |
| Combined | 327/5125 (6.4) | 586/5115 (11.5) | 748/5110 (14.6) | |
BID, twice daily; CV, cardiovascular; MI, myocardial infarction; thieno, thienopyridine; TIMI, Thrombolysis in Myocardial Infarction.
Regression analyses
Exposure–efficacy analysis
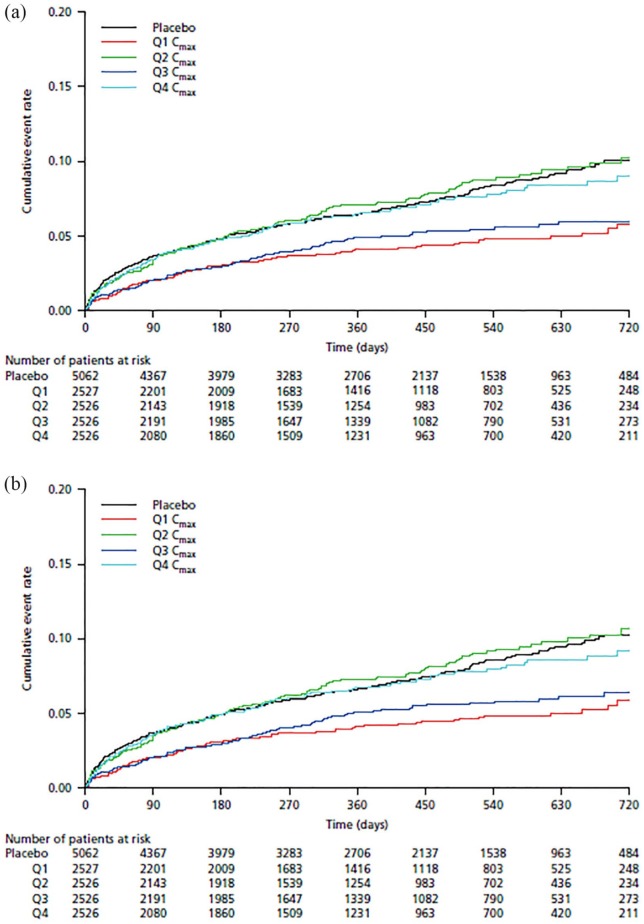
In the univariate regression analysis, the AIC values generated from fitting the exposure metrics AUC0–24, Cmax, or Ctrough were only marginally different. However, Cmax was associated with the lowest AIC value and was therefore selected as the exposure marker for further investigation. The cumulative event rates versus stratified Cmax values are shown in Kaplan–Meier plots (Figure 1). There was no apparent trend between the quartiles of Cmax and the composite efficacy outcomes of MI, ischemic stroke, or nonhemorrhagic CV death [Figure 1(a)] and MI, ischemic stroke, or death from all causes [Figure 1(b)].
Figure 1.
Kaplan–Meier plots of the cumulative event rate of the composite efficacy outcomes of (a) myocardial infarction, ischemic stroke, or nonhemorrhagic cardiovascular death and (b) myocardial infarction, ischemic stroke, or death from all causes versus predicted steady-state maximum concentration of rivaroxaban.
Cmax, steady-state maximum plasma concentration; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile.
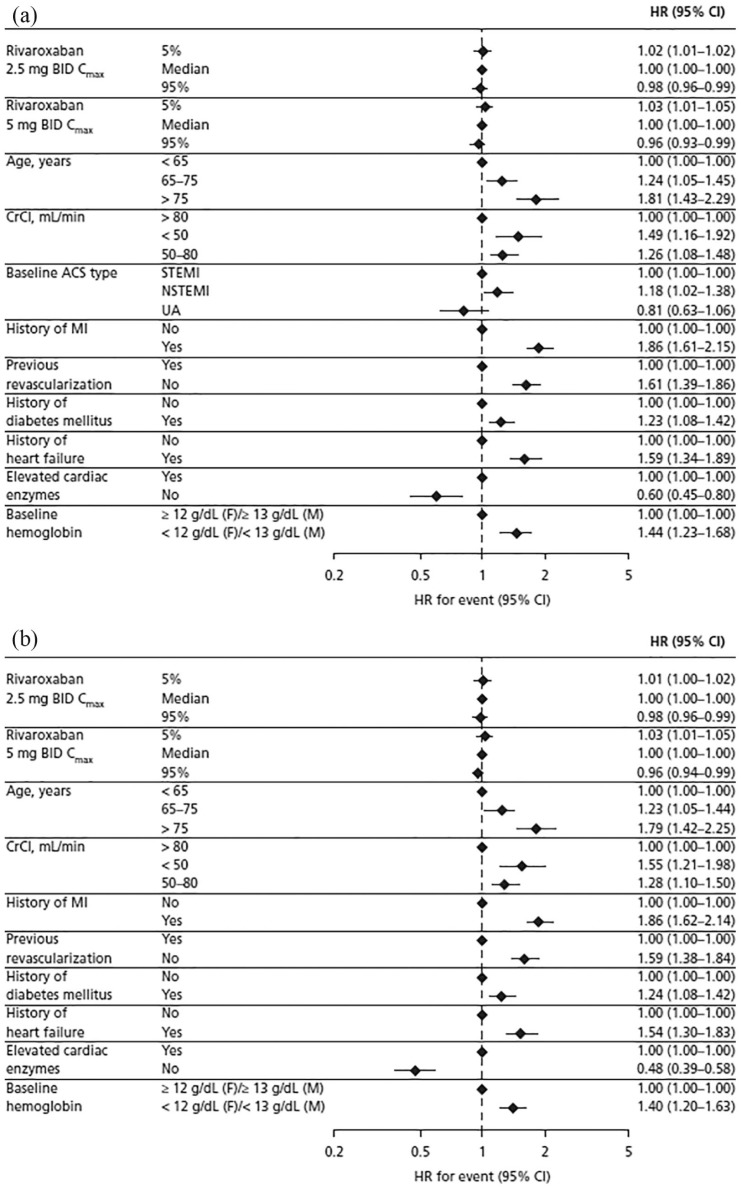
For the composite efficacy outcome of MI, ischemic stroke, or nonhemorrhagic CV death, the final Cox regression model included Cmax, age, and CrCl as forced variables, and the following significant patient characteristics: type of ACS at baseline, history of MI, history of diabetes mellitus, history of heart failure, previous revascularization, elevated cardiac enzymes, and baseline hemoglobin levels (Table S2). All variables displayed a statistically significant association with this efficacy outcome (p ⩽ 0.01; Table S2). The variables with the greatest impact were history of MI [HR, 1.86; 95% confidence interval (CI), 1.61–2.15] and age >75 years versus <65 years [HR, 1.81; 95% CI, 1.43–2.29; Figure 2(a)].
Figure 2.
Hazard ratio for (a) the composite efficacy outcome of myocardial infarction, ischemic stroke, or nonhemorrhagic cardiovascular death and (b) the composite efficacy outcome of myocardial infarction, ischemic stroke, or death from all causes based on results of the final model.
ACS, acute coronary syndrome; BID, twice daily; CI, confidence interval; Cmax, steady-state maximum plasma concentration; CrCl, creatinine clearance; F, female; HR, hazard ratio; M, male; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina.
For the composite efficacy outcome of MI, ischemic stroke, or death from all causes, the final Cox regression model included the same risk factors except for type of ACS (Table S2). The variables with the greatest impact were again history of MI (HR, 1.86; 95% CI, 1.62–2.14) and age >75 years versus <65 years [HR, 1.79; 95% CI, 1.42–2.25;Figure 2(b)].
For both composite efficacy outcomes, the HRs associated with Cmax in the 5th or 95th percentile (versus the median), and therefore the associated risks, were statistically significant (p = 0.002–0.003), but the magnitude of impact was small: the highest HR was 1.03 (95% CI, 1.01–1.05) at the 5th percentile of Cmax for rivaroxaban 5 mg BID, and the lowest HR was 0.96 (95% CI, 0.93–0.99) at the 95th percentile of Cmax for rivaroxaban 5 mg BID (Figure 2). This small but statistically significant effect of exposure is due to the inclusion of the placebo group (zero exposure) in the analysis, and when treatment effect (placebo versus 2.5 mg BID versus 5 mg BID) was added into the final model, Cmax was no longer a significant predictor of the composite efficacy outcomes.
Exposure–safety analysis
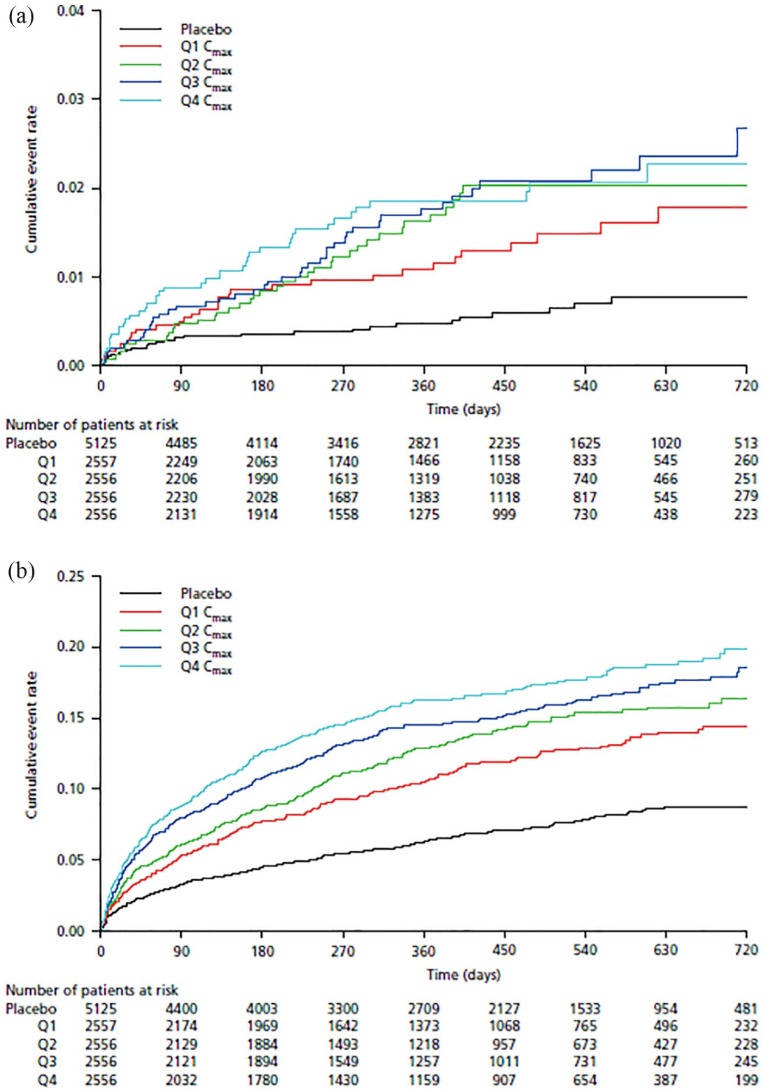
In the univariate regression analysis, AUC0–24, Cmax, and Ctrough had broadly similar AIC values. However, Cmax was associated with the lowest AIC value and was therefore selected for further investigation. The cumulative event rate versus stratified Cmax values are shown in Kaplan–Meier plots, which suggested that the cumulative event rate for TIMI major bleeding [Figure 3(a)] and clinically significant bleeding [Figure 3(b)] increased as the rivaroxaban Cmax level increased.
Figure 3.
Kaplan–Meier plots of the cumulative event rate of the safety outcomes of (a) TIMI major bleeding and (b) clinically significant bleeding versus predicted steady-state maximum concentration of rivaroxaban.
Cmax, steady-state maximum plasma concentration; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; TIMI, Thrombolysis in Myocardial Infarction.
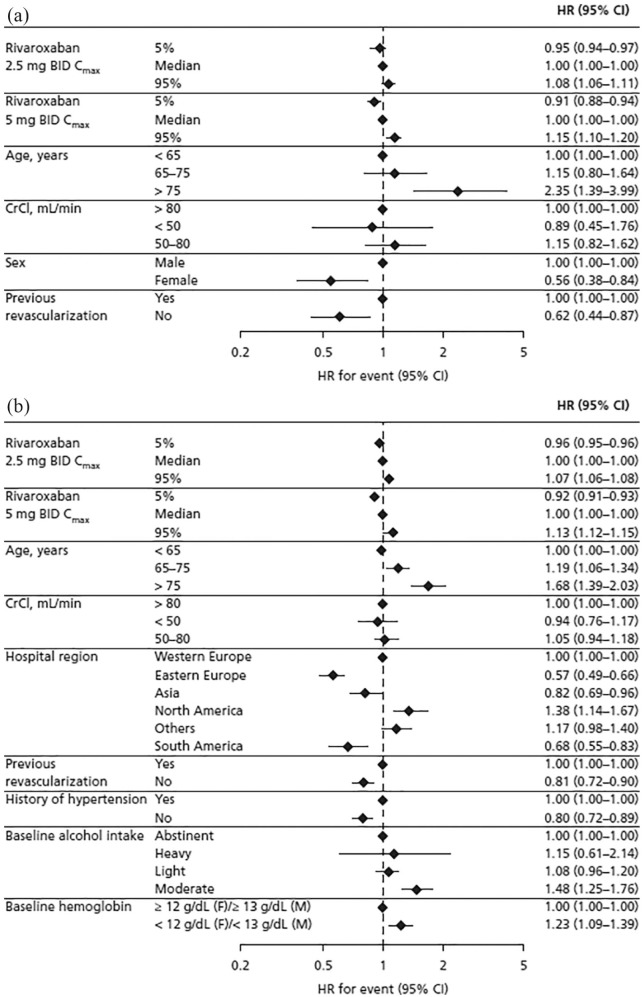
The final exposure–response model for TIMI major bleeding included Cmax, age, and CrCl as forced input variables, and the following significant patient characteristics: sex and previous revascularization (Table S3). Of the forced variables, only Cmax displayed a statistically significant association with TIMI major bleeding; age and CrCl did not display significant associations with this safety outcome (Table S3). Female patients (HR, 0.56; 95% CI, 0.38–0.84) and those without previous revascularization (HR, 0.62; 95% CI, 0.44–0.87) had a reduced likelihood of TIMI major bleeding compared with male patients and patients with previous revascularization, respectively [Figure 4(a)]. In the 2.5 mg BID dose group, Cmax at the 95th percentile (versus the median) was associated with a HR of 1.08 (95% CI, 1.06–1.11) for TIMI major bleeding [Figure 4(a)].
Figure 4.
Hazard ratio for (a) TIMI major bleeding and (b) clinically significant bleeding based on results of the final model.
BID, twice daily; CI, confidence interval; Cmax, steady-state maximum plasma concentration; CrCl, creatinine clearance; F, female; HR, hazard ratio; M, male; TIMI, Thrombolysis in Myocardial Infarction.
The final model for clinically significant bleeding included Cmax, age, and CrCl as forced variables, and the following significant patient characteristics: hospital region, previous revascularization, history of hypertension, alcohol use, and baseline hemoglobin levels (Table S3). Of the forced variables, only age and Cmax displayed a significant association with clinically significant bleeding; CrCl did not display a significant association with this safety outcome (Table S3). Overall, the risk factor with the greatest impact was age >75 years versus <65 years [HR, 1.68; 95% CI, 1.39–2.03; Figure 4(b)]. Patients without previous revascularization (HR, 0.81; 95% CI, 0.72–0.90) and those without history of hypertension (HR, 0.80; 95% CI, 0.72–0.89) were less likely to experience clinically significant bleeding than patients with previous revascularization and those with a history of hypertension, respectively [Figure 4(b)]. Hospital region Eastern Europe versus Western Europe was also associated with a reduced likelihood of this outcome (HR, 0.57; 95% CI, 0.49–0.66). Patients with moderate alcohol intake (HR, 1.48; 95% CI, 1.25–1.76) and those with low [<12 g/dl (female) or <13 g/dl (male)] baseline hemoglobin (HR, 1.23; 95% CI, 1.09–1.39) had a higher likelihood of clinically significant bleeding than those who were abstinent and those with hemoglobin ⩾12 g/dl (female) or ⩾13 g/dl (male), respectively [Figure 4(b)].
For both safety outcomes, the HRs associated with Cmax in the 5th or 95th percentile (versus the median), and therefore the associated risks, were statistically significant (p < 0.00001) but the magnitudes of impact were considered small: the highest HR was 1.15 (95% CI, 1.10–1.20) at the 95th percentile of Cmax for rivaroxaban 5 mg BID, and the lowest HR was 0.91 (95% CI, 0.88–0.94) at the 5th percentile of Cmax for rivaroxaban 5 mg BID. Similar to the exposure–efficacy analysis, this small but statistically significant effect of exposure is due to the inclusion of the placebo group (zero exposure) in the analysis. When treatment effect (placebo versus 2.5 mg BID versus 5 mg BID) was added into the final model, the impact of Cmax on both safety outcomes was attenuated, and Cmax was no longer a significant predictor of the safety outcomes (p = 0.03747 for TIMI major bleeding and p = 0.5463 for clinically significant bleeding).
Expected probability of efficacy or safety events at 1 year of treatment with rivaroxaban
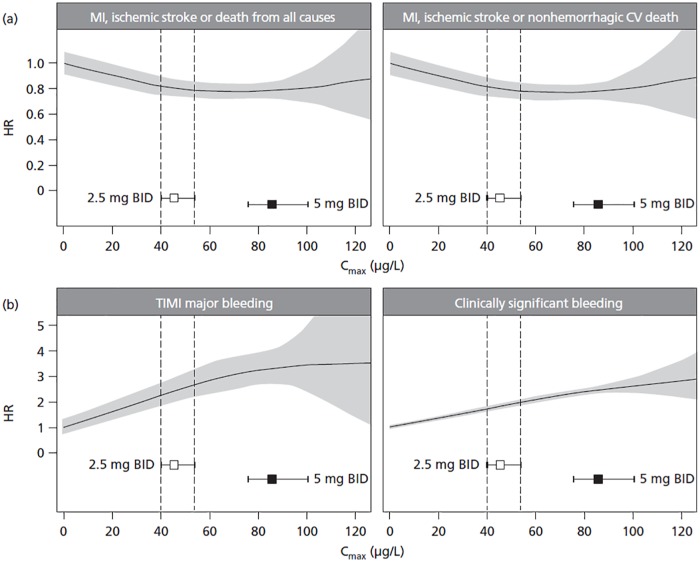
Using the derived final models, the expected hazard for each outcome in a typical patient was estimated and plotted against the range of predicted Cmax, using the placebo group (Cmax set to 0 µg/l) as a reference (Figure 5). For efficacy outcomes, the HR plateaued after about 40 µg/l with no further decrease observed at higher Cmax values. For the safety outcomes, there was a shallow increase in HR over the entire Cmax range. The uncertainty in the predicted hazard increased considerably when Cmax was higher than the predicted 95th percentile of Cmax with rivaroxaban 5 mg BID.
Figure 5.
Expected HR for (a) efficacy and (b) safety outcomes in a typical patient plotted against the range of Cmax. The reference group for calculation of the HRs was the placebo group (Cmax set to 0 µg/l). Black lines represent means, and gray-shaded areas represent 95% confidence intervals for the HR. Squares represent median exposure, and horizontal error bars represent the range between the 5th and 95th percentiles of exposure. Vertical dashed lines label the 5th and 95th percentiles of exposure for the 2.5 mg BID dose group.
BID, twice daily; Cmax, steady-state maximum plasma concentration; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
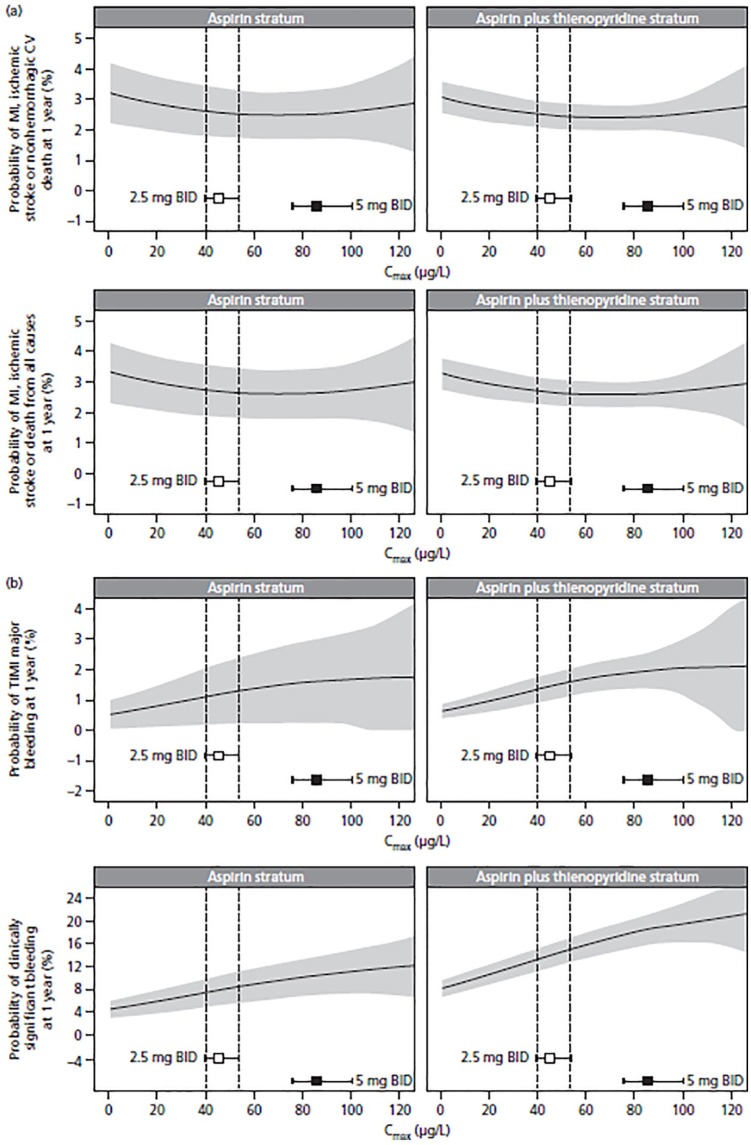
Figure 6 shows the expected probability of having an efficacy or safety event at 1 year of treatment with rivaroxaban 2.5 mg BID in a typical patient presented as a function of increasing rivaroxaban exposure and baseline antiplatelet stratum (aspirin alone or aspirin plus thienopyridine). For efficacy, the maximal effect was observed with concentrations associated with the 2.5 mg BID dose, with no further reductions observed at higher concentrations (i.e. the 5 mg BID dose). At the approved dose of 2.5 mg BID, an increase in rivaroxaban exposure from the median to the 95th percentile was predicted to increase the risk of TIMI major bleeding from 1.1% to 1.3% in the aspirin stratum and from 1.3% to 1.6% in the aspirin plus thienopyridine stratum. The risk of clinically significant bleeding was predicted to increase from 6.5% to 8.2% in the aspirin stratum and from 13% to 15% in the aspirin plus thienopyridine stratum. Similar to Figure 5, the uncertainty in the predicted probability of having an efficacy and safety event increased considerably when Cmax was higher than the predicted 95th percentile of Cmax with rivaroxaban 5 mg BID because very few patients had exposure at those levels.
Figure 6.
Expected probability of (a) efficacy events and (b) safety events at 1 year of treatment with rivaroxaban 2.5 mg BID in a typical patient presented as a function of increasing Cmax and baseline antiplatelet stratum (aspirin alone or aspirin plus thienopyridine). Black lines represent means, and gray-shaded areas represent 95% confidence intervals for the probability. Squares represent median exposure, and horizontal error bars represent the range between the 5th and 95th percentiles of exposure. Vertical dashed lines label the 5th and 95th percentiles of exposure for the 2.5 mg BID dose group.
BID, twice daily; Cmax, steady-state maximum plasma concentration; CV, cardiovascular; MI, myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Discussion
The results of this analysis from the ATLAS ACS 2-TIMI 51 trial demonstrate statistically significant but shallow linear relationships between rivaroxaban exposure and efficacy/safety outcomes in patients with ACS. Within the studied regimen, Increasing rivaroxaban exposure was associated with a reduced risk of thrombotic events and an increased risk of bleeding events, but the magnitude of impact was small. Patient characteristics such as age, history of MI, elevated cardiac enzymes, or history of previous revascularization also had a significant impact on treatment outcomes and the magnitude of impact of patient characteristics on treatment outcomes was greater than that of rivaroxaban exposure.
The steady-state exposure metrics (AUC0–24, Cmax, and Ctrough) predicted using the population PK model for the 5 mg BID rivaroxaban dose were twice the magnitude of those predicted for rivaroxaban 2.5 mg BID, owing to the linear PK characteristics of rivaroxaban at doses of 10 mg or less.13 The distribution of predicted exposure metrics reflected the differences and impact of patient characteristics (age, weight, CrCl, and sex) on rivaroxaban exposure.12 Cmax was used to investigate exposure–response relationships because it had a slightly stronger univariate relationship with both the efficacy and safety outcomes. However, as expected, all three PK metrics (AUC0–24, Cmax, and Ctrough) were highly correlated, and therefore exhibited similar relationships with efficacy and safety outcomes. It should be noted that PK samples were not collected in the ATLAS ACS 2-TIMI 51 study, and because inter- and intra-individual variability cannot be estimated, the variability in the predicted exposures was much lower than would be expected in clinical practice.
In the ATLAS ACS 2-TIMI 51 study, patients receiving concomitant systemic treatment with strong cytochrome P450 (CYP) 3A4 and P-glycoprotein inhibitors (e.g. certain azole antimycotics, such as ketoconazole, and HIV-protease inhibitors, such as ritonavir) were excluded. The apparent clearance of rivaroxaban in patients taking a concomitant moderate or weak CYP3A4 inhibitor or a CYP3A4 inducer has been estimated to be 86.3%, 93.9% and 130% of the apparent clearance in patients not taking comedication, respectively.12 Owing to variation in comedication intake pattern (e.g. duration, dose, regimen, type) during the long-term treatment duration of the study and the estimated small magnitude of comedication impact on exposure, comedication was not included as a covariate on the estimated exposure. This would lead to slightly lower exposure variability but was not expected to impact the subsequent exposure–response analysis.
Despite the narrow concentration range in which to evaluate exposure–response relationships, statistically significant linear relationships with shallow slopes in the expected direction were observed for all exposure parameters for both efficacy and safety outcomes. These relationships were largely driven by inclusion of the placebo group (Cmax set to 0 µg/l) and would be even more shallow or nonexistent if the analyses had been restricted to active treatment groups. Cmax was no longer a significant predictor of the composite efficacy outcomes when treatment effect (placebo versus 2.5 mg BID versus 5 mg BID) was added into the final model. These findings are consistent with observations from the phase II ATLAS ACS-TIMI 46 study, in which 14 active treatment combinations of rivaroxaban and antiplatelet therapy were studied.4 No dose–response relationship for efficacy outcomes was observed in the rivaroxaban BID dosing groups while clear dose–response relationships were observed for safety outcomes in all treatment groups.4 These data support the validity of the applied model and suggest that the lack of rank-ordered efficacy versus Cmax quartiles in Kaplan–Meier plots is not likely caused by the choice of exposure marker.
The decrease in thrombotic events (MI, ischemic stroke, or nonhemorrhagic CV death) that might be achieved by adjusting the rivaroxaban dose to increase the plasma concentration appears to be small; the HR for such a thrombotic event in patients with Cmax in the 5th percentile versus the median (2.5 mg BID dose) was 1.02, which translates into an expected absolute risk reduction of approximately 0.2% over 1 year for the typical patient. Similarly, the decrease in TIMI major bleeding events that might be achieved by decreasing the rivaroxaban dose (and therefore rivaroxaban exposure) appears small; the HR for TIMI major bleeding in patients with Cmax in the 95th percentile versus the median (2.5 mg BID dose) was 1.08, which translates into an absolute risk reduction of approximately 0.2% over 1 year. Therefore, these data suggest that therapeutic drug monitoring of rivaroxaban (i.e. plasma concentration-based dose adjustment) would provide little additional clinical benefit to patients with ACS. Moreover, clinical observations in ATLAS ACS 2-TIMI 51 demonstrated that doses of rivaroxaban 2.5 mg BID showed similar efficacy to doses of 5 mg BID with a lower incidence of bleeding and led to authorization of the 2.5 mg BID regimen for the treatment of patients with ACS in the EU.1
Compared with vitamin K antagonists, within the studied regimen rivaroxaban exposure–response curves for both efficacy and safety are essentially linear and shallow with no threshold of exposure above which bleeding risk accelerated, or below which loss of efficacy occurred. A target therapeutic range for rivaroxaban cannot easily be identified from this exposure–response analysis. In addition, the CIs around the 1-year estimates for both the efficacy and safety outcome event rates were wide for any given rivaroxaban concentration and appeared to overlap in the 2.5 mg BID and 5 mg BID groups, indicating that adjusting the rivaroxaban dose based on measured rivaroxaban concentrations in individual patients is unlikely to be of benefit. Furthermore, patient characteristics such as age and history of MI had a greater impact than rivaroxaban exposure on the occurrence of thrombotic events over a 1-year period. Likewise, age, sex, and revascularization appeared to have a greater impact on TIMI major bleeding than rivaroxaban exposure. These results are supported by exposure–response analyses with edoxaban and apixaban in indications such as stroke prevention in atrial fibrillation and treatment of venous thromboembolism. Similar to the current findings with rivaroxaban, those analyses supported administration of edoxaban and apixaban at approved doses without routine therapeutic drug monitoring.14–17
Strengths of this analysis include the use of data from a large, prospective, double-blind clinical trial that assessed two different doses of rivaroxaban and included a placebo control group. In addition, the patients included in this analysis were at high risk of recurrent ischemic events and bleeding because 50.3% had ST-segment elevation MI, and 93.2% were receiving dual antiplatelet therapy at baseline. Furthermore, blinded central adjudication of all efficacy outcomes was performed to standardize the assessments of efficacy outcomes across all participating sites.
Some limitations of the analysis include the lack of direct PK measurements in the ATLAS ACS 2-TIMI 51 study, which necessitated the use of model-predicted exposure data for the analysis. It is also worth noting that the variability in exposure metrics may be underestimated in this analysis; the covariate model explains the majority, but not all, of the exposure variability between patients. In addition, the range of exposures evaluated was limited to those associated with the 2.5 mg and 5 mg BID doses of rivaroxaban studied in the ATLAS ACS 2-TIMI 51 study, which itself was not designed to evaluate exposure–response relationships and the impact of patient characteristics on the outcomes. These factors may reduce the ability of the analysis to detect a weak exposure–response relationship. However, weak exposure–response relationships are unlikely to prompt dose adjustments.
Conclusion
In summary, the exposure–response modeling results support administration of a fixed 2.5 mg BID dose of rivaroxaban in patients with ACS. The shallow slopes of the exposure–response relationships and the lack of a clear therapeutic window render it unlikely that therapeutic drug monitoring would provide additional information regarding rivaroxaban dose beyond that already provided by patient characteristics in the ACS indication.
Supplemental Material
Supplemental material, Riva_TDM_ACS_Supplemental_material_29Aug18 for Influence of model-predicted rivaroxaban exposure and patient characteristics on efficacy and safety outcomes in patients with acute coronary syndrome by Liping Zhang, Xiaoyu Yan, Partha Nandy, Stefan Willmann, Keith A. A. Fox, Scott D. Berkowitz, Amarnath Sharma, Anne Hermanowski-Vosatka, Stephan Schmidt, Jeffrey I. Weitz, Dirk Garmann and Gary Peters in Therapeutic Advances in Cardiovascular Disease
Acknowledgments
This work was conducted at Bayer AG, Bayer US LLC, and Janssen Research & Development LLC.
Emma Bolton of Oxford PharmaGenesis Ltd., Oxford, UK provided medical writing support, which was funded by Bayer AG, Berlin, Germany. The authors would like to thank Alexander Solms (Bayer AG, Berlin, Germany) and Theodore Spiro (Bayer US LLC, Whippany, NJ, USA)
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: This analysis, including design, collection, analysis, and interpretation of data, was funded by Bayer AG, Berlin, Germany, and Janssen Research & Development LLC, Raritan, NJ, USA. The writing of the report and decision to submit the work for publication were carried out jointly by Bayer AG and Janssen Research & Development LLC. All authors had access to the study data.
Dirk Garmann and Stefan Willmann are employees of Bayer AG; Scott D. Berkowitz is an employee of Bayer US LLC. This work was conducted within the scope of their employment, and no additional payment was received.
Liping Zhang, Partha Nandy, Amarnath Sharma, Anne Hermanowski-Vosatka, and Gary Peters are employees of Janssen Research & Development LLC and own stock in Johnson & Johnson. This work was conducted within the scope of their employment, and no additional payment was received. Xiaoyu Yan was an employee of Janssen Research & Development LLC at the time that this work was carried out and owns stock in Johnson & Johnson. He is currently an employee of The Chinese University of Hong Kong, Shatin, N.T., Hong Kong. This work was conducted within the scope of his employment at Janssen Research and Development LLC, and no additional payment was received.
Stephan Schmidt is a paid consultant for Bayer AG.
Keith A. A. Fox has received grants and honoraria relating to this work from Bayer AG and Janssen Research & Development LLC, and for unrelated work he has received grants from AstraZeneca and honoraria from Sanofi/Regeneron and Verseon.
Jeffrey I. Weitz is a consultant for and has received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Ionis, Janssen, Merck, Novartis, Pfizer, and Portola.
ORCID iDs: Liping Zhang  https://orcid.org/0000-0002-1398-5641
https://orcid.org/0000-0002-1398-5641
Scott D. Berkowitz  https://orcid.org/0000-0002-9428-4408
https://orcid.org/0000-0002-9428-4408
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Liping Zhang, Global Clinical Pharmacology, Janssen Research and Development, LLC, 5 Pauma Valley Ct, Raritan, NJ 08558, USA.
Xiaoyu Yan, Janssen Research & Development, LLC, Raritan, NJ, USA.
Partha Nandy, Janssen Research & Development, LLC, Raritan, NJ, USA.
Stefan Willmann, Bayer AG, Wuppertal, Germany.
Keith A. A. Fox, Centre for Cardiovascular Sciences, The University of Edinburgh, Edinburgh, UK
Scott D. Berkowitz, Bayer US LLC, Whippany, NJ, USA
Amarnath Sharma, Janssen Research & Development, LLC, Raritan, NJ, USA.
Anne Hermanowski-Vosatka, Janssen Research & Development, LLC, Raritan, NJ, USA.
Stephan Schmidt, Department of Pharmaceutics, University of Florida, Orlando, FL, USA.
Jeffrey I. Weitz, McMaster University and the Thrombosis & Atherosclerosis Research Institute, Hamilton, ON, Canada
Dirk Garmann, Bayer AG, Wuppertal, Germany.
Gary Peters, Janssen Research & Development, LLC, Raritan, NJ, USA.
References
- 1. European Medicines Agency. Rivaroxaban summary of product characteristics, www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf (accessed 8 July 2019).
- 2. Mueck W, Stampfuss J, Kubitza D, et al. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014; 53: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samama MM, Contant G, Spiro TE, et al. Laboratory assessment of rivaroxaban: a review. Thromb J 2013; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 2009; 374: 29–38. [DOI] [PubMed] [Google Scholar]
- 5. Gibson CM, Mega JL, Burton P, et al. Rationale and design of the Anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J 2011; 161: 815–821. e6. [DOI] [PubMed] [Google Scholar]
- 6. Weinz C, Schwarz T, Kubitza D, et al. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009; 37: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 7. Clark B. Biology of renal aging in humans. Adv Ren Replace Ther 2000; 7: 11–21. [DOI] [PubMed] [Google Scholar]
- 8. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284: 835–842. [DOI] [PubMed] [Google Scholar]
- 9. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366: 9–19. [DOI] [PubMed] [Google Scholar]
- 10. Fox KA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014; 4: e004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000; 102: 2031–2037. [DOI] [PubMed] [Google Scholar]
- 12. Willmann S, Zhang L, Frede M, et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst Pharmacol 2018; 7: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stampfuss J, Kubitza D, Becka M, et al. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther 2013; 51: 549–561. [DOI] [PubMed] [Google Scholar]
- 14. Byon W, Sweeney K, Frost C, et al. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol 2017; 6: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nyberg J, Karlsson KE, Jonsson S, et al. Edoxaban exposure-response analysis and clinical utility index assessment in patients with symptomatic deep-vein thrombosis or pulmonary embolism. CPT Pharmacometrics Syst Pharmacol 2016; 5: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015; 385: 2288–2295. [DOI] [PubMed] [Google Scholar]
- 17. Yin OQ, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure-response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol 2014; 70: 1339–1351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Riva_TDM_ACS_Supplemental_material_29Aug18 for Influence of model-predicted rivaroxaban exposure and patient characteristics on efficacy and safety outcomes in patients with acute coronary syndrome by Liping Zhang, Xiaoyu Yan, Partha Nandy, Stefan Willmann, Keith A. A. Fox, Scott D. Berkowitz, Amarnath Sharma, Anne Hermanowski-Vosatka, Stephan Schmidt, Jeffrey I. Weitz, Dirk Garmann and Gary Peters in Therapeutic Advances in Cardiovascular Disease