Short abstract
Background
Current data for the use of dimethyl fumarate (DMF) in Japanese patients with relapsing–remitting multiple sclerosis (RRMS) is limited.
Objectives
To assess the efficacy of DMF in Japanese patients with RRMS.
Methods
The phase 3, multinational APEX study (ClinicalTrials.gov identifier: NCT01838668) consisted of two parts: a 24-week double-blind part where subjects were randomized to receive DMF 240 mg or placebo twice daily in East Asian and Eastern European countries, and an open-label extension part where all subjects received DMF. The primary endpoint was the total number of new gadolinium-enhancing lesions in Weeks 12–24. In this interim analysis, we report efficacy data in the Japanese subgroup (DMF n = 56; placebo n = 58) over 72 weeks, including an extension phase.
Results
DMF reduced the total number of new gadolinium-enhancing lesions in Weeks 12–24 by 85% versus placebo (p < 0.0001). At Week 24, the annualized relapse rate was also reduced by 48% with DMF, versus placebo. DMF reduced the probability of relapse from Week 8 and was sustained. The number of gadolinium-enhancing lesions was maintained through 72 weeks.
Conclusions
DMF demonstrated sustained efficacy in this Japanese subgroup. The results were consistent with those observed in studies of DMF enrolling primarily Caucasian patients.
Keywords: APEX, delayed release, dimethyl fumarate, efficacy, Japanese patients, relapsing–remitting multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, immune mediated disease of the central nervous system (CNS) which results in neurodegeneration and neurological disability.1 The majority of individuals with MS (80–85%) have relapsing–remitting (RRMS) disease progression with defined clinical exacerbations of neurological symptoms.2 The global estimation of prevalence is 30 per 100,000 persons.2 A recent study indicated an increasing diagnosis of MS in Japan, with an estimated prevalence of 18.6 per 100,000 persons.3,4
Currently MS is incurable; however, effective strategies utilizing disease-modifying drugs (DMDs) are available to alter the course of disease, manage symptoms, and treat exacerbations.5 In Japan, DMDs available include interferon (IFN)-β (1a and 1b), natalizumab, glatiramer acetate, fingolimod, and dimethyl fumarate (DMF). Traditional DMDs such as IFN-β and glatiramer acetate require subcutaneous or intramuscular injections, which may significantly impact compliance to treatment.5,6 DMF, an oral DMD, has recently been approved in the USA, EU, and Japan for the treatment of RRMS.7
DMF is a derivative of fumaric acid with anti-inflammatory, cytoprotective, and immunomodulating properties. While the mechanism of action of DMF is not fully understood, it is thought to act on the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and the hydroxycarboxylic acid receptor 2 (HCAR2) receptors.8–10 DMF is thought to exert a neuroprotective effect by activating the transcription factor Nrf2, which modulates the expression of antioxidant genes including NADPH quinoline oxidoreductase-1 (NQO-1).11 Its anti-inflammatory effect is thought to be due to inhibition of transcription of the nuclear factor (NF)-κB signaling pathway via HCAR2.12 DMF is hydrolyzed rapidly to its active metabolite monomethyl fumarate (MMF) by esterases in the small intestine.11,12
MMF is an agonist of HCAR2, a G protein-coupled membrane receptor expressed by a variety of immune cells including dendritic cells, microglia, and macrophages. Activation of HCAR2 by MMF causes a switch in the activated microglia from its normal pro-inflammatory activity to an alternate neuroprotective form, thus showing an anti-inflammatory and neuroprotective effects as the other effect of HCAR2, flushing, which is one of the specific adverse events during DMF treatment caused by HCAR2 expressed by keratinocytes and epidermal Langerhans cells, and a signaling pathway, which may modulate microglial activation causing an upregulation of neuroprotective pro-inflammatory markers and subsequently target synaptic dysregulation and be also related to inflammatory cells infiltration in the CNS.11 In concert, these factors may lead to cytoprotective effects in neurons and glial cells and exert immunomodulatory actions.13
The efficacy of DMF was assessed in two pivotal 2-year, double-blind, placebo-controlled, multinational, phase 3 studies, DEFINE (n = 1237) and CONFIRM (n = 1430).14,15 Both studies demonstrated significant improvements in clinical endpoints and magnetic resonance imaging (MRI) measurements compared with placebo. Dosing of 240 mg twice daily and three times daily showed similar efficacy, hence 240 mg twice daily was chosen for dosing.16 Patients enrolled in the DEFINE and CONFIRM studies could also participate in an open-label extension study (ENDORSE). The 5-year interim analysis of ENDORSE demonstrated low clinical and MRI disease activities in patients with RRMS, indicating sustained treatment benefit and an acceptable safety profile with DMF.17
APEX was a phase 3 study conducted in patients with RRMS from East Asia (including Japan) and Eastern Europe to evaluate the efficacy and safety of DMF. Primary results of this study showed that DMF had a favorable benefit–risk profile. Overall, DMF demonstrated improved efficacy in MRI and clinical endpoints, and an acceptable safety profile. There is insufficient data on DMF in Japanese patients, and this is the first randomized clinical trial to include large numbers of Japanese patients. The results on the safety and tolerability of DMF in Japanese patients with RRMS from the 72-week APEX study have already been published.18 Overall, treatment was well tolerated, with a lower incidence of flushing and related symptoms and lower decrease in lymphocyte counts compared with previous studies of DMF that mainly enrolled Caucasian patients.18 This sub-analysis aimed to evaluate the efficacy of DMF in Japanese patients with RRMS enrolled in the APEX study.
Materials and methods
Study design and participants
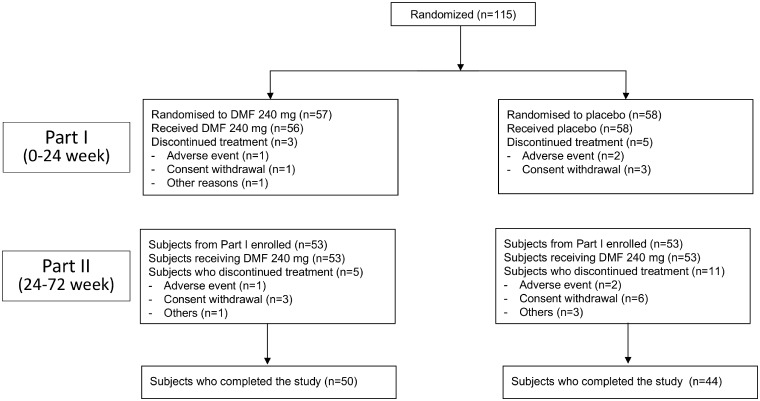
APEX (ClinicalTrials.gov identifier, NCT01838668) was a two-part study conducted at 54 sites located in the Asia-Pacific region (Japan, South Korea, and Taiwan), as well as from the Czech Republic and Poland. In Part I of the study, subjects were randomized using a centralized interactive voice/web response system (IXRS) (1:1) in a double-blinded manner to receive 24 weeks of treatment with oral DMF 240 mg or placebo twice daily. Subjects in the treatment arm were giving a starting dose of DMF 120 mg twice daily, which was increased to a maintenance dose of 240 mg twice daily after 7 days. Those who completed Part I were eligible to continue with Part II, an open-label extension study for 4.5 years in which all subjects are receiving DMF 240 mg twice daily (Figure 1).
Figure 1.
CONSORT diagram of the study design.
Subjects were enrolled in the study if they were aged 18–55 years, with a diagnosis of RRMS, as defined by the McDonald criteria 1–4,19 had an Expanded Disability Status Scale (EDSS) score of 0.0–5.0, and experienced disease activity within the 12 months prior to randomization, evidenced by at least one relapse or gadolinium-enhancing (Gd+) lesion of the brain on an MRI performed within 6 weeks prior randomization. Key exclusion criteria were primary or secondary progressive or progressive relapsing MS,20 diagnosis or history of neuromyelitis optica, prior treatment with natalizumab, fingolimod, azathioprine, plasmapheresis within 6 months prior to randomization, or prior treatment with IFNβ-1a, 1b or glatiramer acetate within 3 months prior to randomization.
The study protocol was approved by the ethics committee at the participating institutions and complied with guidelines and regulations, in accordance with the Declaration of Helsinki. All participants provided written informed consent before any evaluations or procedures were conducted.
Outcomes
The primary endpoint for Part I of APEX was the total number of new Gd+ lesions over four brain MRI scans at Weeks 12, 16, 20 and 24. Secondary endpoints included the total number of new Gd+ lesions from baseline to Week 24 and the number of new or newly enlarging T2 hyperintense lesions at Week 24, compared with baseline. As additional endpoints, the total number of new T1 hypointense lesions at Week 24 compared to baseline and the number of Gd+ lesions at Week 24 was also evaluated. Clinical assessments, including neurological examinations and EDSS scoring, were completed at baseline and at Weeks 12 and 24. The probability of relapse, the proportion of subjects who had a relapse by Week 24, the time to first relapse, and the annualized relapse rate (ARR) were also investigated. The ARR was calculated as the total number of relapses in each treatment group divided by the total number of days on treatment for the group, and the ratio multiplied by 365. Relapses were defined as new or recurrent neurologic symptoms, not associated with fever or infection, lasting 24 h or more, and accompanied by new neurological findings, assessed objectively. The treatment for relapse was 3–5 days of intravenous methylprednisolone, dosed at 1000 mg/day. Efficacy was also assessed in Part II of the study as an additional endpoint using MRI endpoints (total number of new Gd+ lesions, number of new or newly enlarging T2 hyperintense lesions, number of new T1 hypointense lesions) at Week 48, and yearly thereafter. Finally, EDSS evaluations were carried out in Part II at Weeks 36, 48 and every 24 weeks thereafter. Part II of APEX is ongoing; therefore, the data cut-off for this interim analysis was at Week 72 (24 weeks in Part I and 48 weeks in Part II).
MRI measurements of the brain were conducted at baseline and every 4 weeks during the treatment period to measure the number of Gd+ lesions, the T2 lesion volume (all MRI visits), the number of new or newly enlarging T2-hyperintense lesions, and the number of new T1-hypointense lesions (at baseline and Week 24). MRIs were not to be performed within 28 days after a course of steroids. If data were obtained within 28 days, inclusive, following an intravenous methylprednisolone treatment, the observed values at such visits were not used in the analysis to minimize any confounding effect of corticosteroids on Gd+ lesions. To ensure the quality and consistency, MRI capability of all investigational sites was validated by a central MRI reading center (NeuroRx Research). MRI technicians at study sites and the central MRI reading center were blinded to subjects’ treatment assignments.
Statistical analysis
The total (cumulative) number of new Gd+ lesions at Weeks 12, 16, 20, and 24, the number of Gd+ lesions at Week 24, new/newly enlarging T2 hyperintense and T1 hypointense lesions were summarized using descriptive statistics (mean, SD, median, and range) for each treatment group. Comparisons of the total number of new Gd+ lesions between treatment groups were made using negative binomial regression, with adjustment for baseline number of Gd+ lesions. Lesion mean ratio (DMF/placebo) and 95% confidence interval (CI) from the negative binomial regression model and treatment effect (% reduction in lesion mean) were calculated. Sensitivity analysis was performed using a non-parametric method; the Wilcoxon rank sum test was used. Because of the small sample size, formal statistical analysis was performed only on data for the number of Gd+ lesions.
The ARR was presented with its 95% CI and analyzed using negative binomial regression for treatment groups in the model, adjusted for age (≥40 vs. <40 years), baseline EDSS (≤2.0 vs. >2.0), and number of relapses in the 1 year prior to study entry. A post hoc evaluation of EDSS scores was made but not subjected to formal statistical comparison.
Results
Part I
From the total APEX population, 114 subjects from Japan were randomized to receive DMF (n=56) or placebo (n = 58). The baseline demographics and MS disease characteristics of subjects were well balanced between treatment groups (Table 1).
Table 1.
Baseline demographic characteristics of the Japanese patients enrolled in APEX.
| Part I |
Part II |
|||
|---|---|---|---|---|
| Placebo(n = 58) | DMF(n=56) |
Placebo/DMF (n = 53) |
DMF/DMF(n = 53) | |
| Age, years | 36.4 (7.24) | 38.4 (8.16) | 36.1 (7.3) | 38.3 (8.2) |
| Female, n (%) | 46 (79) | 44 (79) | 42 (79) | 41 (77) |
| BMI, kg/m2 | 21.5 (3.6) | 22.1 (3.5) | 21.6 (3.7) | 22.2 (3.6) |
| Time since first MS symptom, years | 7.1 (6.4) | 8.1 (5.9) | – | – |
| Relapses in prior year | 1.3 (0.6) | 1.5 (0.7) | – | – |
| Relapses in last 3 years | 2.3 (1.7) | 2.7 (1.9) | – | – |
| EDSS score | 1.8 (1.3) | 1.9 (1.3) | 1.8 (1.4) | 1.9 (1.4) |
| History of prior treatment for MS, n (%) | 31 (53) | 31 (55) | – | – |
| Presence of Gd+ lesions, n (%) | 25 (43) | 23 (41) | 14 (26)a | 4 (8)b |
| Number of Gd+ lesions | 1.6 (3.3) | 1.3 (2.6) | 0.7 (1.2)a | 0.2 (0.6)b |
| T2 hyperintense lesions volume, cm3 | 8.1 (8.9) | 5.7 (7.3) | 8.3 (10.1)a | 5.8 (7.6)b |
Results presented as mean (SD), unless otherwise stated.
an = 43.
bn = 47.
BMI, body mass index; DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; MS, multiple sclerosis.
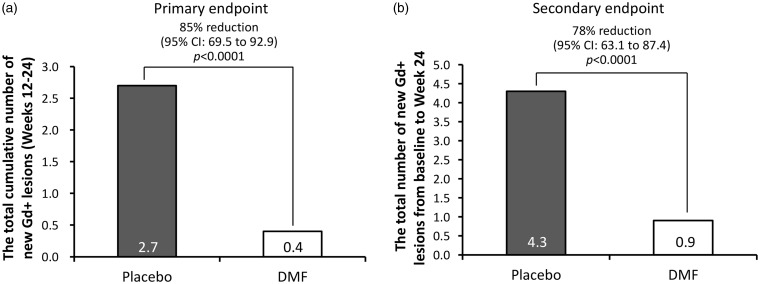
At the primary endpoint, the total number of Gd+ lesions in Weeks 12–24 was 2.7 for placebo and 0.4 for DMF treatment groups, corresponding to an 85% relative reduction (95% CI: 69.5–92.9) in subjects receiving DMF compared with those receiving placebo (p < 0.0001; Figure 2(a)). Approximately 80% of DMF-treated subjects did not develop new Gd+ lesions during Weeks 12–24 compared with 41% of subjects in the placebo group. The secondary endpoint of the total number of new Gd+ lesions from baseline to Week 24 was 4.3 for placebo and 0.9 for the DMF group (78% reduction, 95% CI: 63.1–87.4; p < 0.0001; Figure 2(b)). The adjusted mean number of newly enlarging T2-hyperintense lesions at Week 24 (1.4 in DMF, 3.7 in placebo) were also reduced by 63% (95% CI: 40.2–77.4; Table 2), in subjects receiving DMF versus placebo. There was no difference in the number of new T1 hypointense lesions between DMF and placebo. Furthermore, the risk of relapse at Week 24 was significantly reduced by 56% (95% CI: 13.1–77.4) in the DMF group, compared with placebo (Table 2). The adjusted ARR over 24 weeks were 1.17 (95% CI: 0.79–1.74) in the placebo group and 0.60 (95% CI: 0.36–1.01) in the DMF group. The rate ratio (DMF/placebo) was 0.52 (95% CI: 0.29–0.93), representing a 48% reduction (95% CI: 7.4–71.2) in ARR. Finally, time to first relapse showed a sustained reduced probability of relapse with DMF beginning at Week 8 with a hazard ratio of 0.44 (95% CI: 0.23–0.87).
Figure 2.
The number of new gadolinium-enhancing (Gd+) lesions at (a) Weeks 12–24 and (b) Weeks 0–24.
CI, confidence interval; DMF, dimethyl fumarate.
Table 2.
Summary of efficacy outcomes of the Japanese patients enrolled in APEX.
| Part I | Placebo(n = 58) | DMF(n = 56) | Reduction rate, % (95% CI) | |
|---|---|---|---|---|
| Mean number of new Gd+ lesionsa | ||||
| Weeks 12–24 | 2.7 | 0.4 | 85.3 (69.5, 92.9) | |
| Weeks 0–24 | 4.3 | 0.9 | 78.4 (63.1, 87.4) | |
| Adjusted mean number of new or newly enlarging T2 hyperintense lesions at Week 24b | 3.7 | 1.4 | 63.2 (40.2, 77.4)c | |
| Adjusted mean number of new T1 hypointense lesions | ||||
| Weeks 0–24 | 1.6b | 1.3b | 19.0 (–47.9, 55.6)c | |
| Adjusted ARRd | 1.17 | 0.60 | 48.4 (7.4, 71.2)c | |
| Proportion of patients with relapse at Week 24, % | 45.1 | 26.2 | 55.7 (13.1, 77.4)c | |
|
Placebo/DMF (n = 53) |
DMF/DMF (n = 53) |
|||
|
Part II |
Weeks 24 |
Week 48 |
Week 24 |
Week 48 |
| Mean (SD) number of Gd+ lesionse | 0.7 (1.2) | 0.1 (0.3) | 0.2 (0.6) | 0.3 (1.1) |
| Mean (SD) number of new or newly enlarging T2 hyperintense lesionse | 3.9 (4.5) | 1.3 (2.6) | 1.7 (3.0) | 0.9 (2.5) |
| Mean (SD) number of new T1 hypointense lesionse | 2.0 (3.3) | 1.0 (1.8) | 1.3 (2.2) | 0.3 (0.8) |
|
Week 0–24 |
Week 24–72 |
Week 0–24 |
Week 24–72 |
|
| ARR | 1.2 | 0.41 | 0.60 | 0.35 |
| Proportion of patients relapsed through Week 72, % | 52.7 | 43.5 | ||
aAdjusted for baseline number of Gd+ lesions.
bAdjusted for baseline volume of T2 lesions.
cThe value at Week 24 was adapted from Part I population.
dAdjusted for baseline EDSS (≤2.0 vs >2.0), baseline age (<40 vs ≥40), and number of relapses in the 1 year prior to study entry.
en=44 in placebo/DMF and n=49 in DMF/DMF.
ARR, annualized relapse rate; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing.
Part II
A total of eight subjects from Part I discontinued the study due to adverse events (3 subjects), consent withdrawal (4 subjects), and other causes (1 subject), so 106 Japanese subjects were enrolled in Part II of the study (n = 53 in each treatment group).
In subjects who received placebo in Part I then DMF in Part II (placebo/DMF), the mean (SD) number of Gd+ lesions was 0.7 (1.2) at Week 24 and 0.1 (0.3) at Week 48, while subjects who received DMF in Part I followed by DMF in Part II (DMF/DMF) had a mean of 0.2 (0.6) Gd+ lesions at Week 24 and 0.3 (1.1) at Week 48 (Table 2). The mean number of new or newly enlarging T2 hyperintense lesions was 3.9 (4.5) at Week 24 and 1.3 (2.6) at Week 48 and the mean number of T1 hypointense lesions was 2.0 (3.3) at Week 24 and 1.0 (1.8) at Week 48 in subjects in the placebo/DMF group. The mean number of T2 hyperintense lesions in subjects in the DMF/DMF group was 1.7 (3.0) at Week 24 and 0.9 (2.5) at Week 48, and the mean number of T1 hypointense lesions was 1.3 (2.2) at Week 24 and 0.3 (0.8) at Week 48 (Table 2).
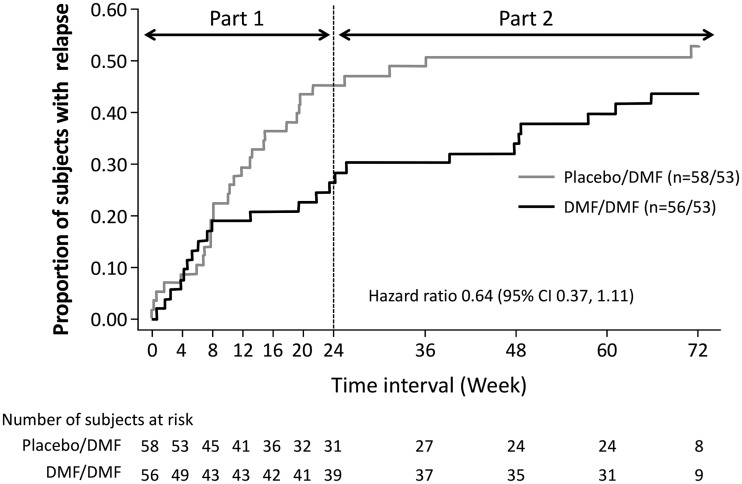
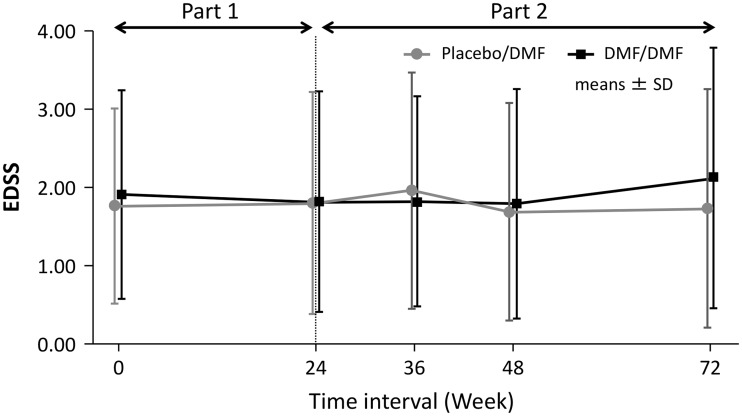
Based on the cumulative proportion of patients experiencing relapse, the introduction of DMF at Week 24 in subjects previously receiving placebo slowed the rate of relapse development (Figure 3). The mean/median change from baseline in the EDSS score was 0.03 ± 0.53/0.00 (–1.0, 2.0) in the placebo group versus –0.05 ± 0.48/0.00 (–1.5, 1.5) in the DMF 240 mg group at Week 12, and –0.05 ± 0.65/0.00 (–2.0, 2.0) in the placebo group versus –0.02 ± 0.7/0.00 (–2.0, 2.0) in the DMF 240 mg group at Week 24 in Part I. The mean/median change from baseline in the EDSS score from Week 24 to Week 72 was –0.20 ± 0.854/0.00 (–2.0, 2.0) in placebo/DMF versus 0.22 ± 1.245/0.00 (–2.0, 6.5) in DMF/DMF. The mean EDSS scores (Figure 4) did not show worsening in either group throughout the 72 weeks in both periods. One patient in each group had disability progression based on the predefined MS disability criteria.
Figure 3.
Kaplan–Meier estimate of the probability of relapse in Parts I and II of the APEX study, up to Week 72, for Japanese subjects in placebo/dimethyl fumarate (DMF) and DMF/DMF groups.
Figure 4.
Expanded Disability Status Scale (EDSS) of Japanese subjects over 72 weeks in both Parts I and II.
DMF, dimethyl fumarate.
Discussion
This interim sub-analysis of APEX investigated the efficacy of DMF in Japanese subjects with RRMS. The results demonstrated the sustained efficacy of DMF on MRI lesions (Gd+ and T2) and clinical endpoints, as measured by relapse rates.
Part I of the study showed that, in Japanese subjects, DMF led to a significant decrease in the cumulative number of new Gd+ lesions on scans obtained from Week 12 to 24 by 85%, compared with placebo. This was consistent with reduction observed in the overall patient population.21 A similar effect on Gd+ lesions was shown for Japanese subjects with RRMS compared with East European patients in APEX study, which emphasized the robustness of the results. The total number of new Gd+ lesions from baseline to Week 24 and the number of new or newly enlarging T2 hyperintense at Week 24, compared with placebo, was also significantly reduced by 78% and 63%, respectively, but the number of new T1 hypointense lesions at Week 24 was not significantly reduced in the DMF versus placebo group. These data are consistent with published literature. In a global phase 2b study, it was found that patients receiving 240 mg three times daily had a 69% reduction of Gd+ lesions and a 48% reduction of T2 hyperintense lesions at Week 24, compared with those receiving placebo.22
At Week 24, the proportion of Japanese patients who relapsed in APEX was lower with DMF than placebo. This was also consistent with published literature. In a phase 2b study, a 32% reduction in ARR was shown for patients who received DMF 240 mg three times daily, compared with patients who received placebo.22 Subjects in the APEX study received DMF twice a day; however, the results may still be comparable as twice daily and three times daily dosing has shown similar efficacy.16
In Japanese subjects who entered Part II of APEX, subjects who switched from placebo to DMF demonstrated a reduction in MRI activity. Among subjects who continued DMF, the effect of DMF on reduction in MRI activity observed in Part I was maintained in Part II. At the end of Week 72 (Part I and Part II), the adjusted ARR was reduced in the placebo/DMF group and further reduced in the DMF/DMF group. Analysis of the proportion of subjects who relapsed during the 72-week period showed that 53% of subjects relapsed in the placebo/DMF group (45% in Part I) and 44% of subjects relapsed in the DMF/DMF group (26% in Part I). Clinical observations in Part II supported the MRI results. The efficacy results in the Japanese subjects were consistent with the overall population and consistent with previous phase 3 studies. The slope of the time-to-first-relapse curve was steeper in the placebo than the DMF group in Part I of the study, but the slope of the placebo curve flattened out in Part II when subjects were switched from placebo to DMF, highlighting the efficacy of DMF in these patients. At Week 72, the probability of relapse in the DMF/DMF group was lower than in the placebo/DMF group, indicating that early initiation of DMF may be beneficial for patients for 72 weeks. The ARR decreased from Part I (Weeks 0–24) to Part II (Weeks 24–72) in both groups, suggesting continued benefit with DMF over time.
In the present study, the median change in EDSS from baseline to Week 24 was 0.00 (–2.0, 2.0) in placebo group and 0.00 (–2.0, 2.0) in the DMF group in Part I; the median EDSS from Week 24 to Week 72 was 0.00 (–2.0, 2.0) in placebo/DMF and 0.00 (–2.0, 6.5) in DMF/DMF in Part II. Since the predefined criteria of EDSS worsening for the study was a change of >0.5, the results of EDSS score showed that majority of the subjects were clinically stable. In addition, given that only one subject in each group had disability progression, these results need to be evaluated in long-term or large sample size studies.
Preclinical studies have shown that DMF, via Nrf2 and HCAR2 pathways, reduces inflammatory responses and exerts cytoprotective and immunomodulatory effects.8,13 These mechanisms may contribute to the beneficial effects of DMF in patients with RRMS, in particular MRI measurements and clinical relapses. Overall, the results from this interim sub-analysis in Japanese patients of the APEX study support DMF as a first-line oral treatment for patients with RRMS, or a potential alternative treatment to injectable DMDs.23 DMF was approved as DMD in patients with MS in Japan based on the results of APEX and pivotal studies (DEFINE and COMFIRM).
The main limitation of the interim analysis was the limited sample size of the Japanese subgroup; due to this, the study was not powered for clinical endpoints. This may be mitigated in future ongoing all case post marketing surveillance studies conducted under real-world conditions, longer follow-up and comprehensive endpoints of the APEX study in Japan. Also, the change from baseline in the number of lesions observed during the study (Gd+, T2 hyperintense, and T1 hypointense) were not statistically analyzed, as an observation period of 24 weeks was not considered sufficient to perform statistical analysis.
In conclusion, the results of this interim sub-analysis of the Japanese patients enrolled in APEX demonstrated sustained efficacy in both MRI measures and clinical outcomes among patients taking DMF. Furthermore, the findings were consistent with those observed in studies of DMF enrolling primarily Caucasian patients.
Acknowledgments
We would like to thank Mimi Chan, PhD, of inScience Communications, Springer Healthcare, who wrote the first draft of this manuscript. Medical writing assistance was funded by Biogen Japan Ltd.
Contributor Information
T Kondo, Department of Neurology, Kansai Medical University Medical Center, Osaka, Japan.
M Hase, Biogen Japan Ltd., Tokyo, Japan.
S Torii, Biogen Japan Ltd., Tokyo, Japan.
Conflicts of Interest
TK received research support from Bayer Yakuhin Ltd., Biogen Japan Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K., and Takeda Pharmaceutical Co. Ltd., and speaker honoraria from Bayer Yakuhin Ltd., Biogen Japan Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Co., and Novartis Pharma K.K. IK received research funding, travel and/or speaker honoraria from Novartis Pharma K.K., Bayer Yakuhin Ltd., Mitsubishi Tanabe Pharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Ono Pharmaceutical Co., Ltd., Alexion Pharmaceuticals, Inc., Japan Blood Products Organization, and Astellas Pharma Inc., and honoraria as a scientific advisory board member honoraria from Biogen Japan Ltd. and Takeda Pharmaceutical Co. Ltd. YO and ST are employees of Biogen Japan. Ltd. and hold stock in Biogen Inc. MH and KH were employees of Biogen Japan and is a stockholder of Biogen Inc. JY and AM were employees of Biogen Inc. and hold stock in Biogen Inc.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Biogen.
References
- 1.Nylander A, Hafler D. Multiple sclerosis. J Clin Invest 2012; 122: 1180--2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Atlas: multiple sclerosis resources in the world 2008 World Health Organization, 2008.
- 3.Houzen H, Kondo K, Horiuchi K, et al. Consistent increase in the prevalence and female ratio of multiple sclerosis over 15 years in northern Japan. Eur J Neurol 2018; 25: 334–339. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima I, Fukazawa T, Ota K, et al. Two subtypes of optic-spinal form of multiple sclerosis in Japan: clinical and laboratory features. J Neurol 2007; 254: 488–492. [DOI] [PubMed] [Google Scholar]
- 5.Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs 2008; 68: 2445–2468. [DOI] [PubMed] [Google Scholar]
- 6.Girouard N, Soucy N. Patient considerations in the management of multiple sclerosis: development and clinical utility of oral agents. Patient Prefer Adherence 2011; 5: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burness CB, Deeks ED. Dimethyl fumarate: a review of its use in patients with relapsing–remitting multiple sclerosis. CNS Drugs 2014; 28: 373–387. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA, Johnson D.A, Kraft AD, et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci 2008; 1147: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134: 678–692. [DOI] [PubMed] [Google Scholar]
- 10.Linker RA, Haghikia A. Dimethyl fumarate in multiple sclerosis: latest developments, evidence and place in therapy. Ther Adv Chronic Dis 2016; 7: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montes DG, Hupperts R, Fraussen J, et al. Dimethyl fumarate treatment in multiple sclerosis: Recent advances in clinical and immunological studies. Autoimmun Rev 2018; 17: 1240–1250. [DOI] [PubMed] [Google Scholar]
- 12.Ruggieri S, Tortorella C, Gasperini C. Pharmacology and clinical efficacy of dimethyl fumarate (BG-12) for treatment of relapsing–remitting multiple sclerosis. Ther Clin Risk Manag 2014; 10: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 2015; 130: 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 15.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 16.Fernández Ó, Giovannoni G, Fox RJ, et al. Efficacy and safety of delayed-release dimethyl fumarate for relapsing–remitting multiple sclerosis in prior interferon users: an integrated analysis of DEFINE and CONFIRM. Clin Ther 2017; 39: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 17.Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: interim analysis of ENDORSE, a randomized extension study. Mult Scler 2017; 23: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochi H, Niino M, Onizuka Y, et al. 72-Week safety and tolerability of dimethyl fumarate in Japanese patients with relapsing–remitting multiple sclerosis: analysis of the randomised, double blind, placebo-controlled, phase III APEX study and its open-label extension. Adv Ther 2018; 35: 1598–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 20.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 21.Saida T, Yamamura T, Kondo T, et al. A randomized placebo-controlled trial of delayed-release dimethyl fumarate in patients with relapsing–remitting multiple sclerosis from East Asia and other countries. BMC Neurol, 2019, 19: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008; 372: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 23.Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther 2017; 6: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]