Abstract
Conventional bone decalcification is a time-consuming process and is therefore unsuitable for clinical applications and time-limited research projects. Consequently, we compared the effect of four different decalcification solutions applied at three different temperatures, and assessed the rate of decalcification and the implications on tissue morphology and antigenicity of mouse and rat tibiae. Bones were decalcified with 10% ethylenediaminetetraacetic acid (EDTA), 10% formic acid, 5% hydrochloric acid, and 5% nitric acid at 4C, 25C, and 37C. Decalcification in both species was fastest in nitric acid at 37C and slowest in EDTA at 4C. Histological and immunohistochemical staining confirmed that the conventional protocols of EDTA at 4C and 25C remain the best option regarding the quality of tissue preservation. Whereas formic acid at 4C is a good alternative saving about 90% of the decalcification time, hydrochloric and nitric acids should be avoided particularly in case of rat tibia. By contrast, due to their smaller size, mouse tibiae had shorter decalcification times and tolerated higher temperatures and exposure to acids much better. In conclusion, this study demonstrated that depending on the specific research question and sample size, alternative decalcification methods could be used to decrease the time of decalcification while maintaining histological accuracy.
Keywords: EDTA, formic acid, hydrochloric acid, nitric acid, bone histology, immunohistochemistry, mineralized tissues, tibia, mouse, rat
Introduction
The histological evaluation of calcified tissues is performed in clinical and research laboratories worldwide and is essential in understanding both anatomic morphology and pathophysiology. Despite decades of improvements in the methods used for histological analyses, performing histology on bones remains a challenge. Complete decalcification, that is, the removal of calcium from the hydroxyapatite matrix of bone, is a time-consuming process that varies from days to months depending on the size of the specimen and the species used.1,2 Moreover, if an inappropriate decalcification method is used, there is a high potential for tissue damage. Consequently, it is very important to select a method that not only decalcifies bone rapidly but also avoids damaging the tissue morphology. The most commonly used decalcification solution is ethylenediaminetetraacetic acid (EDTA), a chelating agent that binds calcium ions from the surface of the hydroxyapatite crystals of the bone, which causes minimal damage to the tissue during the decalcification process.3,4 However, decalcification in EDTA is rather slow, which is why it is considered unsuitable for clinical applications and time-limited research projects. Faster decalcification can be achieved using weak organic (e.g., formic acid) or stronger mineral acids (e.g., hydrochloric and nitric acids), which can be further accelerated by ultrasonication, or temperature, usually via microwave ovens.2,5–11
Mice and rats are the most commonly used vertebrates in basic science research.12,13 They serve as model organisms to study various pathologies to improve treatment strategies for the benefit of patients. The availability of transgenic mice and rats allows for studying the genetic and molecular mechanisms of congenital and acquired bone diseases.14,15 Similarly, rodent models are also used in orthopedic research focusing on injuries and diseases of the skeletal system, such as fracture healing, metabolic diseases (e.g., osteoporosis), and bone cancer. The tibial shaft is the most commonly fractured long bone and is more prone to an open fracture than the femur due to the tibia’s subcutaneous localization, which subjects it to direct injury and increases the risk of infection, delayed union, and nonunion.16 As a result, many closed and open tibial fracture models, in both mice and rats, have been developed to study bone healing.17,18 Most of these studies include various histological analyses, which require decalcification before processing the sample. Despite the improvements in bone decalcification and processing methods for histology, there is a need for a tissue-specific standardization of this process for research and diagnostic purposes, particularly in tibia.19 Previously published studies comparing different decalcification methods for mineralized tissues mostly used teeth and mandibular bone of human and rat origin20–25 or rat femurs.10,26,27 Therefore, the main goal of this study was to evaluate and compare commonly used decalcification methods for mouse and rat tibiae. To investigate this, micro–computed tomography (microCT) was used to accurately measure rates of decalcification for exact demineralization end points and to determine the effects different decalcification solutions and temperatures have on the preservation of tissue morphology and antigenicity of mouse and rat bone tissue.
Methods
Specimen Collection and Decalcification
C57BL/6 mice and Wistar rat tibiae matched by age and gender (4–5 months, males) were harvested from sacrificed animals acquired through the tissue use notification program of the local animal ethics committee, Queensland University of Technology, Australia (#1400000377). After the removal of soft tissue, mouse and rat tibiae were fixed in 4% paraformaldehyde (PFA) at 4C and placed on agitation equipment at 400 rpm for 24 and 48 hr, respectively. A total of three tibiae per treatment group were collected. Four different decalcification solutions were used: 10% EDTA, pH 7.4 (Sigma-Aldrich; Castle Hills, Australia), 10% formic acid (Sigma-Aldrich), 5% hydrochloric acid (Merck; Bayswater, Australia), and 5% nitric acid (Merck). The decalcification rate of the four solutions was compared at 4C, 25C (room temperature), both with agitation, or 37C in a microwave (KOS Multifunctional Microwave Tissue Processor, Milestone S.r.l.; Sorisole, Italy) at 150 W and 400 rpm. Before decalcification, each sample was weighed and placed in 50 ml of decalcifying solution and regularly monitored as indicated in Table 1. EDTA was replaced every 72 hr, whereas the acid solutions were kept unchanged until the decalcification was complete.
Table 1.
Monitoring Frequency for the Different Decalcification Conditions for Mouse and Rat Specimens.
| Decalcification Solution/Temperature | Mouse |
Rat |
||||||
|---|---|---|---|---|---|---|---|---|
| 10% EDTA, pH 7.4 | 10% Formic Acid | 5% HCl | 5% Nitric Acid | 10% EDTA, pH 7.4 | 10% Formic Acid | 5% HCl | 5% Nitric Acid | |
| 4C | 24 hr | 4 hr | 4 hr | 2 hr | 1 week | 12 hr | 4 hr | 1 hr |
| 25C | 24 hr | 4 hr | 4 hr | 2 hr | 1 week | 12 hr | 4 hr | 1 hr |
| 37C | 12 hr | 2 hr | 2 hr | 1 hr | 24 hr | 6 hr | 3 hr | 30 min |
Abbreviation: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid.
Assessment of Decalcification
The weight of each specimen and the pH of the decalcification solution were recorded at each time point before scanning with microCT (μCT 40, SCANCO Medical AG; Brüttisellen, Switzerland) at an isotropic voxel size of 12 (mice) and 16 (rats) µm, an energy of 70 kV, and an integration time of 200 msec. The frequency of testing was determined based on a previous pilot study. Specimens remained in their respective decalcification solutions during microCT scanning. All bones were deemed decalcified when their bone volume (BV) had reached 0% as measured by microCT. On completion of decalcification in acid, the specimens were neutralized using sodium bicarbonate, rinsed in running tap water for 4 hr, and stored in 70% ethanol prior to histological processing.
Histology
Decalcified tissues were processed in a tissue processor (Thermo Fisher Australia Pty Ltd; Mulgrave, Australia) using ascending concentrations of ethanol (70%, 80%, 95%, 3 × 100%), three changes of xylene, and four changes of paraffin (60C) for 40 min per each solution. The samples were sectioned (Leica RM2245, Leica Microsystems Pty Ltd; Macquarie Park, Australia) at a thickness of 5 µm after the specimens were soaked in ice water overnight. Tissue sections were mounted on poly-lysine slides and oven-dried at 45C for a minimum of 5 hr prior to staining. After deparaffinization in xylene and rehydration in graded ethanols (100–50%), hematoxylin and eosin (H&E) staining, Safranin O/Fast Green staining, and immunohistochemistry were performed.
H&E Staining
Following deparaffinization, slides were immersed in Mayer’s hematoxylin for 2 min, differentiated in 0.5% acid alcohol for 30 sec, and then bluing was performed in tap water. Slides were stained with eosin for 30 sec and quickly rinsed in running tap water. Dehydration was performed in ascending concentrations of graded ethanol (50–100%) and cleared by xylene before the slides were coverslipped.
Safranin O/Fast Green Staining
Deparaffinized slides were immersed in Weigert’s hematoxylin for 2 min, differentiated in 2% acid alcohol for 30 sec, stained with 0.05% Fast Green FCF for 7 min, rinsed in 1% acetic acid for 30 sec, and stained in 0.1% Safranin O solution for 7 min. The slides were dehydrated with ethanol and cleared by xylene before being coverslipped.
Immunohistochemistry
After deparaffinization, antigen retrieval was performed using Proteinase K (S3020, Agilent Technologies Australia Pty Ltd; Mulgrave, Australia) for 5 (mice) or 10 min (rats) at room temperature (collagen type I, von Willebrand factor [vWF]) or with heat-mediated antigen retrieval using citrate buffer (pH 6.0) for 12 min at 95C (osterix). Endogenous peroxidase was inhibited using 3% H2O2 (Sigma-Aldrich) for 15 min. Slides were then blocked using 2% bovine serum albumin (Sigma-Aldrich) at room temperature for 60 min. Primary antibodies were applied to the slides at room temperature for 1 hr using a dilution of 1:500 polyclonal rabbit anti–collagen type I antibodies (ab34710, Abcam plc; Cambridge, UK), 1:200 monoclonal rabbit anti-osterix (Sp7) antibodies (ab209484, Abcam plc), or 1:200 polyclonal rabbit anti-human vWF antibodies (IR527, Agilent Technologies Australia Pty Ltd). Following primary antibody incubation, slides were incubated with EnVision+Dual Link System-HRP Rabbit/Mouse secondary antibody (Agilent Technologies Australia Pty Ltd) for 1 hr at room temperature, and antibody binding was visualized with diaminobenzidine tetrahydrochloride chromogen (Agilent Technologies Australia Pty Ltd) substrate for 5 min. After counterstaining with a 1:10 dilution of Mayer’s hematoxylin for 30 sec, the slides were dehydrated through graded ethanols, cleared in xylene, and coverslipped. Negative controls using either a rabbit IgG isotype or omitting the primary antibody were included to validate the stainings.
Microscopy
Brightfield microscopy was performed using Leica DM6 B (Leica Microsystems Inc.; Buffalo Grove, IL). The slides were imaged, and representative regions of interest were selected.
Statistics
Comparisons of the groups were performed using one-way ANOVA. In case of significant differences (p<0.05), comparisons between the groups were further assessed with Bonferroni multiple-comparison test. Data were considered statistically significant at *p<0.05 and highly significant at **p<0.01.
Results
Decalcification Rate
Weight Loss
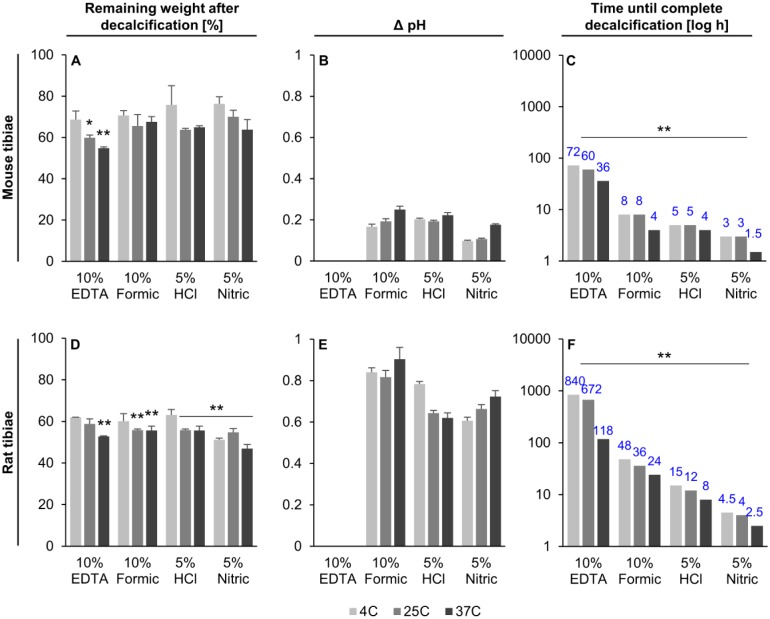
Weight loss was measured during decalcification as it is a commonly used method, although not very accurate, to determine when specimens had reached the end point. Intact mice and rat tibiae on average lost 33.2 ± 7.1% and 43.9 ± 10.4% of their initial weight during the decalcification process, respectively, regardless of the decalcification method used (Fig. 1A and D). The largest weight loss for both species was at 37C, independent of the solution used.
Figure 1.
Effect of the decalcification process on sample weight (A and D), increase in pH of the decalcifying solution (B and E), and the time until complete decalcification (C and F). Blue numbers indicate how many hours it took for complete decalcification in each sample group. Mean ± SD, n=3. Abbreviations: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid. *p<0.05, **p<0.01.
Change of pH
The pH of the solutions was recorded to assess their suitability to serve as an indicator for the progress of decalcification. An increase in pH over time was observed in all setups, except for samples that were decalcified in 10% EDTA, where it remained stable at pH 7.4. In all the other acids, the pH increase ranged between 0.09 and 0.25 for mouse, and between 0.61 and 0.9 for rat tibiae, depending on the temperature and acid used (Fig. 1B and E). In most groups, the samples that were decalcified at 37C showed the highest increase in pH compared with conventional methods. However, a final pH indicating an end point valid for all species could not be identified for any acids used. Therefore, the decalcification end point could not be accurately measured based on pH changes of the solutions.
MicroCT Imaging
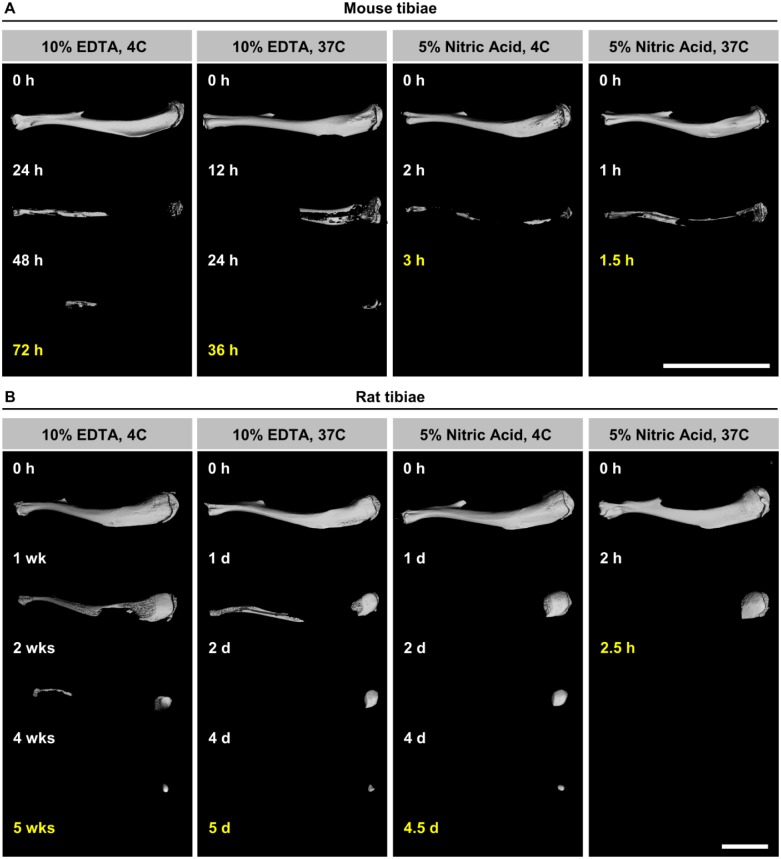
Complete decalcification of specimens was determined using data from microCT scans, which was achieved when the measured BV reached 0%. The slowest decalcification condition for both species was 10% EDTA at 4C, whereas the fastest decalcification was observed when the bones were decalcified with 5% nitric acid at 37C. Mouse (Figs. 1C and 2A) and rat (Figs. 1F and 2B) bone decalcification times ranged from 1.5 to 72 hr and 2.5 to 840 hr (5 weeks), respectively.
Figure 2.
Representative 3D microCT images of mouse (A) and rat (B) tibiae decalcified in 10% EDTA (slowest decalcification) and 5% nitric acid (fastest decalcification) at indicated time points with end points in yellow. Mineralized bone matrix appears white and decalcified bone is not visible. Scale bar = 1 cm. Abbreviations: 3D, three-dimensional; EDTA, ethylenediaminetetraacetic acid; microCT, micro–computed tomography.
MicroCT results demonstrated that an increase in temperature decreased the time until complete decalcification irrespective of the solution used. Overall, the increase in temperature from 4C to 37C resulted in time savings on average of about 42.5 ± 13.0% and 56.8 ± 17.0% for mouse and rat tibiae, respectively. Replacing 10% EDTA with 10% formic acid, 5% hydrochloric acid, or 5% nitric acid decreased the decalcification times by 89%, 93%, and 96% for mouse tibiae and by 94%, 98%, and 99% for rat tibiae. In comparison, an increase in temperature to 25C and 37C led to time savings of 17% and 50% in mouse samples and 20% and 86% in rat samples. In the acid groups, it was noted that decalcification at 37C was completed in half the time of the 4C group in both mouse and rat tibiae. In addition, the bone samples from both species followed the same trend, showing a gradient of decalcification rate, which increased from 10% EDTA to 10% formic acid, to 5% hydrochloric acid, and to 5% nitric acid. The proximal end of the tibia was the last part of the bone to decalcify (Fig. 2A and B).
Preservation of Tissue and Cell Morphology
The tissue and cellular morphology of mouse and rat tibiae decalcified in four different solutions at three different temperatures were compared. H&E was used for general assessment of cell and tissue morphology, whereas Safranin O/Fast Green was used to stain proteoglycans and glycosaminoglycans in cartilage.28 The analysis revealed differences in preservation quality of tissues related to the decalcification solutions and temperatures used.
Mouse
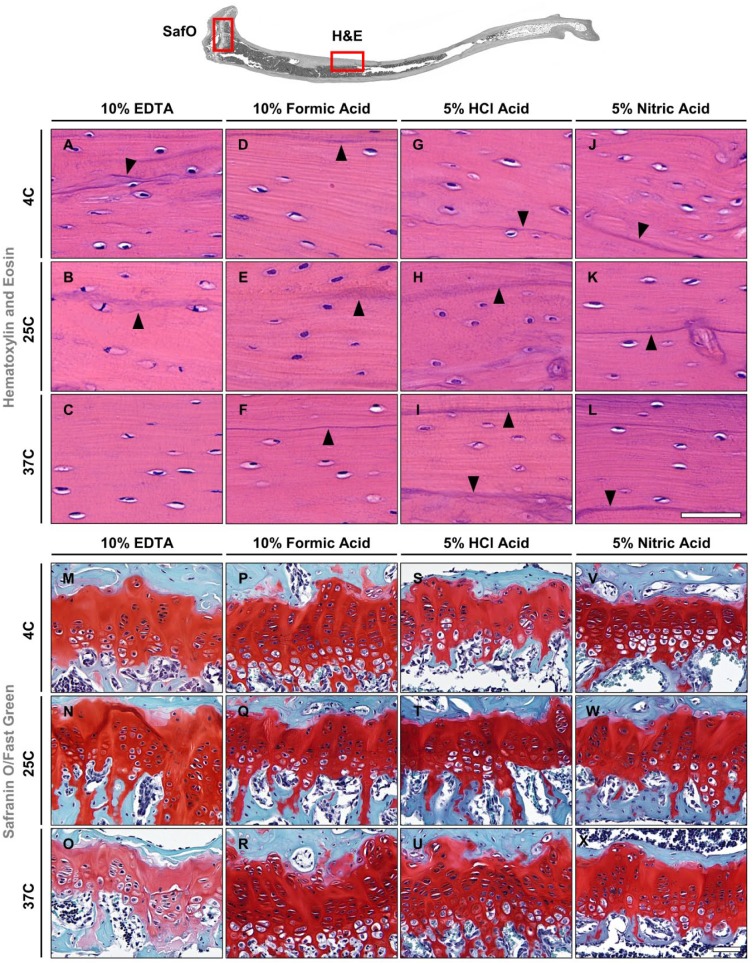
Excellent preservation of the tissue morphology was observed in all mouse tibiae decalcified in EDTA or formic acid at all temperatures. Staining of cell nuclei in bone, cartilage, bone marrow, and blood vessels did not show any signs of deterioration with either H&E or Safranin O/Fast Green stain (Fig. 3A–F, M–R, Supplemental Fig. S1A–F, M–R). In addition, epithelial cell and erythrocyte staining was observed in vascular tissue (Supplemental Fig. S1A–F). H&E-stained sections showed that cement lines, as well as lamellar bone, were intact and clearly defined in both EDTA and formic acid groups (indicated by arrowheads in Fig. 3A–F). Bright red staining of cartilage in the epiphyseal plate and green staining of the bone tissue were observed in Safranin O/Fast Green–stained sections for all decalcification conditions, and both cartilage and bone were well preserved in specimens decalcified in EDTA and formic acid for all conditions (Fig. 3M–R).
Figure 3.
Hematoxylin and eosin (A–L) and Safranin O/Fast Green (M–X) staining of mouse tibiae. Black arrowheads indicate cement lines. Scale bars = 50 μm. Abbreviations: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid.
Different degrees of damage to the tissue morphology were observed in specimens decalcified in hydrochloric or nitric acid for most conditions (Fig. 3G–L, S–X). Cement lines showed a darker hematoxylin stain and appeared less defined (indicated by arrowheads in Fig. 3G–L), whereas the vasculature tissue architecture showed evidence of tissue disruption. In H&E-stained sections, epithelial cells, erythrocytes, and osteocytes in the cortical bone had a slightly smudged and heterogeneous appearance in all hydrochloric acid groups and when using nitric acid at 37C, which resulted in loss of cellular detail. In contrast, osteocyte staining in the nitric acid groups decalcified at 4C and 25C was present in all decalcification conditions (Fig. 3G–L). Interestingly, there were no striking differences observed in the bone marrow, which appeared to be very similar independent of the decalcification protocol (Supplemental Fig. S1M–X). Generally, good tissue preservation of cartilage and bone was detected with Safranin O/Fast Green stain for all decalcification conditions in both hydrochloric and nitric acids; however, the cartilage matrix showed evidence of nonspecific nucleic staining. Instead of dark blue/black, the cell nuclei appeared rather green. Furthermore, in both of these groups, shrinkage of chondrocytes and lacunae was observed, which was less severe in the microwave groups (Fig. 3S–X). In the formic acid group, the cellular morphology still appeared intact, and the morphology of bone tissue did not show any discernible changes between the acid groups. The results for mouse tibiae are summarized in Table 2.
Table 2.
Preservation of Tissue and Cell Morphology of Mouse Tibiae Using Different Decalcification Conditions and Time Savings.
| Condition |
Tissue and Cell Morphology |
Antigenicity |
Time Saving |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decalcification Solution | Temp. (C) | Bone | Cartilage | Osteocytes | Chondrocytes | Bone Marrow Cells | Collagen Type I | Osterix | von Willebrand Factor | (%) |
| 10% EDTA | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 0 |
| 25 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 17 | |
| 37 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 50 | |
| 10% Formic | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 89 |
| acid | 25 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 3 | 89 |
| 37 | 4 | 4 | 4 | 4 | 3 | 3 | 2 | 2 | 94 | |
| 5% HCl | 4 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 93 |
| 25 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 93 | |
| 37 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 94 | |
| 5% Nitric | 4 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 96 |
| acid | 25 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 96 |
| 37 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 98 | |
Grading: 1, poor; 2, fair; 3, good; 4, excellent. Abbreviations: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid; Temp., temperature.
Rat
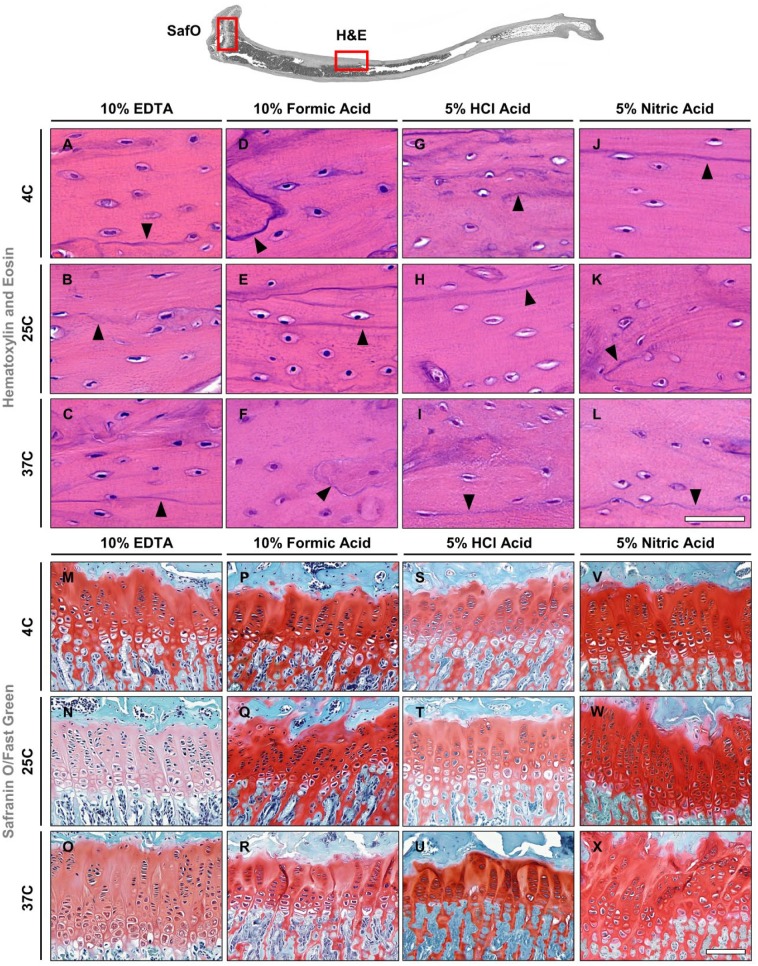
Compared with mouse tibiae, differences in the preservation quality of the tissue and cellular morphology in rat tibiae were found to be more severe between the various decalcification methods. Similar to mouse tibiae, samples decalcified in EDTA at 4C and 25C showed excellent morphology of both tissues and cells (Fig. 4A and B), whereas at 37C the cement lines appeared a bit fainter (Fig. 4C). Formic acid groups had a darker hematoxylin stain of the cortical bone, but in general, very good preservation of tissue and cellular morphology was seen at both 4C and 25C (Fig. 4D and E). An increase in temperature to 37C led to a weaker nuclear stain, less defined cement lines (indicated by arrowheads in Fig. 4A–E, F), and partial loss of the structural integrity of blood vessels in the form of fraying edges (compare Supplemental Fig. S2F with Fig. S2A–E). Tissue and cellular morphology showed different levels of deterioration for all hydrochloric and nitric acid groups (Fig. 4G–L, Supplemental Fig. S2G–L). Most evident was the loss of nuclear staining by hematoxylin in the bone marrow of all these groups (Supplemental Fig. S2S–X) and faded osteocyte staining in the cortical bone in all hydrochloric acid groups (Fig. 4G–I). In contrast, osteocyte staining in the nitric acid groups was observed for all conditions (Fig. 4J–L). Cement line (indicated by arrowheads in Fig. 4G–L), epithelial cell, and erythrocyte staining appeared smudged and were more evident in hydrochloric than in the nitric acid group, which resulted in improved preservation of tissue quality with increasing decalcification temperatures (Fig. 4J–L). Cartilage and bone morphology were well preserved in the EDTA and formic acid–decalcified specimens as evidenced by Safranin O/Fast Green staining (Fig. 4M–R). Epiphyseal cartilage and bone tissue stained bright red and green, respectively, in specimens for all decalcification conditions. Specimens decalcified in hydrochloric and nitric acid showed signs of tissue degradation and cytological damage (Fig. 4S–X). Cell nuclei were observed to stain green instead of black in chondrocytes for the 4C hydrochloric and all nitric acid groups, whereas cell nuclei stained black in all other decalcification conditions. Chondrocyte and cartilage lacunae shrinkage appeared to occur in the hydrochloric and nitric acid groups; however, the shrinkage was less severe in the microwave hydrochloric group. Furthermore, signs of shrinkage were observed in the formic acid group; despite this, the cellular morphology still appeared intact. Bone morphology staining with fast green did not show any discernible changes between the acid groups. Cartilage tissue morphology was well preserved when decalcified in EDTA regardless of the condition used; whereas formic acid revealed minor damage to cytological structures, tissue damage was observed in hydrochloric and nitric acid groups. Table 3 summarizes the results of the decalcification of rat tibiae.
Figure 4.
Hematoxylin and eosin (A–L) and Safranin O/Fast Green (M–X) staining of rat tibiae. Black arrowheads indicate cement lines. Scale bars: A–L = 50 μm; M–X = 100 μm. Abbreviation: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid.
Table 3.
Preservation of Tissue and Cell Morphology of Rat Tibiae Using Different Decalcification Conditions and Time Savings.
| Condition |
Tissue and Cell Morphology |
Antigenicity |
Time Saving |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decalcification Solution | Temp. (C) | Bone | Cartilage | Osteo-cytes | Chondro-cytes | Bone Marrow Cells | Collagen Type I | Osterix | von Willebrand Factor | (%) |
| 10% EDTA | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 0 |
| 25 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 20 | |
| 37 | 3 | 3 | 4 | 3 | 4 | 3 | 3 | 4 | 86 | |
| 10% Formic | 4 | 4 | 4 | 4 | 4 | 3 | 2 | 4 | 3 | 94 |
| acid | 25 | 4 | 4 | 3 | 4 | 3 | 2 | 4 | 4 | 96 |
| 37 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 97 | |
| 5% HCl | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 4 | 98 |
| 25 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | 99 | |
| 37 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 4 | 99 | |
| 5% Nitric | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 99 |
| acid | 25 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 99.5 |
| 37 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 99.7 | |
Grading: 1, poor; 2, fair; 3, good; 4, excellent. Abbreviations: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid; Temp., temperature.
Preservation of Antigenicity in Decalcified Bone Tissues
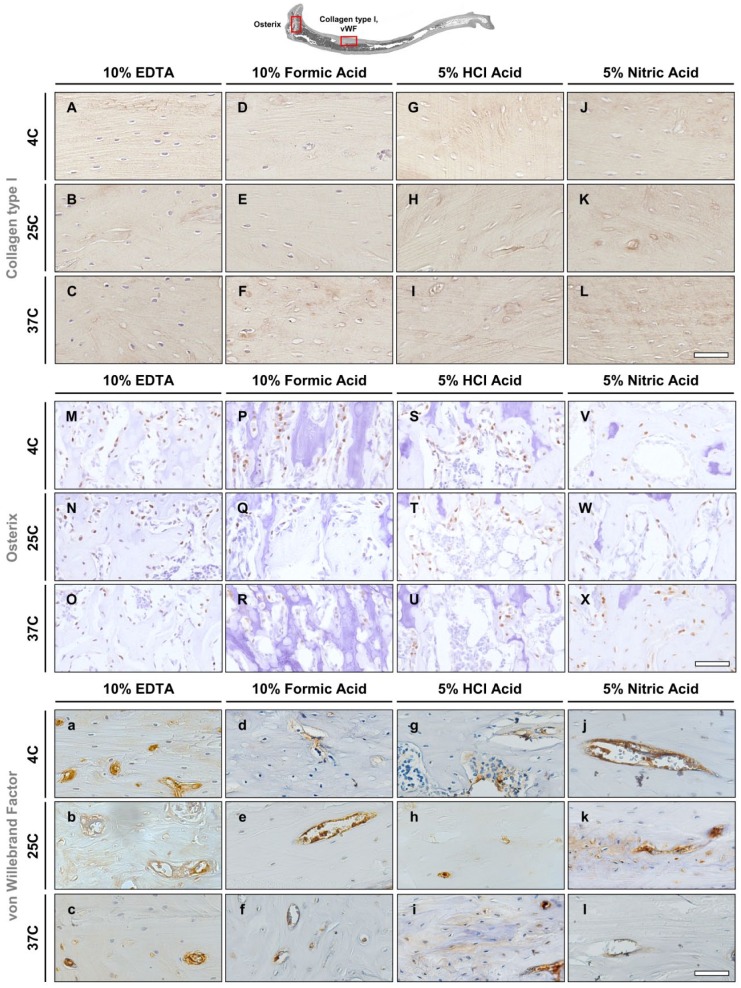
The preservation of antigens in mouse and rat bone tissues after decalcification under 12 different conditions was investigated to evaluate the tissues’ immunoreactivity. Collagen type I, being the most abundant protein in the body and main component of the organic part of bone, osterix, which is an essential transcription factor for osteoblast differentiation and bone formation, and vWF, a marker for vascular endothelial cells, were selected.
Mouse—Collagen Type I
The tissue antigenicity was well preserved in the EDTA and formic acid–decalcified specimens for all conditions. Mouse tibiae in EDTA showed positive staining of collagen type I in the bone matrix. Tissue architecture was well preserved and bone remodeling sites could be identified from the staining in all EDTA and formic acid groups (Fig. 5A–F). In contrast, specimens decalcified by hydrochloric and nitric acid showed evidence of degradation (Fig. 5G–L), which was evidenced by a lack of hematoxylin staining of the cell nuclei. Cement lines were stained in the conventional groups (4C and 25C) and absent in the microwave group (37C). Mouse tibiae specimens decalcified in EDTA at 37C or in formic acid had similar antigenicity preservation and staining patterns as the samples decalcified in EDTA at 4C.
Figure 5.
Immunohistochemistry in mouse tibiae. (A–L) Collagen type I stains the bone matrix. (M–X) Osterix stains nuclei of osteoblasts. (a–l) von Willebrand Factor is a marker for vascular endothelial cells. Scale bars = 50 μm. Abbreviation: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid.
Mouse—Osterix
Positive immunostaining was observed in cell nuclei of osteoblasts in samples from all decalcification groups with no major differences regarding the intensity of the signal (Fig. 5M–X). A slightly fainter staining was observed in samples decalcified in formic, hydrochloric, and nitric acids at 37C (Fig. 5R, U, X).
Mouse—von Willebrand Factor
Mouse tibiae decalcified in EDTA had well-preserved vascular tissue at all decalcification temperatures. Blood vessel endothelium and serum proteins stained positive for vWF (Fig. 5a–c). Vascular tissue was also well preserved in formic acid for all conditions with positive vWF staining detected inside blood vessels (Fig. 5d–f). Vascular tissue antigenicity was retained in all hydrochloric and nitric acid groups at 4C and 25C (Fig. 5g–l). However, endothelial cells had lost cellular detail as nuclei and cytoplasm staining was unable to be identified in hydrochloric acid decalcification at 25C (Fig. 5h) and in nitric acid decalcification at 37C (Fig. 5l). Nonspecific background staining was observed in the bone matrix in all acid groups (formic, hydrochloric, and nitric acid) (Fig. 5d–l). Differences in antigenicity and tissue morphology were noted to occur between EDTA and the acid groups. Compared with EDTA, the acid groups showed evidence of morphological damage and reduced antigenicity. The evaluation of antigen preservation in mouse tibiae is summarized in Table 2.
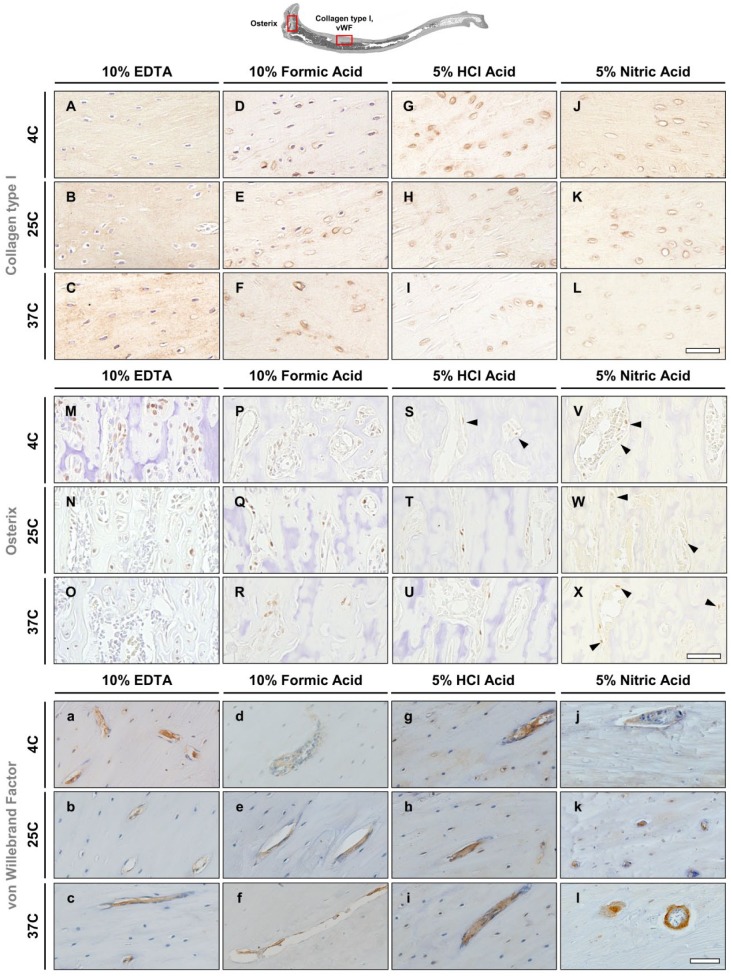
Rat—Collagen Type I
Collagen type I antigenicity was well preserved in rat specimens decalcified in EDTA. Positive immunoreactivity was observed throughout the bone matrix as indicated by brown staining of the bone matrix (Fig. 6A–C). The first signs of tissue deterioration were visible in bone samples decalcified in formic acid especially around cell nuclei, which appeared to increase with the temperature (Fig. 6D–F). Specimens in hydrochloric and nitric acids showed signs of tissue matrix damage as evidenced by partial loss of tissue morphology and nonspecific collagen type I staining (Fig. 6G–L). Deterioration of the lamellar bone matrix was observed in the hydrochloric and nitric acid microwave groups. Furthermore, damage was also observed in the hydrochloric acid groups decalcified at 4C and 25C, although it was possible to distinguish lamellar bone morphology. Although cell nuclei stained well in all EDTA groups as well as in formic acid groups at 4C and 25C, the hydrochloric and nitric acid groups’ cell nuclei staining was absent (Fig. 6G–L). The same was observed in samples decalcified with formic acid at 37C (Fig. 6F). Antigenicity of collagen type I in specimens decalcified by EDTA at 25C and in the microwave at 37C, and the formic acid groups at 4C and 25C did not show any differences to the routine EDTA condition (4C).
Figure 6.
Immunohistochemistry in rat tibiae. (A–L) Collagen type I stains the bone matrix. (M–X) Osterix stains nuclei of osteoblasts. Black arrowheads indicate osterix-positive osteoblasts. (a–l) von Willebrand is a marker for vascular endothelial cells. Scale bars = 50 μm. Abbreviation: EDTA, ethylenediaminetetraacetic acid; HCl, hydrochloric acid.
Rat—Osterix
Positive staining of osterix was detected in samples of all decalcification groups. However, best results were obtained in samples decalcified in EDTA at 4C, followed by EDTA at 25C, formic acid at 4C, and formic acid at 25C (Fig. 6M, N, P, Q). Weaker staining was observed in samples decalcified in EDTA and formic acid at 37C and in hydrochloric acid at 25C (Fig. 6O, R, T). Osterix-positive immunostaining was present but poorly visible in samples decalcified in hydrochloric acid at 4C (indicated by arrowheads in Fig. 6S), as well as in all nitric acid groups (indicated by arrowheads in Fig. 6V–X). No nonspecific background staining was seen in any of the samples; however, all samples decalcified in nitric acid appeared to have a yellow tint (Fig. 6V–X).
Rat—von Willebrand Factor
Blood vessel morphology and serum protein antigenicity of vWF were well preserved in rat tibiae specimens decalcified by EDTA and formic acid regardless of the decalcification conditions. Positive staining was localized to the vasculature and the surrounding endothelium. Circulating vWF within the blood vessels stained positive, indicating that serum protein antigenicity was intact for all conditions in EDTA and in formic acid groups at 25C and 37C (Fig. 6a–c, e, f), whereas it was weaker at 4C (Fig. 6d). Staining for vWF in hydrochloric acid–decalcified specimens revealed similar staining patterns to EDTA and formic acid; however, nonspecific background staining was seen throughout the bone matrix. In addition, positive staining within the vasculature was more intense, although this staining appeared smudged, which hindered identification of endothelium (Fig. 6g–i). Although positive staining was observed in the nitric acid group, the staining intensity appeared diminished or was absent in some of the blood vessels. Furthermore, detail in cellular morphology was lost as nuclei staining was not visible in the endothelium and nonspecific background staining was observed (Fig. 6j–l). Table 3 shows the quality of antigen preservation in the rat tibiae after decalcification using the 12 different protocols.
A summary of the assessment of tissue and cell morphology as well as the preservation of antigenicity between the 12 decalcifying methods for mouse and rat tibiae is presented in Tables 2 and 3, respectively.
Discussion
The main goal of the present study was to compare the effect of four different decalcification solutions applied at three different temperatures and to assess the rate of decalcification and the implications it might have on the quality of tissue morphology and integrity of mouse and rat tibiae. Furthermore, the goal was to determine the best modality to monitor the exact end point of sample decalcification. Commonly used empirical methods, such as bend testing, prick testing with a needle, measuring the weight of the bone until it stops changing, or measuring the pH of the decalcifying solution, were found to be inconclusive, unreliable, and subjective.29,30 In contrast, microCT allows for damage-free precise assessment of bone decalcification and helps to avoid overdecalcification, which can damage the specimen, resulting in poor histological detail.31 The results of this study confirmed that microCT monitoring was the most accurate method to determine the decalcification end point in bone and were consistent within each group (n=3), having only minimal variation in completion times.
An extensive literature search failed to identify any publications regarding the decalcification times for mouse or rat tibiae using different decalcification protocols, but consistent with the current study, EDTA was found to decalcify bone slower than formic and nitric acids.20,23,29 In addition, histological analysis confirmed that decalcification in 10% EDTA at 4C and 25C showed the best results regarding the preservation of cells, tissues, and antigenicity.2,10,23,30,31 Similar findings were also reported in previous studies, where various decalcification solutions were used to demineralize human mandibular bone and teeth,21,32 rat mandibular bone and teeth,24 rat hind paw, fore paw, knee and column,22 and rat femur,10 confirming that the decalcification of mineralized tissues can be a time-consuming process, but is essential to ensure preservation of tissue quality. However, depending on the research question and the bone of interest, faster decalcification protocols could be considered.
To this end, the use of microwaves has become more popular over the last decade to reduce the decalcification time of bones, which is significantly faster compared with conventional methods. For example, Cunningham et al.6 and Pitol et al.1 demonstrated a significant reduction in the decalcification time from 4–7 months to 3–6 weeks in human temporal bones and from 45 days to 48 hr in rat maxillary segments. Both of these studies indicated that tissue morphology and antigenicity were not negatively impacted by the increased temperature. Another key factor also significantly influencing the decalcification time is the sample size.33 For instance, rat tibiae are 5 to 6 times bigger than mouse tibiae, and as the present study’s results showed, decalcification rates for rat tibiae on average were 11, 5.5, 2.5, and 1.5 times longer in 10% EDTA, 10% formic acid, 5% hydrochloric acid, and 5% nitric acid, respectively. Consequently, the longer exposure time of rat tibiae to acid decalcification solutions affected the preservation of tissue quality more severely than mouse tibiae. When stained with H&E or Safranin O/Fast Green, mouse tibiae showed excellent staining results in formic acid, and mostly good results in hydrochloric and nitric acids, especially at 37C, which further shortened the exposure time to the acids used. In samples decalcified in hydrochloric or nitric acid, mainly cell nuclei showed minor signs of damage. By contrast, rat tibiae demonstrated good to excellent results only when decalcified in formic acid, but predominantly fair to poor outcomes of the samples when decalcified using hydrochloric or nitric acid. This was most evident in the cell nuclei of chondrocytes, which appeared pink in H&E and green in Safranin O/Fast Green stains, instead of dark blue. These results were in agreement with previous studies also demonstrating that strong acids degrade nucleic acids in the cell nuclei, thereby reducing the affinity for hematoxylin.2,34 In contrast, EDTA and formic acid–based agents were shown to improve the recovery of nucleic acid quantity and quality significantly when compared with strong acids, such as hydrochloric or nitric acid.35 It is important to note that at certain concentrations (>1 mM) EDTA were shown to deplete magnesium ions and thus inhibit DNA polymerase activity.36 However, to negate the inhibitory effects of EDTA, an additional Mg2+ ion concentration is always added to achieve sufficient PCR activity.
Cartilage stained with Safranin O showed distinct differences in rat tibiae. When decalcified in EDTA, formic acid, or nitric acid, the cartilage showed weaker staining at 37C. These findings complement the results of several other studies, confirming that both fixation and decalcification methods have been associated with proteoglycan and glycosaminoglycan depletion4,26,27,32 reducing the staining intensity of Safranin O, which is directly proportional to the proteoglycan content in normal cartilage.37 Optimal fixation and decalcification conditions for cartilage-containing specimens should always be decided in a pilot study.33 Microwave decalcification at 37C is known to accelerate the decalcification rate1,38 and did not result in negative outcomes for mouse tibiae when acid solutions were used for decalcification. On the contrary, EDTA-decalcified mouse and rat tibiae had a weaker uptake of Safranin O, and a partial loss of tissue integrity was observed at 37C. Adverse effects have also been reported for rat mandibles decalcified at 37C in 10% EDTA.24 Together, these findings indicate that decalcification at 37C is a safe option for small samples such as mouse tibiae but might negatively affect the bone samples that require longer decalcification due to their larger size and is independent of the decalcification solution used.
Many studies investigating the development, injury, or disease of mineralized tissues are also interested in the localization of proteins by immunohistochemistry. Consequently, another important factor to consider when choosing a decalcification protocol is the preservation of antigens. Good-quality immunohistochemistry staining can be a challenge given the special considerations bone processing requires.27,39 Successful detection of antigens depends highly upon choosing the fixation and decalcification method optimized for each specific epitope.39,40
Similar to the classic histological stains, immunohistochemical evaluation of collagen type I, osterix, and vWF in mouse tibiae showed only minor adverse effects caused by either the decalcification solution or temperature. Consistent with previous studies, overall antigen preservation was not compromised in EDTA at 4C and 25C for both mice and rat tibiae.41 Furthermore, decalcification using a microwave (37C) showed comparable levels of tissue preservation and staining specificity. This was in agreement with previously published studies, reporting that decalcification of specimens in EDTA at higher temperatures (37C–45C) is possible, resulting in tissue morphology and antigenicity comparable to conventional conditions (4C and 25C).6,7,42 Targeted chelation of calcium by EDTA may be the reason why the effect on tissue antigenicity is minimal, regardless of the temperature.43 Decalcification by weak organic (formic) and stronger mineral acids (hydrochloric and nitric) produced mixed results regarding the preservation of the tested antigens. For instance, formic acid was previously reported to be acceptable for use by pathology and research labs, as it is able to decalcify bone much faster than EDTA without causing significant damage to the tissue morphology or antigenicity.8,44–46 Other studies have also indicated that fixation was one of the key factors for antigen preservation when decalcifying with formic acid.47,48 Some studies have reported acceptable results obtained with hydrochloric or nitric acid.10,22,26,44 In contrast, the findings from this study were in agreement with Neves et al., who found specific positive staining despite poor tissue morphology, and concluded that hydrochloric acid should be avoided for bone decalcification if preservation of bone matrix and antigenicity is desired.49 Moreover, Athanasou et al. reported that sufficient fixation and controlled decalcification in hydrochloric and nitric acids had no negative effects on diagnostic markers, such as vWF or prostate-specific antigen.46 This was in contrast to the current study, which demonstrated that decalcification in both hydrochloric and nitric acids, despite being carefully controlled, had partially damaged tissue morphology and antigenicity, particularly in rat tibiae, even when decalcification times and exposure to acids were minimal.
The main limitation of this study was that only several routinely used histological stains (H&E and Safranin O/Fast Green) and antibodies (collagen type I, osterix, and vWF) found in the literature were used to determine the preservation quality of tissue morphology comparing different decalcification conditions of mouse and rat tibiae. It is possible that using the same decalcification conditions on different species, different size bone samples, and other histological stains and antibodies, the results would be different. Phosphoproteins, for example, which were not tested in the current study, are known to be poorly preserved in clinical samples after fixation in 10% formalin or 4% PFA, and a majority is lost using standard demineralization protocols.50,51 However, recent studies have shown that using Streck’s tissue fixative50 as well as a newly formulated decalcification solution51 called theralin, which fixes and decalcifies mineralized tissue simultaneously, can significantly improve the preservation of phosphoproteins. Consequently, when using different specimens or histological stains and antibodies from the ones tested in the current study, the decalcification method should be carefully considered to avoid tissue damage. Therefore, it is recommended to perform a pilot study when using different antigens such as osteocalcin, collagen type II, alkaline phosphatase, and CD34, and histological stains such as alcian blue and toluidine blue, to assess the antigenicity and preservation quality of cartilage and bone morphology under the desired decalcification conditions.
In conclusion, this study demonstrated the importance of decalcification protocols taking into account the sample size and how this might affect the rate of decalcification, histological staining, and the antigens of interest if immunohistochemical localization of proteins is required. If tissue and cell morphology are the focus of the study, 10% EDTA at 4C or 25C remains the best option. In case of time constraints, 10% formic acid at 4C might be a good alternative, having about 90% faster decalcification time and good histological and immunohistochemical results, whereas hydrochloric and nitric acids should be avoided. If available, microCT should be used to determine the exact end point of decalcification, thereby ensuring easy sectioning. It is important to note that scanning and analyzing BV by microCT to determine the end point of decalcification is not necessary, as this can be efficiently achieved using a scout view scan. The findings from this study are very important, and should serve as a guideline for histological studies in research and pathology labs for similar size specimens. Furthermore, it will allow researchers to better understand the required times to decalcify bone using different solutions and temperatures, and the implications it might have on tissue morphology and antigenicity.
Supplemental Material
Supplemental material, DS_10.1369_0022155419850099 for Tissue Morphology and Antigenicity in Mouse and Rat Tibia: Comparing 12 Different Decalcification Conditions by Kristofor Bogoevski, Anna Woloszyk, Keith Blackwood, Maria A. Woodruff and Vaida Glatt in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Bridney Lundquist, BS, and Jesse Hernandez, MS (University of Texas Health Science Center at San Antonio), for their technical assistance with histology, immunohistochemistry, and imaging.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: KBogoevski and AW performed the experimental work, analyzed the data, and cowrote the manuscript; KBlackwood sectioned samples and read the manuscript; MAW codesigned experiments and revised the manuscript; VG designed experiments and oversaw the study, reviewed data, and cowrote the manuscript. All authors have read, edited, and approved the final submitted manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by institutional funds from Queensland University of Technology and the University of Texas Health Science Center at San Antonio.
Contributor Information
Kristofor Bogoevski, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia.
Anna Woloszyk, Department of Orthopedic Surgery, University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Keith Blackwood, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia.
Maria A. Woodruff, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia
Vaida Glatt, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Queensland, Australia; Department of Orthopedic Surgery, University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Literature Cited
- 1. Pitol DL, Caetano FH, Lunardi LO. Microwave-induced fast decalcification of rat bone for electron microscopic analysis: an ultrastructural and cytochemical study. Braz Dent J. 2007;18(2):153–7. [DOI] [PubMed] [Google Scholar]
- 2. Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed London: Churchill Livingstone; 2008. [Google Scholar]
- 3. Rosen D. End-point determination in EDTA decalcification using ammonium oxalate. Stain Technol. 1981;56(1):48–9. [DOI] [PubMed] [Google Scholar]
- 4. Kiviranta J, Tammi M, Jurvelin J, Säämänen AM, Helminen HJ. Fixation, decalcification, and tissue processing effects on articular cartilage proteoglycans. Histochemistry. 1984;80(6):569–73. [PubMed] [Google Scholar]
- 5. Matthews JB, Mason GI. Influence of decalcifying agents on immunoreactivity of formalin-fixed, paraffin-embedded tissue. Histochem J. 1984. Jul;16(7):771–87. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham CD, Schulte BA, Bianchi LM, Weber PC, Schmiedt BN. Microwave decalcification of human temporal bones. Laryngoscope. 2001;111(2):278–82. [DOI] [PubMed] [Google Scholar]
- 7. Madden VJ, Henson MM. Rapid decalcification of temporal bones with preservation of ultrastructure. Hear Res. 1997. Sep;111(1–2):76–84. [DOI] [PubMed] [Google Scholar]
- 8. Hatta H, Tsuneyama K, Nomoto K, Hayashi S, Miwa S, Nakajima T, Nishida T, Nakanishi Y, Imura J. A simple and rapid decalcification procedure of skeletal tissues for pathology using an ultrasonic cleaner with D-mannitol and formic acid. Acta Histochem. 2014;116(5):753–7. [DOI] [PubMed] [Google Scholar]
- 9. Mawhinney WH, Richardson E, Malcolm AJ. Control of rapid nitric acid decalcification. J Clin Pathol. 1984. Dec;37(12):1409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Zhu R, Liu C, Ma R, Wang L, Chen B, Li L, Niu J, Zhao D, Mo F, Fu M, Brömme D, Zhang D, Gao S. Evaluation of decalcification techniques for rat femurs using HE and immunohistochemical staining. Biomed Res Int. 2017;2017:9050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaneko M, Tomita T, Nakase T, Takeuchi E, Iwasaki M, Sugamoto K, Yonenobu K, Ochi T. Rapid decalcification using microwaves for in situ hybridization in skeletal tissues. Biotech Histochem. 1999. Jan;74(1):49–54. [DOI] [PubMed] [Google Scholar]
- 12. Goodman J, Chandna A, Roe K. Trends in animal use at US research facilities. J Med Ethics. 2015. Jul;41(7):567–9. [DOI] [PubMed] [Google Scholar]
- 13. Seventh report on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union [Internet]. 2013. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52013DC0859&from=EN
- 14. Rauner M, Thiele S, Fert I, Araujo LM, Layh-Schmitt G, Colbert RA, Hofbauer C, Bernhardt R, Bürki A, Schwiedrzik J, Zysset PK, Pietschmann P, Taurog JD, Breban M, Hofbauer LC. Loss of bone strength in HLA-B27 transgenic rats is characterized by a high bone turnover and is mainly osteoclast-driven. Bone. 2015. Jun;75:183–91. [DOI] [PubMed] [Google Scholar]
- 15. Lietman CD, Marom R, Munivez E, Bertin TK, Jiang M-M, Chen Y, Dawson B, Weis MA, Eyre D, Lee B. A transgenic mouse model of OI type V supports a neomorphic mechanism of the IFITM5 mutation. J Bone Miner Res. 2015. Mar; 30(3):489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Courtney PM, Bernstein J, Ahn J. In brief: closed tibial shaft fractures. Clin Orthop Relat Res. 2011. Dec;469(12):3518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schindeler A, Morse A, Harry L, Godfrey C, Mikulec K, McDonald M, Gasser JA, Little DG. Models of tibial fracture healing in normal and Nf1-deficient mice. J Orthop Res. 2008. Aug;26(8):1053–60. [DOI] [PubMed] [Google Scholar]
- 18. Handool KO, Ibrahim SM, Kaka U, Omar MA, Abu J, Yusoff MSM, Yusof LM. Optimization of a closed rat tibial fracture model. J Exp Orthop. 2018. May;25(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torlakovic EE, Naresh K, Kremer M, van der Walt J, Hyjek E, Porwit A. Call for a European programme in external quality assurance for bone marrow immunohistochemistry; report of a European Bone Marrow Working Group pilot study. J Clin Pathol. 2009;62(6):547–51. [DOI] [PubMed] [Google Scholar]
- 20. Sanjai K, Patil A, Jayaram S, Kumarswamy J, Papaiah L, Krishnan L. Evaluation and comparison of decalcification agents on the human teeth. J Oral Maxillofac Pathol. 2012;16(2):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rehan AD, Saigal S, Bhargava A, Kumar U, Thakur P, Kausar T. Comparison of microwave decalcification with conventional decalcification method by using different decalcifying agents. Int J Res Med Sci. 2017. Jun24;5(7):3126–28. [Google Scholar]
- 22. González-Chávez SA, Pacheco-Tena C, Macías-Vázquez CE, Luévano-Flores E. Assessment of different decalcifying protocols on osteopontin and osteocalcin immunostaining in whole bone specimens of arthritis rat model by confocal immunofluorescence. Int J Clin Exp Pathol. 2013;6(10):1972–83. [PMC free article] [PubMed] [Google Scholar]
- 23. Srinivasyaiah A, Nitin P, Hegde U. Comparison of microwave versus conventional decalcification of teeth using three different decalcifying solutions. J Lab Physicians. 2016;8(2):106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Savi FM, Brierly GI, Baldwin J, Theodoropoulos C, Woodruff MA. Comparison of different decalcification methods using rat mandibles as a model. J Histochem Cytochem. 2017;65(12):705–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thirumal Raj A, Patil S, Rao RS. A comparison of conventional and microwave decalcification and processing of tooth and mandibular bone specimens. J Clin Diagnostic Res. 2016;10(10):ZC121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abrantes AAA, Rafacho A, Rivero ERC, Mariano FV, Siqueira FM, Gondak RO. Tissue integrity, costs and time associated with different agents for histological bone preparation. Microsc Res Tech. 2017;80(4):344–9. [DOI] [PubMed] [Google Scholar]
- 27. Hosoya A, Hoshi K, Sahara N, Ninomiya T, Akahane S, Kawamoto T, Ozawa H. Effects of fixation and decalcification on the immunohistochemical localization of bone matrix proteins in fresh-frozen bone sections. Histochem Cell Biol. 2005; Jun;123(6):639–46. [DOI] [PubMed] [Google Scholar]
- 28. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthr Cartil. 2010;18(Suppl. 3):S113–6. [DOI] [PubMed] [Google Scholar]
- 29. Callis G, Sterchi D. Decalcification of bone: literature review and practical study of various decalcifying agents. Methods, and their effects on bone histology. J Histotechnol. 1998;21(1):49–58. [Google Scholar]
- 30. Srinivasyaiah A, Hegde U, Nagpal B. Decalcification of biopsy tissue. LAP Lambert Academic Publishing; Saarbrücken, Germany; 2016. [Google Scholar]
- 31. Khurana JS, Arguello-Guerra V. Grossing of bone and soft tissue (common specimens and procedures). In: Khurana JS, editor. Bone pathology. Totowa, NJ: Humana Press; 2009. p. 125–28. [Google Scholar]
- 32. Sangeetha R, Uma K, Chandavarkar V. Comparison of routine decalcification methods with microwave decalcification of bone and teeth. J Oral Maxillofac Pathol. 2013. Sep;17(3):386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. An YH, Martin KL. editors. Handbook of histology methods for bone and cartilage. Totowa, NJ: Humana Press; 2003. [Google Scholar]
- 34. Choi S-E, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. J Pathol Transl Med. 2015;49(3):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh VM, Salunga RC, Huang VJ, Tran Y, Erlander M, Plumlee P, Peterson MR. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann Diagn Pathol. 2013. Aug;17(4):322–6. [DOI] [PubMed] [Google Scholar]
- 36. Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors—occurrence, properties and removal. J Appl Microbiol. 2012;113(5):1014–26. [DOI] [PubMed] [Google Scholar]
- 37. Camplejohn KL, Allard SA. Limitations of safranin “O” staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry. 1988;89(2):185–8. [DOI] [PubMed] [Google Scholar]
- 38. Katoh K. Microwave-assisted tissue preparation for rapid fixation, decalcification, antigen retrieval, cryosectioning, and immunostaining. Int J Cell Biol. 2016;2016:7076910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bussolati G, Leonardo E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J Clin Pathol. 2008;61(11):1184–92. [DOI] [PubMed] [Google Scholar]
- 40. Hao Z, Kalscheur VL, Muir P. Decalcification of bone for histochemistry and immunohistochemistry procedures. J Histotechnol. 2002. Mar 18;25(1):33–7. [Google Scholar]
- 41. Chuang SS, Jung YC, Li CY. von Willebrand factor is the most reliable immunohistochemical marker for megakaryocytes of myelodysplastic syndrome and chronic myeloproliferative disorders. Am J Clin Pathol. 2000;113(4):506–11. [DOI] [PubMed] [Google Scholar]
- 42. Louw I, De Beer DP, Du Plessis MJ. Microwave histoprocessing of bone marrow trephine biopsies. Histochem J. 1994;26(11):487–94. [DOI] [PubMed] [Google Scholar]
- 43. Castania VA, Silveira JW, Issy AC, Pitol DL, Castania ML, Neto AD, Del Bel EA, Defino HLA. Advantages of a combined method of decalcification compared to EDTA. Microsc Res Tech. 2015. Feb;78(2):111–8. [DOI] [PubMed] [Google Scholar]
- 44. Mullink H, Henzen-Logmans SC, Tadema TM, Mol JJ, Meijer CJ. Influence of fixation and decalcification on the immunohistochemical staining of cell-specific markers in paraffin-embedded human bone biopsies. J Histochem Cytochem. 1985. Nov;33(11):1103–9. [DOI] [PubMed] [Google Scholar]
- 45. Frank JD, Balena R, Masarachia P, Seedor JG, Cartwright ME. The effects of three different demineralization agents on osteopontin localization in adult rat bone using immunohistochemistry. Histochemistry. 1993;99(4):295–301. [DOI] [PubMed] [Google Scholar]
- 46. Athanasou NA, Quinn J, Heryet A, Woods CG, McGee JO. Effect of decalcification agents on immunoreactivity of cellular antigens. J Clin Pathol. 1987. Aug;40(8):874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Starklint H, Lausten GS, Arnoldi CC. Microvascular obstruction in avascular necrosis. Immunohistochemistry of 14 femoral heads. Acta Orthop Scand. 1995;66(1):9–12. [DOI] [PubMed] [Google Scholar]
- 48. Song SJ, Hutmacher D, Nurcombe V, Cool SM. Temporal expression of proteoglycans in the rat limb during bone healing. Gene. 2006;379:92–100. [DOI] [PubMed] [Google Scholar]
- 49. Neves JDS, Omar NF, Narvaes EAO, Gomes JR, Novaes PD. Influence of different decalcifying agents on EGF and EGFR immunostaining. Acta Histochem. 2011. Jul;113(4):484–8. [DOI] [PubMed] [Google Scholar]
- 50. Burns JA, Li Y, Cheney CA, Ou Y, Franlin-Pfeifer LL, Kuklin N, Zhang Z-Q. Choice of fixative is crucial to successful immunohistochemical detection of phosphoproteins in paraffin-embedded tumor tissues. J Histochem Cytochem. 2009. Mar;57(3):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mueller C, Gambarotti M, Benini S, Picci P, Righi A, Dana SH, Lance H, Virginia L. Unlocking bone for proteomic analysis and FISH. Lab Investig. 2019;99:708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155419850099 for Tissue Morphology and Antigenicity in Mouse and Rat Tibia: Comparing 12 Different Decalcification Conditions by Kristofor Bogoevski, Anna Woloszyk, Keith Blackwood, Maria A. Woodruff and Vaida Glatt in Journal of Histochemistry & Cytochemistry