Abstract
Background:
Limited data are available regarding outcomes for stentless aortic valve reoperation. The reported reoperative mortality has been unacceptably high.
Methods:
Between 1997–2017, a retrospective analysis was performed on 143 patients that underwent open aortic valve reoperations for failed stentless aortic valve bioprostheses. We evaluated both short- and long-term outcomes on this cohort of patients.
Results:
Bicuspid aortic valve was present in 107/143 (75%) of patients at the time of the initial Freestyle procedure and 84% (120/143) patients underwent a modified inclusion aortic root replacement procedure. The interval from first operation to reoperation was 9.0 (5.4, 11.8) years, which was significantly shorter for patients with infectious endocarditis [4.1 (1.8, 7.1) years] compared to patients with structural valvular deterioration [10.4 (8.1, 12.4) years, p < 0.001]. The median age at the time of reoperation was 59 (50, 67) years. Aortic valve reoperation was performed for SVD in 68% cases compared to 32% for infectious prosthetic valve endocarditis. Concomitant surgery included coronary artery bypass (13%), mitral valve surgery (4%), and ascending aorta and arch replacement (42%). The operative mortality (including 30-day and in-hospital mortality) was 1% and 2%, respectively. The composite outcome including myocardial infarction, stroke, new-onset renal failure on hemodialysis, and operative mortality was 4%. The 5- and 10-year KM survival after reoperation for failed stentless valve was 83% (95% CI: 73%, 89%) and 57% (95% CI: 36%, 74%).
Conclusions:
Aortic valve reoperation following stentless valve implantation can be performed with low operative mortality and favorable long-term survival.
Keywords: aortic valve reoperation, stentless aortic valve, prosthetic valve endocarditis, survival, Aortic Valve Replacement, Prosthesis, Reoperation
INTRODUCTION:
Stentless valves are known to have better transvalvular gradients and allow improved left ventricular function secondary to sustained decreases in left ventricular mass (1–3). Hemodynamics at rest and during exercise compared to stented bioprostheses (4) as well as reduced incidences of patient-prosthesis mismatch (5) are also reported as superior in stentless valves. These advantages of stentless valve may result in a survival benefit (1–3). However, utilization of stentless valves remains controversial secondary to the difficulty of implantation and high reoperative mortality rates ranging between 11–21% (6, 7). To date, few studies have reported short- and long-term outcome after aortic valve reoperation following implantation of an initial stentless valve.
Over two decades at our center, 2413 patients have received stentless aortic valves (Freestyle porcine aortic root, Medtronic, Inc.), and as anticipated, patients have returned for aortic valve reoperations. The objective of this study was to evaluate the short- and long-term outcomes of aortic valve reoperation, including perioperative outcomes, operative mortality, and long-term survival following aortic valve reoperation with an initial implantation of a stentless valve. We hypothesized that short- and long-term survival would be favorable for patients that received an open aortic valve reoperation following an initial stentless valve implantation.
MATERIAL AND METHODS
Study Approval and Design
This study was approved by the Institutional Review Board at the University of Michigan Health System (UMHS, Ann Arbor, MI) and was in compliance with Health Insurance Portability and Accountability Act regulations. Society of Thoracic Surgery (STS) data was obtained from the University of Michigan, Michigan Medicine, Department of Cardiac Surgery Data Warehouse to identify the cohort, and medical record review was utilized to supplement and confirm data collection. Operative reports were reviewed by a single cardiac surgeon (BY) to confirm that the patients received an aortic valve reoperation for a failed stentless aortic valve. Long-term survival was obtained through linkage with the National Death Index database through December 31st, 2015 and supplemented with medical record review.
Patient Selection
From 1997–2017, all consecutive patients (n=143) who underwent open reoperative aortic valve replacement (AVR) or aortic root replacement for a previously implanted but failed stentless valve were enrolled in this study. If a patient had more than one stentless valve reoperation, we only included the first reoperation after the initial stentless valve implantation. A stentless valve was used for the first operation when the patient requested a bioprosthesis for aortic valvular pathology, dilated aortic root, and/or occasionally for small left ventricular outflow tract (LVOT)
Operative Technique - Stentless Aortic Valve Reoperation
The current strategy at our center for managing a stentless aortic valve open reoperation includes: (1) AVR as valve-in-valve: if the initial Freestyle porcine aortic root annulus is not calcified and can hold a good size valve (≥23 mm valve), we consider placement of a new stented valve or mechanical valve in the stentless root as valve-in-valve; (2) AVR with or without aortic root patch repair after removing the previous stentless valve: the Freestyle porcine aortic root is explanted if heavy calcification of the porcine aortic root is encountered or if the Freestyle porcine aortic root cannot hold an adequate-sized prosthesis as valve-in-valve. This is a tedious process and more technically challenging than removing a stented valve. After the Freestyle porcine aortic root is removed, if the remaining native aortic root is intact or repairable with a patch, an AVR is then performed; (3) aortic root replacement: if the native aortic root is significantly damaged during the removal of the Freestyle porcine aortic root, we perform a reoperative aortic root replacement using a new Freestyle porcine aortic root either as modified inclusion/inclusion or as a total root with implantations of two coronary buttons. In a small portion (15%) of patients, we also used homograft (5.6%) or mechanical composite valve graft (9.8%) based on the patient’s preference as a redo total root replacement (Bentall procedure).
Statistical Analysis
Descriptive statistics were computed for the study cohort. Continuous variables were summarized by median (interquartile range (IQR) 25 percentile, 75 percentile) and categorical variables were reported as n (%) in frequency tables. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to assess differences in survival between the two groups. All statistical calculations used SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Initial Stentless Aortic Valve Implantation (First Operation)
Seventy-five percent of reoperative patients (107/143) received an initial operation due to disease from bicuspid aortic valve pathology. Eighty-five percent of patients (121/143) had the initial operation performed at the University of Michigan, Michigan Medicine; while 15% (22/143) had the initial operation performed at an outside hospital and were transferred to the University of Michigan, frequently due to prosthetic valve endocarditis. The most common technique for the initial stentless aortic valve implantation was modified inclusion or inclusion root replacement (n=120, 84%). All the patients received Freestyle Porcine Aortic Roots (Medtronic, Inc.) as their first aortic valve operation. (Table 1).
Table 1.
Data of Initial Operation (First Stentless Valve Implantation)
| Variables | N=143 |
|---|---|
| Bicuspid Aortic Valve | 107 (75) |
| Marfan Syndrome | 5 (3.5) |
| First AV Operation | |
| AVR | 11 (8) |
| Aortic Root Replacement | 132 (92) |
| Concomitant Surgery | |
| CABG | 19 (13) |
| Mitral Valve Operation | 6 (4) |
| Ascending Aorta or Arch Replacement | 60 (42) |
| Implantation Technique | |
| Subcoronary | 11 (8) |
| Inclusion/ Modified Inclusion | 120 (84) |
| Total Root Replacement | 12 (8) |
| Location of First AV Operation | |
| University of Michigan | 121 (85) |
| Other Hospital | 22 (15) |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass graft surgery.
Stentless Aortic Valve Reoperation (Second Operation)
There were two primary indications for the stentless valve reoperation: structural valve deterioration (SVD) (n=97, 68%) and prosthetic valve endocarditis (PVE) (n=46, 32%). The interval from first stentless valve implantation and the stentless valve reoperation was 9.0 (5.4, 11.8) years. This timeframe was significantly shorter in the patients with PVE compared to SVD, [4.1 (1.8, 7.1) vs 10.4 (8.1, 12.4) years, p<0.001]. (Table 2) Reoperative aortic root replacement was more commonly utilized than aortic valve replacement (64% vs 36%) in this cohort of patients (Table 3).
Table 2.
Demographics and Pre-Operative Data of Stentless Aortic Valve Reoperation
| Variables | N=143 |
|---|---|
| Patient Age (years) | 59 (50, 67) |
| <50 | 34 (24) |
| 50–59 | 39 (27) |
| ≥60 | 70 (49) |
| Sex (Female) (%) | 27 (19) |
| Pre-existing Comorbidities | |
| Diabetes Mellitus | 24 (17) |
| PAD | 13 (9) |
| CAD | 55 (38.5) |
| Myocardial Infarction (<30 days) | 8 (6) |
| History of Stroke | 19 (13) |
| History of Smoking | 72 (50) |
| COPD | 21 (15) |
| Hypertension | 97 (68) |
| Pre-op Creatinine (mg/dL) | 1 (1, 1) |
| Renal Failure on Dialysis | 6 (4) |
| Pre-operative Atrial Fibrillation | 22 (15) |
| Indications for Reoperation | |
| Aortic Stenosis | 39 (27) |
| Patient-Prosthesis Mismatch | 0 |
| Aortic Insufficiency | 107 (75) |
| Active Endocarditis | 46 (32) |
| Active Endocarditis with Abscess | 19 (13) |
| Interval Between Operations (years) | 9 (5, 12) |
| NYHA Function Class | |
| I | 20 (14) |
| II | 47 (33) |
| III | 55 (38.5) |
| IV | 21 (15) |
| Ejection Fraction | 60 (53, 65) |
| >60% | 87 (61) |
| 40–60% | 45 (31.5) |
| 20–40% | 11 (8) |
| <20% | 0 |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Abbreviations: PAD, peripheral arterial disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Table 3.
Intraoperative Data
| Variables | N=143 |
|---|---|
| Reoperative Procedure | |
| Redo AVR | 52 (36) |
| Redo Root | 91 (64) |
| Timing of Operation | |
| Elective | 78 (55) |
| Urgent | 57 (40) |
| Emergent/ Salvage | 8 (6) |
| Concomitant Procedures | |
| Patch Repair of Aortic Root | 23 (16) |
| CABG | 11 (8) |
| MV Surgery | 23 (16) |
| Ascending or Arch Replacement | 35 (24.5) |
| Type of Re-implanted Aortic Valve | |
| Stented Valve | 51 (36) |
| Freestyle Stentless Valve | 70 (49) |
| Mechanical Valve | 14 (10) |
| Homograft | 8 (6) |
| CPB Time (min) | 236 (171, 285) |
| Cross Clamping Time (min) | 190 (150, 230) |
| PRBC transfusions | 71 (50) |
| PRBC-Intraoperative (units) | 1(0, 3) |
| Pathology of Explanted Valve | |
| Calcified | 59 (41) |
| Leaflet Fracture | 69 (48) |
| Infection | 46 (32) |
| Intra-Operative Mortality | 0 |
Data presented as median (25 %, 75 %) for continuous data and n (%) for categorical data.
Abbreviations: AVR, Aortic Valve Replacement; CABG, coronary artery bypass graft; MV, mitral valve; CPB, cardiopulmonary bypass; PRBC, packed red blood cells.
Short-Term Outcomes
The 30-day mortality was 1% (n=2) and operative mortality was 2% (n=3), which included all mortality within 30 days after the operation and/or in-hospital mortality. No patients suffered from stroke or perioperative myocardial infarction; however, 2% (n=3) of patients suffered from acute renal failure requiring dialysis (Table 4). The operative outcomes for mortality, postoperative complications, and composite outcome were not affected by the techniques utilized for the aortic valve reoperation (including AVR or aortic root replacement).
Table 4.
Post-Operative Outcomes
| Variables | N=143 |
|---|---|
| Hours to Extubation | 9 (5, 20) |
| Reoperation for Bleeding / Tamponade | 2 (1) |
| Blood transfusion | 34 (24) |
| VAD | 0 |
| MI | 0 |
| Stroke | 0 |
| New-onset Renal Failure | 3 (2) |
| Post-op Creatinine (mg/dL) | 1 (1, 1.5) |
| Postoperative Atrial Fibrillation | 37 (26) |
| Complete Heart Block or Pacemaker | 8 (6) |
| ICU Stay (hours) | 63 (43, 95) |
| Hospital Stay (days) | 7 (5, 13) |
| 30-day Mortality | 2 (1) |
| Composite outcome | 6 (4) |
Data presented as median (25 %, 75 %) for continuous data and n (%) for categorical data. Abbreviations: ICU, intensive care unit; MI, myocardial infarction; and End-stage kidney disease; VAD, ventricular assist device. The composite outcome was comprised of operative mortality, postoperative MI, stroke, and new onset renal failure.
Long-Term Survival
The total patient-years of reoperative follow-up was 555 for the cohort. The median follow-up time was 2.5 years. The mortality rate was 3.8% per patient-year (21/555), including those with (4.9%, 13/263) and without (2.7%, 8/292) PVE (p=0.17). The 5- and 10-year KM survival after reoperation of stentless valve was 83% (95% CI: 73%, 89%) and 57% (95% CI: 36%, 74%), respectively (Figure 1), which was better in the patients with SVD compared to patients with PVE, but not significantly (p=0.14) (Figure 2).
Figure 1:
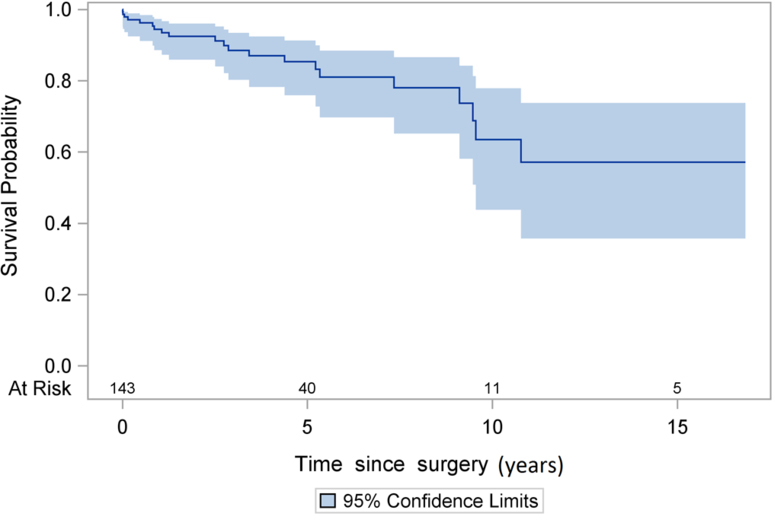
Kaplan-Meier Survival after stentless aortic valve reoperation.
Figure 2:
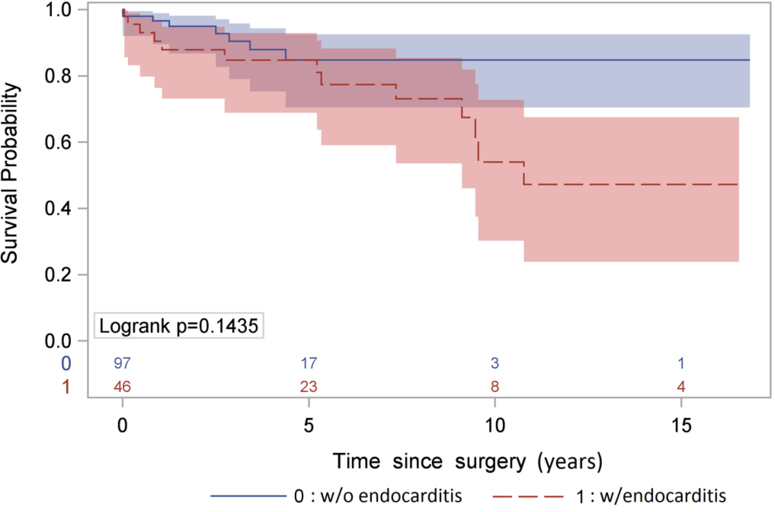
Kaplan-Meier Survival after stentless aortic valve reoperation stratified by the indication of the reoperation: structural valvular deterioration and active prosthetic valve endocarditis.
COMMENT
In this study, we summarize our experience with aortic valve reoperation after first implantation of a stentless aortic valve. The primary findings of this study were as follows. First, the operative mortality and major post-operative complications following aortic valve reoperation were relatively low ranging from 0–2% for all patients. Second, long-term survival was favorable and not significantly different between patients with different indications for reoperation including SVD versus PVE.
Stentless aortic valve reoperation is considered a technical challenge due to dense calcification and extensive adherence of the xenograft. For the majority of patients, the primary method of reoperation requires complete explantation of the stentless xenograft. Following stentless xenograft removal, extensive damage to the aortic root is frequently unveiled and surgeons have to perform a reoperative aortic root replacement, which increases the complexity of the operation. Borger and colleagues (6) reported an 11% (6/57 patients) operative mortality following stentless aortic valve reoperation, in which 63% of patients received reoperative aortic root replacements. Similarly, Boning and colleagues (7) reported a 21% (5/24 patients) 30-day mortality following stentless aortic valve reoperation with primary implantation of a Freestyle porcine aortic root, which was higher than the reported mortality (4%) for a stented aortic valve reoperation. Finch and colleagues reported that 30-day mortality following aortic root reoperation was four times higher than a valve-in-valve stentless reoperation (9). These numbers highlight the challenge to U.S. surgeons when faced with a reoperative stentless xenograft.
We previously reported no 30-day mortality for ten consecutive reoperative cases for a Freestyle stentless aortic valve (10). Herein, we report only three deaths in 143 patients, maintaining a 1% 30-day mortality and 2% operative mortality for a stentless aortic valve reoperation. Our experience differs from the previously mentioned series. Possible reasons for the differences include (1) differences in age and incidence of comorbidities compared to other reports (6, 7), (2) the majority of the first stentless implantations reported herein used the modified inclusion technique (84% of total cases) which makes the reoperative root replacement easier than an initial total root replacement (Bentall procedure), (3) our center sub-specializes in aortic operative procedures that are mostly performed by three primary aortic surgeons. More recently, transcatheter aortic valve replacements (TAVRs) are increasingly utilized for patients considered non-operative candidates for reoperative AVR. In addition, sutureless valve-in- stentless root could be another option for reoperative AVR as valve-in-valve which would decrease the need for re-do root replacement in a heavily calcified degenerative stentless valve.
The unadjusted 5- and 10-year survival in patients with stentless valve after reoperation was 83% and 57% based on Kaplan-Meier analysis (Figure 1). Our findings are similar to those reported by Borger et al. (6) and Finch et al. (9), which indicate a 5-year survival of 79% and a 7-year survival of 76%, respectively. The 5-year survival appeared more favorable in the patients with SVD compared to infectious PVE, although this finding was not statistically significant (Figure 2); however, small sample size and low event rate may account for the lack of statistical significance. We also conducted the analysis of short- and long-term survival after aortic valve reoperation in patients with a stented valve for both SVD and PVE at our center from 1997–2013 (Supplement Figure 1A and 1B). The operative mortality was not different between stented (data shown in supplemental figures) and the stentless groups. The long-term survival in the stented group was similar to previous reports (11–13), but appeared to be worse than that in the stentless group, especially among patients with active PVE (Supplement Figure 1B). However, since the patients in the stentless and stented groups are different patient populations, no definitive conclusion can be determined from the comparison of those two groups on long-term survival.
We acknowledge the limitations of any retrospective study along with the single center experience and potential for selection bias. The small sample size of patients with PVE did not have enough power to differentiate the long-term survival of patients with SVD and those with PVE.
In conclusion, aortic valve reoperation after an initial AVR or aortic root replacement with a stentless valve can be performed with low operative mortality and morbidity and favorable long-term survival. The stentless valve is an acceptable option for AVR or aortic root replacement when indicated, such as bicuspid aortic valve with valvulopathy, aortic root aneurysm, and small LVOT.
Supplementary Material
(A) Kaplan-Meier Survival after stented aortic valve reoperation. The 5- and 10-year survival was 55% (95% CI: 41%, 67%) and 22% (95% CI: 8%, 40%) respectively.
(B) Kaplan-Meier Survival after stented aortic valve reoperation stratified by the indication of the reoperation: structural valvular deterioration (8-year survival: 43% (19%, 64%)) and active prosthetic valve
REFERENCES
- 1.Christ T, Grubitzsch H, Claus B, Heinze G, Dushe S, Konertz W. Hemodynamic behavior of stentless aortic valves in long term follow-up. Journal of cardiothoracic surgery 2014;9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach DS, Kon ND, Dumesnil JG, Sintek CF, Doty DB. Ten-year outcome after aortic valve replacement with the freestyle stentless bioprosthesis. The Annals of thoracic surgery 2005;80(2):480–486; discussion 486–487. [DOI] [PubMed] [Google Scholar]
- 3.Bach DS, Kon ND. Long-term clinical outcomes 15 years after aortic valve replacement with the freestyle stentless aortic bioprosthesis. The Annals of thoracic surgery 2014;97(2):544–551. [DOI] [PubMed] [Google Scholar]
- 4.Fries R, Wendler O, Schieffer H, Schafers HJ. Comparative rest and exercise hemodynamics of 23-mm stentless versus 23-mm stented aortic bioprostheses. The Annals of thoracic surgery 2000;69(3):817–822. [DOI] [PubMed] [Google Scholar]
- 5.Pepper J, Cheng D, Stanbridge R et al. Stentless versus stented bioprosthetic aortic valves: A consensus statement of the international society of minimally invasive cardiothoracic surgery (ismics) 2008. Innovations (Philadelphia, Pa) 2009;4(2):49–60. [DOI] [PubMed] [Google Scholar]
- 6.Borger MA, Prasongsukarn K, Armstrong S, Feindel CM, David TE. Stentless aortic valve reoperations: A surgical challenge. The Annals of thoracic surgery 2007;84(3):737–743; discussion 743–734. [DOI] [PubMed] [Google Scholar]
- 7.Boning A, Niemann B, Ennker I, Richter M, Roth P, Ennker J. Are aortic valve reoperations after primary replacement with stentless heart valve prostheses more demanding than after stented biological prostheses? The Thoracic and cardiovascular surgeon 2014;62(6):475–481. [DOI] [PubMed] [Google Scholar]
- 8.Statistics NCfH. National death index (year range of data used 1997–2013), centers for disease control and prevention. [Google Scholar]
- 9.Finch J, Roussin I, Pepper J. Failing stentless aortic valves: Redo aortic root replacement or valve in a valve? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2013;43(3):495–504. [DOI] [PubMed] [Google Scholar]
- 10.Deeb GM, Smolens IA, Bolling SF, Eppinger MJ, Pagani FD, Prager RL. Reoperation for freestyle stentless aortic valves. Seminars in thoracic and cardiovascular surgery 2001;13(4 Suppl 1):16–23. [PubMed] [Google Scholar]
- 11.David TE, Gavra G, Feindel CM, Regesta T, Armstrong S, Maganti MD. Surgical treatment of active infective endocarditis: A continued challenge. The Journal of thoracic and cardiovascular surgery 2007;133(1):144–149. [DOI] [PubMed] [Google Scholar]
- 12.Musci M, Weng Y, Hubler M et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: Twenty-year single-center experience. The Journal of thoracic and cardiovascular surgery 2010;139(3):665–673. [DOI] [PubMed] [Google Scholar]
- 13.Moon MR, Miller DC, Moore KA et al. Treatment of endocarditis with valve replacement: The question of tissue versus mechanical prosthesis. The Annals of thoracic surgery 2001;71(4):1164–1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Kaplan-Meier Survival after stented aortic valve reoperation. The 5- and 10-year survival was 55% (95% CI: 41%, 67%) and 22% (95% CI: 8%, 40%) respectively.
(B) Kaplan-Meier Survival after stented aortic valve reoperation stratified by the indication of the reoperation: structural valvular deterioration (8-year survival: 43% (19%, 64%)) and active prosthetic valve


