Abstract
Frontotemporal Dementia (FTD) is preceded by a long period of subtle brain changes, occurring in the absence of overt cognitive symptoms, that need to be still fully characterized. Dynamic network analysis based on resting-state magnetic resonance imaging (rs-fMRI) is a potentially powerful tool for the study of preclinical FTD.
In the present study, we employed a “chronnectome” approach (recurring, time-varying patterns of connectivity) to evaluate measures of dynamic connectivity in 472 at-risk FTD subjects from the Genetic Frontotemporal dementia research Initiative (GENFI) cohort.
We considered 249 subjects with FTD-related pathogenetic mutations and 223 mutation non-carriers (HC). Dynamic connectivity was evaluated using independent component analysis and sliding-time window correlation to rs-fMRI data, and meta-state measures of global brain flexibility were extracted.
Results show that presymptomatic FTD exhibits diminished dynamic fluidity, visiting less meta-states, shifting less often across them, and travelling through a narrowed meta-state distance, as compared to HC. Furthermore, global brain flexibility was significantly correlated to the expected age at symptom onset and processing speed performances in mutation carriers.
Dynamic connectivity changes characterize preclinical FTD, arguing for the desynchronization of the inner fluctuations of the brain. These changes antedate clinical symptoms, and might represent an early signature of FTD to be used as a biomarker in clinical trials.
Keywords: Frontotemporal Dementia, mutation, Granulin, Microtuble Associate Protein Tau, C9orf72, resting-state fMRI, dynamic brain functional connectivity, chronnectome
INTRODUCTION
Resting state functional magnetic resonance imaging (rs-fMRI) has become a useful tool to investigate the connectivity changes in neurodegenerative dementias1, 2. Spontaneous brain activity at rest is organized in functionally specialized large-scale networks, that roughly correspond to different functional domains and that are selectively damaged by various neurodegenerative conditions3.
However, previous results make the implicit assumption that the functional coupling among brain regions is static and unchanging over short periods of time4–6.
This concept has since been modified with analytic approaches that capture the fact that the human brain is an interacting dynamic network and its architecture of coupling among brain regions varies across time (termed “the chronnectome”)7–12. Dynamic connectivity studies have demonstrated reoccurring patterns of brain functional connectivity, or functional connectivity “states”, that are reproducible over time and across subjects8, 13, 14. Initial dynamic connectivity studies were based on the assumption that subjects were allowed to be in only one “state” at a given point in time, while recent work has introduced the concept of “meta-states”, suggesting that subjects may be in multiple states to varying degrees at the same point in time7, 15. Thus, for instance, if in the time-course we are able to identify six distinct states of functional dynamic connectivity, at a given point in time each subject will have a weighted probability to be in more than one state15. Meaningful measures of meta-state dynamic fluidity, such as the number of meta-states a subject passes through or the number of switches from one meta-state to another, have been suggested as an intuitive way to characterize global dynamic connectivity behaviour, showing promise for predicting mental states and cognitive performances4, 16. From this point of view, meta-state measures could provide a more “global” information on the effect of an ongoing neurodegenerative process, overcoming the evaluation of single specific brain areas or connectivity pathways, and evaluating global perturbation of the brain activity’s temporal dynamics7, 15.
Frontotemporal Dementia (FTD) is a neurodegenerative disease characterized by behavioural abnormalities, impairment of executive functions and language deficits17, 18 and defined by focal frontotemporal atrophy19. In a significant proportion of the cases, FTD is an inherited autosomal dominant disorder; mutations in the Granulin (GRN), chromosome 9 open reading frame 72 (C9orf72) or Microtuble Associated Protein Tau (MAPT) genes drive up to ~40% of Mendelian cases20. In genetic FTD, the neural substrates associated with the presymptomatic stage need to be fully characterized, even though the perturbation of static large-scale networks mainly involving the frontal regions has already been demonstrated21, 22. Namely, in presymptomatic genetic FTD increased connectivity in medial frontal regions2, 23, especially within the Salience Network, and reduced seed-based connectivity between anterior cingulate cortex and posterior regions of the Default Mode Network21 has been already reported. The evaluation of large-scale structural network topology along with their temporal dynamics might offer a theoretical framework that can contribute to understand the earliest abnormalities in FTD, with the new perspective of whole brain assessment24.
These premises set the stage for the present study, in which we analyzed dynamic brain connectivity in presymptomatic subjects carrying GRN, MAPT or C9orf72 mutations with the purpose a) to assess the chronnectome fingerprint by considering meta-state measures; b) to study the association between chronnectome changes and cognitive performances; and c) to correlate chronnectome changes with expected age at disease onset, to evaluate if meta-state measures are associated with proximity to clinical onset. To this end, we analyzed rs-fMRI data of 472 subjects from the Genetic Frontotemporal Dementia Initiative (GENFI) cohort (http://genfi.org.uk) using a dynamic functional network connectivity (dFNC) approach to investigate the chronnectome in presymptomatic mutations carriers as compared to mutation non-carriers.
METHODS
Subjects.
Data for this study were drawn from the GENFI multicenter cohort study, which consists of 23 research centers in Europe and Canada. Inclusion and exclusion criteria have been previously described25. Local ethics committees approved the study at each site and all participants provided written informed consent according to the Declaration of Helsinki.
We considered asymptomatic participants at risk to carry GRN, C9orf72 or MAPT mutations. Between January 2012 and January 2017, we considered 472 participants, of which 249 were mutation carriers (45 with MAPT, 122 with GRN, and 82 with C9orf72 mutations) and 223 were mutation non-carriers. Subjects were enrolled from 18 centers belonging to the GENFI network (4 remaining centers were excluded from the present project for image artifacts, very low number of included subjects (<2), or for using 1.5T MRI scanner); the MRI parameters for each of the 18 included centers was reported in Supplementary Table 1. Demographic characteristics of mutation carriers and mutation non-carriers are reported in Table 1.
Table 1.
Demographic characteristics of included participants.
| Characteristic | Carriers (n=249) | Non-carriers (n=223) | P-value* |
|---|---|---|---|
| Age (years) | 44.7±11.7 | 47.2±13.2 | 0.042 |
| Female, % | 64.7% | 56.5% | 0.073^ |
| Education (years) | 14.4±3.2 | 14.1±3.3 | 0.242 |
| Years at expected onset (years) | −13.8±11.3 | - | - |
| Cognitive and behavioural assessment | |||
| MMSE | 29.2±1.1 | 29.4±0.9 | 0.633 |
| CBI-R | 4.45±8.2 | 3.3±6.1 | 0.098 |
| TMT-A (Z-scores) | −2.5±64.5 | −10.2±70.3 | 0.075 |
| TMT-B (Z-scores) | −8.2±80.7 | −14.4±69.0 | 0.345 |
Mann-Whitney U test, otherwise specified; ^Chi-Square test; results are expressed as mean±standard deviation, otherwise specified.MMSE: Mini-Mental State Examination; CBI-R: Cambridge Behavioural Inventory Revised version; TMT-A: part A of the Trial Making Test; TMT-B: part B of the Trial Making Test
Estimated years from expected symptom onset in presymptomatic mutation carriers were calculated as the age of the participant at the time of the study assessment minus the mean familial age at symptom onset, as previously reported25.
Included at-risk subjects underwent a careful recording of demographic data and a standardized clinical and neuropsychological assessment (derived from the Uniform Data Set26), as previously published27. We considered tests highly sensitive to identify initial changes in presymptomatic genetic FTD, as previously reported25. Thus, we considered assessment of behavioural symptoms with the Cambridge Behavioural Inventory Revised version (CBI-R)28, general cognitive function with the Mini-Mental State Examination (MMSE)26, and cognitive processing speed and executive functions assessed with the part A and part B of the Trial Making Test (TMT)26, respectively. For each test, apart from the MMSE and CBI-R, we calculated Z scores based on language-specific norms25. Neuropsychological evaluation was harmonized across sites.
MRI acquisition.
MRI protocol was common to all the GENFI sites, and adapted for different scanners; no pre-study phantom harmonization was performed at local level. In summary, T2-weighted echo planar imaging (EPI) sequences sensitized to blood oxygenation level dependent (BOLD) contrast for rs-fMRI were considered in the present study (see Supplementary Table 1 for details on the fMRI protocol used by each site). As the repetition times (TRs, ranging from 2200 ms to 2500 ms) and the volume numbers (ranging from 140 to 200) varied across the GENFI centres, we considered only the first 140 volumes of the EPI images for each subject (mean acquisition time: 311.5±4.95 sec). During scanning, subjects were asked to keep their eyes closed, not to think of anything in particular, and not to fall asleep.
Neuroimaging pre-processing and analysis.
Functional data were pre-processed using the toolbox for Data Processing & Analysis for Brain Imaging (DPABI, http://rfmri.org/dpabi)29 based on the Statistical Parametric Mapping (SPM12) software.
For each subject, the first 2 volumes of the fMRI series were discharged to account for magnetization equilibration. The remaining 138 volumes underwent slice-timing correction and were realigned to the first volume. Any subject who had a maximum displacement in any direction larger than 2.5 mm, or a maximum rotation (x,y,z) larger than 2.5°, was excluded. Data were subsequently spatially normalized to the EPI unified segmentation template in Montreal Neurological Institute coordinates derived from SPM12 software and resampled to 3×3×3 cubic voxels. We preferred a normalization to the EPI template (instead of T1-based normalization) in line with recent data demonstrating that EPI normalization is able to reduce variability across subjects (especially when EPI distortion correction is not applied, as in our case) and boost the effective sample size by 15–25 %. Furthermore, studies assessing distance maps and intra subject variability in multicentre cohorts by EPI normalization are comparable with our data30. Spatial smoothing with an isotropic Gaussian kernel with full-width at half-maximum, 10 mm was applied; this threshold smoothing value was chosen for a number of reasons: 1) we assessed dFC within large areas, which are not usually affected by a relative large spatial smoothing; 2) we adopted in Abrol’s template to estimate the dFC31 (which has been calculated on 7500 healthy subjects) and consequently we opted for similar fMRI pipeline32 (10 mm FWHM); 3) spatial smoothing of 8–10 mm FWHM is recommended to increase sensitivity33.
Functional Networks Decomposition.
The functional imaging data were processed using the GIFT (GIFT toolbox, http://mialab.mrn.org/software/gift)34 and a spatially constrained ICA algorithm35 called Group Information Guided independent component analysis (GIG-ICA) was used to compute spatial maps that corresponded to those from a previous analysis36. In this approach, brain network spatial maps are used as reference templates to calculate functional networks for each individual subject one-by-one by maximizing independence in the context of the spatial constraint. These template maps include the brain networks with a neuronal origin (not artefactual) and assign the remaining data to be noise. We take advantage from the recently published set of 37 spatial maps derived from 7500 healthy subjects as spatial references for our network selection31. We then considered only cortical and subcortical networks, and we discharged cerebellar networks due to incomplete coverage of cerebellum in our sample, thus considering 35 spatial maps. The TR of each subject was entered in GIFT pre-processing, and we accounted for the differences in EPI acquisition protocols among centres. The Infomax approach was applied37 to estimate the independent group components and 35 functional networks were considered (see Supplementary Figure 1 for details). Subject-specific spatial patterns and time-courses were derived using spatial-temporal regression and then converted to Z-scores. The single time courses were detrended (to remove baseline drifts from the scanners and/or physiological pulsations), orthogonalized with respect to 12-motion parameters, despiked (replacement of outlier time points with 3rd order spline fitting to clean neighbouring points) and filtered using a 5th order Butterworth filter (0.01 to 0.15 Hz)32.
Windowed functional network connectivity and correlation patterns decomposition (meta-states).
The dynamic functional network connectivity (dFNC) was achieved using dynamic FNC toolbox implemented in GIFT38. dFNC was assessed using a sliding-window approach to estimate correlation matrices between components for each segment. Segments were defined with a tapered window convolving a rectangle (width=30, TRs=66 sec) with a Gaussian (σ =3) and slide in steps of 1 TR. A LASSO approach with L1 regularization (100 repetitions) was used to compute the covariance between the independent component (IC) time-courses. To obtain the decomposition into connectivity patterns (CPs), the spatial ICA (sICA) approach was applied14. As previously described, the time-courses were discretized (to work over a more tractable space) into 8 bins (positive and negative quartiles) and each timepoint was ended into a meta-state39. The time-courses for sICA CPs were derived from the regression of each subject’s dFNC information at each time window on the group of sICA CPs. During dFNC preprocessing the following covariates of no interest were considered: age, gender, acquisition site, scanner type, family number, genetic status of the proband, mean framewise displacement (calculated for each subject from the estimated motion parameters)40 and the variance associated with them has been regressed out from the windowed dynamic functional network connectivity correlations for each subject at this processing step.
Four indexes of connectivity dynamism were considered: i) the number of distinct meta-states the subjects occupied during their scans (meta-state number); ii) the number of times that subjects switch from one meta-state to another (meta-state changes), iii) the largest distance of two meta-states that subjects occupied (meta-state span), and iv) the overall distance traveled by each subject through the state space (the sum of the L1 distances between successive meta-states, i.e. meta-state total distance).
Statistical analysis.
The assumption of normality for continuous variables was not satisfied for all group combinations, as assessed by Shapiro-Wilk’s test (p<0.05). Thus, comparisons of demographic and clinical characteristics between groups (mutation carriers vs. mutation non-carriers) were assessed by Mann-Whitney U test for continuous variables and χ2 test for categorical variables. Pearson’s correlation was used to assess the relationship between the meta-state measures (meta-state number, meta-state changes, meta-state span and meta-state total distance) and age at expected symptom onset. Finally, partial correlation (considering age as a nuisance variable) was used to test the relationship between meta-state measures and cognitive/behavioural performances (CBI-R, MMSE, TMT-A, TMT-B). All the statistical analysis was performed using IBM SPSS Statistics 22.0 (Chicago, USA) and statistical significance level set at p< 0.05, corrected for multiple comparisons (Benjamini-Hochberg False-Discovery-Rate (FDR) correction41), considering four meta-state measures and four clinical tests, either for direct comparisons (mutations carriers vs mutation non-carriers) and for correlation analyses (between age at expected symptom onset and meta-state measures and between clinical tests and meta-states measures). Finally, we considered each gene (GRN, C9orf72 or MAPT) and we conducted exploratory analyses as preprocessing (Independent Component Analysis, ICA) was applied on all subjects together.
RESULTS
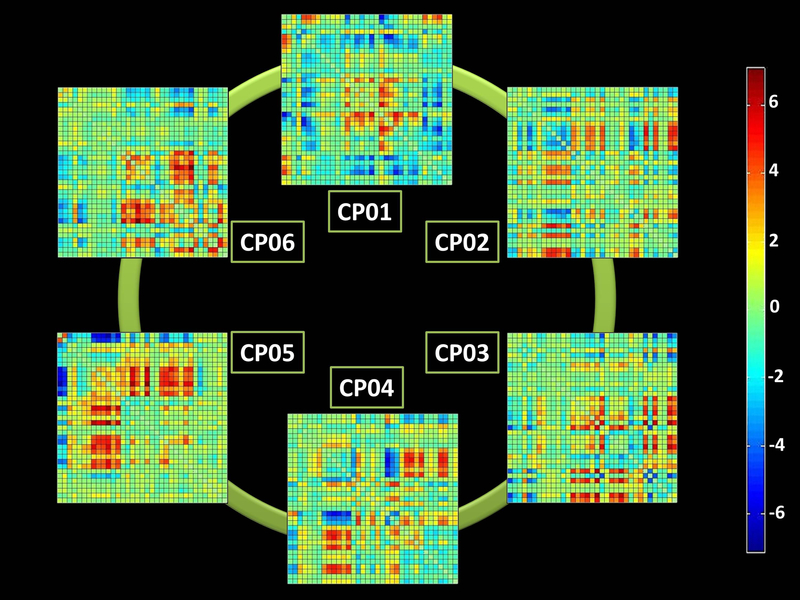
Two hundred-forty nine mutation carriers (82 with C9orf72, 122 with GRN and 45 with MAPT mutations) were considered, compared with 223 mutation non-carriers. Considering clinical and demographic variables, mutation non-carriers were slightly older (47.2±13.2 vs 44.7±11.7, p=0.042), in particular compared with MAPT mutation carriers (see Supplementary Table 2 for details). We considered six connectivity patterns (CPs) of dFNC, which are reported in Figure 1. The colors of each CP represent the direction and the strength of the correlation among the 35 considered network components (red: positive correlation and blue: negative correlation).
Figure 1. The six connectivity patterns (CPs) resulting from the dynamic Functional Network Connectivity (dFNC) analysis.
The six correlations’ matrix (among the 35 considered network components) are reported. The colorbar represents the direction and the strength of each correlation (red: positive correlation, blue: negative correlation).
dFNC was expressed as a weighted sum of the discretized six-dimensional CPs, for each given point in time and for each subject. Mutation carriers exhibited diminished dynamic fluidity, as they occupied a fewer number of meta-states (i.e., meta-state numbers) and changed from one meta-state to another less often (i.e., meta-state changes) than mutation non-carriers (see Table 2). Furthermore, mutation carriers operated over a restricted dynamic range with decreased meta-state total distance, as they travelled less overall distance, between successive meta-states, through the state space than mutation non-carriers (see Table 2). We did not find any difference in meta-state span between groups.
Table 2.
Meta-state measures in the studied groups.
| Variable | Carriers (n=249) | Non-carriers (n=223) | p* |
|---|---|---|---|
| Number of distinct metastates, mean±SD | 51.0±8.9 | 53.1±8.8 | 0.024 |
| Number of meta-state changes, mean±SD | 51.3±8.5 | 53.3±8.1 | 0.024 |
| Meta-state span, mean±SD | 23.0±4.7 | 23.8±4.5 | 0.136 |
| Meta-state total distance, mean±SD | 82.1±17.5 | 86.2±17.3 | 0.027 |
Mann-Whitney U test (carriers vs non-carriers) FDR-corrected for multiple comparisons; SD: standard deviation.
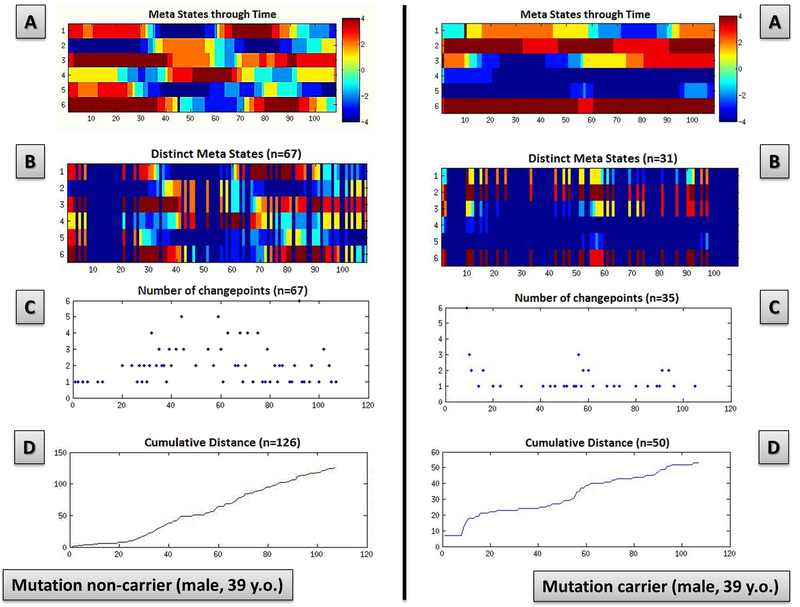
In Figure 2, meta-state dynamics through time, meta-state numbers, meta-state change points, and meta-state total distance in a representative mutation carrier and in a representative mutation non-carrier were reported. A mutation non-carrier subject will show a greater brain dynamism as compared to a representative mutation carrier (panel A), as suggested by the more complex pattern in the former subject, with an higher number of realized meta-states (panel B), meta-state changes (panel C) also travelling a greater overall distance (panel D) compared to a mutation carrier subject.
Figure 2. Meta-state dynamics through time, meta-state numbers, meta-state change points, and meta-state total distance in a representative mutation carrier and in a representative mutation non-carrier.
Meta-state dynamics through time (panel A), meta-state numbers (panel B), meta-state change points (panel C), and meta-state total distance (panel D) in a representative mutation non carrier (left column) and representative mutation carrier (right column).
The colorbar represents the strength of probability to be in each meta-state. X-axis: the six connectivity patterns (Cps) are reported, from 1 to 6; Y-axis: time (seconds, after timecourse discretization in quartiles).
In the exploratory analysis (not corrected for multiple comparisons), we evaluated C9orf72, GRN and MAPT mutation carriers separately, as compared to HC, C9orf72 mutation carriers showed reduced meta-states numbers (p=0.032) and reduced meta-state changes (p=0.041), MAPT mutation carriers had reduced meta-states numbers (p=0.042) and reduced overall meta-state total distance (p=0.046), while we did not find significant findings in regard to GRN mutation carriers, as compared to HC.
The correlation between meta-state measures and age at expected onset in mutation carriers was then considered (FDR-corrected for multiple comparisons). The closer the age at expected symptom onset, the lower the number of meta-states (Pearson’s correlation, r=−0.174, p=0.012), the lower the meta-state changes (r=−0.166, p=0.012), the lower the meta-state span (r=−0.167, p=0.012) and the lower the meta-state total distance (r=−0.141, p=0.027) was found.
Finally, the correlation between meta-state measures and cognitive performance in mutation carriers and non-carriers, considering age at evaluation as a covariate, was assessed (not corrected for multiple comparisons). TMT-A scores (the higher the scores the worse the performances) were inversely correlated with meta-state span (r=−0.143, p=0.024) and meta-state total distance (r=−0.124, p=0.050): interestingly, exploring the correlation of TMT-A scores and meta-state measures, the inverse correlation with meta-state span was primarily related to C9orf72 group (r=−0.281, p=0.011, not corrected for multiple comparisons). No other significant correlation between meta-state measures and neuropsychological/behavioural tests in the three mutation separately were demonstrated. No other significant correlations between meta-state measures and MMSE, CBI-R or TMT-B were found. No significant correlation between meta-state measures and neuropsychological/behavioural tests were evident.
DISCUSSION
In this study, results showed consistent evidence of reduced global flexibility and dynamism in the brain of presymptomatic FTD, which progressively worse with proximity to age at expected symptoms onset. Moreover, the impairment of global inner fluctuations of the brain was well correlated with processing speed performances in asymptomatic subjects carrying pathogenetic FTD mutations.
In the last decade, rs-fMRI has been used to estimate functional brain connectivity, considering regions with temporally coherent brain activity as “functional brain networks”42, 43. Up to now, most studies have relied on two implicit assumptions: the first is a “spatial assumption”, that each brain region participates in exactly one network, and the second is a “temporal assumption”, that the connectivity within each network are essentially static over time14, 44–46. New evidence clearly suggests that the brain is dynamically multistable, and spontaneous low-frequency fluctuations in BOLD fMRI data during the acquisition capture reoccurring patterns (states) of interactions among intrinsic networks at rest (chronnectome)7, 47. This is in line with spontaneous activity fluctuations found in electrophysiological studies48–50. Such findings open a new chapter in the study of neurodegenerative diseases, offering a different perspective to investigate the earliest brain changes, thus considering global brain connectivity instead of either single network connectivity or focal neural damage.
In the present study, we assessed the chronnectome fingerprint in preclinical monogenic FTD by considering meta-states. Meta-states properly describe whole brain flexibility, moving from the concept that each subject may be in a defined “state” of functional dynamic connectivity in at given point in time to the concept that a subject may have a weighted probability to be in more “states” in each given point in time. So, for each subject dynamic connectivity was represented by the sum of different connectivity pattern probability at the same time. From this point of view we explored indexes (meta-state numbers, changes, span, total distance) that were related to the global dynamic properties of the brain rather than to a specific state behaviour. Figure 1 represented the six mean (considering all subjects) correlations’ matrix that were considered simultaneously to define the dynamic connectivity parameters for each subject. On the other hand, Figure 2 showed the matrix of the six CPs through time for each subject: as you can see in the first row (A) a “visual“ difference was present (mutation non-carrier appeared more dynamic than the mutation carrier) but the objective parameters (B, C, D: metastate number, changes, total distance travelled across metastates) were defined considering all the metastates in each point in time simultaneously. This core challenge allows us to better identify early and hidden features of brain disorders, as already demonstrated in schizophrenia15.
Herein, we reported that asymptomatic mutation carriers a) passed through a lower number of distinct meta-states (i.e., lower meta-state number); b) less often switched between meta-states (i.e., lower meta-state changes), and c) switched frequently between two meta-states at close distal boundaries of the state space (i.e., lower mate-state total distance), as compared to mutation non-carriers. Altogether, these findings point to a precocious impairment of the inner fluctuations of the brain with an effect on at-distance networks through a diminished dynamic fluidity (meta-state number and meta-state changes) and a restricted dynamic range (meta-state total distance) in preclinical FTD51.
Prior work has primarily focused on topological differences among networks in FTD, identifying structural and even functional changes of specific brain networks in preclinical disease2, 21, 22, 25, 52–55 with specific neuropathological progression according with the molecular nexopathy paradigm56. In the present work, we suggested that FTD at the early stages also affects whole brain efficiency, providing a complementary and coordinated point of view on the presymptomatic phases of FTD.
Moreover, we reported that the greater the meta-state abnormalities in mutation carriers, the closer the age at expected onset, in line with the progressive changes which in turn lead to symptom onset and structural damage in FTD.
Finally, TMT-A, a test reflecting processing speed skills57, inversely correlated with measures of dynamic range connectivity. This confirms and extends previous hypothesis of a strict link between chronnectome fingerprint and cognition performances58, 59 that needs to be further explored, also considering the strength of correlations (low-moderate) in the mutation-carriers group . With regard to the discrepancy between cognitive (CBI-R) and behavioural (TMT-A in particular) association with meta-state measures it should be noted that the level of behavioural/cognitive symptoms in the preclinical phase of FTD is very low (not fulfilling diagnostic criteria for FTD), making behavioural and neuropsychological tests potentially useful only in the late preclinical phase of FTD (approximately 5 years before) as already demonstrated25. On the other hand, TMT-A, as index of preprocessing speed skill could represent a marker of the “global” brain perturbation in the preclinical phase of FTD, as captured by dynamic brain connectivity approach. Finally, no significant correlations between TMT-A and metastate measures in healthy controls were evident, supporting the idea that this findings in presymptomatic FTD were not primarily age-related.
Our study presents a number of limitations that need to be acknowledged. First, the influence of vigilance (considering that rs-fMRI data were collected eye-closed) was not evaluated35. Second, subject motion is of particular concern in dynamic analyses of rs-fMRI60. To overcome this limit, we included motion parameters estimation as well as mean framewise displacement in the preprocessing and in the statistical design, respectively32, 40. Third, considering the unconstrained nature of the resting-state signal, thought content during the scan represented a significant source of variability, can only be partially evaluated by retrospective questionnaires13, 61. Four , scan time of acquisition was in line with (or even greater than) previous studies13, 14 even though the development of effective dynamic functional connectivity statistical approaches is still an open field, deserving attention in the future15, 62–65.
Despite these limitations, to the best of our knowledge, this is the first study applying chronnectome approach to neurodegenerative dementias. The exploration of time-varying aspects of functional connectivity unveiled aspects of the underappreciated early brain changes in FTD and supported the view that at the very early disease stage FTD is affecting brain as global system, and only in a second phase the pathology involves selective focal regions. Therefore, FTD should be considered less focal than previously thought. These findings may have important implication on clinical grounds, as tracking desynchronization of the inner fluctuations of the brain might be a helpful prognostic marker to be used in future pharmacological and prevention trials and it could be considered a feasible approach to identify novel targets of intervention.
Supplementary Material
Supplementary Figure 1.Network independent components (displayed on a 3D standard template and showed at the most activated sagittal, coronal and axial slices), subsequently used as the reference template for the GIG-ICA analysis (modified by Abrol et al. 31). GIG-ICA: Group Information Guided independent component analysis.
Acknowledgements
We would like to thank Dr. Paolo Semeraro for his valuable support in data preprocessing. This work was supported in part by grants from the NIH (R01REB020407, P20GM103472) as well as NSF grant 1539067.
APPENDIX
List of other GENFI consortium members
Maria Rosario Almeida - Centre of Neurosciences and Cell Biology, Universidade de Coimbra, Coimbra, Portugal
Sarah Anderl-Straub - Ulm University, Ulm, Germany
Christin Andersson - Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Anna Antonell - Alzheimer’s disease and other cognitive disorders unit, Neurology Department, Hospital Clinic, Institut d’Investigacions Biomèdiques, Barcelona, Spain
Andrea Arighi - Neurology Unit, Department of Physiopathology and Transplantation, Fondazione Cà Granda, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale Policlinico, Milan, Italy
Mircea Balasa - Alzheimer’s disease and other cognitive disorders unit, Neurology Department, Hospital Clinic, Institut d’Investigacions Biomèdiques, Barcelona, Spain
Myriam Barandiaran - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
Nuria Bargalló - Radiology Department, Image Diagnosis Center, Hospital Clínic and Magnetic Resonance Image core facility, IDIBAPS, Barcelona, Spain
Robart Bartha - Department of Medical Biophysics, Robarts Research Institute, University of Western Ontario, London, Ontario
Benjamin Bender - Department of Radiology, University of Tuebingen, Tuebingen, Germany
Luisa Benussi - Istituto di Ricovero e Cura a Carattere Scientifico Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Giuliano Binetti - Istituto di Ricovero e Cura a Carattere Scientifico Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Sandra Black - LC Campbell Cognitive Neurology Research Unit, Sunnybrook Research Institute, Toronto, Canada
Martina Bocchetta - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Sergi Borrego-Ecija - Alzheimer’s disease and other cognitive disorders unit, Neurology Department, Hospital Clinic, Institut d’Investigacions Biomèdiques, Barcelona, Spain
Jose Bras - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Rose Bruffaerts - Laboratory for Cognitive Neurology, Department of Neurosciences, KU Leuven, Leuven, Belgium
Paola Caroppo - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
David Cash - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Miguel Castelo-Branco - Neurology Department, Centro Hospitalar e Universitário de Coimbra, Instituto de Ciências Nucleares Aplicadas à Saúde (ICNAS), Portugal
Rhian Convery - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Thomas Cope - University of Cambridge, UK
María de Arriba - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
Giuseppe Di Fede - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Zigor Díaz - ITA Alzheimer, San Sebastian, Spain
Katrina M Dick - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Diana Duro - Faculty of Medicine, Universidade de Coimbra, Coimbra, Portugal
Chiara Fenoglio - Department of Pathophysiology and Transplantation, “Dino Ferrari” Center, University of Milan, Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy
Carlos Ferreira - Instituto Ciências Nucleares Aplicadas à Saúde, Universidade de Coimbra, Coimbra, Portugal
Catarina B. Ferreira - Faculty of Medicine, University of Lisbon, Portugal
Toby Flanagan - University of Manchester, UK
Nick Fox - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Morris Freedman - Division of Neurology, Baycrest Centre for Geriatric Care, University of Toronto, Canada
Giorgio Fumagalli - Neurology Unit, Fondazione Cà Granda, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale Policlinico, Milan, Italy; Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
Alazne Gabilondo - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
Serge Gauthier - Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
Roberta Ghidoni - Istituto di Ricovero e Cura a Carattere Scientifico Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Giorgio Giaccone - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Ana Gorostidi - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
Caroline Greaves - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Rita Guerreiro - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Carolin Heller - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Tobias Hoegen - Department of Neurology, Ludwig-Maximilians-University Munich, Germany
Begoña Indakoetxea - Cognitive Disorders Unit, Department of Neurology, Donostia University Hospital, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
Vesna Jelic - Division of Clinical Geriatrics, Karolinska Institutet, Stockholm, Sweden
Lize Jiskoot - Department of Neurology, Erasmus Medical Center, Rotterdam
Hans-Otto Karnath - Section of Neuropsychology, Department of Cognitive Neurology, Center for Neurology & Hertie-Institute for Clinical Brain Research, Tübingen, Germany
Ron Keren - University Health Network Memory Clinic, Toronto Western Hospital, Toronto, Canada
Maria João Leitão - Centre of Neurosciences and Cell Biology, Universidade de Coimbra, Coimbra, Portugal
Albert Lladó - Alzheimer’s disease and other cognitive disorders unit, Neurology Department, Hospital Clinic, Institut d’Investigacions Biomèdiques, Barcelona, Spain
Gemma Lombardi - Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
Sandra Loosli - Department of Neurology, Ludwig-Maximilians-University Munich, Germany
Carolina Maruta - Lisbon Faculty of Medicine, Language Research Laboratory, Lisbon, Portugal
Simon Mead - MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK
Lieke Meeter - Department of Neurology, Erasmus Medical Center, Rotterdam, Netherlands
Gabriel Miltenberger - Faculty of Medicine, University of Lisbon, Portugal
Rick van Minkelen - Department of Clinical Genetics, Erasmus Medical Center, Rotterdam, Netherlands
Sara Mitchell - LC Campbell Cognitive Neurology Research Unit, Sunnybrook Research Institute, Toronto, Canada
Benedetta Nacmias - Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
Mollie Neason - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Jennifer Nicholas - The London School of Hygiene and Tropical Medicine, London, UK
Linn Öijerstedt - Division of Neurogeriatrics, Karolinska Institutet, Stockholm, Sweden
Jaume Olives - Alzheimer’s disease and other cognitive disorders unit, Neurology Department, Hospital Clinic, Institut d’Investigacions Biomèdiques, Barcelona, Spain
Jessica Panman – Department of Neurology, Erasmus Medical Center, Rotterdam, the Netherlands
Janne Papma - Department of Neurology, Erasmus Medical Center, Rotterdam, the Netherlands
Maximilian Patzig - Department of Neuroradiology, Ludwig-Maximilians-University Munich, Germany
Michela Pievani - Istituto di Ricovero e Cura a Carattere Scientifico Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Sara Prioni - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Catharina Prix - Department of Neurology, Ludwig-Maximilians-University Munich, Germany
Rosa Rademakers - Department of Neurosciences, Mayo Clinic, Jacksonville, Florida
Veronica Redaelli - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Tim Rittman - University of Cambridge, UK
Ekaterina Rogaeva - Tanz Centre for Research in Neurodegenerative Diseases, University of Toronto, Canada
Pedro Rosa-Neto - Translational Neuroimaging Laboratory, McGill University Montreal, Quebec, Canada
Giacomina Rossi - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Martin Rossor - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Beatriz Santiago - Neurology Department, Centro Hospitalar e Universitário de Coimbra, Portugal
Elio Scarpini - Neurology Unit, Department of Physiopathology and Transplantation, Fondazione Cà Granda, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale Policlinico, Milan, Italy
Elisa Semler - Ulm University, Ulm, Germany
Rachelle Shafei - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Christen Shoesmith - Department of Clinical Neurological Sciences, University of Western Ontario, London, Ontario, Canada
Miguel Tábuas-Pereira - Centre of Neurosciences and Cell Biology, Universidade de Coimbra, Coimbra, Portugal
Mikel Tainta - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
David Tang-Wai - University Health Network Memory Clinic, Toronto Western Hospital, Toronto, Canada
David L Thomas - Neuroradiological Academic Unit, UCL Institute of Neurology, UK
Hakan Thonberg - Center for Alzheimer Research, Division of Neurogeriatrics, Karolinska Institutet, Stockholm, Sweden
Carolyn Timberlake - University of Cambridge, UK
Pietro Tiraboschi - Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Neurologico Carlo Besta, Milano, Italy
Philip Vandamme - Neurology Service, University Hospitals Leuven, Belgium; Laboratory for Neurobiology, VIB-KU Leuven Centre for Brain Research, Leuven, Belgium
Mathieu Vandenbulcke - Geriatric Psychiatry Service, University Hospitals Leuven, Belgium; Neuropsychiatry, Department of Neurosciences, KU Leuven, Leuven, Belgium
Michele Veldsman - University of Oxford, UK
Ana Verdelho - Department of Neurosciences, Santa Maria Hospital, University of Lisbon, Portugal
Jorge Villanua - OSATEK Unidad de Donostia, San Sebastian, Gipuzkoa, Spain
Jason Warren - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Carlo Wilke - Hertie Institute for Clinical Brain Research, University of Tuebingen, Tuebingen, Germany
Henrik Zetterberg - Department of Neurodegenerative Disease, UCL Institute of Neurology, UK
Miren Zulaica - Neuroscience Area, Biodonostia Health Research Institute, Paseo Dr Begiristain sn, CP 20014, San Sebastian, Gipuzkoa, Spain
REFERENCES
- 1.Pievani M, Filippini N, van den Heuvel MP, Cappa SF, Frisoni GB. Brain connectivity in neurodegenerative diseases--from phenotype to proteinopathy. Nat Rev Neurol 2014; 10(11): 620–633. [DOI] [PubMed] [Google Scholar]
- 2.Premi E, Formenti A, Gazzina S, Archetti S, Gasparotti R, Padovani A et al. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol 2014; 71(2): 216–221. [DOI] [PubMed] [Google Scholar]
- 3.de Pasquale F, Corbetta M, Betti V, Della Penna S. Cortical cores in network dynamics. Neuroimage 2017. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Liao X, Xia M, He Y. Chronnectome fingerprinting: Identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. 2018; 39(2): 902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006; 103(37): 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 2006; 29(4): 1359–1367. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 2014; 84(2): 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010; 50(1): 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma 2010; 23(5–6): 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, Wallis JD et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci U S A 2010; 107(40): 17356–17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries P A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 2005; 9(10): 474–480. [DOI] [PubMed] [Google Scholar]
- 12.Hillebrand A, Tewarie P, van Dellen E, Yu M, Carbo EW, Douw L et al. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc Natl Acad Sci U S A 2016; 113(14): 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH et al. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp 2017; 38(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 2014; 24(3): 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RL, Yaesoubi M, Turner JA, Mathalon D, Preda A, Pearlson G et al. Higher Dimensional Meta-State Analysis Reveals Reduced Resting fMRI Connectivity Dynamism in Schizophrenia Patients. PLoS One 2016; 11(3): e0149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 2017; 160: 41–54. [DOI] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76(11): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134(Pt 9): 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitwell JL, Jack CR Jr., Boeve BF, Senjem ML, Baker M, Rademakers R et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 2009; 72(9): 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borroni B, Archetti S, Alberici A, Agosti C, Gennarelli M, Bigni B et al. Progranulin genetic variations in frontotemporal lobar degeneration: evidence for low mutation frequency in an Italian clinical series. Neurogenetics 2008; 9(3): 197–205. [DOI] [PubMed] [Google Scholar]
- 21.Dopper EG, Rombouts SA, Jiskoot LC, den Heijer T, de Graaf JR, de Koning I et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 2014; 83(2): e19–26. [DOI] [PubMed] [Google Scholar]
- 22.Premi E, Cauda F, Gasparotti R, Diano M, Archetti S, Padovani A et al. Multimodal FMRI resting-state functional connectivity in granulin mutations: the case of fronto-parietal dementia. PLoS One 2014; 9(9): e106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res 2008; 11(3): 585–595. [DOI] [PubMed] [Google Scholar]
- 24.Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci 2017; 19(1): 17–33. [DOI] [PubMed] [Google Scholar]
- 25.Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015; 14(3): 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006; 20(4): 210–216. [DOI] [PubMed] [Google Scholar]
- 27.Premi E, Grassi M, van Swieten J, Galimberti D, Graff C, Masellis M et al. Cognitive reserve and TMEM106B genotype modulate brain damage in presymptomatic frontotemporal dementia: a GENFI study. Brain 2017; 140(6): 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wear HJ, Wedderburn CJ, Mioshi E, Williams-Gray CH, Mason SL, Barker RA et al. The Cambridge Behavioural Inventory revised. Dement Neuropsychol 2008; 2(2): 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016; 14(3): 339–351. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun VD, Wager TD, Krishnan A, Rosch KS, Seymour KE, Nebel MB et al. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum Brain Mapp 2017; 38(11): 5331–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR et al. Replicability of time-varying connectivity patterns in large resting state fMRI samples. Neuroimage 2017; 163: 160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrol A, Chaze C, Damaraju E, Calhoun VD. The chronnectome: Evaluating replicability of dynamic connectivity patterns in 7500 resting fMRI datasets. Conf Proc IEEE Eng Med Biol Soc 2016; 2016: 5571–5574. [DOI] [PubMed] [Google Scholar]
- 33.Mikl M, Marecek R, Hlustik P, Pavlicova M, Drastich A, Chlebus P et al. Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging 2008; 26(4): 490–503. [DOI] [PubMed] [Google Scholar]
- 34.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 2001; 14(3): 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Ong JL, Patanaik A, Zhou J. Spontaneous eyelid closures link vigilance fluctuation with fMRI dynamic connectivity states. 2016; 113(34): 9653–9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y, Allen EA, He H, Sui J, Wu L, Calhoun VD. Artifact removal in the context of group ICA: A comparison of single-subject and group approaches. Hum Brain Mapp 2016; 37(3): 1005–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 1995; 7(6): 1129–1159. [DOI] [PubMed] [Google Scholar]
- 38.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin 2014; 5: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller RL, Yaesoubi M, Calhoun VD. Higher dimensional analysis shows reduced dynamism of time-varying network connectivity in schizophrenia patients. Conf Proc IEEE Eng Med Biol Soc 2014; 2014: 3837–3840. [DOI] [PubMed] [Google Scholar]
- 40.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59(3): 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57 1995; 1: 289–300. [Google Scholar]
- 42.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 2009; 29(6): 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 2008; 39(4): 1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 2013; 34(9): 2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciric R, Nomi JS, Uddin LQ, Satpute AB. Contextual connectivity: A framework for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci Rep 2017; 7(1): 6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faghiri A, Stephen JM, Wang YP, Wilson TW. Changing brain connectivity dynamics: From early childhood to adulthood. 2018; 39(3): 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onton J, Makeig S. Information-based modeling of event-related brain dynamics. Prog Brain Res 2006; 159: 99–120. [DOI] [PubMed] [Google Scholar]
- 48.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 1996; 273(5283): 1868–1871. [DOI] [PubMed] [Google Scholar]
- 49.Pascual-Marqui RD, Michel CM, Lehmann D. Segmentation of brain electrical activity into microstates: model estimation and validation. IEEE Trans Biomed Eng 1995; 42(7): 658–665. [DOI] [PubMed] [Google Scholar]
- 50.Yanagawa T, Mogi K. Analysis of ongoing dynamics in neural networks. Neurosci Res 2009; 64(2): 177–184. [DOI] [PubMed] [Google Scholar]
- 51.Warren JD, Rohrer JD, Schott JM, Fox NC, Hardy J, Rossor MN. Molecular nexopathies: a new paradigm of neurodegenerative disease. Trends Neurosci 2013; 36(10): 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno F, Sala-Llonch R, Barandiaran M, Sanchez-Valle R, Estanga A, Bartres-Faz D et al. Distinctive age-related temporal cortical thinning in asymptomatic granulin gene mutation carriers. Neurobiol Aging 2013; 34(5): 1462–1468. [DOI] [PubMed] [Google Scholar]
- 53.Pievani M, Paternico D, Benussi L, Binetti G, Orlandini A, Cobelli M et al. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement 2014; 10(5 Suppl): S354–S363.e351. [DOI] [PubMed] [Google Scholar]
- 54.Caroppo P, Habert MO, Durrleman S, Funkiewiez A, Perlbarg V, Hahn V et al. Lateral Temporal Lobe: An Early Imaging Marker of the Presymptomatic GRN Disease? J Alzheimers Dis 2015; 47(3): 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Premi E, Cauda F, Costa T, Diano M, Gazzina S, Gualeni V et al. Looking for Neuroimaging Markers in Frontotemporal Lobar Degeneration Clinical Trials: A Multi-Voxel Pattern Analysis Study in Granulin Disease. J Alzheimers Dis 2016; 51(1): 249–262. [DOI] [PubMed] [Google Scholar]
- 56.Warren JD, Rohrer JD, Hardy J. Disintegrating brain networks: from syndromes to molecular nexopathies. Neuron 2012; 73(6): 1060–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc 2006; 1(5): 2277–2281. [DOI] [PubMed] [Google Scholar]
- 58.Jia H, Hu X, Deshpande G. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect 2014; 4(9): 741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct Global Brain Dynamics and Spatiotemporal Organization of the Salience Network. PLoS Biol 2016; 14(6): e1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chee MW. Proc Natl Acad Sci U S A. [Google Scholar]
- 61.O’Callaghan C, Shine JM, Lewis SJ, Andrews-Hanna JR, Irish M. Shaped by our thoughts--a new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn 2015; 93: 1–10. [DOI] [PubMed] [Google Scholar]
- 62.Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK et al. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 2016; 127: 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakil S, Lee CH, Keilholz SD. Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. Neuroimage 2016; 133: 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shine JM, Koyejo O, Bell PT, Gorgolewski KJ, Gilat M, Poldrack RA. Estimation of dynamic functional connectivity using Multiplication of Temporal Derivatives. Neuroimage 2015; 122: 399–407. [DOI] [PubMed] [Google Scholar]
- 65.Yaesoubi M, Allen EA, Miller RL, Calhoun VD. Dynamic coherence analysis of resting fMRI data to jointly capture state-based phase, frequency, and time-domain information. Neuroimage 2015; 120: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1.Network independent components (displayed on a 3D standard template and showed at the most activated sagittal, coronal and axial slices), subsequently used as the reference template for the GIG-ICA analysis (modified by Abrol et al. 31). GIG-ICA: Group Information Guided independent component analysis.