Abstract
The disassembly of the clathrin lattice surrounding coated vesicles is the obligatory last step in their life cycle. It is mediated by the coordinated recruitment of auxilin and Hsc70, an ATP-driven molecular clamp. Here, we describe the preparation of reagents and the single-particle fluorescence microscopy imaging assay in which we visualize directly the Hsc70-driven uncoating of synthetic clathrin coats or clathrin-coated vesicles.
Keywords: Clathrin-coated vesicle, Auxilin, Microfluidics, TIRF microscopy, Clathrin uncoating
1. Introduction
The disassembly of the clathrin coat occurs shortly after the scission of the coated vesicle from the donor membrane and represents a key step in the life cycle of a clathrin-coated vesicle (CCV) [1–4]. The uncoating reaction depends on ATP hydrolysis [5–7], and is jointly mediated by interactions by the molecular chaperone heat shock cognate protein 70 (Hsc70) that binds to the unstructured C-terminal tail of clathrin heavy chain with its cochaperone auxilin [8] also recruited to the coated vesicle [3, 5, 9]. Ensemble in vitro measurements of the uncoating reaction of natural coated vesicles or clathrin coats (CCs) assembled from purified clathrin and adaptor proteins (AP-2) have resolved key molecular steps of the process [5, 7]. We recently developed a fluorescence microscopy method to observe the uncoating reaction at the level of individual synthetic clathrin coats reconstituted with the AP-2 adaptor complex [8]. Here, we describe in detail use of this method to follow the uncoating mediated by recombinant Hsc70 and auxilin of fluorescently tagged CCs or of in vitro reconstituted CCVs. Fluorescent clathrin trimers reconstituted from recombinant heavy chain and light chain labeled with a fluorophore were either mixed with the adaptor protein complex AP-2 for assembly into CCs (Fig. 1a) or mixed with AP-2 and extruded liposomes with tyrosine-motif (YQRL) containing peptidolipid for assembly into CCVs (Fig. 1b). The clathrin-coated structures were immobilized at the bottom of a microfluidic channel device on a passivated glass coverslip coated with a monoclonal antibody specific for clathrin light chain A (Fig. 2) and appeared as bright diffraction-limited spots in the clathrin channel. In the case of reconstituted CCVs, the signal from the lipid vesicle stained with membrane-binding dyes was detected at the locations corresponding to immobilized CCVs, whereby different color dyes were used to distinguish vesicles made with different lipid compositions (Fig. 1c). ATP-loaded Hsc70 labeled with a different fluorophore together with auxilin were then injected into the microfluidic channel to initiate the uncoating reaction (Fig. 2); the time course of the fluorescence signals associated with single clathrin coats and Hsc70 are then recorded using multiwavelength time-lapse total internal reflection fluorescence (TIRF) microscopy (Fig. 2). Traces of clathrin intensity were extracted from the fluorescence movies at each location corresponding to a single coat, whereby the fluorescence intensity was converted into number of molecules using a calibration value determined by single-molecule photobleaching. The resulting clathrin traces showed the process of clathrin uncoating for individual clathrin structures (Fig. 3). Likewise, traces of Hsc70 association with the coats were determined from the colocalized signals of Hsc70 at the location of each clathrin coat. The loss of clathrin fluorescence and amount of recruited Hsc70 were determined from several hundred coats imaged in the field of view allowing us to extract kinetic parameters of Hsc70 binding and clathrin uncoating [8].
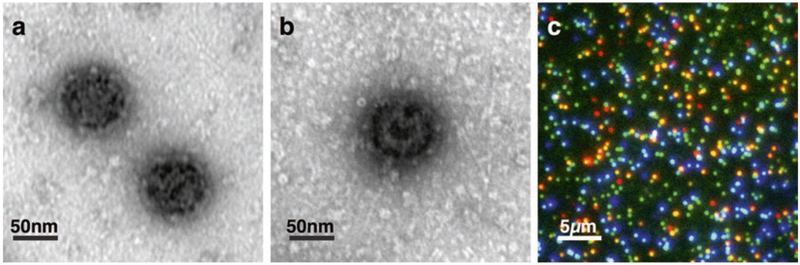
Fig. 1.
Transmission electron microscopy (TEM) and total internal reflection (TIRF) images of in vitro assembled clathrin coats and clathrin-coated vesicles. (a) Negative stain TEM images of clathrin coats (CCs) and (b) a clathrin coated vesicle (CCV). (c) Multiplexed visualization of CCV components using TIRF microscopy. Empty clathrin coats are shown in green, clathrin coats surrounding liposomes containing PI(3)P, PI(4,5)P2, DOPC, and YQRL containing peptidolipid are shown in orange, and clathrin coats surrounding liposomes containing PI(5) P, PI(4,5)P2, DOPC, and YQRL containing peptidolipid are shown in cyan. The clathrin light chain A is labeled with Alexa Fluor 488 (visualized in green color), PI(3)P and PI(5)P containing liposomes are labeled with DiI and DiD lipid dyes (visualized in red and blue colors), respectively
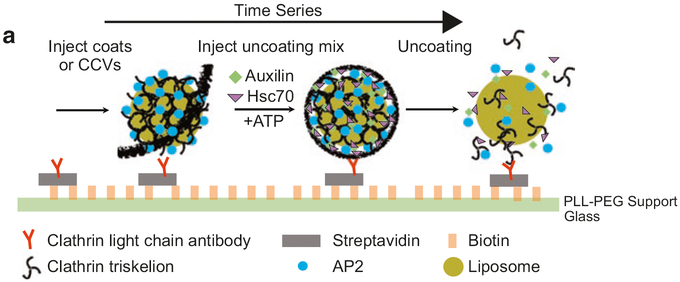
Fig. 2.
Schematic of surface chemistry and uncoating assay. Glass coverslips are coated with a layer of a copolymer composed of poly-l-lysine and biotinylated poly(ethylene glycol) (PLL-PEG) to minimize nonspecific adsorption of proteins to the surface. The surface is then modified with streptavidin followed by the biotinylated monoclonal antibody CVC.6 directed against mutated rat clathrin light chain A. In vitro assembled CCs clathrin coats or CCVs are then captured onto the surface via the antibody (shown here for CCV). Uncoating reagents (Hsc70, auxilin, ATP) are then added to the immobilized clathrin coats or CCV to initiate the uncoating reaction. Different components of the reaction can be labeled with spectrally distinct fluorophores to allow observation of their arrival and/or departure at the locations of individual coats/CCVs by multiwavelength TIRF microscopy
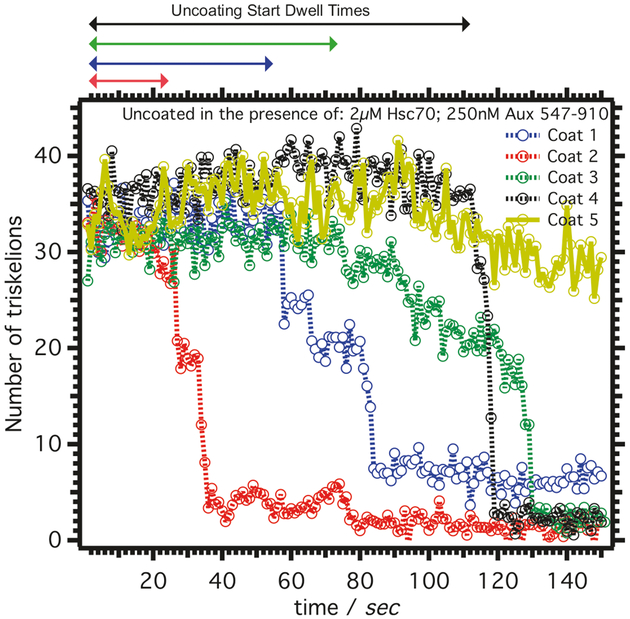
Fig. 3.
Kinetics of clathrin-coat disassembly. Single clathrin-coat disassembly tracings after injecting ΔPTEN-Auxilin, Hsc70 with ATP. The stochastic behavior illustrated by differences in the dwell time to start the clathrin lattice disassembly (monitored via labeled clathrin light chain A signal) is shown for five different traces
2. Materials
2.1. Equipment
Shaker for baculovirus cultures (28C, dark).
Sonicator.
Metal beaker.
Bacterial shaker.
FPLC.
Ultracentrifuge Sorvall or equivalent.
Benchtop centrifuge for pelleting bacterial/baculovirus cultures.
Spectrophotometer.
Tissue grinder (e.g., Potter Homogenizer or similar).
0.2 μm syringe filters.
Baffled flask for bacterial culture.
Spinner flask for baculovirus culture.
SDS-PAGE gel electrophoresis setup.
Kitchen blender.
Argon-flow sample dryer.
Electron microscope.
Vacuum desiccator.
Laboratory oven (70 °C).
Hot plate.
Headway spin coater (or similar).
Laurell Spin Coater (or similar).
THINKY ARE-250 Mixer.
Technics Plasma etcher 500-II (or similar).
Micropuncher to make holes in PDMS.
Glow discharge plasma chamber.
Access to clean-room facility.
2.2. Reagents for Expression and Purification of Clathrin Heavy Chain
pFastBac-based plasmid encoding the heavy chain [10].
DH10Bac bacterial cells, and Sf9 and High Five insect cells (ThermoFisher) (Ref. 10).
LB agar containing 7 μg/mL gentamycin, 10 μg/mL tetracycline, 50 μg/mL kanamycin, 100 μg/mL Bluo-Gal, and 40 μg/mL isopropyl β-d −1-thiogalactopyranoside (IPTG).
Sf900-II (Gibco/ThermoFisher), and serum-free medium for insect cell culture (either Excell 420 (Sigma-Aldrich) [10] or alternatively Express Five (Gibco/ThermoFisher).
Lysis Buffer 1: 50 mM Tris–HCl pH 8.0, 300 mM NaCl, 0.5 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraace-tic acid (EDTA), 0.05 mg/mL RNase A.
Cage Formation Buffer: 20 mM Mes pH 6.2, 2 mM CaCl2, 0.02% NaN3, 0.5 mM DTT.
Buffer A: 50 mM Mes pH 6.5, 100 mM NaCl, 1 mM EGTA, 0.5 mM MgCl2, 0.02% NaN3, 0.5 mM DTT.
Buffer B: 2.4 M Tris–HCl pH 7.4, 0.04% NaN3, 1 mM DTT.
Column Buffer 1(filter before use): 0.5 M Tris–HCl pH 7.4, 0.02% NaN3, 0.5 mM DTT.
Low phosphate buffer (filter before use): 10 mM NaH2PO4 pH 7.1, 100 mM NaCl, 0.02 NaN3, 0.5 mM DTT.
High phosphate buffer (filter before use): 500 mM NaH2PO4 pH 7.1, 100 mM NaCl, 0.02 NaN3, 0.5 mM DTT.
Alkaline Buffer: 200 mM NaOH with 1% SDS.
Isopropanol.
Tris–EDTA buffer: 10 mM Tris–HCl, pH 7.5, 1 mM EDTA.
PMSF (stock concentration) or PEFA (stock concentration).
0.5 M EDTA stock solution, pH 8.0.
HiLoad 26/600 Superdex 200 gel filtration column (GE Life Sciences).
Econo-Pac CHT-II Ceramic Hydroxyapatite column, 5 mL (Bio-Rad).
2.3. Reagents for Expression, Purification, and Labeling of Clathrin Light Chain
Construct/bacterial cells: pET28 LCA D203E C218S/E. coli BL21 DE3 [8].
LB medium containing kanamycin (30 μg/mL).
1 M IPTG.
Lysis buffer 2: 20 mM BisTris pH 6.0 (see Note 1), 0.5 mM DTT, 1 mM EDTA, Complete protease inhibitor (see Note 2).
Mono Q 5/50 GL column, 1 mL bed volume (GE Life Sciences).
Buffer C (filter before use): 20 mM BisTris pH 6.0, 0.5 mM DTT.
Buffer D (filter before use): 20 mM BisTris pH 6.0, 1 M NaCl, 0.5 mM DTT.
Dialysis tubing or capsules (10 kDa MWCO).
Labeling buffer: 20 mM Tris–HCl pH 7.4, 1 mM EDTA, 0.2 mM tris(2-carboxyethyl)phosphine (TCEP).
Thiol-reactive dye, e.g., Alexa Fluor® 488 C5 Maleimide.
2-Mercaptoethanol.
0.5 M DTT.
Centrifugal filtration device (MWCO 10 kDa).
Glycerol.
2.4. Reagents for Expression and Purification of ΔPTEN-Auxilin
Construct/bacterial cells: pGEX auxilin 547–910 (N-terminal GST tag, thrombin cleavage site)/E. coli BL21 DE3 [11].
LB medium containing ampicillin (0.1 mg/mL).
1 M isopropyl β-d-1-thiogalactopyranoside (IPTG).
Glutathione agarose.
Lysis buffer 3: 20 mM HEPES pH 7.6, 100 mM KCl, 0.2 mM EDTA, 20% glycerol, 1% Triton, 0.5 mM DTT, Complete protease inhibitor (Roche Life Science).
Wash buffer 1: 25 mM Tris–HCl pH 7, 40 mM NaCl, 0.1 mM EDTA, 0.2 mM TCEP.
Elution Buffer 1: 20 mM glutathione in wash buffer; make fresh; adjust pH to 8 with NaOH.
0.5 U/μL thrombin, frozen in 50 μL aliquots.
Buffer E (filter before use): 50 mM MES, pH 6.7, 1 mM EDTA, 0.2 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP).
Buffer F (filter before use): 50 mM MES, pH 6.7, 500 mM NaCl, 1 mM EDTA, 0.2 mM TCEP.
Mono S HR 5/5 column, 1 mL bed volume (GE Life Sciences).
2.5. Reagents for Expression, Purification, and Labeling of Hsc70
Construct/bacterial cells: pProEX Hsc70 (N-terminal hexahistidine tag, TEV cleavage site, C-terminal Gly-Cys extension)/E. coli BL21 DE3.
LB medium containing 0.1 mg/mL ampicillin.
1 M IPTG.
Lysis buffer 4: 50 mM Tris–HCl pH 7.5, 300 mM NaCl, complete protease inhibitor tablet.
Cobalt affinity resin (e.g., Talon; (Clontech), or similar).
Wash buffer 2: 50 mM Tris–HCl pH 7.5, 300 mM NaCl, 10 mM imidazole.
Elution buffer 2: 50 mM Tris–HCl pH 7.5, 300 mM NaCl, 80 mM imidazole.
0.5 M EDTA solution, pH 8.
0.5 M DTT.
TEV protease.
0.5 M Tris(2-carboxyethyl) phosphine (TCEP) stock solution.
100 mM ATP.
Centrifugal filtration device, 30 kDa MWCO.
Superose 6 HR 10/30 column.
Column buffer 2: 20 mM imidazole pH 6.8, 100 mM KCl, 2 mM MgCl2, 0.1 mM ATP.
2.6. Reagents for Purification of AP-2 Adaptor Protein
4 fresh calf brains.
50 mM phenylmethane sulfonyl fluoride (PMSF) solution in ethanol.
Buffer G: 50 mM MES, pH 6.9, 100 mM NaCl, 1 mM EGTA, 0.5 mM MgCl2, 0.02% NaN3, 0.5 mM DTT.
Buffer H (filter before use): 2.4 M Tris–HCl, pH 7.4, 0.04% NaN3, 1 mM DTT.
12.5% Ficoll/12.5% sucrose in Buffer G.
Column Buffer 3: 0.5 M Tris–HCl, pH 7.4, 0.02% NaN3, 0.5 mM DTT.
Sepharose CL-4B column (length = 1 m, diameter = 3 cm, volume = 700 mL).
0.5 M Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′- tetraacetic acid (EGTA) pH 8.
Low phosphate buffer: 10 mM KH2PO4; pH 7.1, 100 mM KCl, 0.02% NaN3, 0.1% 2-mercaptoethanol.
High phosphate buffer (see Note 3): 500 mM KH2PO4; pH 7.1, 100 mM KCl, 0.02% NaN3, 0.1% 2-mercaptoethanol.
Ceramic hydroxyapatite column (2 mL bed volume, Type I (see Note 4), 20 microns).
AP buffer: 100 mM MES, pH 7.0, 150 mM NaCl, 1 mM EDTA, 0.02% NaN3, 0.5 mM DTT.
2.7. Reagents for In Vitro Reconstitution of Fluorescent Clathrin Coat ormation
Recombinant clathrin heavy chain trimers.
Labeled recombinant clathrin LCA.
AP-2 adaptor complex purified from bovine brain.
Coat Formation Buffer (50 mM MES pH 6.5, 100 mM NaCl, 2 mM EDTA, 0.5 mM DTT) and/or Coated-Vesicle Formation Buffer (80 mM MES pH 6.5, 20 mM NaCl, 2 mM EDTA, 0.4 mM DTT).
Dialysis cassette, 10 kDa MWCO.
2.8. Reagents for Fabrication of Liposomes Used for Reconstitution of Clathrin-Coated Vesicles
- Lipids:
- Maleimide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE).
- 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC).
- 18:1 PI(3)P 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myoinositol-3′-phosphate) (ammonium salt).
- 18:1 PI(4)P 1,2-dioleoyl-sn-glycero-3-phospho-(1′-myoinositol-4′-phosphate) (ammonium salt).
- PI(4,5)P2 l-α-phosphatidylinositol-4,5-bisphosphate (Brain, Porcine) (ammonium salt).
- Lipid Dyes:
- DiD’ oil; DiIC18(5) oil (1,1′-dioctadecyl-3,3,3′,3’-tetramethylindodicarbocyanine perchlorate)
- Dil Stain (1,1′-dioctadecyl-3,3,3′,3’-tetramethylindocar bocyanine perchlorate (‘DiI’; DiIC18(3))).
YQRL containing peptide (CKVTRRPKASDYQRLNL), lyophilized powder.
20 mM HEPES pH 7.4.
Dimethyl sulfoxide (DMSO).
10 mM β-mercaptoethanol.
Chloroform, methanol, and water (4:3:2.25 v/v mixture).
Avanti Lipid film extruder set: mini-extruder, two syringes (250 μL), and 50 nm pore size polycarbonate membrane.
2.9. Reagents for Assembly of Coats onto Liposomes
Coated-vesicle Formation Buffer: (80 mM MES pH 6.5, 20 mM NaCl, 2 mM EDTA, 0.4 mM DTT).
Extruded liposomes that are 50–80 nm in diameter (determined by negative stain EM).
Recombinant clathrin heavy chain triskelia.
Fluorescently labeled recombinant clathrin LCA.
AP-2 adaptor complex isolated from bovine brain (see step 3).
Dialysis cassette, 10 kDa MWCO.
2.10. Reagents for Fabrication and Assembly of Microfluidic Flow Cells
Silicon Wafer: 3″ N/Ph Orient. [111], 380umP, Mech Grade for Spin Coating or similar.
MicroChem SU-8 2050; Permanent epoxy negative photoresist.
MicroChem SU-8 developer.
TFOCS (Tridecafluoro-1,1,2,2, tetrahydrooctyl-1-trichloro silane).
Methanol.
Acetone.
Isopropanol.
Polydimethylsiloxane (PDMS): 184 sylgard elastomer and curing agent.
2.11. Reagents for Surface Chemistry on Glass Coverslips
Glass coverslips (#1.5).
Toluene.
Dichloromethane.
Ethanol.
1:1 v/v ethanol–37% hydrochloric acid.
1 M NaOH.
Poly-l-lysine(20 kDa) conjugated with PEG(2 kDa) and PEG-Biotin (3.4 kDa) (SuSoS AG, Switzerland).
2.12. Reagents for Capture of Clathrin Coats on the Surface of Coverslips
1 mg/mL streptavidin in PBS.
Blocking solution: 20 mM Tris–HCl pH 7.5, 2 mM EDTA, 50 mM NaCl.
Biotinylated CVC.6 clathrin light chain A mouse monoclonal antibody.
2.13. Reagents and Microscope for Single-Particle Fluorescence Imaging Uncoating Assay
Uncoating Buffer:20 mM imidazole pH 6.8, 100 mM KCl, 2 mM MgCl2, 5 mM protocatechuic acid, 50 nM protocatechuate-3,4-dioxygenase, 2 mM Trolox, 8 mM 4-nitrobenzyl alcohol.
Uncoating Mix: 1 μM Hsc70, 5 mM ATP, 10 mM MgCl2, 25 nM ΔPTEN-auxilin1 in Uncoating Buffer.
- Inverted fluorescence microscope equipped with the following hardware (Fig. 4).
- Objective: 100×, 1.46NA TIRF oil objective, with an additional 2× magnification lens (spatial sampling necessary for point source model fitting for data collected using 16 μm2 camera pixel size).
- Stage controller (optional): Piezo ‘Z’ PZ-2000, X&Y Stage MS-2000 (Applied Scientific Instrumentation or similar).
- TIRF slider unit: (Carl Zeiss Microimaging, Inc. or similar).
- Lasers: (λ = 488, 561, 647; 40–50 mW coupled with fiber optics to TIRF slider).
- Camera: EMCCD (Photometrics, Andor or similar), 5122 imaging array (16 μm2) creating an image pixel size of 0.08 μm.
Fig. 4.
Microscope configuration. Schematic representation of the setup used for the single particle uncoating assay including the components for TIRF microscopy and the microfluidic flow cell
3. Methodology
3.1. Expression and Purification of Clathrin Heavy Chain
3.1.1. Expression in Insect Cells
-
1
This procedure uses the Bac-to-Bac system (Invitrogen/ThermoFisher) to recombinantly express rat clathrin heavy chain based on a pFastBac-based plasmid encoding the heavy chain [10].
-
2
Transform DH10Bac cells with the clathrin heavy chain plasmid, and grow the cells on plates containing the appropriate antibiotics (gentamycin, tetracycline, kanamycin), Bluo-Gal, and IPTG. Completing blue–white screening, after 48 h, restreak a white colony. From this second plate, use a single colony to grow a 4 mL culture in LB medium containing the appropriate antibiotics (gentamycin, tetracycline, kanamycin). Isolate the bacmid DNA sample: lyse the cells with alkaline buffer, add 800 μL isopropanol, incubate on ice for 10 min., centrifuge at 18k × g, and wash the pellet with 70% ethanol. Finally, air-dry the pellet in a fume hood, and resuspend it with Tris–EDTA buffer.
-
3
Prepare Sf9 cells using Sf900-II medium. Transfect cells in 6-well plates with the clathrin heavy chain bacmid according to the manufacturer’s protocol.
-
4
After 3–5 days, isolate the cell supernatant to obtain a baculo-virus stock.
-
5
Amplify the virus stock by infecting Sf9 cells and isolating the cell supernatant after considerable cell death is observed by Trypan Blue staining. Complete a second round of amplification.
-
6
Express clathrin heavy chain by using High Five cells grown in spinner flasks and Express Five or Excell 420 medium.
-
7
Prepare the cells to a density of 1.2–1.5 × 106 cells/mL (typically 2 × 500 mL), and infect them with the baculovirus stock (typically using an amount of the stock equal to 1% of the culture volume see Note 5).
-
8
Incubate in spinner flask for 2.5–3 days @ 28 °C.
-
9
Spin cells at 1500 rpm for 10 min in conical plastic bottles.
3.1.2. Cell Lysis and Purification of Clathrin Heavy Chain
-
10
Add one Complete tablet to 25 mL ice-cold Lysis Buffer 1. Resuspend the cells by quickly vortexing with 10 mL of the buffer, and then add remainder of the buffer.
-
11
Sonicate the material on ice for five periods of 60″ (with pauses of 120″) in a metal beaker.
-
12
Ultracentrifuge the lysate at 4 °C (e.g., TLX 100.4 rotor for 20 min at 438,813.5 × g).
-
13
Dialyze the supernatant in Cage Formation Buffer o/n at 4 °C; exchange once.
-
14
Spin the sample for 10 min at 1000 rpm in a table-top centrifuge; withdraw most of the supernatant (except for 2–3 mL), vortex, and spin again at 3000 rpm to recover more supernatant.
-
15
Ultracentrifuge the supernatant at 4 °C (e.g., Type 60 Ti for 1 h at 203,347.1 × g).
-
16
Supplement Buffer A with 2 mM PMSF or 0.6 mg/mL PEFA, and 3 mM EDTA. Using this solution, resuspend the pellet from step 9 initially by pipetting, and then using a tissue grinder (Potter homogenizer; 5 strokes).
-
17
Add Buffer B (half the volume of that for Buffer A used in step 10), mix, and shake gently at room temperature for 15 min on a rocker.
-
18
Ultracentrifuge the sample in a TLA 100.4 for 20 min at 541,700 × g and 4 °C.
-
19
Filter the supernatant (0.2 μ syringe filter), and load onto a gel filtration (Sephacryl S-500) column equilibrated with Column Buffer.
3.1.3. Polishing Purification Step by Hydroxyapatite Chromatography
Use an FPLC at room temperature.
Wash the column with high phosphate buffer, and equilibrate it with low phosphate buffer.
Inject the sample onto the column, and wash by using five column volumes of low phosphate buffer.
Add 15 μL100 mM EGTA solution to the receiving tubes.
Elute the sample using a gradient of 0–100% high phosphate buffer.
Pool fractions that contain clathrin according to SDS-PAGE, and add glycerol to 20%. Aliquot the sample into 500 μL portions, and freeze by submerging the aliquots in liquid nitrogen for storage at −80 °C. Approximate expected concentration is 1 mg/mL.
3.2. Expression, Purification, and Labeling of Clathrin Light Chain
3.2.1. Expression in Bacteria
-
1
Inoculate 100 mL LB medium containing kanamycin (30 μg/mL) with a colony of BL21(DE3) bacteria transformed with a pET28-based vector for expression of rat light chain a1 (LCA) with mutations D203E and C218S (see Note 6). Grow an overnight culture at 37 °C with shaking at 250 rpm.
-
2
Inoculate 1 L LB medium containing kanamycin (30 μg/mL) in baffled flasks with 10 mL of saturated culture. Grow at 37 °C with shaking at 250 rpm until the OD(600) reaches approximately 0.5 (2.5–3 h).
-
3
Induce expression by addition of 0.6 mL 1 M IPTG (final concentration of 0.6 mM), and continue growth at 37 °C and 250 rpm for 3 h.
-
4
Pellet cells by centrifugation (5000 rpm, 4500 × g, 10 min, 4 °C).
-
5
Resuspend the bacterial cell pellet in cold Lysis Buffer 2 (volume ~20 mL).
-
6
Place suspension into conical flask and immerse in boiling water for 6 min, then chill on ice. The solution becomes turbid. Add fresh DTT solution to a concentration of 0.5 mM.
-
7
Centrifuge to pellet denatured protein and cell debris using a 60Ti rotor, 292,819.8 × g, 30 min, 4 °C. The supernatant contains the light chain.
-
8
Dialyze the supernatant against 1 L Lysis Buffer 2 (without protease inhibitors) at 4 °C overnight.
3.2.2. Purification of LCA Using Anion Exchange Chromatography
-
9
Wash MonoQ column with Buffer D, and the column with Buffer C.
-
10
Load filtered supernatant (0.2 μm syringe filter) into Superloop and inject onto column at a flow rate of 1 mL/min.
-
11
Elute the labeled light chain using a linear gradient from 0% to 32% Buffer D over 20 column volumes with a flow rate of 1 mL/min.
-
12
Add EDTA to each fraction to a final concentration of 1 mM.
-
13
Identify fractions containing LCA by SDS-PAGE. Concentrate combine fractions by centrifugal ultrafiltration. Approximate concentration of purified protein should be 1.5 mg/mL.
3.2.3. Labeling of LCA
-
9
Dialyze the protein solution against Labeling Buffer.
-
10
Determine the concentration of the protein from its absorbance at 280 nm (with the molar extinction coefficient of 32,430). The concentration in the labeling reaction should be between 3–6 mg/mL.
-
11
Add a 10 mM solution of the maleimide-functionalized fluorophore (see Note 7) to obtain a molar excess between 4:1 and 8:1. Mix and allow to react in the dark at room temperature for 2 h.
-
12
Quench the unreacted dye by addition of 2-mercaptoethanol to a final concentration of 10 mM.
-
13
Dilute the solution tenfold with Buffer C (without DTT) and remove the excess dye by centrifugal ultrafiltration. Repeat this process until the flow-through is colorless.
-
14
The labeled protein can be further purified with the chromatography conditions as described above in step 11 using buffers without DTT and a linear gradient from 0–65% Buffer D over 40 column volumes.
-
15
Measure the UV-visible absorption spectrum of the sample. Determine the protein concentration and degree of labeling by using the extinction coefficient of the protein at 280 nm calculated from its sequence, the extinction coefficient of the fluorophore as provided by the manufacturer, and the correction factor for absorption of the fluorophore at 280 nm. Add glycerol up to 20% final concentration to the protein solution, divide into aliquots, and freeze in liquid nitrogen for storage at −80 °C.
3.3. Expression and Purification of ΔPTEN-Auxilin
3.3.1. Expression in Bacteria
-
1
Inoculate 100 mL LB medium containing ampicillin (0.1 mg/mL) with a colony of BL21(DE3) bacteria transformed with a pGEX vector for expression of auxilin 547–910. Grow an overnight culture at 37 °C with shaking at 250 rpm.
-
2
Inoculate 1 L LB medium containing ampicillin (1 mg/mL) in baffled flasks with 10 mL o/n culture and grow at 37 °C with shaking at 250 rpm until the OD(600) reaches approximately 0.6–1.
-
3
Induce by addition of 0.25 mL 1 M IPTG (final concentration of 0.25 mM). Expression at 37 °C, 250 rpm, 4 h.
-
4
Pellet cells by centrifugation (5000 rpm, 4500 × g, 10 min, 4 °C).
-
5
Resuspend in 15 mL Lysis Buffer 3.
-
6
Lyse cells by sonication on ice.
-
7
Centrifuge the lysate for 30 min at 30,900 × g in a JA-17 rotor at 4 °C.
3.3.2. Purification of GST-Tagged Auxilin
-
8
Place 2 mL of glutathione–agarose slurry into a 15 mL conical tube, pellet beads (1500 rpm, 500 × g, 5 min), resuspend in 10 mL Lysis Buffer 3 to wash, pellet, decant supernatant. Repeat washing step. Resuspend beads in 0.5 mL Lysis Buffer 3.
-
9
Transfer beads into the lysate. Rotate end-over-end for 2 h.
-
10
Pour into column, let drain, collect flow-through.
-
11
Wash with 5–10 bed volumes of Lysis Buffer 3.
-
12
Wash with 5–10 bed volumes of Wash Buffer 1, let drain.
-
13
Place cap on column outlet; add 0.5 mL Elution Buffer 1, incubate for 5 min at RT, collect eluted proteins.
-
14
Repeat elution step five times.
-
15
Bradford assay or SDS PAGE to identify fractions with protein.
3.3.3. Removal of the GST Tag
-
8
Test digestion: (a) 20 μL GST-aux + 0.5 μL thrombin, (b) 20 μL GST-aux + 0.5 μL 1:4 dilution of thrombin in Elution Buffer 1. Incubate at room temperature and withdraw 10 μL samples after 1 h and 2 h. Boil samples with 10 μL Laemmli buffer and analyze on SDS PAGE with Coomassie staining.
-
9
Digestion: Scale up according to the results of the test digestion. Quench reaction by addition of complete protease inhibitor.
-
10
Add equal volume of Buffer E, adjust pH to ~6.6 (pH paper) by addition of 1 N HCl.
3.3.4. Separation of Auxilin from GST via Ion Exchange Chromatography
-
8
Spin protein solution (10 min, 18,000 × g) to remove aggregated material.
-
9
Inject protein solution onto Mono S column equilibrated with Buffer E.
-
10
Elute by running a linear gradient from 0% Buffer F to 80% Buffer F over 40 column volumes at a flow rate of 1 mL/min (GST expected in the flow through; auxilin appears early in the gradient).
-
11
SDS PAGE to identify fractions containing auxilin, combine fractions, concentrate if needed (centrifugal filter, 10 kD MWCO).
-
12
Add 20% glycerol to the protein solution, divide into aliquots and freeze in liquid nitrogen for storage at −80 °C. Approximate protein yield should be 0.1–0.5 mg/mL.
3.4. Expression, Purification and Labeling of Hsc70
3.4.1. Expression in Bacteria
-
1
Inoculate 100 mL LB medium containing ampicillin (0.1 mg/mL) with a colony of BL21(DE3) bacteria transformed with a vector for expression of Hsc70 containing a Gly-Cys extension at the C-terminus and an N-terminal, TEV cleavable hexahistidine-tag. Grow an overnight culture at 37 °C with shaking at 250 rpm.
-
2
Inoculate 1 L LB medium containing ampicillin (0.1 mg/mL) in baffled flasks with 10 mL o/n culture and grow at 37 °C with shaking at 250 rpm until the OD(600) reaches approximately 0.3.
-
3
Transfer the cultures to 16 °C and allow to grow until the OD(600) reaches 0.4.
-
4
Induce by addition of IPTG (final concentration of 0.1 mM). Expression at 16 °C, 250 rpm, overnight.
-
5
Pellet cells by centrifugation (5000 rpm, 4500 × g, 10 min, 4 °C).
-
6
Resuspend the bacterial cell pellet in cold Lysis Buffer 4 (volume ~25 mL).
-
7
Break cells using a microfluidizer or by sonication.
-
8
Remove cell debris by centrifugation (45 Ti, 185,511.4 × g, 4 °C, 45 min).
3.4.2. Purification of Hexahistidine-Tagged Hsc70 Using Immobilized Metal Affinity Chromatography
-
9
Bind His-tagged protein in the soluble fraction (high speed supernatant) to Cobalt affinity resin (2 mL settled bed volume, equilibrated with Lysis Buffer 4) by head over head rotation at 4 °C for 2 h.
-
10
Pour beads into column, let drain.
-
11
Washing steps: (a) Lysis Buffer 4 (without protease inhibitor, 10 mL), (b) Wash Buffer 2 (10 mL).
-
12
Elute with Elution Buffer 2. Collect 0.5 mL fractions in tubes containing EDTA solution (final concentration of 2 mM).
-
13
SDS PAGE to identify fractions containing Hsc70.
3.4.3. TEV Protease Treatment to Remove the Hexahistidine Tag
-
9
Add DTT to a final concentration of 2 mM. Add TEV protease to a final concentration of 0.05 mg/mL.
-
10
Digest for 5 h at room temperature.
-
11
Dialyze against Lysis Buffer 4 (without protease inhibitor).
-
12
Incubate protein with Cobalt affinity resin (1 mL settled bed volume, equilibrated with Lysis Buffer 4) by head-over-head rotation at 4 °C for 2 h. TEV protease and Hsc70 with uncleaved hexahistidine tag binds to the beads during this step.
-
13
Pour beads into column, let drain and collect the flow-through (containing Hsc70 without hexahistidine tag).
-
14
Add 0.1 mM TCEP, 1 mM MgCl2, and 0.1 mM ATP (final concentrations) and concentrate using a centrifugal filtration device (30 kDa MWCO). Centrifuge to remove aggregates (benchtop centrifuge, 20,000 × g, 4 °C, 10 min). Expected concentration ~2 mg/mL. Samples can be stored at −80 °C.
3.4.4. Fluorescent Labeling of Hsc70
-
9
Add a thiol-reactive fluorophore (e.g., Alexa Fluor-C5-maleimide) in a ratio of 5:1 (fluorophore–protein, mol/mol) to the Hsc70 solution and incubate for 80 min at room temperature.
-
10
Quench unreacted fluorophore with 2-mercaptoethanol (final concentration of ~10 mM).
-
11
Separate the labeled Hsc70 from the free fluorophore by gel filtration on a Superose 6 HR 10/30 column operated at 0.5 mL/min with column buffer.
-
12
Identify and combine fractions containing labeled Hsc70 and concentrate by centrifugal ultrafiltration as described above in step 19.
-
13
Determine the protein concentration and degree of labeling using UV-visible absorption spectroscopy and the formula provided by the manufacturer. Add 20% glycerol to the protein solution, divide into aliquots and freeze in liquid nitrogen for storage at −80 °C.
3.5. Isolation of AP-2 Adaptor Protein (See Note 8)
3.5.1. Day 1 (4 °C Room): Isolation of CCV from Brain and Dissociation of the Clathrin Coat at High Tris Concentration
-
1
On the day prior to preparation: Store Buffer G, centrifuge bottles, and blender at 4 °C. Prepare Buffer B and store at room temperature. On the morning of preparation: Precool centrifuges to 4 °C. Prepare Ficoll/sucrose solution. Prepare Column Buffer 3 and store at room temperature.
-
2
Process brains immediately upon delivery. Cut the brains in halves, and wash them in a 4 L beaker containing cold tap water and ice.
-
3
Add 400 mL of Buffer G to the blender.
-
4
Keeping brains in ice-cold water, remove white matter and large vessels, cut remainder into cubes (~2.5 cm in each dimension), and place in blender. It is not essential to get rid of all white matter; just cut off the brainstem.
-
5
Add 10 mL of 50 mM PMSF solution, blend on a low setting for 1 min, then on a high setting for another 2 min. The sample should look homogeneous with a pinkish color.
-
6
Spin in a JA-10 rotor (Beckman J2-HS centrifuge) for 30 min at 17,650 × g and 4 °C.
-
7
Carefully pour off supernatants.
-
8
Add 60 mL of Buffer G to remaining pellets and shake vigorously to resuspend; pour suspended pellets into blender and blend on a low setting for 1 min.
-
9
Spin the resuspended pellets in JA-14 rotor in Beckman J2-HS centrifuge for 45 min at 30,000 × g and 4 °C.
-
10
Load the supernatants into Type 45 Ti tubes (70 mL).
-
11
Spin in Type 45 Ti rotor (Beckman) for 60 min at 224,500 × g and 4 °C.
-
12
Following each spin, pour off and discard the supernatant. Resuspend 5 to 6 pellets by adding 10 mL Buffer G to only one tube, and then swirl to dislodge pellet. Pour this into next tube and remove that pellet. Repeat until all pellets are in solution. Add Buffer G until the total volume is 20 mL. Add PMSF from 50 mM stock (1:100, 200 μL).
-
13
Homogenize pellets with 5–10 strokes using a motor driven Potter homogenizer; make sure that no particulate is left.
-
14
Collect homogenate in a 50 mL conical tube. Wash pestle and glass homogenizer with Buffer G. Add this to homogenate and, if necessary, add more Buffer G until total volume is 25 mL. Add PMSF (1:200).
-
15
Add 25 mL of 12.5% Ficoll/12.5% sucrose to the homogenate. Mix well by repeated inversion.
-
16
Repeat steps 15–18 with remaining pellets.
-
17
Pour these mixtures into four 25 mL ultracentrifuge tubes.
-
18
Spin in Type 60 rotor (Beckman) for 25 min at 90,400 × g and 4 °C.
-
19
Collect supernatants.
-
20
Divide into four Type 45 Ti tubes (i.e., 70 mL tubes), fill to ~15 mL/supernatant per tube and fill them to the top with Buffer G. Mix by repeated inversion.
-
21
Spin in a Type 45 Ti rotor (Beckman) for 1 h. at 224,500 × g and 4 °C.
-
22
Discard supernatants. Add 1 mL of Buffer G to only one tube, resuspend pellet, transfer suspension into a second tube and use it to resuspend the pellet in the second tube. Transfer into homogenizer. Use another 1 mL of Buffer G to rinse both tubes. Repeat procedure with remaining tubes until all pellets are resuspended. Total volume must not exceed 10 mL.
-
23
Homogenize pellets (coated vesicles) as in step 16.
-
24
Pour into a 50 mL conical tube. Add Buffer G until volume is 10 mL, if necessary.
-
25
Add 5 mL of Buffer H and 300 μL of 50 mM PMSF to initiate depolymerization of coats.
-
26
Mix well by repeated inversion and leave overnight at RT.
3.5.2. Day 2 (Room Temperature): Gel Filtration to Separate Clathrin from Adaptor Proteins (APs)
-
27
Put 15 mL sample into a Type 60 Ti tube (25 mL tube volume). Fill to the top with water to reduce Tris concentration to approx. 0.4 M.
-
28
Spin for 1 h at 203,300 × g and room temperature in Type 60 Ti rotor (Beckman).
-
29
Carefully remove and save the supernatant except for ~2 mL on top of the fluffy pellet. Collect this remaining volume, spin it again in the TLA-100.4 rotor (TLX centrifuge; Beckman) for 20 min at 541,700 × g and room temperature, and add it to the rest. Pass the solution through a syringe filter (0.2 μm) and store it in a 50 mL conical tube.
-
30
Load the sample onto a Sepharose CL-4B column and run at 1 mL/min. Collect fractions of 5 mL. Clathrin appears as a broad peak after the void, followed by a small peak containing AP-1 and AP-2.
-
31
Pool clathrin fractions, add EGTA to a final concentration of 1 mM and store at 4 °C. Pool AP fractions and store at 4 °C overnight.
3.5.3. Day 3 (4 °C Room): Hydroxyapatite Chromatography to Separate AP-1 and AP-2
-
27
Connect the hydroxyapatite column to the FPLC system at 4 °C. Put a 0.2 μm syringe filter at the inlet of the column. Operate at 1 mL/min for all steps. Wash the column with 20 mL of high phosphate buffer, and equilibrate it with low phosphate buffer.
-
28
During the equilibration phase, load the 50 mL Superloop with the AP sample.
-
29
Inject the APs, and continue running low phosphate buffer.
-
30
Elute AP-1 and AP-2 from the column using the following gradient program: 0–60% high phosphate buffer over 24 mL, and then a step to 100% high phosphate buffer, with continued elution for 20 mL (see Note 9).
-
31
Pool AP-1 fractions and AP-2 fractions separately, and dialyze them against 1 L of AP buffer overnight, and for a few more hours after exchanging the buffer (4 °C).
-
32
Add glycerol to 20%, and freeze the samples for storage at −80 °C.
3.6. In Vitro Reconstitution of Fluorescent Clathrin and Fluorescent Coat Formation
-
1
Mix a solution of recombinant clathrin heavy chain with a solution of labeled clathrin LCA at a molar ratio of heavy chain– LCA = 1:2.4 and incubate at room temperature for 40 min. Total protein concentration should be 1 mg/mL.
-
2
Add a solution of AP-2 to the clathrin solution prepared in step 1 to a ratio of clathrin–AP-2 of 3:1 (w/w). Mix the solution and transfer to a dialysis capsule (see Note 10).
-
3
Dialyze overnight against Coat Formation Buffer at 4 °C. Replace Coat Formation Buffer and dialyze for an additional 3–4 h.
-
4
Transfer the coat solution to a 1.5 mL centrifuge tube, centrifuge to remove larger aggregates (benchtop centrifuge, 15,000 RCF, 4 °C, 10 min).
-
5
Transfer supernatant to fresh centrifuge tube, centrifuge to collect coats (TLA-100.4, 228,887.3 × g, 4 °C, 12–16 min).
-
6
Immediately withdraw supernatant with a 1 mL pipette. The pellet should have a hemispherical dome shape and be colorless and translucent.
-
7
Wash carefully with Coat Formation Buffer around the pellet.
-
8
Add Clathrin Coat Formation Buffer or Coated-Vesicle Formation Buffer to the tube (use a volume of 120 μL for a pellet with a diameter of ~3 mm), allow to stand at room temperature for 10–15 min in the dark, then slowly wash buffer over the pellet using a micropipettor to resuspend, avoiding foaming.
-
9
Coats can be stored frozen at −80 °C in the presence of 20% glycerol (snap-freeze in liquid nitrogen).
3.7. Liposome Preparation
3.7.1. YQRL-Peptidolipid Synthesis
-
10
Prepare a mix of 20 mg/mL of YQRL containing peptide (CKVTRRPKASDYQRLNL; prepared in 20 mM HEPES buffer pH 7.4), DMSO, and maleimide-DOPE (1:1:2 v/v mixture respectively).
-
11
Vortex the mixture at 1000 rpm for 2 h at room temperature.
-
12
Add 10 mM β-mercaptoethanol to the coupling reaction in order to quench any unreacted groups.
-
13
Extract the YQRL peptidolipid by adding chloroform, methanol, and water (4:3:2.25 v/v mixture) and centrifuging at 1000 rpm for 5 min.
-
14
Dry the organic phase containing the peptidolipid under argon and stored in a sealed argon atmosphere containing vial.
-
15
Resuspend the films in chloroform and methanol mixture (2:1) at 2 mg/mL (based on the mass in the sealed lipids vials) prior to use for liposome lipid film prep.
3.7.2. Phosphoinositide Specific Lipid Films
-
10
Mix the desired lipid composition (default Molar percent: 2% PI(4,5)P2, 5% PI(4)P or PI(3)P, 3% YQRL-DOPE peptidolipid, 0.1% DiI or DiD lipid dye, 86.9% DOPC) in 20:9:1 chloroform–methanol–water
-
11
Dry the mixture under argon to prepare composition specific lipid films and store as 300 μmol aliquots at −20 °C in argon purged sealed chambers until the expiration of the lipids as specified by the manufacturer.
3.7.3. Lipid Film Extrusion
-
10
Immediately prior to reconstitution of the clathrin-coated vesicles, hydrate the lipid film aliquots by adding 300 μL of Coat Formation Buffer and letting sit at room temperature for 3 min.
-
11
Vortex the mixture using a benchtop mixer at max speed for 30 s. Let sit for 60 s and repeat this step.
-
12
Assemble the mini-extruder (membrane supports and 50 nm pore size polycarbonate membrane), and inject 200 μL of Coat Formation Buffer through the extruder.
-
13
Aspirate the solution into the extrusion syringe and inject through the extruder ~20–30 passes to generate liposomes with a 50–80 nm diameter.
3.8. Reconstitution of Clathrin-Coated Vesicles (CCV)
3.8.1. Day 1
-
1
Clathrin-coats composed of clathrin and AP-2 self-assemble around liposomes (containing YQRL-peptidolipid and PI(4,5) P2.)
-
2
Premix clathrin heavy chain and fluorescently labeled light chain (1:3 mol/mol ratio) at room temperature for 20 min. Minimum volume required is 300 μL.
-
3
Add AP2 (3:1 w/w clathrin:AP2) and extruded liposomes (15 μL of extruded liposomes per 100 μg of Clathrin heavy chain, with 2% PI(4,5)P2, 5% PI(4)P or PI(3)P, 3% YQRL-DOPE peptidolipid, 0.1% DiI or DiD lipid dye, 86.9% DOPC) to the clathrin heavy chain and light chain mixture.
-
4
Using a 200 μL pipette to gently mix the solution without introducing bubbles or foam.
-
5
Transfer the above solution into a mini dialysis cassette (10 kDa MWCO) and dialyze at 4 °C overnight against Coated-vesicle Formation Buffer.
3.8.2. Day 2
-
6
Replace the dialysis buffer and redialyze for an additional 4 h at 4 °C.
-
7
Transfer the dialyzed CCV mixture into an 1.5 mL tube and spin down at max speed on a benchtop centrifuge at 4 °C for 10 min in order to remove large aggregates.
-
8
Transfer the supernatant into a fresh tube and centrifuged in a TLA-100.4 at 265,500 × g for 30 min at 4 °C.
-
9
Withdraw and discard the supernatant.
-
10
Using a micropipettor resuspend the CCV containing pellet in Coated-vesicle Formation Buffer (in 100 μL of buffer per 100 μg of clathrin heavy chain; avoid foaming) and centrifuge a second time at 265,500 × g for 30 min at 4 °C.
-
11
Withdraw and discard the supernatant.
-
12
Resuspend the final pellet in Coated-vesicle Formation Buffer and use the CCVs immediately for best results.
3.9. Fabrication and Assembly of Microfluidic Flow Cells in a Clean Room Facility
3.9.1. Channel Design
-
1
To prevent channels from collapsing or sagging, the height (h) and width (w) of channels, and space (s) between channels should correspond to the following optimized values: h = 50 μm (variable and controlled below using the spin speed and viscosity of the photoresist); w = 200 μm; s = 500 μm.
-
2
Design the mask (using CAD program of your choice) while optimizing the number of channels per chip, and number of chips per silicon wafer (default: 5–7 channels per chip, 10–13 chips per wafer, typical channel length 10 mm).
3.9.2. Fabrication and Photolithography: (3–5 Wafers Prepared in Parallel in a Clean Room)
-
3
Pour negative photoresist on silicon wafer, and spin-coat for 50 μm (or desired) thickness.
-
4
Place mask over the silicon wafer with prebaked (on a hot plate) photoresist and expose to UV (duration based on thickness of the photoresist).
-
5
Develop (to remove non-UV exposed photoresist) wafer to create a master wafer, using developing times specified by the manufacturer.
3.9.3. Passivatethe Master Wafer: (Ensure That the Surface Is Nonsticky)
-
3
Place wafer in vacuum desiccator with a petri dish containing TFOCS (tridecafluoro-1,1,2,2, tetrahydrooctyl-1-trichlorosilane) for 30 min to deposit a monolayer.
-
4
This step is critical to make sure that PDMS does not stick to the master wafer.
-
5
Repeat the passivation process after casting >20–30 PDMS molds.
3.9.4. PDMS Molding
-
3
Pour and mix PDMS and curing agent in 10:1 ratio and degas to remove bubbles.
-
4
Cure the PDMS mold for at least 6 h at 70 °C.
-
5
Carefully peel the cured PDMS mold from the master wafer, and cover with adhesive Scotch tape.
-
6
Using micropuncher, punch holes at the channel edges to create an inlet and outlet (see Note 11).
3.9.5. Device Assembly
-
3
Cut and shape the PDMS device with inlet and outlet punched holes to fit the imaging coverslip.
-
4
Plasma treat (by glow discharging) precleaned coverslip and PDMS for 20–40 s at 0.35 mBar pO2 (using purged oxygen plasma; air plasma may also be used, however, bonding conditions should be optimized since the quality of adhesion varies based on atmospheric compositions, humidity levels, power, etc.; see Note 12) [12].
-
5
Immediately join the two plasma exposed surfaces, press gently to remove any air bubbles and heat for 5 min at 100 °C on a hot plate to improve bonding strength.
-
6
Functionalize devices immediately using PLL-PEG solution, or store devices in clean room until ready for use.
3.10. Surface Chemistry on Glass Coverslips
Sequentially rinse the glass coverslips for 20 min in the following solvents in series: toluene, dichloromethane, ethanol, ethanol–hydrochloric acid (1:1 v/v), and Milli-Q water. Store coverslips in water until ready to assemble the PDMS device (see Subheading3.9.5 above).
- The freshly bonded chips are functionalized by the following series of channel infusions (injected volumes for channel washes should be at least 100× the channel volume):
- Flushing 1 mL of 1 M NaOH at 200 μL/min.
- Flushing 2 mL of ddH2O at 200 μL/min.
- Immediately followed by PLL-PEG/PLL-PEG-Biotin solution 20 μL at 10 μL/min and let sit for 5 min.
- Wash free reagents from the channels by flowing 100 μL of ddH2O at 20 μL/min.
- Functionalize the PLL-PEG-Biotin groups by flowing in 20 μL of streptavidin in Blocking Solution at 5 μL/min and let sit for 5 min (20 μL of 1 mg/mL streptavidin in PBS + 80 μL 20 mM Tris–HCl pH 7.5, 2 mM EDTA, 50 mM NaCl).
- Wash excess Streptavidin by flowing in 100 μL of ddH2O at 20 μL/min.
- Functionalize the inert layer of PLL–PEG–Biotin– Streptavidin in the channels with biotin-labeled CVC.6 clathrin light chain A mouse monoclonal antibody by flowing in 20 μL of 10 μg/mL at 10 μL/min and let sit for 5 min.
3.11. Single-Particle Uncoating Assay
3.11.1. Microscope Calibration (See Note 13)
TIRF Angle
-
1
If the TIRF angle of the microscope needs to be calibrated for quantitative imaging, prepare a coverslip with immobilized fluorescent beads (100–170 nm diameter).
-
2
Add ~200 μL of MilliQ water containing fluorescent beads, and place on the microscope in order to calibrate the TIRF angle.
-
3
Set the TIRF slider angle such that the laser illumination is passing straight through the objective (warning: laser illumination will no longer be confined within the microscope).
-
4
Set the laser power and exposure such that the emission counts from the immobilized beads are ~2–5% of the EMCCD’s max dynamic range.
-
5
At this point, you will observe a mix of immobilized beads and free-floating beads.
-
6
Optimize the evanescent filed: Adjust the TIRF slider angle to maximize the signal from the immobilized beads and minimize signal from the floating beads. Ideally, set the angle between 70 and 80% of maximum TIRF, as this will reduce the background fluorescence from injected solutions.
Single-Molecule Calibration
-
7
Prepare a coverslip with immobilized fluorophores in the femtomolar to picomolar-range required to resolve single fluorophores (labeled clathrin LCA adsorbs to glass coverslips cleaned as in Subheading 3.10, step 1 followed by glow discharge; oxygen plasma is not required; however, conditions should be optimized since the extent of adsorption varies depending on atmospheric compositions, humidity levels, etc.).
-
8
Adjust the power and exposure such that you are able to detect the signal from single fluorophores with a signal-to-noise ratio of 5 or higher. Fix the power and image and subsequently bleach the fluorophores; repeat this process at different exposures and extract the single bleaching step-sizes in order to generate a single molecule fluorescence signal calibration curve.
-
9
Adjust the laser power such that imaging is carried out with one fluorophore sensitivity with exposures of 150–200 ms.
3.11.2. Capture of Clathrin Coats or Reconstituted Coated Vesicles on the Surface of Functionalized Coverslips
Inject 15 μL of Clathrin Coats (CC) or Clathrin Coated Vesicles (CCVs) (in Coat Formation Buffer or Clathrin Coated Vesicle Formation Buffer respectively) at 10 μL/min into the CVC.6 antibody functionalized channel and let sit for 1–5 min (the concentration of CC or CCV may be titrated if the field-of-view is saturated, or conversely too few CC or CCVs are bound).
Wash the free CC or CCVs using the Uncoating Buffer.
Subsequently, inject the Uncoating Mix at 20 μL/min for 150 s into the channel to disassemble the CCs or CCVs.
3.11.3. Fluorescence Microscopy of Uncoating:
- Prior to the uncoating assay:
- Calculate the injection tubing volume and the time needed for injected uncoating reagent mix to enter the microfluidic channel by injecting a testing fluorescent solution (such as fluorescein).
- Calibrate the TIRF angle (see above subheading “TIRF-Angle calibration”) of the microscope and ensure the system is configured between 70% and maximum TIRF angle.
- Uncoating Assay:
- Start microscope acquisition at 1 Hz using 50 ms exposure for each of the excited channels 10 s prior to when the uncoating mixture enters into the channel and end acquisition after 150 s.
- Monitor the signal of fluorescent clathrin excited using the 488 nm laser.
- If required, monitor the signal of fluorescent Hsc70 generated by exciting it with the appropriate laser.
- If required, monitor the signal of the fluorescent lipid dye in liposomes using the appropriate laser.
- Quantify signals from CCs, or CCVs using a fixed position 2D point source detector [13].
- Using single-molecule intensity calibration (see Subheading 3.11, steps 7–9) for the fluorescent clathrin light chain A, convert the recorded signal amplitude for the clathrin coats to the number of molecules.
4. Notes
Adjust the pH of the BisTris buffer at room temperature. The pH of the solution at 4 °C is approximately pH 6.5.
Add DTT and Complete protease inhibitor from stock solutions (stored at −20 °C) just before using the buffer.
The potassium salt is used because it has higher solubility at 4 °C than the sodium salt.
The separation of AP-2 from AP-180 is improved with type I ceramic hydroxyapatite compared to type II.
We determine the amount of viral stock to use for batch production based on an experiment completed with High Five cells in well-plates: wells are treated with a series of volumes of virus stock, and protein production is assessed by SDS-PAGE and Coomassie staining.
The cDNA of rat clathrin light chain a1 was cloned into the pET28 expression vector using the NcoI and EcoRI restriction sites to allow expression without tags. The point mutations were introduced using site-directed mutagenesis. The mutation D203E restores the epitope recognized by the monoclonal antibody CVC.6 (which is used to capture clathrin coats onto the glass coverslip surface for imaging). The mutation C218S removes one of the two cysteines in the light-chain sequence to allow site-specific labeling at C187 with thiolreactive reagents.
The dye can be stored in aliquots at −20 °C for an extended period of time (>1 year). Prepare a 10 mM stock solution of the dye in water, divide into aliquots (e.g., 10 μL in 0.2 mL microcentrifuge tubes) and evaporate to dryness for storage in an airtight container containing a desiccant. The dye aliquots can be redissolved in water or buffer and added to the desired concentration to the protein solution for labeling.
Biochemistry 23, 4420–4426 (1984).
The elution profiles for AP-1 and AP-2 tend to vary considerably from one purification to the other; AP-1 is eluted first and tends to be eluted from the column in three to four 1 mL fractions. The step to 100% buffer B in the gradient is meant to coincide with the elution of AP-2 and was included to elute AP-2 at high concentration from the column. The fractions containing the APs need to be verified by SDS-PAGE.
The volume of the solution for coat formation is typically 0.5 mL. The concentration of clathrin heavy chain should be around 1 mg/mL.
Use only nitrile gloves, not latex, when handling PDMS to preserve adhesive functionality.
Over exposure to plasma may damage surfaces and affect bonding strength.
Caution: Please make sure that appropriate laser safety precautions are observed during the microscope calibration and imaging experiments.
Acknowledgments
T.B. was supported by grants from the Australian Research Council (DP130100936, FT 1001004) and the National Health and Medical Research Council (1098870, 1100771).
The Kirchhausen laboratory was generously supported by grants from Biogen and by National Institutes of Health Grants R01 GM075252.
References
- 1.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T (2004) Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605 [DOI] [PubMed] [Google Scholar]
- 2.Lee DW, Wu X, Eisenberg E, Greene LE (2006) Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci 119:3502–3512 [DOI] [PubMed] [Google Scholar]
- 3.Massol RH, Boll W, Griffin AM, Kirchhausen T (2006) A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A 103: 10265–10270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrifield CJ, Perrais D, Zenisek D (2005) Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606 [DOI] [PubMed] [Google Scholar]
- 5.Barouch W, Prasad K, Greene L, Eisenberg E (1997) Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry 36:4303–4308 [DOI] [PubMed] [Google Scholar]
- 6.Braell WA, Schlossman DM, Schmid SL, Rothman JE (1984) Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol 99:734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378:632–635 [DOI] [PubMed] [Google Scholar]
- 8.Bocking T, Aguet F, Harrison SC, Kirchhausen T (2011) Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating. Nat Struct Mol Biol 18:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T (2004) Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432:649–653 [DOI] [PubMed] [Google Scholar]
- 10.Rapoport I, Boll W, Yu A, Bocking T, Kirchhausen T (2008) A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction. Mol Biol Cell 19:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T (2004) Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432:573–579 [DOI] [PubMed] [Google Scholar]
- 12.Millare B, Thomas M, Ferreira A, Xu H, Holesinger M, Vullev VI (2008) Dependence of the quality of adhesion between poly(dimethylsiloxane) and glass surfaces on the conditions of treatment with oxygen plasma. Langmuir 24:13218–13224 [DOI] [PubMed] [Google Scholar]
- 13.Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G (2013) Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 26:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]