Abstract
OBJECTIVES
The BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction during TAVR) investigational device exemption trial was a prospective, multicenter, single-arm safety and feasibility study.
BACKGROUND
Coronary artery obstruction is a rare but devastating complication of transcatheter aortic valve replacement (TAVR). Current stent-based preventative strategies are suboptimal. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR (BASILICA) is a novel transcatheter technique performed immediately before TAVR to prevent coronary artery obstruction.
METHODS
Subjects with severe native or bioprosthetic aortic valve disease at high or extreme risk for surgery, and at high risk of coronary artery obstruction, were included. The primary success endpoint was successful BASILICA and TAVR without coronary obstruction or reintervention. The primary safety endpoint was freedom from major adverse cardiovascular events. Data were independently monitored. Endpoints were independently adjudicated. A core laboratory analyzed computed tomography images.
RESULTS
Between February 2018 and July 2018, 30 subjects were enrolled. Primary success was met in 28 (93%) subjects. BASILICA traversal and laceration was successful in 35 of 37 (95%) attempted leaflets. There was 100% freedom from coronary obstruction and reintervention. Primary safety was met in 21 (70%), driven by 6 (20%) major vascular complications related to TAVR but not BASILICA. There was 1 death at 30 days. There was 1 (3%) disabling stroke and 2 (7%) nondisabling strokes. Transient hemodynamic compromise was rare (7%) and resolved promptly with TAVR.
CONCLUSIONS
BASILICA was feasible in both native and bioprosthetic valves. Hemodynamic compromise was uncommon. Safety was acceptable and needs confirmation in larger studies. BASILICA appears effective in preventing coronary artery obstruction from TAVR in subjects at high risk. (J Am Coll Cardiol Intv 2019;12:1240–52) Published by Elsevier on behalf of the American College of Cardiology Foundation.
Keywords: bioprosthetic heart valve failure, coronary artery obstruction, structural heart disease, transcatheter aortic valve replacement, transcatheter electrosurgery
Transcatheter aortic valve replacement (TAVR) is an effective treatment for patients with severe aortic stenosis or failing bioprosthetic valves (1–3). Coronary artery obstruction is a rare but devastating complication of TAVR, with an overall incidence of 0.7% but 30-day mortality of 41% (4). The incidence is higher (2.3%) in bioprosthetic surgical valves (5), and does not account for patients excluded from TAVR for concern of this complication. Coronary obstruction occurs when unresected diseased leaflets are displaced toward the coronary artery ostia or sinotubular junction during transcatheter valve deployment.
Predicting coronary obstruction is imprecise. From the available data, those at highest risk are female, with coronary ostial height of <10 mm, sinus of Valsalva width of <30 mm, and those with previous aortic bioprostheses, particularly with externally mounted leaflets or stentless surgical valves, and with virtual transcatheter heart valve to coronary distance (VTC) of <4 mm (4,5).
Preventive strategies have to date involved pre-positioning a guidewire or stent in the threatened coronary artery and, after TAVR, deploying the stent in the ostium and “snorkeling” it alongside the TAVR valve into the aorta (6). This may be a suboptimal solution, as these stents are prone to extrinsic compression, deformation, and thrombosis, causing delayed thrombotic coronary occlusion with often challenging percutaneous bailout (7).
Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR (BASILICA) is a transcatheter procedure performed immediately before TAVR. The target aortic leaflet is split using focused radiofrequency energy directed by catheters and guide-wires. The BASILICA technique is derived from the earlier LAMPOON (Intentional Laceration of the Anterior Mitral leaflet to Prevent left ventricular Outflow ObstructioN during transcatheter mitral valve implantation) technique (8,9). Early in vitro, animal, and clinical BASILICA experience in 7 patients (10) showed that the split leaflets splay away from the coronary ostia during TAVR and that flow is maintained through the open cells of the TAVR valve. The purpose of this study was to systematically assess the early safety and efficacy of the BASILICA procedure.
METHODS
TRIAL DESIGN AND OVERSIGHT.
The BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction during TAVR) investigational device exemption (IDE) trial () was a prospective, single-arm, multicenter study of the BASILICA procedure, with independent on-site source-data verification and data monitoring, independent endpoint adjudication, and central core laboratory analysis of baseline and post-procedure images. The trial was designed by the investigators and sponsored by the senior author (R.J.L.) on behalf of the National Heart, Lung, and Blood Institute (NHLBI). Sites were not reimbursed for research activities. The U.S. Food and Drug Administration granted IDE for the study under the Early Feasibility pathway. The Institutional Review Board at each site and at the NHLBI approved the study protocol. The NHLBI Data Safety Monitoring Board provided study oversight. The NHLBI was the data-coordinating center. A Clinical Event Committee independently adjudicated the primary endpoints, all strokes, and all deaths, and determined relatedness to the BASILICA procedure and to TAVR. The authors have full custody of the data and the senior author had final responsibility for the decision to submit for publication.
SUBJECTS.
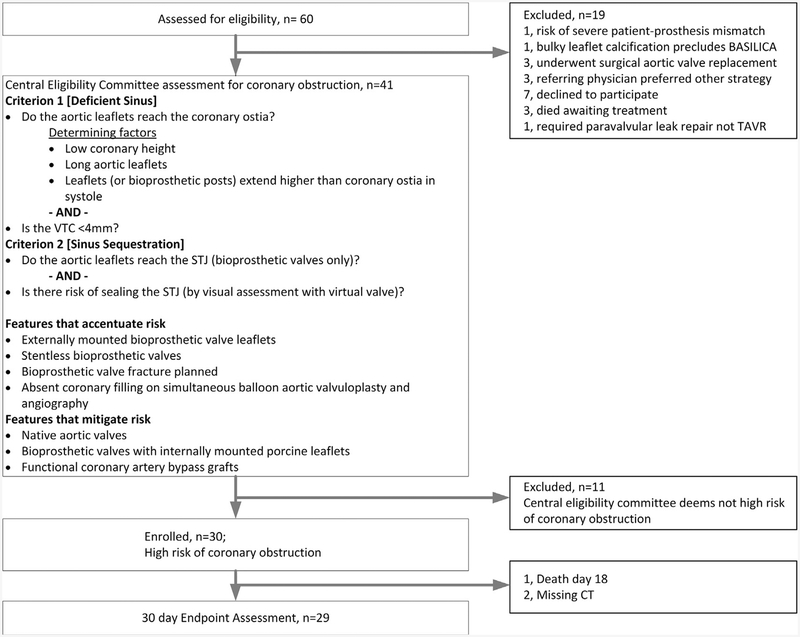
Between February 15, 2018, and July 31, 2018, 30 subjects with symptomatic severe aortic stenosis or bioprosthetic aortic valve failure were enrolled at 4 centers in the United States (see Figure 1 and the Online Appendix for study enrollment details). Adult patients were included if they were considered to be at high or prohibitive risk for surgical aortic valve replacement on the basis of clinical assessments by the institutional multidisciplinary heart team and considered at high risk of developing coronary artery obstruction from TAVR as determined by a central eligibility committee (see Figure 1). Coronary artery height, sinus width, VTC, sinotubular junction height and diameter, leaflet length and thickness, bioprosthetic valve type, prior coronary artery bypass grafts, and balloon valvuloplasty with simultaneous aortography were all considered, where appropriate, to determine coronary obstruction risk. Patients with severe calcified masses on the target aortic leaflets and those not expected to survive beyond 12 months despite TAVR were excluded. The complete list of inclusion and exclusion criteria is provided in the Online Appendix. All subjects consented to participate in writing.
FIGURE 1. Trial Enrollment.
Sixty patients were screened and 30 were enrolled. All patients completed follow-up. One patient declined 30-day computed tomography (CT) and 1 died before 30 days. BASILICA = bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR; STJ = sinotubular junction; TAVR = transcatheter aortic valve replacement; VTC = virtual transcatheter heart valve to coronary distance.
BASILICA TECHNIQUE.
The technique is described elsewhere (10,11) and is demonstrated in the Central Illustration and Online Video 1. Briefly, the BASILICA target leaflet or leaflets are chosen depending on the coronary artery at risk of obstruction. Fluoroscopic projection angles for the target aortic leaflets are planned on cardiac computed tomography (CT). The BASILICA procedure is performed under general anesthesia or moderate sedation. The procedure uses standard cardiovascular catheterization equipment. Two guiding catheters are used per target leaflet. For single leaflet or solo BASILICA, no additional vascular access is required beyond the 2 sheaths required for TAVR deployment and angiography. For double leaflet or doppio BASILICA, the sheath used for angiography is upsized to 12- to 14-F to house 2 side-by-side 6- to 8-F catheters. The guiding catheters are positioned on either side of the aortic leaflet, with a traversal guidewire (Astato XS 20, Asahi, Japan) in the aortic root and snare (Amplatz Gooseneck, AGA Medical Corporation, Minneapolis, Minnesota) in the left ventricular outflow tract, respectively. The guidewire is insulated in a microcatheter (Piggyback Wire Convertor, Teleflex, Wayne, Pennsylvania) to confine the electrical current to the tip, and electrified using a radiofrequency generator (ForceFx Val-leylab, Minneapolis, Minnesota) to perforate the base of the target leaflet. The guidewire is snared in the left ventricular outflow tract and externalized to form a loop through the leaflet, between the 2 guiding catheters. The guidewire shaft is shaped to confine electrical contact with leaflet tissue, and then further electrified under tension to lacerate the leaflet down the centerline. The split leaflet typically splays in systole and coapts in diastole. The BASILICA system is disconnected and removed from the body, and then TAVR is performed as usual. The TAVR valve displaces the leaflets outwards but the split leaflets splay away from the coronary ostia, maintaining coronary flow.
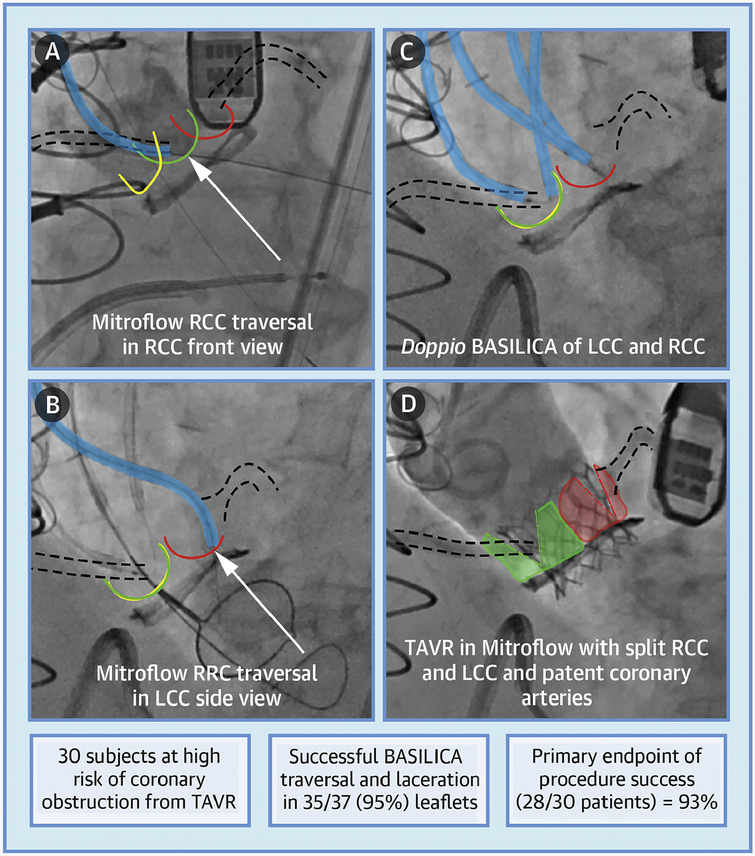
CENTRAL ILLUSTRATION. BASILICA Representative Example and Trial Results.
Khan, J.M. et al. J Am Coll Cardiol Intv. 2019;12(13):1240–52.
A representative subject with failed Mitroflow valve and at high risk of coronary obstruction who underwent doppio bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR (BASILICA) and transcatheter aortic valve replacement (TAVR) (Online Video 1). (A) The guidewire traverses the right coronary cusp (RCC). (B) The guidewire traverses the left coronary cusp (LCC) into a snare in the left ventricular outflow tract. (C) BASILICA guidewire loops are formed through the base of both right and left cusps, ready for radiofrequency-assisted laceration. (D) Aortic root angiography demonstrates flow to both coronaries through the split Mitroflow leaflets after TAVR with a SAPIEN 3 valve. The RCC is highlighted in green, LCC highlighted in red, noncoronary cusp highlighted in yellow, BASILICA catheters highlighted in blue, coronary arteries outlined by dashed lines, and arrow points indicate traversal target.
STUDY ENDPOINTS.
The prespecified primary endpoint was procedure success, measured at exit from the catheterization laboratory, and required all the following: successful BASILICA traversal and laceration of the intended leaflet(s); successful access, delivery, and retrieval of the BASILICA device system; successful TAVR device implantation; absence of procedural mortality; absence of coronary artery obstruction; and freedom from emergency cardiac surgery or reintervention related to the BASILICA TAVR procedure, including attempted implantation of coronary stents to treat TAVR-induced coronary artery obstruction. The prespecified primary safety endpoint was freedom from major adverse clinical events according to Valve Academic Research Consortium (VARC)-2 early safety at 30 days, which is a composite of all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury (stage 2 or 3), coronary artery obstruction requiring intervention, major vascular complications, and valve-related dysfunction requiring repeat procedure. Secondary endpoints included hemodynamic instability caused by BASILICA before TAVR, TAVR thrombosis on follow-up CT or echocardiography, hemolytic anemia, and BASILICA-related technical failure, including embolism, mitral valve injury, off-target traversal, and coronary artery injury. The complete list of endpoints is provided in the Online Appendix.
IMAGING.
Transesophageal or transthoracic echocardiograms were performed at baseline, intra-procedure, discharge, and 30-days. Contrast-enhanced electro-cardiogram-gated multislice CT scans were performed at baseline and post-procedure. Images were analyzed by the NHLBI core laboratory using dedicated 4-dimensional CT software, 3mensio version 9.1 (Pie Medical, Maastricht, the Netherlands). The VTC was measured using the Vancouver method (12). A virtual valve cylinder with a diameter of the selected valve being implanted was simulated in position and the distance to the coronary ostia measured in the short and long axes. For SAPIEN valves (Edwards Lifesciences, Irvine, California), the nominal valve diameter was used for the virtual valve (a 23 SAPIEN 3 valve was simulated with a valve with diameter 23 mm). For Evolut R/Pro valves (Medtronic, Minneapolis, Minnesota), a “constrained” diameter was used as follows: 20 mm, 23 mm, and 26 mm for 23, 26, and 29 Evolut valves, respectively. Follow-up CT was performed to measure observed valve to coronary artery distances; and to evaluate coronary patency; hypoattenuated leaflet thickening (HALT), defined as an area of hypodensity on TAVR valve leaflets on CT; and hypoattenuation affecting motion, defined as reduced leaflet motion in the presence of HALT (13). Leaflet calcium volumes were measured on contrast CT using dedicated software (3mensio) after performing automation-assisted segmentation of the aortic valve leaflets, using established methodology (14).
STATISTICAL ANALYSIS.
All analyses were based on the intention-to-treat principle with data from all enrolled patients. The sample size of 30 subjects was not derived statistically. Baseline subject and procedural characteristics were summarized as median and interquartile range for continuous variables and count and proportion for categorical variables. McNemar’s test and paired t test were used to assess the difference in the proportion of New York Heart Association (NYHA) functional class and Kansas City Cardiomyopathy Questionnaire (KCCQ) quality of life measure between baseline and 30-day visits, respectively. Pearson’s correlation and Bland-Altman plot were used to assess the correlation and agreement between predicted VTC on pre-procedure CT and observed measurements on post-procedure CT. Statistical analyses were performed using R statistical software 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
subject and procedure details.
Baseline subject characteristics are shown in Table 1. Subjects were typically elderly, with high surgical risk and comorbidity, and in NYHA functional class III or IV. Of note, 80% were women, and 23% had a prior stroke. 43% had native aortic stenosis and 57% had bio-prosthetic aortic valve failure. Procedure characteristics are shown in Table 2. SAPIEN 3 valves were used in 53% and Evolut R/Pro in 47%. TAVR access was transfemoral in 77%, transcaval 20%, and percutaneous axillary in 3%. The bioprosthetic valve frame was intentionally fractured (15) with high-pressure balloon inflation in 10%. A Sentinel device (Claret Medical, Santa Rosa, California) was used for cerebral protection in 43% and embolic debris was recovered in 46% of those cases, though no systematic inspection technique was mandated. General anesthesia and transesophageal echocardiography were used in 90% and moderate
TABLE 1.
Baseline Characteristics (n = 30)
| Age, yrs | 76 (69–82) |
| Female | 24 (80) |
| Comorbidities | |
| Prior stroke | 7 (23) |
| Prior myocardial infarction | 5 (17) |
| Coronary artery disease | 19 (63) |
| Peripheral artery disease | 7 (23) |
| Diabetes | 12 (40) |
| End-stage kidney disease on dialysis | 3 (10) |
| Severe pulmonary disease | 12 (40) |
| Liver cirrhosis | 2 (7) |
| Hypertension | 26 (87) |
| Atrial fibrillation | 11 (37) |
| Prior endocarditis | 0 (0) |
| Prior rheumatic fever | 1 (3) |
| Prior percutaneous revascularization | 11 (37) |
| Prior coronary artery bypass surgery | 7 (23) |
| ≥2 prior cardiac surgeries | 4 (13) |
| Pacemaker or ICD | 6 (20) |
| NYHA functional class III or IV | 26 (87) |
| Aspirin or P2Y12 inhibitor | 23 (77) |
| Oral anticoagulant | 8 (27) |
| Frail | 14 (47) |
| STS predicted risk of mortality | 6 (3–15) |
| KCCQ-12 summary score | 23 (16–40) |
| TAVR setting | |
| Native | 13 (43) |
| Bioprosthetic | 17 (57) |
| Aortic valve dysfunction | |
| Aortic stenosis | 24 (80) |
| Aortic regurgitation | 1 (3) |
| Mixed | 5 (17) |
| LVEF <30% | 4 (13) |
| AV peak velocity, m/s | 4 (4–5) |
| AV mean gradient, mm Hg | 43 (37–53) |
| AVA, cm2 | 0.7 (0.6–0.8) |
Values are median (interquartile range) or n (%).
AV = aortic valve; AVA = aortic valve area; ICD = implantable cardioverterdefibrillator; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
TABLE 2.
Procedure Characteristics
| Valve type | |
| SAPIEN 3 | 16 (53) |
| Evolut R | 11 (37) |
| Evolut Pro | 3 (10) |
| Valve nominal size | |
| 20 mm | 4 (13) |
| 23 mm | 17 (57) |
| 26 mm | 6 (20) |
| 29 mm | 3 (10) |
| Access for TAVR | |
| Transfemoral | 23 (77) |
| Transcaval | 6 (20) |
| Percutaneous axillary | 1 (3) |
| Target cusp | |
| Left solo | 18 (60) |
| Right solo | 5 (17) |
| Doppio | 7 (23) |
| Sentinel cerebral protection | 13 (43) |
| Balloon pre-dilation | 5 (17) |
| Balloon post-dilation | 7 (23) |
| Bioprosthetic valve fracture | 3 (10) |
| General anesthesia | 27 (90) |
| Moderate sedation | 3 (10) |
| Total procedure time (access to hemostasis), min | 113 (98–162) |
| BASILICA time, solo (catheter introduction to laceration), min |
73 (58–88) |
| BASILICA time, doppio (catheter introduction to laceration), min |
123 (106–137) |
| Time from BASILICA to TAVR, min | 9 (7–16) |
| Fluoroscopy time, min | 75 (57–111) |
| Contrast volume, ml | 143 (101–226) |
Values are n (%) or median (interquartile range)
BASILICA = bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR; TAVR = transcatheter aortic valve replacement.
coronary obstruction risk.
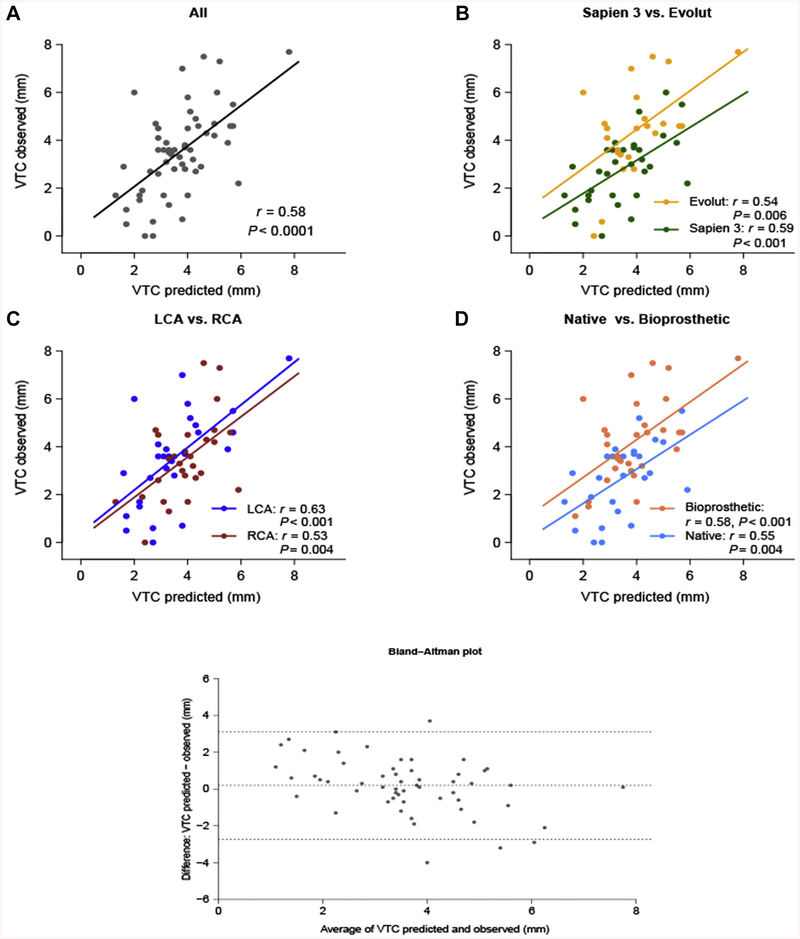
The target BASILICA leaflet was solo in 77% of subjects (left only in 60%, right only in 17%) and doppio (both leaflets) in 23%. The risk profile of the target leaflets and associated coronary arteries compared with the nontarget control leaflets is shown in Table 3. Individual risk for each patient is presented in Online Table 1. A total of 95% of target leaflets fit at least 2 of 3 best-available CT risk criteria for coronary obstruction-namely coronary height <10 mm, sinus width <30 mm, and VTC <4 mm (4,5). The predicted VTC on pre-procedure CT and observed transcatheter heart valve-to-coronary ostia measurements on postprocedure CT correlated well overall (Figure 2). Observed valve-to-coronary distance tended to be smaller than predicted VTC in SAPIEN 3 valves and in native annuli.
TABLE 3.
Coronary Obstruction Risk
| Risk Prediction | Total Coronary Leaflets (N = 60) | Native (N = 26) | Bioprosthetic (N = 34) | |||
|---|---|---|---|---|---|---|
| BASILICA Leaflet (n = 37) |
Non-BASILICA Control Leaflet (n = 23) |
BASILICA Leaflet (n = 14) |
Non-BASILICA Control Leaflet (n = 12) |
BASILICA Leaflet (n = 23) |
Non-BASILICA Control Leaflet (n = 11) |
|
| Coronary height, mm | 7.2 (5.2–9.7) | 10.4 (6.2–12.1) | 9.8 (7.9–11.8) | 11.7 (10.4–12.4) | 6.3 (3.8–8.4) | 5.6 (4.6–10.7) |
| Coronary height <10 mm | 28 (76) | 9 (24) | 7 (50) | 1 (8) | 21 (91) | 8 (73) |
| Sinus width, mm | 25.9 (24.8–29.0) | 27.9 (24.8–31.4) | 26.5 (24.7–29.6) | 28.4 (24.8–31.1) | 25.5 (24.9–28.6) | 27.9 (25.5–32.1) |
| Leaflet above coronary ostium | 34 (92) | 13 (57) | 12 (86) | 4 (33) | 22 (96) | 9 (82) |
| Leaflet above STJ | 18 (49) | 6 (26) | 0 (0) | 0 (0) | 18 (78) | 6 (55) |
| Calcium volume, mm3 | 94 (33–298) | 127 (85–228) | 57 (29–96) | 118 (91–190) | 136 (60–395) | 164 (75–230) |
| VTC, mm | 3.3 (2.7–4.0) | 4.3 (3.4–5.2) | 2.9 (2.4–3.7) | 4.2 (3.4–4.8) | 3.7 (2.9–4.1) | 4.6 (3.6–5.3) |
| VTC <4 mm | 27 (73) | 9 (39) | 13 (93) | 5 (42) | 14 (61) | 4 (36) |
| VTC <3 mm | 14 (38) | 3 (13) | 7 (50) | 2 (17) | 7 (30) | 1 (9) |
| THV to STJ, mm | 2.2 (0.5–3.1) | 2.6 (1.1–4.0) | 1.2 (0.1–2.2) | 2.0 (0.5–2.8) | 2.6 (1.6–3.4) | 3.5 (2.5–5.8) |
| THV to STJ <3 mm | 27 (73) | 15 (65) | 13 (93) | 10 (83) | 14 (61) | 5 (46) |
| Post-procedure risk assessment | ||||||
| VTC, mm | 3.3 (1.7–4.5) | 3.6 (2.8–4.7) | 2.3 (0.6–3.7) | 3.2 (2.6–3.8) | 3.6 (3.0–4.6) | 4.7 (3.9–6.0) |
| VTC <4 mm | 24 (69) | 12 (57) | 13 (93) | 9 (75) | 11 (52) | 3 (33) |
| VTC <3 mm | 14 (40) | 7 (33) | 9 (64) | 6 (50) | 5 (24) | 1 (11) |
| THV to STJ, mm | 1.4 (0–2.2) | 1.3 (0–2.2) | 0 (0–0.7) | 0.4 (0–1.1) | 1.8 (0.9–2.7) | 2.1 (1.6–2.9) |
| THV to STJ <3 mm | 28 (90) | 16 (89) | 11 (100) | 10 (100) | 17 (85) | 6 (75) |
Values are median (interquartile range) or n (%).
BASILICA = bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR; STJ = sinotubular junction; THV =transcatheter heart valve; VTC = virtual transcatheter heart valve to coronary distance.
FIGURE 2. Correlation Between Predicted and Observed Valve-to-Coronary Distance.
(A) The overall correlation coefficient was 0.58, with (C) good correlation across left and right cusps. Observed virtual transcatheter heart valve to coronary distance (VTC) was smaller than predicted (B) for SAPIEN 3 valves and (D) in native aortic stenosis. (B, D) There were few outliers when using Evolut valves in surgical bioprostheses in which prediction underestimated VTC. The Bland-Altman plot demonstrates no systematic difference (bias) between predicted and observed VTC. LCA = left coronary artery; RCA = right coronary artery.
PROCEDURE OUTCOMES.
The adjudicated endpoints are shown in Table 4. The primary endpoint of procedure success was met in 93% of subjects. Leaflet traversal was successful in 35 of 37 (95%) of target leaflets. Laceration was successful in all leaflets traversed. All subjects survived their procedure with successful implantation of the first TAVR device. There were no cases of coronary obstruction. There were no cases requiring reintervention or surgery. In the 2 cases in which leaflet traversal was not successful, coronary stents were pre-positioned, of which 1 was deployed after TAVR and the other removed due to low probability of obstruction on angiographic assessment after TAVR. We attribute the 2 traversal failures to confluent leaflet calcification at the crossing target at the nadir of the leaflet.
TABLE 4.
Clinical Outcomes
| Primary efficacy endpoint (exit from catdeter laboratory)* | (n = 30) |
| Successful BASILICA traversal and laceration | 28 (93) |
| Immediate survival | 30 (100) |
| Successful first TAVR device implantation | 30 (100) |
| Coronary obstruction | 0 (0) |
| Freedom from emergency surgery or reintervention related to BASILICA or TAVR | 30 (100) |
| Technical success (all of above) | 28 (93) |
| Primary safety endpoint (30 days)* | (n = 30) |
| All death | 1 (3) |
| Cardiovascular | 1 (3) |
| Noncardiovascular | 0 |
| All stroke | 3(10) |
| Disabling | 1 (3) |
| Nondisabling | 2 (7) |
| Life threatening bleeding | 2 (7) |
| Clearly related to BASILICA | 0 |
| Potentially related to BASILICA | 0 |
| Not related to BASILICA | 2 (7) |
| Major vascular complication | 6 (20) |
| Clearly related to BASILICA | 0 |
| Potentially related to BASILICA | 0 |
| Not related to BASILICA | 6 (20) |
| AKI stage 2/3 | 1 (3) |
| Coronary artery obstruction | 0 (0) |
| Valve-related dysfunction requiring repeat procedure | 0 (0) |
| VARC-2 early safety (all of above) | 21 (70) |
| Secondary endpoints (30 days) | (n = 30) |
| Secondary myocardial infarction | 1 (3) |
| Major cardiac structural complication | 0 (0) |
| Hemolytic anemia | 0 (0) |
| Endocarditis | 0 (0) |
| New pacemaker | 2 (7) |
| Need for second valve | 0 (0) |
| PVL > mild | 0 (0) |
| Cardiac tamponade | 0 (0) |
| Nontarget BASILICA traversal (left atrial entry) | 1 (3) |
| Hemodynamic instability from laceration requiring vasopressors | 2(7) |
| Embolic debris recovered if cerebral protection used | 6/13 (46) |
| Coronaries obstructed on follow-up CT | 0/28 (0) |
| 30-day mean gradient >20 mm Hg | 9/28 (32) |
| HALT on follow-up CT | 3/28 (11) |
| HAM on follow-up CT | 1/28 (4) |
Values are n (%) or n/N (%).
Clinical Event Committee adjudicated.
AKI = acute kidney injury; ICA = bioprosthetic or native aortic scallop intentional laceration; CT = computed tomography; HALT = hypoattenuated leaflet thickening; HAM = hypoattenuation affecting motion; PVL = paravalvular leak; VARC = Valve Academic Research Consortium.
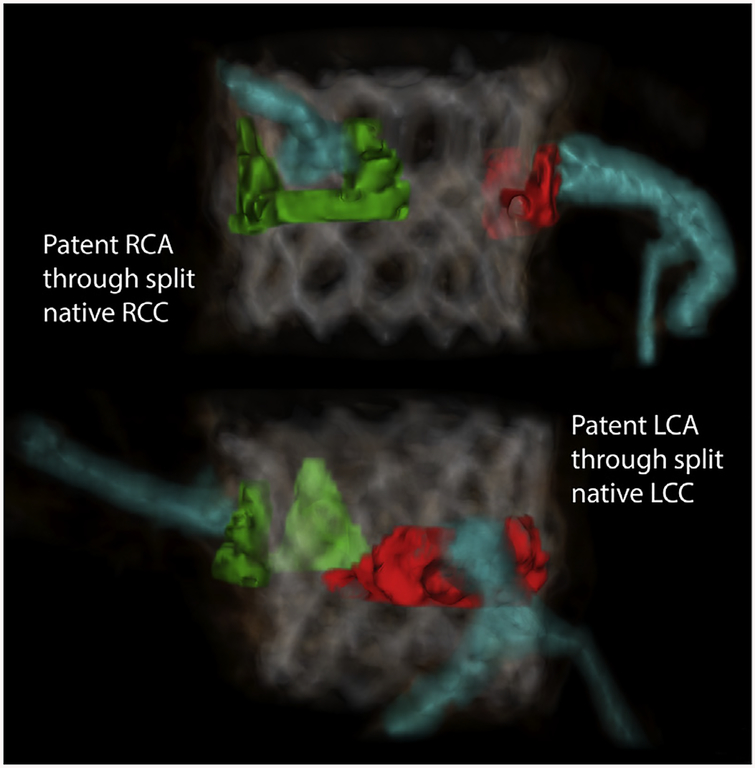
Figure 3 and Online Video 2 show a manually segmented post-procedure CT of a typical subject, demonstrating split native leaflets parting away from otherwise threatened left and right coronary ostia.
FIGURE 3. Three-dimensional CT Reconstruction After Doppio Native BASILICA.
Native leaflets (LCC in red, RCC in green) and coronary arteries (blue) are manually segmented and colorized on a CT acquired after BASILICA and TAVR. Both left and right coronary leaflets are seen split and parted away from the coronary ostia. (Online Video 2) The leaflet configuration suggests that both coronary ostia would have obstructed without BASILICA. Abbreviations as in Figures 1 and 2.
The primary endpoint of early safety was met in 70% of subjects at 30 days (Table 4). This was mostly driven by 6 TAVR-related major vascular complications: 1 retroperitoneal bleed from femoral access; 1 transcaval bleed requiring covered stent placement, both of which qualified as VARC-2 life-threatening bleeds; 2 groin hematomas; 1 groin bleed without hematoma; and 1 ischemic limb requiring femoral artery thromboendarterectomy.
There was 1 death in a subject who developed a severe inflammatory response at induction of anesthesia despite a technically successful procedure, leading to multiple organ failure on a background of multiple brain metastases. Care was withdrawn and the subject died on post-procedure day 18.
There was 1 (3%) disabling stroke and 2 (7%) non-disabling strokes, detailed in Table 5. Two of the 3 subjects had baseline central neurological pathology, including the subject that died, making the clinical and radiological diagnosis ambiguous. Cerebral protection was used in 1 and no debris was recovered.
TABLE 5.
Procedural Stroke
| Subject 1 | Subject 2 | Subject 3 | |
|---|---|---|---|
| Neurological symptoms and sequelae | Mild bilateral hand weakness, resolved at 30 days | Confounded by baseline central and peripheral neuropathy; subjective left leg weakness, resolved at 30 days | Confounded by acute encephalopathy from anesthesia-related hypotension; fluctuating neurology with right hand weakness |
| Disability (by modified Rankin score) | Nondisabling | Nondisabling | Disabling |
| MRI | MRI-DWI multiple small bilateral foci of diffusion restriction | MRI-DWI multiple small bilateral foci of diffusion restriction | MRI-DWI confounded by multiple brain metastases and global ischemia |
| Setting | Mitroflow stenosis, doppio BASILICA L+R | Native aortic stenosis, solo BASILICA L | Mitroflow stenosis, solo BASILICA L |
| TAVR valve | SAPIEN 3 | SAPIEN 3 | Evolut R |
| Pre-dilatation/post-dilatation | No/no | Yes/yes | No/yes |
| Calcium volume of BASILICA leaflet | LCC 182, RCC 215 | LCC 95 | LCC 93 |
| Sentinel cerebral protection used; debris retrieved | No; N/A | Yes; no debris | No; N/A |
| HALT on CT | No | Yes | No |
DWI - diffusion-weighted imaging; L - left; LCC - left coronary cusp; MRI - magnetic resonance imaging; N/A - not applicable; R - right; RCC - right coronary cusp; other abbreviations as in Table 4.
OTHER CLINICAL ENDPOINTS.
Mean arterial pressures for each subject at baseline, before and after BASILICA, and after TAVR are shown in Online Figure 1. Transient hypotension after laceration was seen in 2 subjects. This resolved promptly with TAVR. There was 1 off-target guidewire traversal into the left atrium. The guidewire was withdrawn and traversal reattempted without clinical sequelae. One subject went into ventricular fibrillation after left leaflet guidewire traversal requiring cardioversion. No subject had greater than mild paravalvular leak. Two subjects had permanent pacemaker implantation. Mean aortic valve gradient fell from 44 ± 16 mm Hg to 18 ± 7 mm Hg at 30 days. Gradients were similar between bioprosthetic and native valve TAVR subjects. A total of 29% had severe patient-prosthesis mismatch (indexed effective orifice area <0.65 cm2/m2), and 38% had moderate prosthesis-patient mismatch (indexed effective orifice area 0.65 to 0.85 cm2/m2).
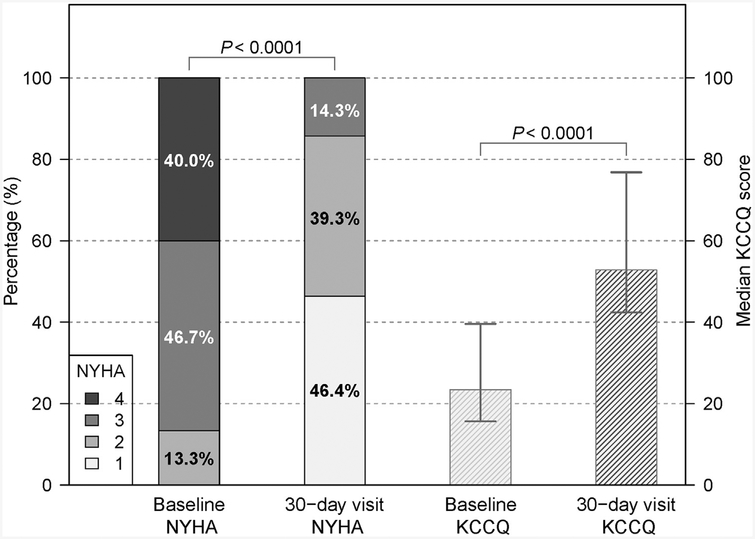
There was improvement in subject NYHA functional class and KCCQ quality of life scores (16) (Figure 4). At 30-day visit, the median KCCQ summary score was 52.9 (interquartile range: 42.5 to 76.8) with 14% subjects in NYHA functional class III to IV, compared with a median KCCQ of 23.4 (interquartile range: 15.6 to 39–6) and 87% subjects in NYHA functional class III to IV at baseline (both p < 0.0001). HALT was seen in 3 (11%) subjects on follow-up CT, all in SAPIEN 3 valves, and on the noncoronary leaflet in all. One of these subjects had hypoattenuation affecting motion. No HALT was seen on TAVR leaflets adjacent to leaflets split by BASILICA.
FIGURE 4. Symptom and Quality of Life Improvement.
There was an improvement in New York Heart Association (NYHA) functional class and Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 30 days compared with baseline. Error bars represent the interquartile range.
DISCUSSION
The purpose of this IDE trial was to determine feasibility, early safety, and efficacy of TAVR with BASILICA in subjects who might obstruct their coronary arteries with stand-alone TAVR. BASILICA-TAVR met the composite primary endpoint of procedure success in 93% Crucially, despite the high predicted risk, 100% of subjects were free of coronary artery obstruction after TAVR, with no evidence of coronary obstruction on angiography, CT, electrocardiogram, or echocardiogram, or clinically at 30 days.
Guidewire traversal failed in 2 leaflets. These cases demonstrate that confluent heavy leaflet calcification at the nadir of the target aortic leaflets may be an obstacle to leaflet traversal using radiofrequency energy. This is similar to the experience using guidewire electrification in transcaval access for TAVR (17,18). In all cases where leaflet traversal was successful, laceration was able to be performed.
Three (10%) subjects had neurological events (3% disabling stroke). The neurological event rate from TAVR in contemporary adjudicated trials in intermediate-risk subjects was 6.4% (3.2% disabling stroke) in the PARTNER 2 (Placement of Aortic Transcatheter Valves) trial (1) and 4–5% (1.2% disabling stroke) in SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trial (2), and in high-risk subjects in the SENTINEL (Cerebral Protection in Transcatheter Aortic Valve Replacement) trial was 5.6% with cerebral protection and 9.1% without (19). Whether there is an excess risk of stroke from BASILICA cannot be determined from this small sample size of high- and extreme-risk subjects, 23% of whom had a previous stroke and 23% of whom had severe vascular disease requiring alternative TAVR access. Two of the 3 subjects experiencing stroke had ambiguous findings related to baseline neurological comorbidity including a central demyelinating syndrome and metastatic brain cancer. Embolic stroke from BASILICA may theoretically be caused by multiple catheter manipulations and leaflet laceration releasing calcific debris. Stroke risk for BASILICA-TAVR needs to be assessed in larger prospective trials and registries.
Hemodynamic instability was uncommon but tolerated after BASILICA (7%) and resolved promptly with TAVR. Another concern is the VARC-2 major vascular complication rate (20%), which was higher than seen in contemporary registries-4.1% in for SAPIEN 3 in the SOURCE 3 trial (20) and 6.5% for Evolut R in the FORWARD trial (21). This may reflect the smaller anatomies in patients at risk of coronary obstruction, with increased the risk of vascular complications. All were independently adjudicated as related to TAVR and not related to BASILICA. Small anatomies are also reflected in the high postprocedural gradients and higher rates of severe and moderate prosthesis-patient mismatch compared with those recently reported from the TVT (Transcatheter Valve Therapy) registry (12% and 25%, respectively) (22) but similar to those seen in the Valve-in-Valve registry (23). Overall, the results of this early feasibility study suggest an acceptable early safety profile for BASILICA in high-risk subjects.
Subclinical leaflet thrombosis rates (11%) were similar to those in observational registries (13% in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and Its Treatment With Anticoagulation) and SAVORY (Subclinical Aortic Valve Bioprosthesis Thrombosis Assessed With 4D CT) registries (24) and clinical trials (14% in Low Risk TAVR trial) (3). Interestingly, no HALT was seen in TAVR leaflets adjacent to lacerated aortic leaflets. This generates a testable hypothesis that BASILICA might reduce subclinical leaflet thrombosis by promoting sinus washout and reducing stasis (25).
There is an unmet clinical need for high surgical risk patients at risk of coronary obstruction from TAVR. Coronary protection with pre-positioned guidewires and coronary stents is potentially hazardous (7). “Snorkel” stents, implanted to create a channel between TAVR valve and coronary sinus, are at risk of deformation and thrombosis due to constant mechanical pressures from the TAVR valve and poor blood flow in the obstructed neosinus. Future coronary access may be compromised, and patients are committed to long-term thromboprophylaxis and potential late bleeding complications. Furthermore, absence of coronary obstruction while a guidewire is down the coronary artery may be falsely reassuring, and the coronary artery may obstruct once the leaflet is unpinned after guidewire withdrawal. Additionally, coronary obstruction may occur remotely from the index procedure (7).
PROCEDURE LIMITATIONS.
High-quality CT image acquisition is required to assess for a calcium-free target at the base of the leaflet for guidewire traversal. If there appears to be confluent leaflet calcium, there may be a low likelihood of success, and if BASILICA is attempted, we recommend it is only tried briefly and by an experienced operator.
Careful procedure planning and image-guided execution is required to align the laceration in front of the coronary artery ostium. If the laceration is not aligned, obstruction may occur from a portion of the leaflet.
The poor correlation between predicted and post hoc observed VTC probably reflects unpredictable TAVR implantation characteristics (e.g., device selection, implantation depth, canting angle, and device flaring), as well as variable surgical bioprosthesis characteristics (leaflet material, frame geometry, and frame expansion after TAVR).
STUDY LIMITATIONS.
Further study is required to enhance the specificity of coronary obstruction risk prediction. Coronary obstruction is a rare complication and it is possible that some of the subjects undergoing BASILICA may not have obstructed their coronary arteries with standalone TAVR. Indeed, 1 subject did not obstruct their coronary artery after failed BASILICA. Acknowledging these limitations, all subjects were at high risk of coronary obstruction from TAVR by currently available criteria, as determined by the central eligibility committee based on pre-procedural CT imaging including simulation of the transcatheter heart valve and assessing the relation to the coronary arteries and aortic sinuses. Indeed, the predicted risk correlated well with observed measurements on follow-up CT. In this study, the coronary obstruction risk appeared to be underestimated in SAPIEN 3 valves and native annuli. In a few cases of Evolut valves implanted into bioprostheses, expansion of the valve was less than predicted.
No control arm was possible in this study as TAVR-related coronary obstruction has a high mortality and these subjects had no suitable alternatives that could provide clinical equipoise for a randomized comparison.
This study was designed to test early feasibility. Whether there are excess cardiovascular events related to BASILICA need to be addressed in larger studies.
FUTURE DIRECTIONS.
Further registry data are required to increase the sensitivity and specificity of coronary artery obstruction risk prediction, including analysis of high-quality pre- and post-procedure CT. This trial should inform further study. We do not believe that we have equipoise to compare BASILICA against alternatives such as snorkel stenting. One possible investigation might be a comparison of BASILICA and TAVR against surgical aortic valve replacement among patients at high or extreme surgical risk.
Dedicated catheter tools may make BASILICA into a relatively easy and swift adjunct to TAVR. At present, the BASILICA procedure is a novel technique and should be limited to high-volume centers and be performed with appropriate education and proctoring.
The method may be used to lacerate bicuspid aortic leaflets (Bi-SILiCA [Bicuspid Scallop Intentional Laceration to Circularize the Annulus]) and may enhance transcatheter heart valve circularity and improve TAVR outcomes in this difficult cohort of patients. We have reported this technique previously (26) and it needs to be evaluated in a prospective trial.
CONCLUSIONS
In subjects at high risk of coronary artery obstruction from TAVR, BASILICA demonstrated feasibility in both native and surgical bioprosthetic valves in a prospective, independently adjudicated, IDE early feasibility trial. Hemodynamic compromise was rare, and resolved with TAVR. Safety was acceptable and needs to be confirmed in larger studies. BASILICA appears effective in preventing coronary obstruction from TAVR in subjects at high risk.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Coronary artery obstruction from the residual diseased aortic leaflets is a rare and devastating complication of TAVR. BASILICA is a transcatheter technique that slices the aortic valve leaflets to prevent coronary obstruction, with proof of principal demonstrated in animal and first-in-human studies.
WHAT IS NEW?
The BASILICA IDE clinical trial demonstrated feasibility in native and bioprosthetic aortic valves, with an acceptable safety profile, and appeared effective in preventing coronary artery obstruction in subjects at high risk.
WHAT IS NEXT?
This early feasibility study should instigate larger prospective studies to further assess the safety of BASILICA as an adjunct to TAVR. BASILICA may be offered to enable TAVR in patients at risk of coronary artery obstruction at experienced centers.
ACKNOWLEDGMENTS
The authors thank clinical and research staff at University of Washington (Christopher Rumer, Data Manager; Ikki Komatsu, MD; Gabriel Aldea, MD; G. Burkhard Mackensen, MD, PhD; James M. McCabe, MD); Emory University (Lauren Wheeler; James Lee, RN; Patricia Keegan, DNP; Elizabeth Charles, Research Coordinator; Kristy Pitts, Research Coordinator; Kimberly McWhorter, Research Coordinator; Hima Patel, Research Coordinator; Jennifer James, Research Coordinator); Medstar Washington Hospital Center (Petros Okubagzi, Clinical Research Director; Dorion Hoffmeister, Research Coordinator); Henry Ford Hospital (Ashish Solanki, Research Coordinator); and independent Data Monitors (Olha Katynska; Valeriy Matveev; Artur Karapetyan). We thank the Medstar Clinical Events Adjudication Committee (Hector Garcia-Garcia, Soloman Beyene, Kayode Kuku, and Viana Azizi); and the National Heart, Lung, and Blood Institute Computed Tomography Core Laboratory.
This work was supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL006040–7), and by the intramural programs of the participating centers. Dr. Greenbaum has served as a proctor for Edwards Lifesdences, Medtronic, and Abbott Vascular; and a consultant for Transmural Systems. Dr. Babaliaros has served as a consultant for Edwards Lifesdences and Abbott Vascular; and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesdences, Abbott Vascular, Medtronic, St. Jude Medical, and Boston Scientific.
ABBREVIATIONS AND ACRONYMS
- BASILICA
bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR
- HALT
hypoattenuated leaflet thickening
- IDE
investigational device exemption
- KCCGt
Kansas City Cardiomyopathy Questionnaire
- NHLBI
National Heart, Lung, and Blood Institute
- NYHA
New Yorlt Heart Association
- TAVR
transcatheter aortic valve replacement
- VARC
Valve Academic Research Consortium
- VTC
virtual transcatheter valve to coronary distance
Footnotes
APPENDIX For a supplemental figure, table, videos, selection criteria, trial endpoints, and enrolling sites, please see the online version of this paper.
REFERENCES
- 1.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–20. [DOI] [PubMed] [Google Scholar]
- 2.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376:1321–31. [DOI] [PubMed] [Google Scholar]
- 3.Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018;72:2095–105. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–62. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro HB, Rodes-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687–95. [DOI] [PubMed] [Google Scholar]
- 6.Abramowitz Y, Chakravarty T, Jilaihawi H, et al. Clinical impact of coronary protection during transcatheter aortic valve implantation: first reported series of patients. Eurointervention 2015; 11:572–81. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour RJ, Tanaka A, Finkelstein A, et al. Delayed coronary obstruction after transcatheter aortic valve replacement. J Am Coll Cardiol 2018; 71:1513–24. [DOI] [PubMed] [Google Scholar]
- 8.Khan JM, Rogers T, Schenke WH, et at. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. J Am Coll Cardiol Intv 2016;9:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaliaros VC, Greenbaum AB, Khan JM, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first-in-human experience. J Am Coll Cardiol Intv 2017;10:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement: concept to first-in-human. J Am Coll Cardiol Intv 2018;11:677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing coronary obstruction during TAVR: From CT to BASILICA. J Am Coll Cardiol Intv 2019; 12:1197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanke P, Soon J, Dvir D, et al. Computed tomography assessment for transcatheter aortic valve in valve implantation: the vancouver approach to predict anatomical risk for coronary obstruction and other considerations. Journal of cardiovascular computed tomography 2016;10: 491–9. [DOI] [PubMed] [Google Scholar]
- 13.Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015;373: 2015–24. [DOI] [PubMed] [Google Scholar]
- 14.Khalique OK, Hahn RT, Gad a H, et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. J Am Coll Cardiol Intv 2014;7: 885–94. [DOI] [PubMed] [Google Scholar]
- 15.Chhatriwalla AK, Allen KB, Saxon JT, et al. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ Cardiovasc Interv 2017;10:e005216. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenbaum AB, Babaliaros VC, Chen MY, et al. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol 2017;69:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederman RJ, Greenbaum AB, Rogers T, Khan JM, Fusari M, Chen MY. Anatomic suitability for transcaval access based on computed tomography. J Am Coll Cardiol Intv 2017;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol 2017;69:367–77. [DOI] [PubMed] [Google Scholar]
- 20.Wendler O, Schymik G, Treede H, et al. SOURCE 3 registry: design and 30-day results of the european postapproval registry of the latest generation of the SAPIEN 3 transcatheter heart valve. Circulation 2017;135: 1123–32. [DOI] [PubMed] [Google Scholar]
- 21.Grube E, Van Mieghem NM, Bleiziffer S, et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: the international FORWARD study. J Am Coll Cardiol 2017;70:845–53. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann HC, Daneshvar SA, Fonarow GC, et al. Prosthesis-patient mismatch in patients undergoing transcatheter aortic valve replacement: from the STS/ACC TVT registry. J Am Coll Cardiol 2018;72:2701–11. [DOI] [PubMed] [Google Scholar]
- 23.Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA: the journal of the American Medical Association 2014;312:162–70. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarty T, Sondergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389: 2383–92. [DOI] [PubMed] [Google Scholar]
- 25.Midha PA, Raghav V, Sharma R, et al. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 2017; 136: 1598–609. [DOI] [PubMed] [Google Scholar]
- 26.Kamioka N, Lederman RJ, Khan JM, et al. BI-SILICA during transcatheter aortic valve replacement for noncalcific aortic insufficiency: initial human experience. J Am Coll Cardiol Intv 2018;11:2237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.