Key Points
Question
Are ideal cardiovascular health metrics (ICVHMs) associated with lower risks of cardiovascular disease among patients with prediabetes or diabetes?
Findings
In this cohort study of 111 765 Chinese participants, compared with participants with normal glucose regulation, participants with prediabetes or diabetes who achieved at least 5 ICVHMs had lower or no increased risks of cardiovascular disease events compared with participants with normal glucose regulation.
Meaning
Our findings emphasize the importance of promoting the adherence to ICVHMs in the prevention of cardiovascular disease events among patients with prediabetes or diabetes.
Abstract
Importance
Whether optimal cardiovascular health metrics may counteract the risk of cardiovascular events among patients with prediabetes or diabetes is unclear.
Objective
To investigate the associations of ideal cardiovascular health metrics (ICVHMs) with subsequent development of cardiovascular disease (CVD) among participants with prediabetes or diabetes as compared with participants with normal glucose regulation.
Design, Setting, and Participants
The China Cardiometabolic Disease and Cancer Cohort Study was a nationwide, population-based, prospective cohort study of 20 communities from various geographic regions in China. The study included 111 765 participants who were free from CVD or cancer at baseline. Data were analyzed between 2011 and 2016.
Exposures
Prediabetes and diabetes were defined according to the American Diabetes Association 2010 criteria. Seven ICVHMs were adapted from the American Heart Association recommendations.
Main Outcomes and Measures
The composite of incident fatal or nonfatal CVD, including cardiovascular death, myocardial infarction, stroke, and hospitalized or treated heart failure.
Results
Of the 111 765 participants, 24 881 (22.3%) had normal glucose regulation, 61 024 (54.6%) had prediabetes, and 25 860 (23.1%) had diabetes. Mean (SD) age ranged from 52.9 (8.6) years to 59.4 (8.7) years. Compared with participants with normal glucose regulation, among participants with prediabetes, the multivariable-adjusted hazard ratio for CVD was 1.34 (95% CI, 1.16-1.55) for participants who had 1 ICVHM or less and 0.57 (95% CI, 0.43-0.75) for participants who had at least 5 ICVHMs; among participants with diabetes, the hazard ratios for CVD were 2.05 (95% CI, 1.76-2.38) and 0.80 (95% CI, 0.56-1.15) for participants who had 1 ICVHM or less and at least 5 ICVHMs, respectively. Such pattern of association between ICVHMs and CVD was more prominent for participants younger than 55 years (prediabetes and at least 5 ICVHMs: hazard ratio [HR], 0.32; 95% CI, 0.16-0.63; 1 ICVHM or less: HR, 1.58; 95% CI, 1.13-2.21; diabetes and at least 5 ICVHMs: HR, 0.99; 95% CI, 0.44-2.26; 1 ICVHM or less: HR, 2.46; 95% CI, 1.71-3.54; compared with normal glucose regulation) than for participants 65 years or older (prediabetes and at least 5 ICVHMs: HR, 0.80; 95% CI, 0.50-1.26; 1 ICVHM or less: HR, 1.01; 95% CI, 0.79-1.31; diabetes and at least 5 ICVHMs: HR, 0.79; 95% CI, 0.46-1.35; 1 ICVHM or less: HR, 1.73; 95% CI, 1.36-2.22, compared with normal glucose regulation; P values for interaction ≤.02). Additionally, the hazard ratio for CVD per additional ICVHM was 0.82 (95% CI, 0.79-0.86) among participants with prediabetes and was 0.85 (95% CI, 0.80-0.89) among participants with diabetes.
Conclusions and Relevance
Participants with prediabetes or diabetes who had 5 or more ICVHMs exhibited lower or no significant excess CVD risks compared with the participants with normal glucose regulation.
This study investigates the associations of ideal cardiovascular health metrics with subsequent development of cardiovascular disease among participants with prediabetes or diabetes compared with Chinese adults with normal glucose regulation.
Introduction
Type 2 diabetes poses a major threat to public health. According to the data from the International Diabetes Federation, 1 in 11 adults aged 20 to 79 years had diabetes globally in 2017 (425 million), and China has become the epicenter of the diabetes epidemic.1 Cardiovascular complications are the leading cause of disability and mortality among patients with type 2 diabetes.2,3,4,5 Diabetes confers about a 2-fold excess risk of cardiovascular disease (CVD) events independent of conventional risk factors.6,7 Moreover, among patients with type 2 diabetes, CVD occurs earlier and with greater severity compared with individuals without diabetes.8 In addition, as a high-risk state for diabetes, prediabetes is also associated with a modest increase in the risk of CVD.9,10 Given the epidemic proportion of prediabetes worldwide and especially in China and the United States,1 if a causal relation exists, such modest increase in risk might still translate into substantial burden of CVD events.9
In 2010, the American Heart Association (AHA) suggested 7 ideal cardiovascular health metrics (ICVHMs), including healthy lifestyle behaviors and ideal metabolic measures, to promote optimal cardiovascular health in the population.11 Following the recommendations of the AHA, several population-based studies have supported a beneficial effect of ideal cardiovascular health on CVD events.12,13,14 Such evidence has been derived from general populations so far. Individuals with prediabetes or diabetes are at higher risks of developing CVD than those with normal glucose regulation.6,7,9,10 However, whether patients with prediabetes or diabetes who achieved a greater number of ICVHMs may have lower risk for CVD is unclear.
To this end, in a nationwide prospective population-based study, we investigated the individual and combined associations of ICVHMs with the risk of CVD events among individuals with prediabetes or diabetes compared with the risk of CVD events among individuals with normal glucose regulation.
Methods
Study Design and Population
The China Cardiometabolic Disease and Cancer Cohort (4C) Study is a multicenter, population-based, prospective cohort study.15 During 2011 to 2012, 193 846 men and women 40 years or older were recruited from local resident registration systems of 20 communities from various geographic regions in China to represent the general population. During 2014 to 2016, all participants were invited to attend an in-person visit, and 170 240 participants (87.8%) were followed up. At baseline and the follow-up visit, demographic characteristics, lifestyle and dietary factors, and medical history were collected by using standard questionnaires. Anthropometric and blood pressure measurements, oral glucose tolerance tests, and blood samples were obtained following a standard protocol. The analysis included 111 765 participants who had complete baseline information on cardiovascular health metrics and diabetes status and were free from CVD or cancer at baseline. This study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University. All study participants provided written informed consent.
Data Collection
Data collection was performed in local community clinics by trained study personnel according to a standard protocol at baseline and the follow-up visit. A questionnaire comprising information on demographic characteristics, lifestyle factors (including cigarette smoking and alcohol drinking), family history, and medical history was administered by trained interviewers. Current alcohol drinker was defined as a person who drank alcohol regularly in the past 6 months. Education attainment was categorized as less than high school and high school or more. A food frequency questionnaire was used to collect habitual dietary intake by asking the consumption frequency and portion size of typical food items during the previous 12 months. The International Physical Activity Questionnaire was used to assess physical activity.16 Height and body weight were measured according to a standard protocol, and body mass index (BMI) was calculated as the weight in kilograms divided by height in meters squared.
Three blood pressure measurements were obtained from each participant by using an automated electronic device (OMRON Model HEM-752 FUZZY) in a seated position after at least a 5-minute quiet rest. Participants were required to avoid alcohol, coffee, tea, smoking, and exercise at least 30 minutes before the blood pressure measurement. The 3 readings were averaged for the analysis.
All participants underwent an oral glucose tolerance test after an overnight fast of at least 10 hours, and blood samples were collected at 0 and 2 hours during the test. Fasting and 2-hour plasma glucose concentrations were measured locally using a glucose oxidase or hexokinase method within 2 hours after blood sample collection under a stringent quality control program. Finger capillary whole-blood samples were collected by the Hemoglobin Capillary Collection System (Bio-Rad Laboratories) and were stored at 2°C to 8°C and shipped to the central laboratory in the Shanghai Institute of Endocrine and Metabolic Diseases, which was certificated by the National Glycohemoglobin Standardization Program and the College of American Pathologists Laboratory Accreditation Program. Glycated hemoglobin A1c (HbA1c) was measured within 4 weeks after collection by high-performance liquid chromatography using the VARIANT II Hemoglobin Testing System (Bio-Rad Laboratories). The capillary HbA1c values and the venous values from whole-blood samples collected using ethylene diamine tetraacetic acid dipotassium tubes were highly correlated (r = 0.99) in a validation subsample.17 Total cholesterol was measured at the central laboratory using an autoanalyzer (ARCHITECT ci16200 analyzer; Abbott Laboratories).
Assessment of Prediabetes and Diabetes
Baseline prediabetes and diabetes were defined according to the American Diabetes Association 2010 criteria.18 Prediabetes was defined as fasting plasma glucose levels between 100.90 mg/dL and 124.32 mg/dL (to convert to millimoles per liter, multiply by 0.0555), 2-hour plasma glucose levels between 140.54 mg/dL and 198.20 mg/dL, or HbA1c concentrations between 5.7% and 6.4% in participants without a prior diagnosis of diabetes (to convert to proportion of total hemoglobin, multiply by 198.20). Diabetes was defined as fasting plasma glucose level of at least 126.13 mg/dL, 2-hour plasma glucose level of at least 200 mg/dL, HbA1c level of at least 6.5%, or by a self-reported previous diagnosis by health care professionals.
Definition of ICVHMs
Seven ICVHMs were adapted from the recommendations of the Goals and Metrics Committee of the Strategic Planning Task Force of the AHA11: never smoked or quit smoking more than 12 months prior, BMI less than 23, physical activity at goal (at least 150 minutes/week moderate intensity or 75 minutes/week vigorous intensity or 150 minutes/week moderate plus vigorous intensity), fruit and vegetable intake at least 4.5 cups/d, total cholesterol less than 200 mg/dL (untreated; to convert to millimoles per liter, multiply by 0.0259), blood pressure less than 120/80 mm Hg (untreated), and ideal HbA1c level (<5.7% for prediabetes or <6.5% for diabetes) (eTable 1 in the Supplement). We defined an ideal BMI by a BMI cutoff point of less than 23, which has been proposed as a more reasonable threshold to define a normal BMI for Asian individuals,19,20 and we have validated that a BMI of at least 23 was associated with greater risks of CVD events and elevated HbA1c than a BMI of at least 24 or at least 25 as compared with their corresponding normal BMI in this study cohort (eTable 2 in the Supplement). We used fruit and vegetable intake to evaluate a healthy diet because data on whole grain and sodium consumption were not collected, and fruits and vegetables are primary dietary components highlighted by the AHA 2020 Strategic Impact Goals and have been associated with lower risks of CVD.21,22 We replaced fasting plasma glucose with HbA1c as one metric to estimate glucose metabolism in participants with prediabetes or diabetes, in accordance with the stringent HbA1c goals of the current American Diabetes Association clinical practice recommendations.23
Ascertainment of Cardiovascular Events
The outcome of this study was the composite of incident fatal or nonfatal CVD events including cardiovascular death, myocardial infarction, stroke, and hospitalized or treated heart failure. Myocardial infarction was defined by characteristic changes in levels of troponin T and creatine-kinase-MB isoform, symptoms of myocardial ischemia, changes in electrocardiogram results, or a combination of them. Stroke was defined as a fixed neurologic deficit at least 24 hours because of a presumed vascular cause. Heart failure was identified by hospitalization or an emergency department visit requiring a treatment with infusion therapy for a clinical syndrome presenting with multiple signs and symptoms in consistence with cardiac decompensation or inadequate cardiac pump function. Deaths and clinical outcomes were collected from local vital registries of the National Disease Surveillance Point System and National Health Insurance System. Two members of the outcome adjudication committee independently verified each clinical event and assigned potential causes of death, and discrepancies were adjudicated by discussion involving other members of the committee. All members of the committee were unaware of the baseline risk factors of study participants.
Statistical Analysis
Baseline characteristics of participants with normal glucose regulation, prediabetes, and diabetes were summarized as means with standard deviation for continuous variables and percentages for categorical variables. Person-time for each participant was calculated from the date of enrollment to the date of CVD diagnosis, death, or the end of follow-up, whichever came first. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals for CVD events, with multivariable adjustment for age, sex, education attainment, family history of diabetes, and family history of CVD. We first estimated the risk of CVD events according to individual and combined ICVHMs among participants with prediabetes or diabetes compared with participants with normal glucose regulation. In the analysis of the association between each component of the ICVHMs and CVD events, we further adjusted for all other ICVHMs. As a next analysis, we stratified participants into subgroups according to age and sex and examined the association between ICVHMs and CVD risk among participants with prediabetes or diabetes as compared with participants with normal glucose regulation in each age or sex subgroup. Among participants with prediabetes or diabetes, we also analyzed the risk of CVD events according to individual and combined ICVHMs. To test whether the pattern of association varies across stratifications, we estimated multiplicative interactions by including the product term (exposure × stratification variable) in the models. We replicated the main analyses for nonfatal CVD events and CVD mortality in secondary analyses. All reported P values are nominal and 2-sided, and a P value of less than .05 was considered statistically significant. All statistical analyses were performed by using SAS software, version 9.2 (SAS Institute Inc).
Results
Of the 111 765 participants, 24 881 (22.3%) had normal glucose regulation, 61 024 (54.6%) had prediabetes, and 25 860 (23.1%) had diabetes. Mean (SD) age ranged from 52.9 (8.6) years to 59.4 (8.7) years. Baseline characteristics of the study participants according to glucose tolerance status are shown in Table 1. Compared with participants with normal glucose regulation, participants with prediabetes or diabetes were older, had higher proportions of men, had lower level of education attainment, were more likely to have family history of diabetes, and had poorer metabolic profiles and less number of ICVHMs.
Table 1. Baseline Characteristics of Participants With Normal Glucose Regulation, Prediabetes, and Diabetes (N = 111 765)a.
| Baseline Characteristic | No. (%) | ||
|---|---|---|---|
| Normal Glucose Regulation | Prediabetes | Diabetes | |
| Participants | 24 881 (22.3) | 61 024 (54.6) | 25 860 (23.1) |
| Age, mean (SD), y | 52.9 (8.6) | 56.5 (8.7) | 59.4 (8.7) |
| Men | 7872 (31.6) | 20 018 (32.8) | 9945 (38.5) |
| High school or greater education | 9962 (40.0) | 22 895 (37.5) | 9135 (35.3) |
| Current alcohol drinker | 2487 (10.2) | 6501 (10.9) | 2767 (10.9) |
| Family history of diabetes | 2530 (10.2) | 7400 (12.1) | 5346 (20.7) |
| Family history of CVD | 4216 (16.9) | 10 495 (17.2) | 4189 (16.2) |
| Smoking status | |||
| Current | 4818 (19.4) | 11 128 (18.2) | 4997 (19.3) |
| Former, quit ≤12 mo | 136 (0.6) | 378 (0.6) | 228 (0.9) |
| Never or quit >12 mo | 19 927 (80.1) | 49 518 (81.2) | 20 635 (79.8) |
| BMI levels | |||
| <23 | 11 007 (44.2) | 20 731 (34.0) | 5852 (22.6) |
| ≥23 to <25 | 6253 (25.1) | 14 830 (24.3) | 5850 (22.6) |
| ≥25 | 7621 (30.6) | 25 463 (41.7) | 14 158 (54.8) |
| Physical activity at goal | 3219 (12.9) | 9027 (14.8) | 3559 (13.8) |
| Fruit and vegetable intake, mean (SD), cup/d | 4.1 (5.6) | 4.6 (4.5) | 4.6 (10.2) |
| Total cholesterol, mean (SD), mg/dL | 185.0 (40.6) | 192.4 (44.0) | 196.6 (46.9) |
| Systolic blood pressure, mean (SD), mm Hg | 128 (20) | 132 (20) | 138 (21) |
| Diastolic blood pressure, mean (SD), mm Hg | 77 (11) | 78 (11) | 80 (11) |
| Glycated hemoglobin A1c, mean (SD), % | 5.3 (0.3) | 5.8 (0.3) | 7.1 (1.5) |
| ICVHMs, No. | |||
| ≤1 | 494 (2.0) | 9004 (14.8) | 4865 (18.8) |
| 2 | 3296 (13.3) | 16 710 (27.4) | 8036 (31.1) |
| 3 | 6754 (27.2) | 17 937 (29.4) | 7450 (28.8) |
| 4 | 7279 (29.3) | 11 641 (19.1) | 3893 (15.1) |
| ≥5 | 7058 (28.4) | 5732 (9.4) | 1616 (6.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICVHMs, ideal cardiovascular health metrics.
SI conversion factors: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; glycated hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
Percentages may not sum to 100% owing to rounding. The number of missing values is 2135 for alcohol drinkers.
During a mean follow-up of 3.8 years (406 065 person-years), we documented 2882 CVD events (2.6%): 526 participants died of cardiovascular cause, 431 had myocardial infarction, 1781 had stroke, and 204 had heart failure; participants may have experienced more than 1 CVD event. Compared with normal glucose regulation, prediabetes (unadjusted HR, 1.23; 95% CI, 1.11-1.37; adjusted HR, 0.98; 95% CI, 0.88-1.09) and diabetes (unadjusted HR, 2.45; 95% CI, 2.20-2.73; adjusted HR, 1.56; 95% CI, 1.40-1.75) were associated with relatively higher risks of CVD events (eTable 3 in the Supplement).
As shown in Table 2, compared with participants with normal glucose regulation, participants with prediabetes who had 1 of these ICVHMs exhibited significantly lower risk for CVD: ideal BMI (HR, 0.84; 95% CI, 0.72-0.98), physical activity at goal (HR, 0.78; 95% CI, 0.64-0.95), healthy diet (HR, 0.82; 95% CI, 0.71-0.95), ideal total cholesterol (HR, 0.86; 95% CI, 0.75-0.99), and ideal blood pressure (HR, 0.58; 95% CI, 0.48-0.71). Compared with participants with normal glucose regulation, among participants with prediabetes, the HR for CVD gradually decreased from 1.34 (95% CI, 1.16-1.55) in those who had 1 ICVHM or less to 0.57 (95% CI, 0.43-0.75) in those who had more than 5 ICVHMs. Participants with diabetes who had ideal blood pressure had a marginally lower risk for CVD (HR, 0.91; 95% CI, 0.71-1.18), and the risk for CVD decreased with an increasing number of ICVHMs (≤1 ICVHM: HR, 2.05; 95% CI, 1.76-2.38; ≥5 ICVHMs: HR, 0.80; 95% CI, 0.56-1.15), as compared with participants with normal glucose regulation.
Table 2. Hazard Ratio (95% CI) of CVD Events According to Individual and Combined ICVHMs Among Participants With Prediabetes or Diabetes as Compared With the Overall Participants With Normal Glucose Regulationa.
| Category | Person-Years | No. of Cases | HR (95% CI)b |
|---|---|---|---|
| Normal glucose regulation | 92 244 | 453 | 1 [Reference] |
| Prediabetes | |||
| Ideal smoking status | 178 928 | 1017 | 0.89 (0.78-1.02) |
| Ideal BMI | 74 792 | 383 | 0.84 (0.72-0.98) |
| Physical activity at goal | 32 903 | 157 | 0.78 (0.64-0.95) |
| Healthy diet | 101 941 | 508 | 0.82 (0.71-0.95) |
| Untreated total cholesterol <200 mg/dL | 122 210 | 632 | 0.86 (0.75-0.99) |
| Untreated blood pressure <120/80 mm Hg | 58 472 | 156 | 0.58 (0.48-0.71) |
| Glycated hemoglobin A1c <5.7% | 50 843 | 311 | 1.03 (0.89-1.19) |
| No. of ICVHMs | |||
| ≤1 | 32 967 | 307 | 1.34 (1.16-1.55) |
| 2 | 60 272 | 436 | 1.08 (0.95-1.23) |
| 3 | 64 473 | 351 | 0.89 (0.78-1.03) |
| 4 | 42 146 | 174 | 0.75 (0.63-0.89) |
| ≥5 | 20 906 | 54 | 0.57 (0.43-0.75) |
| Diabetes | |||
| Ideal smoking status | 74 324 | 846 | 1.33 (1.16-1.52) |
| Ideal BMI | 20 848 | 235 | 1.41 (1.19-1.66) |
| Physical activity at goal | 12 868 | 137 | 1.31 (1.07-1.61) |
| Healthy diet | 41 844 | 450 | 1.31 (1.13-1.52) |
| Untreated total cholesterol <200 mg/dL | 45 014 | 483 | 1.33 (1.15-1.53) |
| Untreated blood pressure <120/80 mm Hg | 13 418 | 71 | 0.91 (0.71-1.18) |
| Glycated hemoglobin A1c <6.5% | 31 659 | 337 | 1.31 (1.13-1.51) |
| No. of ICVHMs | |||
| ≤1 | 17 554 | 272 | 2.05 (1.76-2.38) |
| 2 | 28 819 | 366 | 1.63 (1.42-1.88) |
| 3 | 26 777 | 305 | 1.48 (1.27-1.71) |
| 4 | 14 000 | 133 | 1.29 (1.07-1.57) |
| ≥5 | 5909 | 31 | 0.80 (0.56-1.15) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; ICVHMs, ideal cardiovascular health metrics.
SI conversion factors: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; glycated hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
111 765 participants (24 881 with normal glucose regulation, 61 024 with prediabetes, and 25 860 with diabetes) were included in the analysis.
Adjusted for age, sex, education attainment (less than high school or high school or greater), family history of diabetes (yes or no), and family history of CVD (yes or no). Individual cardiovascular health metrics were mutually adjusted.
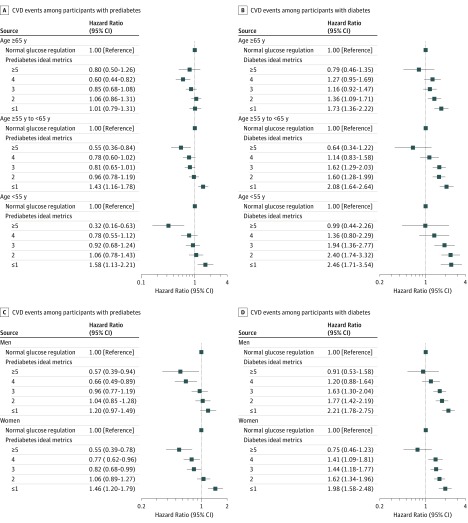
Among participants with prediabetes, the magnitude of the incremental risk for CVD associated with fewer ICVHMs was greatest among participants who were younger than 55 years (≥5 ICVHMs: HR, 0.32; 95% CI, 0.16-0.63; ≤1 ICVHM: HR, 1.58; 95% CI, 1.13-2.21) and was lowest among participants who were 65 years or older (≥5 ICVHMs: HR, 0.80; 95% CI, 0.50-1.26; ≤1 ICVHM: HR, 1.01; 95% CI, 0.79-1.31; P for interaction = .02; Figure 1A). Similar age-related interactions with the number of ICVHMs were observed among participants with diabetes compared with those with normal glucose regulation (younger than 55 years and ≥5 ICVHMs: HR, 0.99; 95% CI, 0.44-2.26; ≤1 ICVHM: HR, 2.46; 95% CI, 1.71-3.54; 65 years or older and ≥5 ICVHMs: HR, 0.79; 95% CI, 0.46-1.35; ≤1 ICVHM: HR, 1.73; 95% CI, 1.36-2.22; P for interaction <.001; Figure 1B). No statistically significant interaction was observed with sex (Figure 1C and D).
Figure 1. Hazard Ratio (95% CI) of Cardiovascular Disease (CVD) Events in Relation to Number of Ideal Cardiovascular Health Metrics (ICVHMs) Among Participants With Prediabetes or Diabetes Compared With the Overall Participants With Normal Glucose Regulation, According to Age and Sex Categories.
A total of 111 765 participants (24 881 with normal glucose regulation, 61 024 with prediabetes, and 25 860 with diabetes) were included in the analysis. Hazard ratios (95% CI) were adjusted for age, sex, education attainment (less than high school or high school or greater), family history of diabetes (yes or no), and family history of CVD (yes or no). Interaction between the combination of glucose tolerance status with number of ICVHMs and age on CVD: P for interaction = .02 (A); P for interaction <.001 (B). Interaction between the combination of glucose tolerance status with number of ICVHMs and sex on CVD: P for interaction = .59 (C); P for interaction = .35 (D).
Among participants with prediabetes, compared with participants who had 1 ideal metric or none, the HR for CVD was 0.80 (95% CI, 0.69-0.93), 0.66 (95% CI, 0.56-0.77), 0.55 (95% CI, 0.46-0.67), and 0.42 (95% CI, 0.32- 0.57) for participants who had 2, 3, 4, and at least 5 ideal metrics, respectively, and was 0.82 (95% CI, 0.79-0.86) per 1-number increment in ICVHMs. A similar pattern was observed among participants with diabetes; each 1-number increment in ICVHMs was associated with an HR of 0.85 (95% CI, 0.80-0.89) for CVD (Table 3). The graded association of more ICVHMs with lower risk for CVD events was consistent across normal glucose regulation, prediabetes, and diabetes strata (eTable 4 in the Supplement).
Table 3. Hazard Ratio (95% CI) of CVD Events According to Individual and Combined ICVHMs Among Participants With Prediabetes or Diabetesa.
| Category | Person-Years | No. of Cases | HR (95% CI)b |
|---|---|---|---|
| Prediabetes | |||
| Smoking status | |||
| Nonideal | 41 835 | 305 | 1 [Reference] |
| Ideal | 178 928 | 1017 | 0.81 (0.69-0.95) |
| BMI | |||
| Nonideal | 145 971 | 939 | 1 [Reference] |
| Ideal | 74 792 | 383 | 0.84 (0.74-0.95) |
| Physical activity | |||
| Nonideal | 187 860 | 1165 | 1 [Reference] |
| Ideal | 32 903 | 157 | 0.81 (0.69-0.96) |
| Healthy diet | |||
| Nonideal | 118 821 | 814 | 1 [Reference] |
| Ideal | 101 941 | 508 | 0.83 (0.74-0.93) |
| Total cholesterol | |||
| Nonideal | 98 553 | 690 | 1 [Reference] |
| Ideal | 122 210 | 632 | 0.83 (0.75-0.93) |
| Blood pressure | |||
| Nonideal | 162 291 | 1166 | 1 [Reference] |
| Ideal | 58 472 | 156 | 0.58 (0.49-0.69) |
| Glycated hemoglobin A1c | |||
| Nonideal | 169 920 | 1011 | 1 [Reference] |
| Ideal | 50 843 | 311 | 1.12 (0.98-1.27) |
| ICVHMs, No. | |||
| ≤1 | 32 967 | 307 | 1 [Reference] |
| 2 | 60 272 | 436 | 0.80 (0.69-0.93) |
| 3 | 64 473 | 351 | 0.66 (0.56-0.77) |
| 4 | 42 146 | 174 | 0.55 (0.46-0.67) |
| ≥5 | 20 906 | 54 | 0.42 (0.32-0.57) |
| Each 1-number increment in ICVHMs | NA | NA | 0.82 (0.79-0.86) |
| Diabetes | |||
| Smoking status | |||
| Nonideal | 18 734 | 261 | 1 [Reference] |
| Ideal | 74 324 | 846 | 0.81 (0.69-0.96) |
| BMI | |||
| Nonideal | 72 210 | 872 | 1 [Reference] |
| Ideal | 20 848 | 235 | 0.99 (0.85-1.14) |
| Physical activity | |||
| Nonideal | 80 190 | 970 | 1 [Reference] |
| Ideal | 12 868 | 137 | 0.91 (0.76-1.08) |
| Healthy diet | |||
| Nonideal | 51 214 | 657 | 1 [Reference] |
| Ideal | 41 844 | 450 | 0.89 (0.78-0.99) |
| Total cholesterol | |||
| Nonideal | 48 044 | 624 | 1 [Reference] |
| Ideal | 45 014 | 483 | 0.83 (0.74-0.94) |
| Blood pressure | |||
| Nonideal | 79 641 | 1036 | 1 [Reference] |
| Ideal | 13 417 | 71 | 0.54 (0.42-0.69) |
| Glycated hemoglobin A1c | |||
| Nonideal | 61 400 | 770 | 1 [Reference] |
| Ideal | 31 658 | 337 | 0.85 (0.74-0.96) |
| No. of ICVHMs | |||
| ≤1 | 17 554 | 272 | 1 [Reference] |
| 2 | 28 819 | 366 | 0.81 (0.69-0.95) |
| 3 | 26 777 | 305 | 0.74 (0.63-0.87) |
| 4 | 14 000 | 133 | 0.64 (0.52-0.79) |
| ≥5 | 5909 | 31 | 0.39 (0.27-0.56) |
| Each 1-number increment in ICVHMs | NA | NA | 0.85 (0.80-0.89) |
Abbreviations: BMI, body mass index; HR, hazard ratio; ICVHMs, ideal cardiovascular health metrics; NA, not applicable.
61 024 participants with prediabetes and 25 860 with diabetes were included in the analysis.
Adjusted for age, sex, education attainment (less than high school, high school or greater), family history of diabetes (yes or no), family history of CVD (yes or no), and diabetes duration (for participants with diabetes only). Individual cardiovascular health metrics were mutually adjusted.
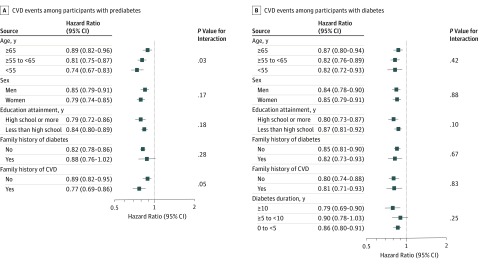
Consistent results were observed in strata of age, sex, education attainment, family history of diabetes, family history of CVD, and diabetes duration (for participants with diabetes only) (Figure 2). We observed an interaction of each 1-number increment in ICVHMs with age on CVD events among participants with prediabetes. The HR for CVD was 0.89 (95% CI, 0.82-0.96) among participants who were 65 years or older and 0.74 (95% CI, 0.67-0.83) among participants who were younger than 55 years (P for interaction = .03). There were no other significant interactions. Secondary analyses showed more significant results for nonfatal CVD events but less significant results for CVD mortality (eTables 5 and 6 in the Supplement).
Figure 2. Hazard Ratio (95% CI) of Cardiovascular Disease (CVD) Events per 1-Number Increment in Ideal Cardiovascular Health Metrics (ICVHMs) According to Stratification Categories Among Participants With Prediabetes or Diabetes.
A total of 61 024 participants with prediabetes and 25 860 with diabetes were included in the analysis. Hazard ratios (95% CI) were adjusted for age, sex, education attainment (less than high school or high school or greater), family history of diabetes (yes or no), family history of CVD (yes or no), and diabetes duration (for participants with diabetes only).
Discussion
In this Chinese nationwide prospective cohort study of 111 765 adults, participants with prediabetes or diabetes who had 5 or more ICVHMs exhibited lower or no significant excess risk of CVD events compared with those with normal glucose regulation. Compared with 1 ICVHM or none, 5 or more ideal metrics were associated with 58% and 61% lower CVD risks among participants with prediabetes and diabetes, respectively. Each additional ICVHM was associated with 18% and 15% lower risks of CVD events among participants with prediabetes and diabetes, respectively.
To the best of our knowledge, this study is the first to comprehensively investigate the association of ICVHMs with CVD events among individuals with diabetes or prediabetes. Randomized clinical trials evaluating cardiovascular effects of multifactorial interventions in individuals with impaired glucose tolerance or type 2 diabetes are sparse and yield mixed results.24,25,26,27,28 In the Steno-2 Study27 of 160 patients with type 2 diabetes and microalbuminuria, implementations of behavior modification and pharmacologic therapy significantly reduced CVD risk by more than 50% after a follow-up of 7.8 years. In contrast, results from the Look AHEAD (Action for Health in Diabetes)26 of 5145 overweight or obese individuals with type 2 diabetes showed that intensive lifestyle intervention focused on weight loss did not reduce CVD events over a median of 9.6 years of follow-up. Prospective observational studies with regard to cardiovascular effects among patients with diabetes are limited and have mainly assessed lifestyle behaviors.29,30,31 In this study, taking advantage of the AHA ICVHMs, we analyzed a comprehensive cardiovascular risk factor profile to ascertain its association with CVD risk. Importantly, we found that patients with prediabetes or diabetes who had 5 or more ICVHMs appeared to have no significant excess or even lower risk of CVD events compared with the overall population with normal glucose regulation. In view of the rising epidemic of prediabetes and diabetes globally and in China, our findings emphasize the importance of an early intervention targeting both lifestyle behaviors and metabolic profiles among individuals with prediabetes and diabetes for the prevention of CVD.
Interestingly, we detected that among younger individuals, patients with prediabetes who had 5 or more ICVHMs showed lower CVD risk than individuals with normal glucose regulation, while such an association was not seen among older individuals. We speculate that the high prevalence of comorbidities and medications for chronic diseases among the elderly population may compromise the benefit of the ICVHMs.14 However, our results did indicate that even among the elderly individuals with prediabetes or diabetes, each additional ICVHM was associated with substantially lower risk of CVD (11% to 13%), highlighting the benefit of achieving and maintaining an ideal cardiovascular health across the life course.32
With regard to each component of cardiovascular health metrics, an ideal blood pressure was predominantly associated with lower CVD risks among individuals with prediabetes or diabetes. Surprisingly, an ideal HbA1c level was not independently associated with the CVD risk among individuals with prediabetes. In 2018, the Whitehall II Study10 of 5427 participants aged 50 to 79 years reported that the risk of CVD associated with prediabetes defined by HbA1c was absolutely eliminated after adjustment for cardiovascular risk factors such as smoking, cholesterol levels, and systolic blood pressure, suggesting that the excess risk of CVD among individuals with prediabetes may be mainly explained by the clustering of other cardiovascular risk factors associated with hyperglycemia. Together with existing evidence, our findings suggest that the primary prevention of CVD in individuals with prediabetes or diabetes should focus on a combination of cardiovascular risk factors.
Strengths and Limitations
The strengths of this study included the large nationwide sample size, the prospective study design, the well-validated definitions of the CVD outcomes, and the comprehensive analyses including individuals with normal glucose regulation, prediabetes, or diabetes. Our study also has notable limitations. First, the relatively short follow-up duration might limit the statistical power for CVD events. Second, the cardiovascular health metrics were evaluated only at baseline, so changes in the metrics over time could not be accounted for in this study. Participants who were excluded owing to missing data on cardiovascular health metrics included a slightly higher proportion of men, so a selection bias may exist. The results, then, should be interpreted cautiously. Third, we evaluated an ideal diet mainly on the basis of fruit and vegetable intake, and the absence of comprehensive dietary data may underestimate the actual association of a healthy diet with CVD events. Fourth, we could not fully rule out all the residual and unmeasured confounders, such as genetic predisposition, medications, and psychological status and possible reverse causation.
Conclusions
In this Chinese nationwide prospective population-based study, patients with prediabetes or diabetes who achieved a greater number of ICVHMs exhibited lower or no significant excess risks of CVD events compared with individuals with normal glucose regulation. In addition, among patients with prediabetes or diabetes, each additional ICVHM was associated with at least 15% lower risk of CVD. Our findings emphasize the importance of promoting the adherence to ICVHMs in the prevention of CVD events among patients with prediabetes or diabetes.
eTable 1. Definitions of 7 Ideal Cardiovascular Health Metrics in Participants With Prediabetes or Diabetes
eTable 2. Risk of CVD Events and High HbA1c According to BMI Categories Defined by Different Cutoff Points
eTable 3. Hazard Ratio (95% CI) of CVD Events Among Participants With Prediabetes or Diabetes, as Compared With Participants With Normal Glucose Regulation
eTable 4. Hazard Ratio (95% CI) of CVD Events According to Combined Ideal Cardiovascular Health Metrics Among Participants With Normal Glucose Regulation, Prediabetes, and Diabetes
eTable 5. Hazard Ratio (95% CI) of Non-fatal CVD Events and CVD Mortality According to Individual and Combined Ideal Cardiovascular Health Metrics Among Participants With Prediabetes or Diabetes Compared With Participants With Normal Glucose Regulation
eTable 6. Hazard Ratio (95% CI) of Non-fatal CVD Events and CVD Mortality According to Individual and Combined Ideal Cardiovascular Health Metrics Among Participants With Prediabetes or Diabetes
References
- 1.International Diabetes Federation IDF diabetes atlas: 8th edition. http://www.diabetesatlas.org/. Accessed October 31, 2018.
- 2.American Diabetes Association 9: Cardiovascular disease and risk management. Diabetes Care. 2017;40(suppl 1):S75-S87. doi: 10.2337/dc17-S012 [DOI] [PubMed] [Google Scholar]
- 3.Kearney M. Improving outcomes in patients with type 2 diabetes mellitus and chronic heart failure: new hope. J Diabetes. 2018;10(10):799-800. doi: 10.1111/1753-0407.12791 [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: a perspective on the concept of metabolic memory. J Diabetes. 2017;9(2):141-148. doi: 10.1111/1753-0407.12475 [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434-449. doi: 10.1111/1753-0407.12521 [DOI] [PubMed] [Google Scholar]
- 6.Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-2222. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. ; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829-841. doi: 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88-98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55(13):1310-1317. doi: 10.1016/j.jacc.2009.10.060 [DOI] [PubMed] [Google Scholar]
- 10.Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II Study. Diabetes Care. 2018;41(4):899-906. doi: 10.2337/dc17-2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690-1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273-1283. doi: 10.1001/jama.2012.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaye B, Canonico M, Perier MC, et al. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the Three-City Study. J Am Coll Cardiol. 2017;69(25):3015-3026. doi: 10.1016/j.jacc.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 15.Lu J, He J, Li M, et al. ; 4C Study Group . Predictive values of fasting glucose, postload glucose, and hemoglobin A1c on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;dc181390. doi: 10.2337/dc18-1390 [DOI] [PubMed] [Google Scholar]
- 16.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Wang L, He J, et al. ; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948-959. doi: 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69. doi: 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization, International Association for the Study of Obesity, International Obesity Task Force The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney, Australia: Health Communications Australia Pty Ltd; 2000. [Google Scholar]
- 20.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38(1):150-158. doi: 10.2337/dc14-2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987-2015. doi: 10.1016/S0140-6736(13)61097-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du H, Li L, Bennett D, et al. ; China Kadoorie Biobank Study . Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374(14):1332-1343. doi: 10.1056/NEJMoa1501451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(suppl 1):S55-S64. doi: 10.2337/dc18-S006 [DOI] [PubMed] [Google Scholar]
- 24.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474-480. doi: 10.1016/S2213-8587(14)70057-9 [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Bolin P, Brancati FL, et al. ; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregg EW, Jakicic JM, Blackburn G, et al. ; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913-921. doi: 10.1016/S2213-8587(16)30162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383-393. doi: 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 28.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829-840. doi: 10.1016/S0140-6736(07)61303-8 [DOI] [PubMed] [Google Scholar]
- 29.Long GH, Cooper AJ, Wareham NJ, Griffin SJ, Simmons RK. Healthy behavior change and cardiovascular outcomes in newly diagnosed type 2 diabetic patients: a cohort analysis of the ADDITION-Cambridge study. Diabetes Care. 2014;37(6):1712-1720. doi: 10.2337/dc13-1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Li Y, Hu Y, et al. Influence of lifestyle on incident cardiovascular disease and mortality in patients with diabetes mellitus. J Am Coll Cardiol. 2018;71(25):2867-2876. doi: 10.1016/j.jacc.2018.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633-644. doi: 10.1056/NEJMoa1800256 [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;(18):39033-39038.30423391 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of 7 Ideal Cardiovascular Health Metrics in Participants With Prediabetes or Diabetes
eTable 2. Risk of CVD Events and High HbA1c According to BMI Categories Defined by Different Cutoff Points
eTable 3. Hazard Ratio (95% CI) of CVD Events Among Participants With Prediabetes or Diabetes, as Compared With Participants With Normal Glucose Regulation
eTable 4. Hazard Ratio (95% CI) of CVD Events According to Combined Ideal Cardiovascular Health Metrics Among Participants With Normal Glucose Regulation, Prediabetes, and Diabetes
eTable 5. Hazard Ratio (95% CI) of Non-fatal CVD Events and CVD Mortality According to Individual and Combined Ideal Cardiovascular Health Metrics Among Participants With Prediabetes or Diabetes Compared With Participants With Normal Glucose Regulation
eTable 6. Hazard Ratio (95% CI) of Non-fatal CVD Events and CVD Mortality According to Individual and Combined Ideal Cardiovascular Health Metrics Among Participants With Prediabetes or Diabetes