Abstract
Background:
We sought to identify predictors of asthma development following severe early childhood RSV bronchiolitis. Different definitions of asthma were also compared.
Methods:
This longitudinal, observational study (N=343) followed patients (<2 years old) from a placebo-controlled trial (N=979) of montelukast after RSV bronchiolitis to identify clinical, demographic, or biochemical predictors of asthma, atopic disorders, and chronic asthma therapy use at 6 years of age. Asthma (primary definition) was based on parental identification of wheeze at 6 AND 12 months before 6 years of age; definitions based on physician diagnosis as well as parental identification of wheeze at 6 OR 12 months (to consider seasonal effect) were also assessed. Post-hoc analyses evaluated agreement among asthma diagnosis criteria.
Results:
Prevalence of asthma (primary definition by parental identification), asthma (physician diagnosis), atopic disorders, and chronic asthma therapy use (parental identification) was 6.1%, 22.4%, 36.2%, and 14.5%, respectively. Predictors for asthma (primary definition) included male gender, a relative with asthma, and RAST positive for dog dander; and for physician diagnosis of asthma, high severity score for RSV bronchiolitis, high respiratory rate, and asthma diagnosis before enrollment. Predictors of atopic disorders included allergic rhinitis before enrollment, a relative with asthma, and the plasma biomarkers IL-5, IL-16, and IL-18. Predictors of chronic asthma therapy use included asthma diagnosis before enrollment and geographic region (Europe and Africa). Only 42% of patients with asthma (primary definition) also met the asthma definition by physician diagnosis and chronic asthma therapy use.
Conclusion:
Among children with early RSV bronchiolitis, hereditary factors (i.e., having a relative with asthma) and RSV bronchiolitis severity were predictors of asthma and atopic disorders at 6 years of age. Of interest, there was poor agreement among the asthma definitions evaluated.
Keywords: Respiratory Syncytial Virus, Asthma, RSV Bronchiolitis, Asthma Diagnosis
Introduction
Respiratory syncytial virus (RSV) infection is a common cause of lower respiratory tract illness in infants and young children 1. Recurrent wheezing and asthma-like symptoms after more severe bronchiolitis have been reported 2, although it is uncertain whether RSV bronchiolitis causes damage to developing lungs or severe symptoms are an indication of inherently diminished lung function and increased bronchial reactivity, or both 3,4.
Recurrent episodes of lower airway obstruction may persist for months and sometimes years after the acute RSV infection has resolved 5,6. Previous research demonstrated that treatment with the Cys LT inhibitor, montelukast, after acute RSV bronchiolitis may alleviate reactive airway disease symptoms 7. A subsequent 24-week study in 979 pediatric patients (3–24 months old) with severe RSV bronchiolitis, however, found no overall differences for montelukast vs. placebo in the percentage of symptom-free days, although an effect with montelukast on symptom-free days was observed among patients with more persistent symptoms 8.
Early identification of respiratory disorders can improve health outcomes in children 9. Therefore, the identification of at-risk patients may be clinically important. Clinical, immunologic, and genetic factors have previously been associated with asthma or reactive airway disease subsequent to viral infection early in life; clinical and demographic risk factors include severe RSV-induced bronchiolitis, recurrent postbronchiolitis wheeze, no breast feeding, and airflow limitation during the initial RSV-induced bronchiolitis episode10,11,12,13. Biomarkers have also been associated with asthma and include inflammatory mediators (such as urinary LTE4, serum eosinophil count, and exhaled nitric oxide) and sputum composition (such as sputum eosinophils), 14.
In this observational study, we sought to identify predictors of asthma development following severe RSV infection. The study cohort is a clinical trial population who were followed longitudinally after completion of the 24-week trial (N=343 children) until they reached 6 years of age8. We evaluated factors such as demographics and the nature of patients’ RSV bronchiolitis (e.g., severity of symptoms and length of hospital stay) in order to assess their predictive value for asthma and atopic disorders later in childhood (i.e., 6 years of age). Previous research has identified pro-inflammatory cytokines such as Interleukin (IL)-13 as being associated with asthma 15 and cytokines such as IL-8, IL-10, and TNF-α have been associated with severe RSV bronchiolitis 16,17,18. In this study, we collected samples to test for a large number of biomarkers, selected on the basis of potential markers identified from a review of the literature 19, which provided an opportunity to assess cytokines previously associated with asthma or RSV infection or to assess other cytokines and markers of eosinophilic inflammation that could have predictive value for asthma and atopic disorders later in childhood. Additional analyses compared the agreement of the diagnostic criteria used to identify asthma in this study.
Materials and Methods
This prospective, observational, longitudinal follow-up study (Sponsor Protocol MK-0476–374, Clinical Trials Registry # ) enrolled patients who completed a 24-week, placebo-controlled study of montelukast in the treatment of respiratory symptoms following RSV bronchiolitis 8. It was conducted at 38 sites in Africa (5), Asia (7), Australia (1), Europe (11), North America (6), and South America (8) between December 2007 and October 2011. The study was approved by local independent ethics committees and principles of Good Clinical Practice were followed. Before enrollment, parents/legal guardians of patients provided written informed consent.
Patients
Patient inclusion criteria for the 24-week study were previously published 8 and are briefly summarized here. Patients were 3- to 24-month-old children with their first or second episode of “severe” RSV bronchiolitis (i.e., hospitalized for ≥24 hours, or requiring urgent medical care with a respiratory severity score similar to those hospitalized for ≥24 hours) 8. Patients were to exhibit two of the following symptoms: respiratory rate ≥ 40 breaths/minute; cough; wheezing; audible rales; crackles; and/or rhonchi; and/or paradoxical chest movements (retractions). Prescribed medications were allowed as needed.
Study Design
Asthma at 6 years of age was defined by responses to a standardized questionnaire consistent with the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire 20. This study was initiated 14 months after completion of the 24-week study. The standardized questionnaire was interviewer-administered by the study coordinator to parents/legal guardians every 6 months following enrollment (during clinic visits or via telephone) until 6 years of age. Blood samples were collected for biomarker evaluation, both at baseline (of the present study) and at the final clinic visit at 6 years of age. Study medication was not provided for this observational study. The sample size of the study was driven primarily by the original 24-week study and was expected to be approximately 400 patients.
Asthma and Atopic Disease Outcomes
Questionnaires were administered every 6 months during the study. For the primary definition of asthma, “YES” was given as the response to the question ‘Has your child had wheezing or whistling in the chest in the past 6 AND 12 months?’ prior to the age of 6 years (i.e., a positive response was given to this question when questionnaires were administered at the time points of 6 months and 12 months prior to the child reaching 6 years of age).
Atopic disorders were defined as a ‘YES’ to the following questions: ‘In the past 6/12 months, has your child had a problem with sneezing or a runny or blocked nose when he/she did not have a cold or the flu?’ and/or a ‘YES’ to the questions ‘Has your child had an itchy rash which was coming and going at any time in the past 6/12 months?’ and ‘Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or eyes?’. Use of chronic asthma therapy was defined as controller therapy used to treat asthma for at least one day in the period of 12 months prior to reaching the age of 6 years; this included inhaled corticosteroids (ICS), long-acting beta2-agonists (LABAs), leukotriene modifiers, and methylxanthines.
A post-hoc analysis of the Physician’s Clinical Diagnosis of Asthma and Atopic Disorders (allergic rhinitis and/or atopic dermatitis) was also performed. Physician’s Clinical Diagnosis of Asthma and Atopic Disorders were assessed at each clinic visit during the study; physicians had access to patient history, symptoms, medication use, physical examination, and spirometry measurements for assessment of asthma, allergic rhinitis, and atopic dermatitis. For asthma, physicians completed a questionnaire that asked if, in their opinion based on international guidelines for the diagnosis of asthma, patients had asthma (i.e., definitely does not have, possibly has, probably has, or definitely has asthma). For allergic rhinitis and atopic dermatitis, physicians completed a questionnaire that asked if, in their opinion, patients had either of these conditions (i.e., does not have, has).
Blood and urine were collected at baseline (Visit 1) and final visit (Visit 10) at 6 years of age. Potential biomarkers (including RAST) were measured at the beginning of the present follow-up study, when patients were at varying ages (ranges from 2 to 5.5 years old) and at 6 years of age. Supplement 1 includes the list of analytes that were evaluated.
The peripheral blood eosinophil count and RAST were analyzed using routine clinical assays, while the plasma analytes were analyzed by a specialized biomarker testing laboratory (Rules-Based Medicine, Inc., Austin, TX) using research-use-only protein immunoassays. The peripheral blood eosinophil count was analyzed by the central laboratory for the study (Covance Central Laboratory Services, Indianapolis, IN) using the Siemens Advia 2120i automated hematology system and Perox reaction chamber. The RAST were analyzed by a referral laboratory (Viracor-IBT Laboratories, Lee’s Summit, MO) using the ImmunoCap by Phadia.
Comparison of Asthma and Atopic Disorders among Placebo Patients with an External Pediatric Cohort
The incidence of asthma, allergic rhinitis, and atopic dermatitis in this study who were on placebo in the original 24-week study was compared to an external pediatric cohort studied in the ISAAC Phase 3 survey by Asher et al21. This external cohort, whose parents were surveyed to assess the worldwide prevalence of asthma, allergic rhinoconjunctivitis, and eczema, consisted of 6–7 year old children from either new random samples of schools in defined geographic areas in Africa, the Asia-Pacific region, Eastern Mediterranean, the Indian subcontinent, Latin America, North America, Europe, and Oceania (i.e., Australia and New Zealand) or the same schools chosen at random in the ISAAC Phase One baseline survey.
Post-Hoc Analyses of Diagnostic Asthma Outcome Criteria
A post-hoc analysis was done to compare asthma diagnosis at 6 years of age by: (1) the primary definition of asthma (by parental identification) in this study based on the question assessing presence of wheeze or whistling in the chest in the past 6 AND 12 months; (2) study physician-based diagnosis (based on the Physician’s Clinical Asthma Diagnosis); and (3) diagnosis defined from current use of chronic controller asthma treatment. Comparisons were also made using a sensitivity definition of asthma (i.e., Has your child had wheezing or whistling in the chest in the past 6 OR 12 months).
A combined endpoint was evaluated that required a ‘YES’ to ≥ 1 of the following criteria: 1) Primary definition of asthma; 2) Physician’s Clinical Asthma Diagnosis; 3) Use of Chronic Asthma Therapy. Additionally, the percentage of patients who had a parent report of an asthma diagnosis by a physician was collected.
Statistical Methodology
All analyses were exploratory and focused on estimation. A forward stepwise logistic regression was applied to identify baseline characteristics that were predictors of asthma, atopic disorders, and use of chronic asthma therapy at 6 years of age. A significance level of 0.15 was required for entering an explanatory variable into (and staying in) the model. For each of the prognostic factors identified, an odds ratio relative to the outcomes of asthma, atopic disorders, and use of chronic asthma therapy, as well as their 95% confidence interval (CI), were presented by a univariate logistic regression model. A similar stepwise logistic regression model was applied for the post-hoc analysis of asthma outcome determined by the Physician’s Clinical Asthma Diagnosis. All analyses were adjusted for age.
A similar approach was used for identifying biomarkers, as collected at baseline of the present study, associated with the incidence of asthma and atopic disorder at 6 years of age. The stepwise forward regression analysis considered 49 biomarkers (listed in Supplement 1). Biomarkers with skewed distribution were normalized (by log transformation) before entering the logistic regression as a covariate. Some biomarkers were analyzed as binary parameters (e.g., RAST parameters) due to their distribution. A final logistic regression model included biomarkers selected by the forward stepwise regression approach (multivariate model). Adjusted odds ratios with 95% CI from the final logistic regression model for selected biomarkers relative to the incidence of asthma and atopic disorders were provided. Biomarkers significant at 0.05, after applying False Discovery Rate to adjust for multiplicity, were identified. Biomarker predictors were also identified for the Physician’s Clinical Asthma Diagnosis at 6 years of age (post-hoc analysis).
Interpretation of the selected prognostic parameters by the forward stepwise regression approach should be made with caution as confounding may play a role.
Odds ratios (95% CI) for the effect of montelukast vs. placebo during the 24-week study on asthma or atopic disorder development were estimated by a logistic regression model, including region, persistence of symptoms, interruption length between end of the 24-week study and start of the current study, and the significant prognostic variables as identified from the forward regression analysis in the evaluation of baseline characteristics as factors.
Finally, the difference in the percentage of patients with asthma and atopic disorders was estimated between the 24-week study placebo group and appropriate external pediatric cohorts. For the comparison with the external cohort, 95% confidence intervals for the difference in proportions were estimated by exact method derived from the binomial distribution.
Results
Patient Characteristics
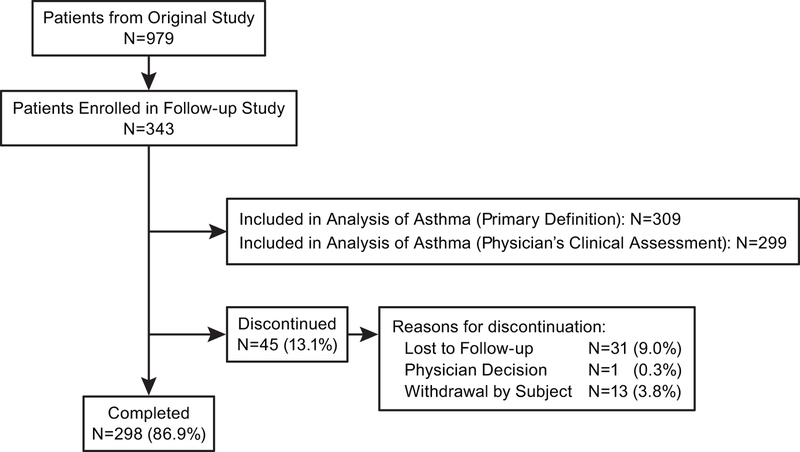
There were 343 patients participating in this study (Figure 1). Of these, 309 patients had questionnaire data and were included in the primary analysis; 298 patients completed the study. Baseline patient characteristics are presented in Table 1. The population participating in this trial was proportionally similar with regard to demographics to the population included in the original 24-week clinical trial (Supplement 2).
Figure 1.
Accounting of patients included in follow up study. Study sites with ≥3 children completing the 24-week clinical trial were invited to participate in the follow-up study.
Table 1 –
Baseline Patient Characteristics
| Placebo | Montelukast | Total | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Patients in population | 117 | 226 | 343 | |||
| Gender | ||||||
| Male | 67 | (57.3) | 133 | (58.8) | 200 | (58.3) |
| Female | 50 | (42.7) | 93 | (41.2) | 143 | (41.7) |
| Age at Entry into Original 24-week Study (in months) | ||||||
| 3 to ≤ 6 | 69 | (59.0) | 113 | (50.0) | 182 | (53.1) |
| 7 to ≤ 12 | 29 | (24.8) | 80 | (35.4) | 109 | (31.8) |
| 13 to ≤24 | 19 | (16.2) | 33 | (14.6) | 52 | (15.2) |
| Mean | 7.5 | 8.0 | 7.8 | |||
| SD | 4.9 | 4.6 | 4.7 | |||
| Median | 6 | 6.5 | 6 | |||
| Range | 3 to 23 | 3 to 23 | 3 to 23 | |||
| Either Parent Smoked in Presence of the Child* | ||||||
| No | 101 | (86.3) | 194 | (86.2) | 295 | (86.3) |
| Yes | 16 | (13.7) | 31 | (13.8) | 47 | (13.7) |
| Age (in months) | ||||||
| 24 to < 36 | 24 | (20.5) | 41 | (18.1) | 65 | (19.0) |
| 36 to < 48 | 47 | (40.2) | 81 | (35.8) | 128 | (37.3) |
| 48 to < 60 | 34 | (29.1) | 79 | (35.0) | 113 | (32.9) |
| 60 to < 72 | 12 | (10.3) | 25 | (11.1) | 37 | (10.8) |
| Mean | 45.4 | 45.9 | 45.7 | |||
| SD | 9.7 | 10.1 | 10.0 | |||
| Median | 45.0 | 45.0 | 45.0 | |||
| Range | 27 to 68 | 26 to 67 | 26 to 68 | |||
| Race | ||||||
| American Indian or Alaska Native | 0 | (0.0) | 1 | (0.4) | 1 | (0.3) |
| Asian | 17 | (14.5) | 38 | (16.8) | 55 | (16.0) |
| Black or African American | 2 | (1.7) | 6 | (2.7) | 8 | (2.3) |
| Multi-racial | 15 | (12.8) | 14 | (6.2) | 29 | (8.5) |
| White | 83 | (70.9) | 167 | (73.9) | 250 | (72.9) |
| Ethnicity | ||||||
| Hispanic or Latino | 55 | (47.0) | 100 | (44.2) | 155 | (45.2) |
| Not Hispanic or Latino | 62 | (53.0) | 126 | (55.8) | 188 | (54.8) |
Treatment groups presented refer to study drug taken in original 24-week study; Montelukast 4 mg and Montelukast 8 mg treatment groups are combined into one treatment group (Montelukast). There was no study drug in this study.
At entry into original 24-week study
Prevalence of Asthma, Atopic Disorders, and Use of Chronic Asthma Therapy
The overall prevalence of asthma (primary definition) in children at 6 years of age was 6.1% (n=19). The prevalence of atopic disorders and use of chronic asthma therapy in children at 6 years of age was 36.2% and 14.5%, respectively. The Physician’s Clinical Asthma Diagnosis identified 22.4% of patients as having asthma (response of “probably has asthma” [6.7%] or “definitely has asthma” [15.7%]) at 6 years of age.
Factors Identified as Predictors of Asthma (Primary Definition), Atopic Disorders, and Use of Chronic Asthma Therapy
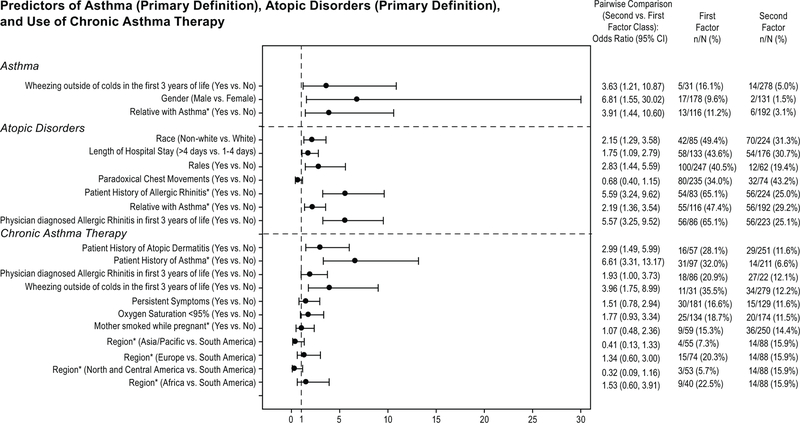
Predictors for asthma at 6 years of age (p <0.05) were gender (male vs. female), having a relative (i.e., parents, grandparents, or siblings) with asthma (yes). Predictors for current atopic disorders (p <0.05) were patient history of allergic rhinitis (yes) and relative with asthma (yes). Predictors for use of chronic asthma therapy (p <0.05) included patient history of asthma (yes) and geographic region (Europe and Africa vs. South America). Odds ratios (95% CI) for demographic predictors are shown in Figure 2.
Figure 2.
Odds ratios (95% CI) for pairwise comparisons of demographic factors predictive of asthma (primary definition), atopic disorders (primary definition), and use of chronic asthma therapy. An odds ratio between 0 and 1 indicates a lower incidence in the first class listed compared to the second class listed. An odds ratio of 1 indicates no difference and an odds ratio between 1 and infinity indicates a higher incidence of the first class mentioned compared to the second one.
For binary biomarkers, dog dander allergen RAST (out of the ten allergens tested, see Supplement) was selected by the stepwise regression as a predictive factor for asthma; a higher prevalence of asthma was observed for positive RAST (> 0.1 kIU/L (6/37 [16.2%]) versus negative RAST (≤ 0.1 kIU /L (11/246 [4.5%]), with an adjusted odds ratio of 4.13 (1.43, 11.97). Biomarker predictors of atopic disorders (p <0.05) included interleukin (IL)-18, IL-16, and IL-5. For IL-16 and IL-5, patients with asthma had a somewhat higher average value compared to patients without asthma (adjusted odds ratios were 1.67 [1.27, 2.19] and 1.62 [1.22, 2.14] for IL-16 and IL-5, respectively). For IL-18, patients with asthma had a somewhat lower average value compared to patients without asthma (adjusted odds ratio was 0.62 [0.46, 0.83]).
Factors Identified as Predictors of Asthma (per Physician’s Clinical Assessment)
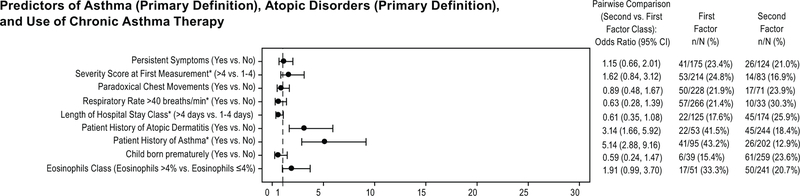
Parameters identified as demographic predictors (p <0.05) for asthma (Physician’s Clinical Asthma Diagnosis) included RSV bronchiolitis severity score at first measurement (symptoms at baseline hospitalization assessment during the 24-week study), respiratory rate > 40 breaths/min (during the 24-week study), length of hospitalization (during the 24-week study), and previous history of asthma (i.e., patient was diagnosed after the 24-week study, but before inclusion in the present follow-up study). Odds ratios (95% CI) for demographic factors found to be predictors of asthma according to the Physician’s Clinical Asthma Diagnosis are in Figure 3.
Figure 3.
Odds ratios (95% CI) for pairwise comparisons of demographic factors predictive of Asthma (Physician’s Clinical Asthma Diagnosis). An odds ratio between 0 and 1 indicates a lower incidence in the first class listed compared to the second class listed. An odds ratio of 1 indicates no difference and an odds ratio between 1 and infinity indicates a higher incidence of the first class mentioned compared to the second one.
Biomarker predictors of Physician’s Clinical Asthma Diagnosis (p <0.05) included the continuous parameters IL-3, IL-13, and pregnancy-associated plasma protein (PAPP-A), and the binary biomarker granulocyte macrophage colony-stimulating factor (GM-CSF). For IL-3 and PAPP-A, patients with asthma had a somewhat higher average value compared to patients without asthma (adjusted odds ratios were 3.22 [1.71, 6.05] and 1.84 [1.22, 2.78] for IL-3 and PAPP-A, respectively). For IL-13, patients with asthma had a somewhat lower average value compared to patients without asthma (adjusted odds ratio was 0.26 [0.10, 0.65]). For the binary biomarker, a lower GM-CSF showed a higher likelihood of asthma with odds ratio of 0.45 (0.22, 0.92) for the comparison of the binary classes ≤5.7 pg/mL (prevalence of asthma of 46/182 [25.3%]) and >5.7 pg/mL (prevalence of asthma of 16/90 [17.8%]).
Evaluation of the Effect of Montelukast Treatment
The prevalence of asthma during this follow-up period was 5.7% for patients who had received placebo and 6.4% for patients who had received montelukast during the 24-week study (odds ratio of 0.90 [0.31, 2.57]). For the subgroup of patients with persistent symptoms, the odds ratio for the treatment effect was 0.21 (0.04, 1.03). For the subgroup of patients without persistent symptoms, the odds ratio of the treatment effect was 4.14 (0.49, 35.23). Due to the low observed incidence of asthma using the primary definition, the reported results should be interpreted with caution.
In the post-hoc analysis of patients with asthma using a definition based on the Physician’s Clinical Asthma Diagnosis, the proportion of patients with asthma was 24.3% for patients who had received placebo and 21.4% for patients who had received montelukast in the 24-week study (odds ratio of 1.04 [0.54, 2.01]). For the subgroup of patients with persistent symptoms in this longitudinal follow-up study, the odds ratio for the treatment effect was 0.70 (0.30, 1.63). For the subgroup of patients without persistent symptoms, the odds ratio of the treatment effect was 3.31 (0.89, 12.27). No relationship between asthma at age 6 years and treatment from the original study was observed.
At 6 years of age, the proportion of patients with atopic disorders (allergic rhinitis and/or atopic dermatitis) was 39.6% for patients who had received placebo and 34.5% for patients who had received montelukast in the 24-week study (odds ratio of 0.69 [0.39, 1.21]). For the subgroup of patients with persistent symptoms, the odds ratio for the treatment effect was 0.44 (0.20, 0.94). For the subgroup of patients without persistent symptoms, the odds ratio of the treatment effect was 1.30 (0.53, 3.19). No relationship between atopic disorders at 6 years of age and treatment from the original study was observed.
Comparison of Asthma and Atopic Disorders among Placebo Patients with an External Pediatric Cohort
In the external pediatric cohort (ISAAC Phase 3) by Asher et al21 the prevalence of asthma (9.8%), allergic rhinitis (6.3%), and atopic dermatitis (5.2%) were reported for children 6 to 7 years of age. In comparison, the subgroup of patients in this study who were in the placebo group of the 24-week study had a lower prevalence of asthma (5.7%) and a higher prevalence of allergic rhinitis (34.9%) and atopic dermatitis (14.1%) at 6 years of age.
Evaluation of Asthma Diagnostic Criteria
Our primary definition of asthma (parent-reported wheeze at 6 AND 12 months prior to 6 years of age) led to the lowest proportion of patients as being diagnosed with asthma at 6 years of age (6.1%) compared with other diagnostic criteria that were considered (Table 2). The sensitivity definition (i.e., parent-reported wheeze at 6 OR 12 months) resulted in a prevalence of 23.2%, which was comparable to the Physician’s Clinical Asthma Diagnosis (22.4%). Use of Chronic Asthma Therapy and Parent Report of Physician-Diagnosed Asthma resulted in asthma prevalence of 14.5% and 15.5%, respectively. The Combined Endpoint led to a 30% asthma prevalence.
Table 2 –
Operational definition of six asthma outcomes and study population prevalence of each.
| Outcome | Operational Definition | n/N | % |
|---|---|---|---|
| 1. Primary asthma trial definition of wheezing in the last 6 and 12 months | ‘YES’to the questionnaire item “Has your child had wheezing or whistling in the chest in the past 6/12 months?” (YES at both timepoints prior to 6 years of age) |
19/309 | 6.1 |
| 2. Wheezing in last 12 months (sensitivity definition) | ‘YES’ to the questionnaire item “Has your child had wheezing or whistling in the chest in the past 6/12 months?” (YES to at least one of last 2 timepoints prior to 6 years of age) – sensitivity analysis |
72/310 | 23.2 |
| 3. Physician’s Clinical Asthma Diagnosis | Response of “patient probably or definitely had asthma” on Physician’s Clinical Assessment | 67/299 | 22.4 |
| 4. Use of Chronic Asthma Therapy | Any use of controller therapy for asthma (e.g., inhaled corticosteroids, leukotriene modifiers, long-acting beta-agonists, theophylline, cromones) | 45/310 | 14.5 |
| 5. Parent report of physician-diagnosed asthma | Confirmatory response to the questionnaire item “Has a doctor ever told you your child had asthma in the past 6/12 months?” (YES on at least one of last 2 timepoints prior to 6 years of age) |
48/310 | 15.5 |
| 6. Combined asthma endpoint | YES if response was “yes” on items 1, 3, and/or 4 | 93/310 | 30.0 |
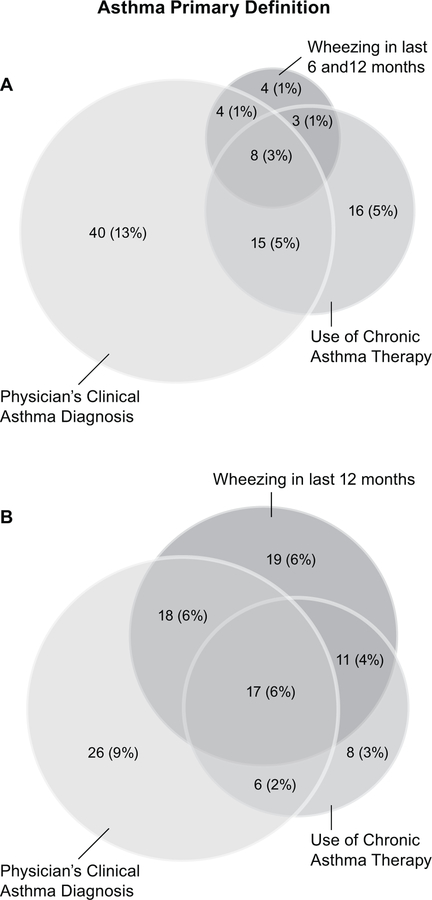
There was little agreement among the different definitions of asthma (Figure 4). Among patients who were identified as having asthma using the primary definition (N=19), only 8 of these patients were also identified as having asthma according to the Physician’s Clinical Asthma Diagnosis and Use of Chronic Asthma Therapy. Although the sensitivity definition increased the number of patients diagnosed with asthma from 19 (6.1%) to 72 (23.2%), the correlation with other diagnostic methods was not substantially improved (Figure 4). The Physician’s Clinical Asthma Diagnosis and Use of Chronic Asthma Therapy also had poor agreement with the other definitions evaluated.
Figure 4.
Venn diagrams demonstrating weak correlation of asthma diagnoses according to different definitions of asthma. (A) Number (%) of patients with asthma according to questionnaire (primary definition), Physician’s clinical asthma diagnosis, or Use of chronic asthma therapy; (B) Number (%) of patients with asthma according to questionnaire (wheezing in last 12 months), Physician’s clinical asthma diagnosis, or Use of chronic asthma therapy. Circles are drawn to approximate scale.
Discussion
Our study was undertaken in order to take advantage of an opportunity to longitudinally follow up, until 6 years of age, a clinical trial population who had RSV bronchiolitis in the first 2 years of life and were treated with an intervention that had been hypothesized to decrease RSV-mediated wheezing. In particular, we were interested in predictors of asthma development (primarily defined as ‘wheezing at 6 AND 12 months prior to 6 years of age), atopic disorders, and the use of chronic asthma therapy.
Predictive factors for asthma according to our primary definition included having a relative with asthma, RAST positivity for dog dander, and male gender. However, the overall incidence of asthma according to the primary definition was very low. The Physician’s Clinical Asthma Diagnosis was associated with a prevalence more consistent with an external pediatric cohort 21 and previous research in patients who had RSV bronchiolitis early in life that shows a prevalence of approximately 20% 22,23. Predictive factors for diagnosis according to this second definition included those related to more severe manifestations observable by the physician during the initial bout with RSV bronchiolitis (i.e., high RSV bronchiolitis severity score at the time of hospitalization, high respiratory rate, length of hospital stay), and a diagnosis of asthma that was made after the original study ended but before this follow-up study was initiated. The following biomarkers were also predictive factors associated with the Physician’s Clinical Asthma Diagnosis: IL-3, IL-13, PAPP-A, and GS-CSF, which have previously been shown to be related to lung function, development of asthma, and asthma severity 24,15,25,26,27,28.
Predictive factors for Chronic Asthma Therapy Use included asthma diagnosis made between the 24-week study and this follow-up study, and geographic location (Europe and Africa). Geographic location as a predictor may reflect local prescribing behavior, but diagnosis of asthma after the 24-week study likely reflects continuing respiratory symptoms following RSV bronchiolitis. Further, we examined atopic disorders at 6 years of age, predictors for which included diagnosis of allergic rhinitis between the 24-week study and this follow-up study, having a relative with asthma, IL-18, IL-16, and IL-5, which are biomarkers associated with asthma and allergic rhinitis 29,30.
We sought to identify predictors that may contribute to a better understanding of the mechanisms of asthma development, and the identification of high-risk infants, either for targeted follow-up, or enrollment in primary prevention trials. Demographic and clinical observations in this study point to both familial history and the severity of bronchiolitis as factors that may contribute to asthma development. With regard to the secondary definition according to a physician’s diagnosis of asthma, clinical observations of greater RSV bronchiolitis severity do, indeed, indicate that this is a factor that may lead to a greater likelihood for asthma later in childhood. However, whether RSV is a causal factor for asthma or it is an exaggerated response to RSV that reflects an individual’s predisposition for asthma, or both, remains controversial 31. Previous research asserts that inherent bronchial hyper-responsiveness precedes severe bronchiolitis from respiratory infections 31,32. Further, corticosteroid use in RSV bronchiolitis has demonstrated a lack of long-term benefit from anti-inflammatory treatment 33. Similarly, our results showed no benefit from montelukast treatment following RSV bronchiolitis on the outcome of asthma or atopic disorders. Thus, these results suggest that reducing inflammatory processes, at least with leukotriene modifying drugs following RSV bronchiolitis, is unlikely to have an impact on the development of asthma and atopic diseases later in childhood.
Our analysis of biomarkers showed that IL-3, IL-13, PAPP-A, and GS-CSF were predictive of a Physician’s Clinical Asthma Diagnosis. Previous studies have sought to determine biomarkers that can predict severity of RSV bronchiolitis 16,18. These studies found that high levels of IL-6, IL-1β, and IL-8 detected in nasopharyngeal aspirates (NPA) of patients infected with RSV was positively correlated with high RSV disease severity. In this study, IL-6 and IL-8 were evaluated, but were not found to be predictors for the primary or secondary definitions of asthma. It should be noted, however, that one of the factors that were predictive of the Physician’s Clinical Asthma Diagnosis in this study was elevated levels of IL-3. A recent small study that analyzed cytokine and chemokine profiles of the upper and lower airways during RSV bronchiolitis in infants (0–9 months of age) found that elevated IL-3 levels were positively correlated with a greater risk for increased recurrent wheezing subsequent to RSV infection34.
Our data should be interpreted as hypothesis generating and the results of this study should be viewed with caution due to the large number of comparisons that were made. Although a multiplicity adjustment was used for the biomarker analyses, there is a potential for a false-positive finding with regard to the biomarker evaluation due to the large number of biomarkers that were evaluated. Additionally, we used False Discovery Rate (FDR) controlling procedures for statistical correction of multiple comparisons. This method was selected due to the large number of biomarkers analyzed and the observational character of this study; however, it should be noted that the FDR provides a weaker control of the type I error compared to the conventional more conservative familywise error rate (FWER) procedures. There were other limitations associated with the observational character of the study. The nonrandom nature of the initial cohort may have introduced selection bias. Additionally, subjects in this study were not excluded for long-term treatment with montelukast and/or ICS therapy, and, although some of these medications were used by only a small proportion of the children in this study, they could have had an impact on the measured cytokines (4.7% of patients used montelukast; 14.3% of patients used inhaled budesonide, 3.8% of patients used inhaled fluticasone propionate, 3.2% of patients used inhaled fluticasone propionate + salmeterol xinafoate, and 14.6% of patients used systemic corticosteroids).
An important observation made in this study that was an additional limitation was that our primary definition of asthma resulted in an unexpectedly low incidence of asthma. The definition of wheezing at both time points was highly specific but lacked sensitivity and may have discounted those whose asthma symptoms are seasonal. Also, the predictive factors detected in this study did not overlap between the primary definition and the Physician’s Clinical Asthma Diagnosis due to the different populations who were identified as having asthma by the different criteria. We observed very little agreement among the different diagnostic criteria for asthma considered in this study. This was the case even when we considered a sensitivity definition of asthma where wheeze was to be observed at either 6 OR 12 months prior to the patient reaching 6 years of age. Our primary definition was based on parental identification of wheeze, which has previously been characterized as being problematic due to the likelihood that healthcare professionals and parents may misidentify respiratory sounds in children 35. Therefore, this study likely would have benefited from diagnostic criteria that were not based on the identification of wheeze.
With regard to the evaluation of agreement among diagnostic criteria, it should be noted that we did not have a gold standard definition by which to compare the sensitivity and specificity of these various outcome definitions, which had been done in previous evaluations that sought to validate epidemiological questionnaires 36,37,38,39. However, similar results were reported in a large study of the Danish national birth cohort where poor agreement was observed among parental reports of doctor diagnoses, diagnoses from a hospitalization registry, and use of asthma medication recorded by a prescription registry 40. These data and the inconsistencies observed in the predictors identified by the two different criteria used in this study suggest the need for improved standardized diagnostic methods to yield more generalizable outcomes for researchers.
Another limitation is that this was a follow-up to a previous clinical trial, and, although demographics were similar in this study to those from the original study, biases may have been introduced in how patients were recruited for this study. An aspect of the follow-up study that should be considered is that children began the follow-up study at different ages and the period of time that children were not participating in either trial varied.
In summary, our study found factors related to both inherent risk of asthma as well as high RSV bronchiolitis severity to be predictive of asthma (according to parental identification of wheeze and physician diagnosis) and atopic disorders at the age of 6 years in children who had severe RSV bronchiolitis in early childhood. Factors predictive of asthma later in life that were associated with severe RSV bronchiolitis included high RSV bronchiolitis severity score at the time of hospitalization, high respiratory rate, and length of hospital stay, as well as elevated IL-3 levels. Factors associated with inherent risk that were predictive of asthma in this study included having a relative with asthma and biomarkers associated with asthma development such as IL-13, PAPP-A, and GS-CSF, as well as IL-18, IL-16, and IL-5 in patients with allergic rhinitis. More research is needed to better answer the question of whether these patients have an inherent predisposition for respiratory distress leading to both asthma and high RSV bronchiolitis severity (as well as the predictors identified in this study related to high RSV bronchiolitis severity) or if the RSV infection causes physical changes to children who would otherwise not have respiratory distress or asthma later in life. While there is likely a shared genetic predisposition to both asthma and the severity of their bronchiolitis, this study cannot determine whether there is a causal relationship between RSV and asthma. We also had several limitations in our study that led us to observe that our primary asthma diagnostic criterion, which was based on parental reporting of wheeze, lacked sensitivity and had poor agreement with other diagnostic criteria that were considered. These data indicate a need for further research to improve the ability of researchers to appropriately generalize the results of their clinical studies to a real-world setting.
Supplementary Material
Acknowledgements
The authors thank Jennifer Pawlowski, MS (Merck & Co., Inc., Kenilworth, NJ) for editorial and administrative assistance with the manuscript.
Funding: This study was funded by Merck & Co., Inc., Kenilworth, NJ.
Footnotes
(Clinical Trials Registry # )
Conflicts of Interests
SL, HG, LN, AM, HP, BK, and TR are or were employees of Merck & Co., Inc., Kenilworth, NJ.
TH and ME report no conflicts of interest.
Reference List
- 1.Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46: 324–347. [DOI] [PubMed] [Google Scholar]
- 2.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999; 354: 541–545. [DOI] [PubMed] [Google Scholar]
- 3.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006; 368: 312–322. [DOI] [PubMed] [Google Scholar]
- 4.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008; 178: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson M, Bennet R, Nilsson A. Wheezing following lower respiratory tract infections with respiratory syncytial virus and influenza A in infancy. Pediatr Allergy Immunol 2000; 11: 193–197. [DOI] [PubMed] [Google Scholar]
- 6.Schauer U, Hoffjan S, Bittscheidt J, Kochling A, Hemmis S, Bongartz S, Stephan V. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J 2002; 20: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard H A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med 2003; 167: 379–383. [DOI] [PubMed] [Google Scholar]
- 8.Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice MP, Marchal JL, Dass SB, Reiss TF, Knorr BA. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med 2008; 178: 854–860. [DOI] [PubMed] [Google Scholar]
- 9.Speight AN, Lee DA, Hey EN. Underdiagnosis and undertreatment of asthma in childhood. Br Med J (Clin Res Ed) 1983; 286: 1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bont L, Van Aalderen WM, Versteegh J, Brus F, Draaisma JT, Pekelharing-Berghuis M, Diemen-Steenvoorde RA, Kimpen JL. Airflow limitation during respiratory syncytial virus lower respiratory tract infection predicts recurrent wheezing. Pediatr Infect Dis J 2001; 20: 277–282. [DOI] [PubMed] [Google Scholar]
- 11.Ermers MJ, Hoebee B, Hodemaekers HM, Kimman TG, Kimpen JL, Bont L. IL-13 genetic polymorphism identifies children with late wheezing after respiratory syncytial virus infection. J Allergy Clin Immunol 2007; 119: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 12.Everard ML. The relationship between respiratory syncytial virus infections and the development of wheezing and asthma in children. Curr Opin Allergy Clin Immunol 2006; 6: 56–61. [DOI] [PubMed] [Google Scholar]
- 13.Kotaniemi-Syrjanen A, Laatikainen A, Waris M, Reijonen TM, Vainionpaa R, Korppi M. Respiratory syncytial virus infection in children hospitalized for wheezing: virus-specific studies from infancy to preschool years. Acta Paediatr 2005; 94: 159–165. [DOI] [PubMed] [Google Scholar]
- 14.Deykin A Biomarker-driven care in asthma: are we there? J Allergy Clin Immunol 2006; 118: 565–568. [DOI] [PubMed] [Google Scholar]
- 15.Hacha J, Tomlinson K, Maertens L, Paulissen G, Rocks N, Foidart JM, Noel A, Palframan R, Gueders M, Cataldo DD. Nebulized anti-IL-13 monoclonal antibody Fab’ fragment reduces allergen-induced asthma. Am J Respir Cell Mol Biol 2012; 47: 709–717. [DOI] [PubMed] [Google Scholar]
- 16.Diaz PV, Valdivia G, Gaggero AA, Bono MR, Zepeda G, Rivas M, Uasapud P, Pinto RA, Boza ML, Guerrero J. Pro-Inflammatory Cytokines in Nasopharyngeal Aspirate From Hospitalized Children With Respiratory Syncytial Virus Infection With or Without Rhinovirus Bronchiolitis, and Use of the Cytokines as Predictors of Illness Severity. Medicine (Baltimore) 2015; 94: e1512- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornsleth A, Loland L, Larsen LB. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol 2001; 21: 163–170. [DOI] [PubMed] [Google Scholar]
- 18.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sanchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J 1999; 18: 115–122. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000; 162: 1403–1406. [DOI] [PubMed] [Google Scholar]
- 20.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. [DOI] [PubMed] [Google Scholar]
- 21.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–743. [DOI] [PubMed] [Google Scholar]
- 22.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005; 16: 386–392. [DOI] [PubMed] [Google Scholar]
- 23.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161: 1501–1507. [DOI] [PubMed] [Google Scholar]
- 24.Coskun A, Balbay O, Duran S, Annakkaya AN, Bulut I, Yavuz O, Kurt E. Pregnancy-associated plasma protein-A and asthma. Adv Ther 2007; 24: 362–367. [DOI] [PubMed] [Google Scholar]
- 25.Landgraf RG, Jancar S. Endothelin A receptor antagonist modulates lymphocyte and eosinophil infiltration, hyperreactivity and mucus in murine asthma. Int Immunopharmacol 2008; %20;8: 1748–1753. [DOI] [PubMed] [Google Scholar]
- 26.Polikepahad S, Moore RM, Venugopal CS. Endothelins and airways--a short review. Res Commun Mol Pathol Pharmacol 2006; 119: 3–51. [PubMed] [Google Scholar]
- 27.Saha S, Doe C, Mistry V, Siddiqui S, Parker D, Sleeman M, Cohen ES, Brightling CE. Granulocyte-macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax 2009; 64: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolley KL, Adelroth E, Woolley MJ, Ramis I, Abrams JS, Jordana M, O’Byrne PM. Interleukin-3 in bronchial biopsies from nonasthmatics and patients with mild and allergen-induced asthma. Am J Respir Crit Care Med 1996; 153: 350–355. [DOI] [PubMed] [Google Scholar]
- 29.Baumann R, Rabaszowski M, Stenin I, Tilgner L, Gaertner-Akerboom M, Scheckenbach K, Wiltfang J, Chaker A, Schipper J, Wagenmann M. Nasal levels of soluble IL-33R ST2 and IL-16 in allergic rhinitis: inverse correlation trends with disease severity. Clin Exp Allergy 2013; 43: 1134–1143. [DOI] [PubMed] [Google Scholar]
- 30.Kawayama T, Okamoto M, Imaoka H, Kato S, Young HA, Hoshino T. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res 2012; 32: 443–449. [DOI] [PubMed] [Google Scholar]
- 31.Chawes BL, Poorisrisak P, Johnston SL, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol 2012; 130: 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisgaard H, Jensen SM, Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med 2012; 185: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 33.Zomer-Kooijker K, van der Ent CK, Ermers MJ, Rovers MM, Bont LJ. Lack of Long-term Effects of High-dose Inhaled Beclomethasone for Respiratory Syncytial Virus Bronchiolitis: A Randomized Placebo-controlled Trial. Pediatr Infect Dis J 2014; 33: 19–23. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand P, Lay MK, Piedimonte G, Brockmann PE, Palavecino CE, Hernandez J, Leon MA, Kalergis AM, Bueno SM. Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing. Cytokine 2015; 76: 417–423. [DOI] [PubMed] [Google Scholar]
- 35.Skytt N, Bonnelykke K, Bisgaard H. “To wheeze or not to wheeze”: That is not the question. J Allergy Clin Immunol 2012; 130: 403–407. [DOI] [PubMed] [Google Scholar]
- 36.Remes ST, Pekkanen J, Remes K, Salonen RO, Korppi M. In search of childhood asthma: questionnaire, tests of bronchial hyperresponsiveness, and clinical evaluation. Thorax 2002; 57: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponsonby AL, Couper D, Dwyer T, Carmichael A, Wood-Baker R. Exercise-induced bronchial hyperresponsiveness and parental ISAAC questionnaire responses. Eur Respir J 1996; 9: 1356–1362. [DOI] [PubMed] [Google Scholar]
- 38.Shaw R, Woodman K, Ayson M, Dibdin S, Winkelmann R, Crane J, Beasley R, Pearce N. Measuring the prevalence of bronchial hyper-responsiveness in children. Int J Epidemiol 1995; 24: 597–602. [DOI] [PubMed] [Google Scholar]
- 39.Kim MH, Kwon JW, Kim HB, Song Y, Yu J, Kim WK, Kim BJ, Lee SY, Kim KW, Ji HM et al. Parent-reported ISAAC written questionnaire may underestimate the prevalence of asthma in children aged 10–12 years. Pediatr Pulmonol 2012; 47: 36–43. [DOI] [PubMed] [Google Scholar]
- 40.Hansen S, Strom M, Maslova E, Mortensen EL, Granstrom C, Olsen SF. A comparison of three methods to measure asthma in epidemiologic studies: results from the Danish National Birth Cohort. PLoS One 2012; 7: e36328- [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.