Abstract
Purpose
Conditional survival (CS) estimates provide important prognostic information for clinicians and patients who have survived a period after diagnosis. In this study we performed a contemporary evaluation of conditional survival among colon cancer patients and created a browser-based tool for real-time determination of conditional survival expectancies.
Patients and Methods
Patients with colon adenocarcinoma diagnosed between 1988 and 2000 were identified from the Surveillance Epidemiology End Results (SEER) registry. Conditional survival estimates were calculated by using the multiplicative law of probability after adjustment for age; sex; ethnicity; grade; and American Joint Commission on Cancer, sixth edition stage. A browser-based calculator was constructed.
Results
A total of 83,419 patients were analyzed. As the time alive after initial treatment increased from 0 to 5 years, significant improvements in CS were observed for patients in all stages except stage I, which was associated with good CS even at diagnosis and which reflected the high likelihood of cure. Notably, adjusted 5-year CS rates improved from 42% to 80% for stage IIIC cancers and from 5% to 48% for stage IV cancers during the first 5 years. Differences in cancer-related CS at diagnosis were identified on the basis of age, ethnicity, and grade, but these differences decreased over time. A browser-based CS calculator was implemented by using the multivariate survival model (concordance index, 0.81).
Conclusion
For patients with colon cancer who survive over time, 5-year, cancer-specific CS improved dramatically, and the greatest improvements were among patients with poorer initial prognoses. These prognostic data are critical to inform patients for non–treatment-related life decisions and to inform treating physicians for planning of follow-up and surveillance strategies.
INTRODUCTION
Colon cancer affects approximately 107,000 new patients each year, and colorectal cancer is the second leading cause of cancer death.1 Survival probability among patients with colon cancer is dependent on the stage of disease at the time of diagnosis. Overall 5-year survival rates usually are assessed at the time of diagnosis and range from 95% for stage I cancers to 11% for stage IV cancers. However, it is not as widely appreciated that, for patients who survive for a duration after the diagnosis, survival probabilities change over time. This information is important to patients who must make important life decisions and in whom uncertainty about the future remains an important factor affecting subsequent quality of life. In turn, this information will benefit health providers as they counsel their patients and tailor subsequent surveillance and management strategies.2 Conditional survival is defined as the survival probability that is calculated after a given length of survival and includes only individuals who have survived to a predefined time of interest.
Calculation of conditional survival requires long-term follow-up, as the probabilities are based on expected survival after a defined period of time. However, conditional survival may be a more accurate measure of survival probability among surviving cancer patients, especially when the initial prognosis is poor, such as with advanced stage colorectal cancer.
Large, population-based tumor registries are ideally suited for such an analysis; thus, several authors have reported studies on conditional survival for various disease sites, including breast, CNS, lung, and other gastrointestinal cancers.3–7 However, prior reports in colon cancer have utilized data from an older cohort of patients from an era before the recommendation of adjuvant chemotherapy for stage III cancers and before the development of modern, systemically active agents for stage IV cancers.6 The analyses have performed univariate comparisons of risk categories without adjustments for covariate effects. Additionally, prior studies have reported conditional survival estimates that were based on American Joint Commission on Cancer (AJCC), third edition, staging systems; these have limited applicability today, when the AJCC, sixth edition, cancer staging system is the standard.
The purpose of this study was to perform a contemporary evaluation of the risk-adjusted conditional survival of patients with colon cancer by using the population-based, SEER (Surveillance, Epidemiology, and End Results) registry data set.
PATIENTS AND METHODS
Data Source and Occurrence Identification
Data from the SEER17 program of the National Cancer Institute (released in 2008) were utilized.8 SEER collects cancer incidence and survival data from 18 regional, population-based registries that cover approximately 26% of the US population. During the study inclusion period (through 2000), SEER captured incident occurrences in approximately 14% of the US population. The SEER registry routinely collects data on patient demographics, primary tumor site, tumor morphology, disease stage at diagnosis (per the AJCC since 1988), first course of treatment, and patient follow-up for vital status. SEER does not collect data on chemotherapy; therefore, these data were not evaluated in this study.
Registry patients eligible for this cohort included those with adenocarcinoma of the colon (ICD-O-3 codes 8000, 8010, 8020-1, 8040, 8141, 8143-4, 8147, 8121-1, 8220-1, 8260-3, 8440, 8480-1, 8490, 8560, and 8570-4) who were diagnosed from January 1988 through December 2000. The study dates were chosen because 1988 was the first year SEER coded data elements necessary for restaging by using the AJCC, sixth edition, and 2000 was the last year of diagnosis that had at least 5 years of actual follow-up. On the basis of these data elements, the AJCC, sixth edition, stage assignment was determined for each occurrence. The landmark trials that established fluorouracil-based adjuvant chemotherapy as the treatment standard for patients with node-positive colon cancer were reported during the early part of the study period.10,11
Exclusion criteria included age younger than 18 years or older than 90 years, in situ disease, and lack of histology or overall survival information. Occurrences also were excluded if the cancer-reporting source was a nursing home, hospice, autopsy, or death certificate; if the incident diagnosis of colon cancer was not the first and only occurrence of malignant disease; if tumor grade was unknown; if survival time was less than 1 month; or if incomplete data regarding tumor and nodal stage precluded AJCC, sixth edition, stage assignment.
Statistical Analysis
Survival outcomes for all patients in the study cohort were determined by using SEER data through December 2005. The SEER registry also codes cause of death; therefore, cancer-specific survival was evaluated. Occurrences were censored if death was a result of something other than colorectal cancer or if the patient was alive at follow-up.
Cumulative diseases-specific survival (DSS) probabilities were estimated by employing the Kaplan-Meier method. Relative survival also was determined as an alternative approach to DSS. The multiplicative law of probability was used to calculate the conditional survival estimates among patients with a minimum of 5 years of actual follow-up, as previously described.7 Conditional survival represents the probability that a patient with cancer will survive an additional number of years (x), given that the patient has already survived a given number of years (y). For example, to compute the x-year conditional survival for patients who have survived y years, the (x + y) –year disease-specific survival is divided by the y-year disease-specific survival.
To evaluate the simultaneous effect of multiple variables on survival, multivariate Cox regression analysis was performed by using the Breslow method for ties. Covariates adjusted in the prediction model were based on clinically relevant factors, including age (< 50, 50 to 59, 60 to 69, 70 to 79, or ≥ 80 years); sex (male or female); ethnicity (white, black or other); tumor grade (well differentiated, moderately differentiated, poorly differentiated, or undifferentiated); and AJCC, sixth edition. We also tested the additional inclusion of the following variables on the improvement of model prediction: SEER region, year of diagnosis, marital status, and tumor location. The proportional hazards assumption was verified graphically on the basis of Schoenfeld residuals.12
Adjusted survival functions stratified by age, sex, ethnicity, tumor grade, and stage were reported at years 1 through 10, and this formed the basis for adjusted conditional survival. For example, conditional survival probabilities stratified by sex were calculated on the basis of the adjusted survival function for men and women and were controlled for the influence from other covariates in the final model. We compared the rate of change in conditional survival over time by using linear regression.
By using the final model, a browser-based application was developed to predict individualized disease-specific survival and conditional survival. Model performance was internally validated by both discrimination and calibration.13 Discrimination was evaluated by using the Harrell concordance index, which quantifies the ability of the model to predict who will experience the event first among a pair of randomly selected patients in the study cohort.14,15 A concordance index of 0.5 represents random chance, whereas a concordance index of 1.0 represents a perfect prediction model. We evaluated the calibration graphically and by using bootstrapping with 200 resamples and at least 10,000 patients per interval, in which patients were grouped by model-predicted, 5-year DSS and then were plotted against the actual 5-year DSS.
Statistical analyses were performed with Stata MP, version 10.0 (release 2007; Stata, College Station, TX). Rates of change for conditional survival were examined by using Prism, version 5.02 (GraphPad Software, La Jolla, CA). R (http://www.r-project.org/) with the Design module was used to generate bootstrap-corrected concordance indices and calibration plots. Because the study used preexisting data with no personal identifiers, this study was exempt from review by our institutional review board.
RESULTS
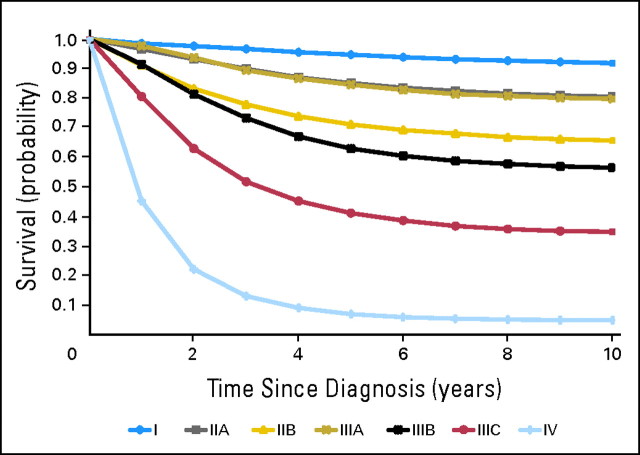
A total of 176,331 patients were diagnosed with colon cancer between 1988 and 2000. After stepwise case exclusion (Appendix Table A1, online only), a total of 83,419 patients remained for analysis. Patients were categorized into age less than 50 years (n = 6,886), 50 to 59 years (n = 10,952), 60 to 69 years (n = 19,963), 70 to 79 years (n = 27,254), and ≥ 80 years (n = 18,364). The baseline patient and tumor characteristics are listed in Table 1. The median age of the cohort was 71 years, (interquartile range, 61 to 79 years). The Kaplan-Meier unadjusted, 10-year, DSS probabilities for patients diagnosed with colon cancer stratified by AJCC, sixth edition, stage are shown in Figure 1. Follow-up after cancer diagnosis for the entire cohort was at least 5 years or until death with a median follow-up of 87 months (interquartile range, 63 to 127 months) for the entire cohort.
Table 1.
Demographic and Clinical Characteristics of Patients Diagnosed With Colon Cancer From 1988 to 2000
| Characteristic | Patients (N = 83,419) |
Final Model Analysis |
Likelihood Ratio χ2 Statistic* | ||
|---|---|---|---|---|---|
| No. | % | Adjusted HR | 95% CI | ||
| Age at diagnosis, years | 1,240.68 | ||||
| < 50† | 6,886 | 8 | 1 | ||
| 50-59 | 10,952 | 13 | 1.14 | 1.08 to 1.19 | |
| 60-69 | 19,963 | 24 | 1.28 | 1.23 to 1.34 | |
| 70-79 | 27,254 | 33 | 1.49 | 1.42 to 1.55 | |
| ≥ 80 | 18,364 | 22 | 1.96 | 1.87 to 2.05 | |
| Sex | 36.72 | ||||
| Male† | 38,874 | 47 | 1 | ||
| Female | 44,545 | 53 | 0.93 | 0.91 to 0.95 | |
| Ethnicity | 129.16 | ||||
| White† | 69,046 | 83 | 1 | ||
| Black | 7,936 | 9 | 1.18 | 1.13 to 1.22 | |
| Other | 6,437 | 8 | 0.87 | 0.83 to 0.91 | |
| Tumor stage | 39,100.38 | ||||
| I† | 12,890 | 16 | 1 | ||
| IIA | 24,070 | 29 | 2.48 | 2.30 to 2.67 | |
| IIB | 5,169 | 6 | 5.13 | 4.72 to 5.58 | |
| IIIA | 1,824 | 2 | 2.76 | 2.42 to 3.14 | |
| IIIB | 14,176 | 17 | 6.74 | 6.27 to 7.25 | |
| IIIC | 7,793 | 9 | 12.84 | 11.93 to 13.83 | |
| IV | 17,497 | 21 | 43.65 | 40.69 to 46.82 | |
| Tumor grade | 684.27 | ||||
| Well differentiated† | 7,492 | 9 | 1 | ||
| Moderately differentiated | 5,668 | 68 | 1.11 | 1.06 to 1.16 | |
| Poorly differentiated and undifferentiated | 19,239 | 23 | 1.52 | 1.45 to 1.60 | |
Abbreviation: HR, hazard ratio.
All P < .01.
Reference group.
Fig 1.
Unadjusted American Joint Commission on Cancer disease-specific survival for patients with colon cancer who were diagnosed between 1988 and 2000.
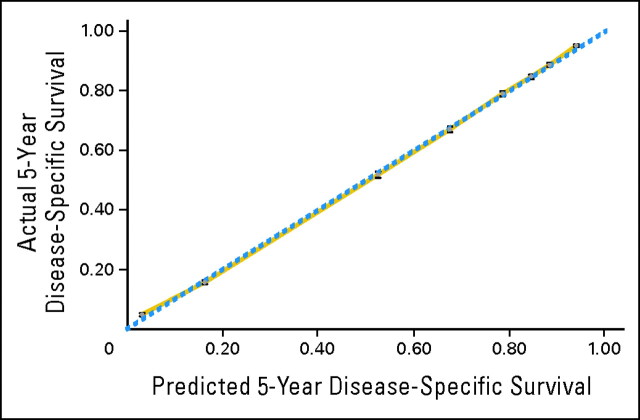
The final model used for the adjusted analyses is shown in Table 1. The bootstrap-corrected concordance index was 0.816. The additional inclusion of variables (ie, SEER region, year of diagnosis, marital status, and tumor location) did not improve the model performance or prediction accuracy. Therefore, the most parsimonious model was utilized for subsequent analyses and browser-based conditional survival calculator development. The calibration curve indicated good agreement between the predicted and observed outcomes of the final model, as shown in Figure 2.
Fig 2.
Overfitting-corrected calibration plot with Efron's bootstrap and with 200 resamples and at least 10,000 patients per interval. Solid gold line represents bias-corrected calibration. Dashed blue line represents the perfect calibration in which predicted outcome corresponds perfectly with actual ones.
The 5-year, crude and adjusted, disease-specific conditional survival probabilities stratified by stage at diagnosis for patients who survived up to 10 years—given that patients have already survived 1 to 5 years—are shown in Figure 3. Significant differences existed by stage at diagnosis. Although the initial survival probabilities were poorest for patients with more advanced stages of disease, these patients experienced the greatest improvement in conditional survival as time elapsed from diagnosis (PΔ slope, trend < .0001). By the fifth year after diagnosis, the adjusted, 5-year, conditional survival probability was ≥ 80% for all disease stages except for stage IV. These conditional survival estimates continued to improve through the eighth year and, in fact, were not greater than 90% until years 6 and 8 for stages IIIB and IIIC, respectively. At 8 years, the adjusted, 5-year, conditional survival probability was 72% for stage IV disease (data not shown).
Fig 3.
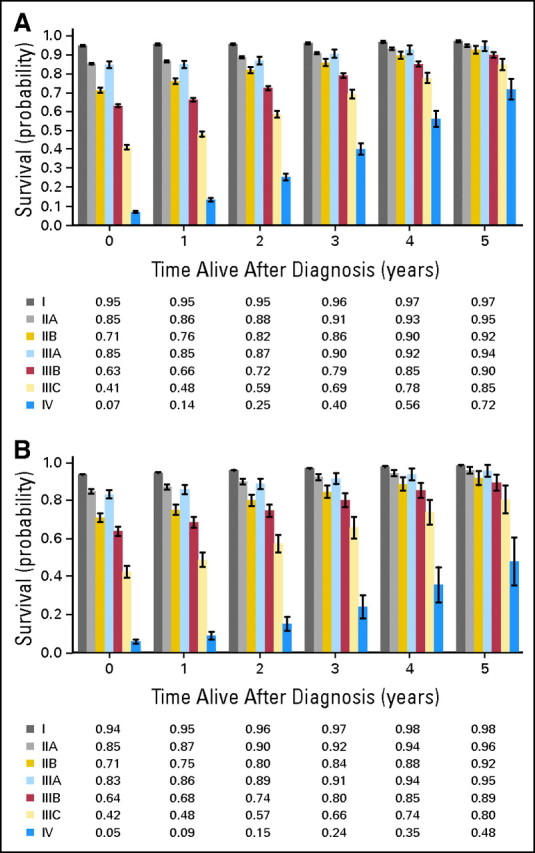
(A) Crude and (B) adjusted 5-year, conditional, disease-specific survival by American Joint Commission on Cancer stage adjusted for age, sex, ethnicity, and tumor grade. Bars represent the 95% CIs. The x-axis represents patients who have already survived 0 to 5 years.
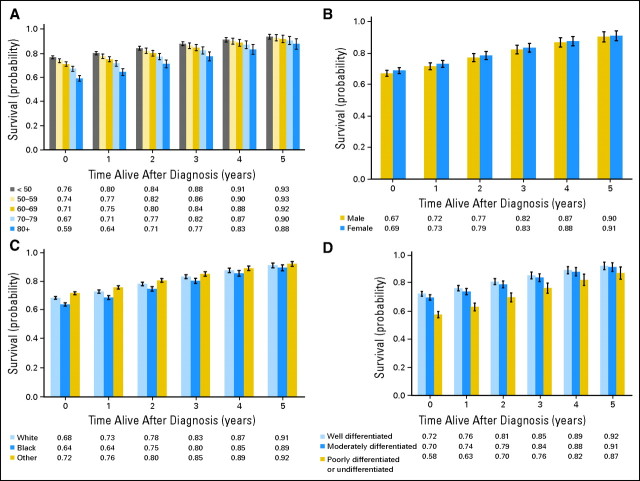
To evaluate the individual contribution of the different patient and tumor characteristics on conditional survival outcomes, patients were separately stratified by age at diagnosis, sex, tumor grade, and ethnicity after analysis was adjusted for the other covariates. Age at diagnosis, ethnicity, tumor grade, and sex all were significant multivariate determinants of 5-year survival expectancy at diagnosis (Table 1). However age, ethnicity, and tumor grade—but not sex—determined changes in conditional survival over time. The absolute difference in adjusted, 5-year, conditional survival expectancy between patients younger than 50 years and patients age 80 years or older decreased from 17% at the time of diagnosis to 5% by the fifth year after diagnosis (PΔ slope < .01; Fig 4). Black patients were noted to have the poorest 5-year conditional survival probabilities at diagnosis (64% v 68% for white patients, v 72% for other ethnicities, and v > 90% for Asian patients). By year 5, this gap reduced to 2% when compared with white patients (PΔ slope = .04) and 3% when compared with others (PΔ slope < .01). Similarly, the difference in adjusted, 5-year, conditional survival between well-differentiated and poorly differentiated or undifferentiated tumors decreased from 14% at diagnosis to 5% at year 5 (PΔ slope< .0001; Fig 4), but no difference was observed between well-differentiated and moderately differentiated tumors (PΔ slope = .27). Sex was not a significant determinant of changes in conditional survival over time (PΔ slope = .50).
Fig 4.
Adjusted 5-year, conditional, disease-specific survival by (A) age, (B) sex, (C) ethnicity, and (D) grade. Bars represent the 95% CIs. The x-axis represents patients who have already survived 0 to 5 years.
The browser-based conditional survival calculator has been implemented and is available at www3.mdanderson.org/coloncalculator.
DISCUSSION
In this study of 83,419 patients diagnosed with colon cancer between 1988 and 2000, a strong relationship between colon cancer–related survival probability and the time elapsed since diagnosis was identified. Thus, conditional survival estimates provide a much more useful and clinically reliable estimate of survival probability for patients who have survived a period of time after diagnosis of colon cancer than do initial survival estimates. This is especially true for those patients with more advanced stages of cancers whose initial survival probabilities were low.
Stage at diagnosis is the strongest single predictor of initial survival estimates among patients with colon cancer and varies widely, ranging from 7% for stage IV cancers to 95% for stage I cancers. However, over time, the crude conditional survival estimates improve markedly; by year 5 after diagnosis, the 5-year conditional survival probability is 72% for stage IV disease. This study confirms the findings of previous studies, which have shown the greatest improvements in conditional survival in patients who had initially the poorest prognosis. In fact, conditional survival estimates for advanced stages (ie, IIIB, IIIC, and IV) continue to improve through year 8 and do not level off until beyond year 5, which indicates that the underlying hazard continues to change beyond the standard 5-year period of follow-up.
Factors other than stage, including age, sex, ethnicity, and tumor grade, also were evaluated after adjustment for the covariates. Each of these factors has been observed previously to be a potentially important determinant of outcome in colon cancer.16–18 Of these factors and after adjustment, all but sex were significant determinants of initial prognosis; however, over time, each of the factors had decreasing influences. These trends are similar to those seen with other disease sites. Notably, with respect to ethnic survival disparities, this study shows that the differences decrease over time, which suggests that addressing treatment disparities at diagnosis may have a great impact on equalizing long-term survival outcomes.
A unique feature of this analysis is the use of multivariate modeling to adjust for covariate influences on conditional survival estimates not previously done for colon cancer cancer.6 Particularly for the more advanced stages at diagnosis, and compared with the unadjusted analyses, adjustment resulted in smaller improvements in conditional survival probabilities over time but more accurate modeling of the true observed outcomes, as shown in the calibration curve. To evaluate the potential effect of cause-of-death coding error within SEER, we confirmed the findings of this study by evaluating relative conditional survival; we identified no difference in the overall results. Identical trends in the relative conditional survival probabilities over time were observed (data not shown). We have, however, reported the DSS, because it provides the ability to determine actual point estimates of survival for individual patients; these estimates, for most patients and clinicians, are easier to interpret when compared with a survival probability relative to the general population.
Studies of conditional survival may provide valuable information to patients and their treating physicians and may serve a meaningful role in cancer survivorship research. There are a number of issues in cancer survivorship that are related to the expectation of future survival, including fear of recurrence, which can be the single largest concern among patients surviving from cancer and among their caregivers.19–22 Particularly among patients with advanced stage disease, an understanding of the degree of improvements in conditional survival over time may have an impact on reducing the level of anxiety and its effects on quality of life. Furthermore, the time point in years after diagnosis in which the conditional survival expectancy reaches a plateau may be considered the duration of time necessary to observe patients to establish cure. This has been shown to vary for different primary disease sites and has been advocated as a potentially important end point in clinical trials design.4,23 For colon cancer, the plateau occurs earlier for early-stage disease but occurs late for advanced disease. A 90% threshold may be appropriate for stage III colon cancers, for which this conditional survival expectancy is observed at 3, 6, and 8 years for stage IIIA, IIIB, and IIIC disease, respectively. After 8 years, the adjusted, 5-year, conditional survival was still less than 75% among patients with stage IV cancers. Thus, these findings would suggest that the standard 5-year period of follow-up may be longer than necessary for patients with earlier-stage cancers (eg, stages I, IIA, IIIA) and may be insufficient for patients with more advanced-stage disease (eg, stages IIIC, IV). Thus, use of conditional survival data to inform surveillance guidelines may have significant impacts on health care utilization costs. These findings also add to a recent report of pooled data from 18 randomized trials of adjuvant fluorouracil therapy in high-risk, stages II and III colon cancer (ie, Adjuvant Colon Cancer End Points group), which identified recurrence rates less than 1.5% at 5 years and less than 0.5% by 8 years. On the basis of these findings, the authors proposed that adjuvant trials for colon cancer do not require continued follow-up for recurrence beyond 5 years.24 However, this study suggests that patients with colon cancer are a heterogeneous group in whom the risk for disease-related death differs by cancer substage.
By utilizing the SEER data–based model developed in this study, we constructed a novel, Web site–based, conditional survival calculator to yield a point estimate of the conditional DSS probability adjusted for age, sex, ethnicity, grade, and stage at a given time after diagnosis. This tool may be accessed by a personal computer or by Web site–enabled cellular phone, which permits clinicians to use it in real time to better inform patients of their prognoses over time from diagnoses and to be able to quantify the survival expectancies. It can be modified easily to reflect temporally related changes in survival outcomes among the population of patients with colon cancer. However, there are limitations associated with the model that should be considered for any individual patient. The study entry period was before the modern expansion of chemotherapeutic agents for both adjuvant and palliative treatment of colon cancer and of indications for surgical resection of metastatic disease.25 Therefore, the model potentially underestimates survival probabilities related to improved cure rates from modern treatments. However, as the follow-up period was through 2005, a number of patients did benefit from the modern therapies. Additional improvements in model performance may be possible with the addition of collaborative staging elements, such as serum carcinoembryonic antigen levels, or with chemotherapy data by using SEER-Medicare–linked data, as we have planned. In the future, we also will test our model performance in external validation by using institutional databases that provide a better reflection of the incorporation of multidisciplinary treatment strategies, including systemic agents and surgery for metastasis.
In summary, we report on covariate-adjusted conditional survival outcomes among patients with colon cancer by utilizing data from the SEER registry, and we have created a unique, internet-based electronic tool that can be used in real time to inform patients who have survived some time from their original diagnoses. Calculation of conditional survival may direct health care providers to an individualized surveillance period and thereby may result in cost efficacy. Furthermore, we show that improvements in conditional survival probabilities continue for a number of years after initial treatment and that the duration of time during which these improvements occur is stage dependent. This information is important for patients and their healthcare providers, who must make important life decisions and grapple with important issues, such as disease recurrence, and who must make decisions regarding duration and intensity of follow-up after initial cancer therapy.
Acknowledgment
We thank Irma Medrano for her technical assistance, and the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute and cancer registrars nationwide for establishing and maintaining the SEER registry.
Appendix
Table A1.
Stepwise Case Ascertainment for Analysis
| Parameter | Patients |
|
|---|---|---|
| No. Overall | % Who Remained in Cohort | |
| Start: colon cancer occurrences diagnosed 1988 to 2000 | 176,379 | 100.00 |
| After excluding number of primaries greater than 1 | 125782 | 71.31 |
| After excluding unknown tumor stage and stage 0 | 97,833 | 55.47 |
| After excluding cell type not determined, not stated, or not applicable and undifferentiated | 88,377 | 50.11 |
| After excluding diagnosed occurrences that were not microscopically confirmed and unknown | 88,366 | 50.10 |
| After excluding occurrences in patients age younger than 18 and older than 90 years | 86,033 | 48.78 |
| After excluding occurrences diagnosed in nursing/convalescent home/hospice, by autopsy, and by death certificate only | 85,962 | 48.74 |
| After excluding inappropriate histology occurrences (adenocarcinoma only) | 85,477 | 48.46 |
| After excluding survival time equal to zero | 83,572 | 47.38 |
| After exclude unknown ethnicity | 83,419 | 47.30 |
Footnotes
Supported by an American Society of Clinical Oncology Career Development Award (G.J.C), and the Zell Family Foundation.
Presented in part at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, January 25-27, 2008, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: George J. Chang
Financial support: George J. Chang
Collection and assembly of data: George J. Chang, Chung-Yuan Hu
Data analysis and interpretation: George J. Chang, Chung-Yuan Hu
Manuscript writing: George J. Chang, Chung-Yuan Hu, Cathy Eng, Miguel A. Rodriguez-Bigas
Final approval of manuscript: George J. Chang, Chung-Yuan Hu, Cathy Eng, John M. Skibber, Miguel A. Rodriguez-Bigas
REFERENCES
- 1. Jemal A Siegel R Ward E , etal: Cancer statistics, 2008 CA Cancer J Clin 58: 71– 96,2008 [DOI] [PubMed] [Google Scholar]
- 2. Ferrell BR Dow KH Leigh S , etal: Quality of life in long-term cancer survivors Oncol Nurs Forum 22: 915– 922,1995 [PubMed] [Google Scholar]
- 3. Choi M Fuller CD Thomas CR Jr , etal: Conditional survival in ovarian cancer: Results from the SEER dataset 1988–2001 Gynecol Oncol 109: 203– 209,2008 [DOI] [PubMed] [Google Scholar]
- 4. Fuller CD Wang SJ Thomas CR Jr , etal: Conditional survival in head and neck squamous cell carcinoma: Results from the SEER dataset 1973–1998 Cancer 109: 1331– 1343,2007 [DOI] [PubMed] [Google Scholar]
- 5. Kato I, Severson RK, Schwartz AG: Conditional median survival of patients with advanced carcinoma: Surveillance, epidemiology, and end results data Cancer 92: 2211– 2219,2001 [DOI] [PubMed] [Google Scholar]
- 6. Merrill RM, Henson DE, Ries LA: Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon Dis Colon Rectum 41: 1097– 1106,1998 [DOI] [PubMed] [Google Scholar]
- 7. Wang SJ Emery R Fuller CD , etal: Conditional survival in gastric cancer: A SEER database analysis Gastric Cancer 10: 153– 158,2007 [DOI] [PubMed] [Google Scholar]
- 8.Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2008. Surveillance, Epidemiology, and End Results (SEER): SEER Program Limited-Use Data, 1973-2005. [Google Scholar]
- 9. Reference deleted.
- 10. Moertel CG Fleming TR Macdonald JS , etal: Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma N Engl J Med 322: 352– 358,1990 [DOI] [PubMed] [Google Scholar]
- 11. Laurie JA Moertel CG Fleming TR , etal: Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil–The North Central Cancer Treatment Group and the Mayo Clinic J Clin Oncol 7: 1447– 1456,1989 [DOI] [PubMed] [Google Scholar]
- 12. Schoenfeld D: Partial residuals for the proportional hazards regression model Biometrika 69: 239– 241,1982 [Google Scholar]
- 13. Freedman AN Seminara D Gail MH , etal: Cancer risk prediction models: A workshop on development, evaluation, and application J Natl Cancer Inst 97: 715– 723,2005 [DOI] [PubMed] [Google Scholar]
- 14. Harrell FE Jr Califf RM Pryor DB , etal: Evaluating the yield of medical tests JAMA 247: 2543– 2546,1982 [PubMed] [Google Scholar]
- 15. Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors Stat Med 15: 361– 387,1996 [DOI] [PubMed] [Google Scholar]
- 16. O'Connell JB, Maggard MA, Ko CY: Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging J Natl Cancer Inst 96: 1420– 1425,2004 [DOI] [PubMed] [Google Scholar]
- 17. Le H Ziogas A Lipkin SM , etal: Effects of socioeconomic status and treatment disparities in colorectal cancer survival Cancer Epidemiol Biomarkers Prev 17: 1950– 1962,2008 [DOI] [PubMed] [Google Scholar]
- 18. Coleman MP Quaresma M Berrino F , etal: Cancer survival in five continents: A worldwide population-based study (CONCORD) Lancet Oncol 9: 730– 756,2008 [DOI] [PubMed] [Google Scholar]
- 19. Alfano CM, Rowland JH: Recovery issues in cancer survivorship: A new challenge for supportive care Cancer J 12: 432– 443,2006 [DOI] [PubMed] [Google Scholar]
- 20. Kattlove H, Winn RJ: Ongoing care of patients after primary treatment for their cancer CA Cancer J Clin 53: 172– 196,2003 [DOI] [PubMed] [Google Scholar]
- 21. Spencer SM Lehman JM Wynings C , etal: Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients Health Psychol 18: 159– 168,1999 [DOI] [PubMed] [Google Scholar]
- 22. Mellon S Kershaw TS Northouse LL , etal: A family-based model to predict fear of recurrence for cancer survivors and their caregivers Psychooncology 16: 214– 223,2007 [DOI] [PubMed] [Google Scholar]
- 23. Wang SJ Fuller CD Kim JS , etal: Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer J Clin Oncol 26: 2112– 2117,2008 [DOI] [PubMed] [Google Scholar]
- 24. Sargent D Sobrero A Grothey A , etal: Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials J Clin Oncol 27: 872– 877,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kopetz S Chang GJ Overman MJ , etal: Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy J Clin Oncol 27: 3677– 3683,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]