Abstract
Disposal of membrane proteins in the late secretory pathway is thought to be exclusively facilitated by ESCRT‐dependent lysosomal degradation. In this issue of The EMBO Journal, Schmidt et al define a previously uncharacterized endosome and Golgi‐associated degradation (EGAD) pathway. This pathway, which has remarkable similarities to ERAD in the endoplasmic reticulum, operates in post‐ER organelles via the proteasome and contributes to lipid homeostasis in eukaryotic cells.
Subject Categories: Membrane & Intracellular Transport; Post-translational Modifications, Proteolysis & Proteomics
Membrane proteins define a large and diverse class of proteins in eukaryotic cells. At the plasma membrane and at the surface of cellular organelles, membrane proteins play a myriad of functions, from nutrient uptake and sensing to protein and lipid synthesis and trafficking.
With such important and widespread functions, the activity and abundance of membrane proteins must be tightly regulated. Scrutiny of membrane proteins starts during their biogenesis in the endoplasmic reticulum (ER). To ensure that only folded and functional membrane proteins traffic to other cellular membranes, the ER is equipped with a stringent quality control system called ER‐associated degradation (ERAD; Ruggiano et al, 2014). All membrane protein molecules failing to properly fold become ERAD substrates. Upon recognition, substrates are ubiquitinated, translocated to the cytoplasm, and membrane‐extracted by the AAA+ ATPase Cdc48 before their full release into the cytoplasm for degradation by the 26S proteasome. Central to the execution of these ERAD steps are E3 ubiquitin ligase complexes embedded in the membrane of the ER. In yeast, where ERAD is best characterized, there are three ERAD E3 ligase complexes—the Hrd1, Doa10, and Asi1 complexes—exhibiting different substrate specificities and localizing to distinct ER domains (Foresti et al, 2014; Ruggiano et al, 2014). Together, these ERAD branches prevent the accumulation of misfolded and mislocalized membrane proteins.
ERAD also controls the abundance of some folded proteins, with their degradation being regulated by metabolic signals. The best‐characterized examples of substrates controlled in this manner are the enzymes 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase (HMGR) and squalene monooxygenase (SM), essential for sterol biosynthesis at the ER (Hampton et al, 1996; Foresti et al, 2013). In sterol‐depleted cells, both enzymes are relatively long‐lived, but upon accumulation of specific sterol metabolites in the ER membrane, both HMGR and SM are quickly degraded via Hrd1 and Doa10 ERAD complexes, respectively (Hampton et al, 1996; Foresti et al, 2013; Ruggiano et al, 2014). Regulated degradation of HMGR and SM was shown to be important for sterol homeostasis from yeast to man, exemplifying important functions of ERAD beyond protein quality control (Ruggiano et al, 2014). Whether ERAD also participates in homeostatic regulation of other lipids such as glycerophospholipids or sphingolipids, whose biosynthesis is at least in part localized to the ER, remains unclear.
In post‐ER compartments, disposal of membrane proteins was thought to depend only on lysosomal degradation, through a process involving the ESCRT (endosomal sorting complexes required for transport) machinery (Migliano & Teis, 2018). Ubiquitinated membrane proteins at the surface of endosomes/multivesicular bodies (MVBs) are recognized by ESCRT complexes that promote their sorting and internalization into intraluminal vesicles, a process that also requires the AAA+ ATPase Vps4. Subsequent MVB fusion with the lysosome results in the release of intraluminal vesicles in the hydrolytic lumen of the lysosome, and ultimately in the degradation of their content (Migliano & Teis, 2018).
In this issue of The EMBO Journal, Schmidt et al (2019) set out to identify additional mechanisms of protein degradation operating in post‐ER compartments. They hypothesized that components of such a system would become essential in the absence of a functional ESCRT pathway, such as in yeast cells lacking the Vps4 ATPase. Using a genetic approach, the authors identified Tul1, an E3 ubiquitin ligase and a subunit of the Dsc (defective in SREBP cleavage) complex. Other Dsc complex subunits also became essential in cells lacking ESCRTs, suggesting a critical function of the whole Dsc complex under these conditions.
The Dsc complex was originally discovered in the fission yeast Schizosaccharomyces pombe, where it contains six components: the membrane proteins Tul1, Dsc2, Dsc3, Dsc4, and Ubx3, and the cytosolic AAA+ ATPase Cdc48 (Stewart et al, 2011). Interestingly, the membrane components of the Dsc complex share structural homology with the ERAD Hrd1 E3 ligase complex. In fission yeast, the Dsc complex has been characterized mostly for its role in activation of the transcription factor Sre1, homologous to the mammalian sterol regulatory element‐binding protein (SREBP). SREBPs are transcription factors synthesized as an inactive precursor bound to the ER membrane. Under conditions of low sterol (in mammals) or oxygen (in fission yeast) levels, SREBP cleavage‐activating protein (SCAP) promotes the traffic of SREBPs from ER to Golgi for cleavage, resulting in release of the active SREBP transcription factor (Stewart et al, 2011). Sre1 activation in the Golgi requires ubiquitination by the Dsc E3 ligase complex, followed by proteolytic cleavage by the rhomboid intramembrane protease Rbd2 assisted by Cdc48 (Stewart et al, 2011; Hwang et al, 2016). In the absence of Rbd2, however, the Dsc complex targets the immature SREBP precursor for proteasomal degradation (Hwang et al, 2016). This observation is at odds with work in budding yeast, where Tul1 substrates appeared to be degraded exclusively via the lysosome (Migliano & Teis, 2018). Moreover, a recent study (Yang et al, 2018) describing two mutually exclusive Dsc cofactors, Vld1 and Gld1, has defined Dsc complexes with distinct subcellular distribution: While Gld1‐containing Dsc complexes localize to Golgi/endosomes, Vld1‐containing complexes are found at the membrane of the vacuole, the yeast equivalent of the lysosome (Yang et al, 2018).
To identify Dsc complex substrates whose turnover is independent of lysosomes, Schmidt et al resorted to quantitative proteomics. Following up on one substrate, Orm2, they showed that Tul1 and all other Dsc subunits were necessary for Orm2 degradation. Interestingly, Vld1 was dispensable and Orm2 degradation depended exclusively on Gld1. Consistently, deletion of Tul1 or other Dsc subunits resulted in Orm2 accumulation at Golgi/endosomes. Orm2 degradation also required Tul1‐dependent ubiquitination, Cdc48‐mediated membrane extraction, and proteasome activity, a sequence of events highly reminiscent of ERAD, but in this case, operating in a post‐ER compartment at the level of Golgi/endosomes. This mode of membrane protein degradation by the Dsc complex was coined EGAD for endosome/Golgi‐associated degradation (Fig 1) (Schmidt et al, 2019).
Figure 1. Comparison of ER‐associated (ERAD) and endosome/Golgi‐associated (EGAD) degradation pathways for membrane proteins.
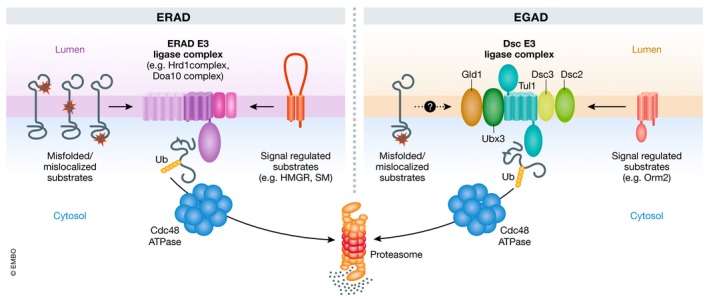
See text for details.
Orm2 is an integral membrane protein predominantly localized to the ER, where it negatively regulates yeast sphingolipid biosynthesis, a function that it shares with its paralog Orm1. Orm1 and Orm2 bind and inhibit serine:palmitoyl‐coenzyme A transferase (SPT), the first enzyme in sphingolipid biosynthesis (Breslow et al, 2010). In response to low sphingolipid levels, Orm1 and Orm2 are inactivated via TORC2‐Ypk1‐mediated phosphorylation, relieving SPT inhibition and allowing sphingolipid synthesis (Roelants et al, 2011). Phosphorylated Orm2 is exported from the ER and, as it reaches Golgi/endosomes, is degraded by EGAD. Indeed, non‐phosphorylatable Orm2 mutants cannot be detected outside of the ER and, as a consequence, are largely resistant to EGAD, and display lower levels of sphingolipid precursors such as ceramides. A comparable sphingolipid biosynthesis defect is also observed upon expression of ubiquitination‐resistant Orm2 mutants and in tul1∆ cells, indicating that both Orm2 phosphorylation and endosome/Golgi‐associated degradation contribute to regulation of SPT activity. This role of EGAD in controlling sphingolipid levels through regulated Orm2 degradation has similarities to the mechanism by which ERAD maintains sterol homeostasis (Fig 1).
Interestingly, Orm1 is not an EGAD substrate but appears to be regulated only via phosphorylation. It is unclear whether this is because phosphorylated Orm1 is not efficiently exported from the ER, or because it is not recognized by the Dsc complex. Also, how Orm2 phosphorylation triggers its export is still unknown. A simple possibility is that phosphorylated Orm2 has reduced affinity for SPT, facilitating its trafficking out of the ER. Importantly, as regulated degradation of other proteins such as HMGR and SM occurs in the ER, it is intriguing that Orm2 needs to traffic to Golgi/endosomes for its degradation. Given that the final stages of sphingolipid synthesis occur in the Golgi, one may speculate that Orm2 turnover in the Golgi may be coupled to an additional regulatory step, such as the binding or sensing of a sphingolipid metabolic intermediate.
Characterization of Orm2 degradation was important in uncovering and outlining the features of EGAD. However, it is unlikely to be its sole (or even main) substrate. Other potential Dsc substrates have been identified, and their future characterization will bring new insight into EGAD. Additionally, understanding how Dsc complex substrates are selected merits further investigation. It will also be important to clarify whether the Dsc complex plays a general role in quality control, for example, by degrading substrates that escape ERAD surveillance or undergo misfolding in post‐ER compartments. Such a role of EGAD in degradation of misfolded proteins would further strengthen its resemblance to ERAD.
The EMBO Journal (2019) 38: e102679
See also: O Schmidt et al (August 2019)
References
- Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463: 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, Ruggiano A, Hannibal‐Bach HK, Ejsing CS, Carvalho P (2013) Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. Elife 2: e00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, Rodriguez‐Vaello V, Funaya C, Carvalho P (2014) Quality control of inner nuclear membrane proteins by the Asi complex. Science 346: 751–755 [DOI] [PubMed] [Google Scholar]
- Hampton RY, Gardner RG, Rine J (1996) Role of 26S proteasome and HRD genes in the degradation of 3‐hydroxy‐3‐methylglutaryl‐CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell 7: 2029–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Ribbens D, Raychaudhuri S, Cairns L, Gu H, Frost A, Urban S, Espenshade PJ (2016) A Golgi rhomboid protease Rbd2 recruits Cdc48 to cleave yeast SREBP. EMBO J 35: 2332–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliano SM, Teis D (2018) ESCRT and membrane protein ubiquitination. Prog Mol Subcell Biol 57: 107–135 [DOI] [PubMed] [Google Scholar]
- Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J (2011) Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae . Proc Natl Acad Sci USA 108: 19222–19227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiano A, Foresti O, Carvalho P (2014) ER‐associated degradation: protein quality control and beyond. J Cell Biol 204: 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O, Weyer Y, Baumann V, Widerin MA, Eising S, Angelova M, Schleiffer A, Kremser L, Lindner H, Peter M et al (2019) Endosome and Golgi‐associated degradation (EGAD) of membrane proteins regulates sphingolipid metabolism. EMBO J 38: e101433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EV, Nwosu CC, Tong Z, Roguev A, Cummins TD, Kim D‐U, Hayles J, Park H‐O, Hoe K‐L , Powell DW et al (2011) Yeast SREBP cleavage activation requires the Golgi Dsc E3 ligase complex. Mol Cell 42: 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Arines FM, Zhang W, Li M (2018) Sorting of a multi‐subunit ubiquitin ligase complex in the endolysosome system. Elife 7: e33116 [DOI] [PMC free article] [PubMed] [Google Scholar]


