Abstract
ATF6 is a major signal transducer for cellular reprogramming in response to protein mis‐folding in the endoplasmic reticulum. However, the mechanism by which ATF6 senses unfolded proteins and becomes activated is not yet known. In this issue of The EMBO Journal, Oka et al show that ERp18, a single‐domain member of the protein disulfide isomerase family, interacts preferentially with ATF6 under stress conditions and regulates ATF6 transport to the Golgi apparatus. Furthermore, ERp18 impacts the ATF6 cleavage product generated in the Golgi, ultimately determining whether or not ATF6 becomes a functional transcription factor and induces the unfolded protein response.
Subject Categories: Membrane & Intracellular Transport, Protein Biosynthesis & Quality Control
A widespread regulatory mechanism in biology is the proteolytic cleavage of activated membrane‐tethered signaling proteins to release fragments that enter the cell nucleus and initiate appropriate transcriptional responses. This general mechanism is shared by the Notch cell‐surface receptors, which function in cell–cell communication, and by stress and sterol sensors in the endoplasmic reticulum (ER). Similarities are found between the sterol‐sensing system, which activates lipogenesis, and the stress sensor ATF6, which controls one branch of the metazoan unfolded protein response (UPR). Specifically, both the sterol regulatory element‐binding protein (SREBP) and ATF6 are ER‐resident proteins, unless cholesterol levels drop or unfolded proteins accumulate, respectively. If these events occur, SREBP and ATF6 are trafficked to the Golgi apparatus, where they are cleaved by two successive proteases, S1P and S2P, producing the relevant transcription factors.
Despite their shared use of Golgi trafficking and S1P/S2P cleavage, the SREBP and ATF6 pathways differ in many aspects. They also differ in the degree to which they are understood mechanistically. Over many years of pioneering research by the team of Brown and Goldstein and others, the players in the sterol‐sensing pathway have been identified and assigned functions (Brown et al, 2018). Briefly, SREBP is found in complex with a protein called SCAP, which promotes ER retention of SREBP in the presence of cholesterol. When cholesterol is scarce, however, SCAP instead escorts SREBP to the Golgi. In contrast to the well‐established role of SCAP in the sterol‐sensing pathway, the identities and functions of ATF6 partner proteins are ambiguous. ATF6 appears to be retained in the ER by association with BiP (Shen et al, 2002), an abundant ER chaperone, but how BiP is released for ATF6 activation is not yet clear. A thrombospondin family protein is proposed to escort ATF6 to the Golgi in cardiac tissue (Lynch et al, 2012), but the generality of this mechanism has not been established. Notably, even the fundamental signals that activate ATF6 are still being discovered, it was recently reported that ATF6 not only senses unfolded proteins but is also directly responsive to intermediates in sphingolipid synthesis (Tam et al, 2018). This finding reinforces the now widely appreciated notion that lipid homeostasis and balanced protein biosynthesis go hand‐in‐hand. Nevertheless, there is more work to be done to build a comprehensive molecular mechanistic model for ATF6 signal sensing at the level of that for SREBP.
Oka et al (2019) took on this challenge by seeking proteins that associate with ATF6 only upon ER stress induction. No analogous factor is known for the SREBP pathway, since SCAP associates with SREBP both in the presence and absence of cholesterol (but in different conformational states; Brown et al, 2018). Nevertheless, since BiP dissociates from ATF6 under stress, it is reasonable to hypothesize that another protein may then come into play. Over the background of common chaperones present at high concentrations in the ER, which were pulled out in the screen regardless of stress induction, Oka et al found that a diminutive and functionally unassigned member of the protein disulfide isomerase (PDI) family associates with ATF6 specifically under stress. This PDI protein is ERp18, product of the TXNDC12 gene. The importance of ERp18 in the ATF6 activation pathway was validated by showing that ERp18 knockout blunts the induction of messenger RNAs on the ATF6 branch of the UPR.
ERp18 was characterized biochemically in 2003 (Alanen et al, 2003), but no biological role had yet been assigned to it. ERp18 and the proteins Agr2 and Agr3 constitute a PDI subgroup of single thioredoxin‐domain proteins. Agr2 and Agr3 are the closest ERp18 paralogs, but the Agr proteins are more closely related to one another than to ERp18. Both Agr2 and Agr3 have the motif Cys‐X‐X‐Ser in their active sites, whereas ERp18 has a Cys‐X‐X‐Cys motif.
The discovery of a PDI family representative in the screen for proteins engaging ATF6 under proteotoxic stress was not entirely surprising. Because most cysteines in proteins that pass through the ER are neatly tucked away in disulfide bonds or buried in protein cores, the persistence of free, reactive cysteines is one sign of folding gone wrong. The status of cysteine amino acids, and perhaps the entire thiol/disulfide redox balance in the ER lumen, is thus potentially relevant to unfolded protein stress sensing by ATF6. Correspondingly, ATF6, unlike SREBP, appears to have a redox sensor in its luminal domain. This sensor consists of two cysteines that mediate covalent dimerization or intramolecular disulfide bonding within ATF6 under normal conditions, whereas reduction of disulfides correlates with extent of ATF6 activation (Nadanaka et al, 2007). The observed disulfide‐mediated interactions between ERp18 and ATF6 may indicate a role for ERp18 in reducing the ATF6 luminal disulfides under stress conditions. Interestingly, disulfide‐bonded dimers of the ERp18‐like protein Agr2 were reported to disappear, like the ATF6 disulfide‐mediated dimers, upon ER stress induction (Maurel et al, 2019).
Oka et al did more than identify ERp18 as a player in ATF6 reduction and activation. They also mapped the stress‐sensitive interaction between ERp18 and ATF6 to a particular ATF6 cysteine and clarified the interpretation of various disulfide‐bonded states of ATF6 as they appear on polyacrylamide gels. Perhaps most importantly, however, they strengthened the notion that generating the transcription factor fragment from ATF6 requires more than just getting the precursor to the Golgi. A number of disparate observations previously suggested that aberrant cleavage of ATF6 can occur. For example, sodium salicylate causes ATF6a to be trafficked to the Golgi without being cleaved to generate the active transcription factor (Mügge & Silva, 2017), and viral infection can lead to ATF6 cleavage into unproductive products (Jheng et al, 2018). The current work emphasizes that ATF6 activation is regulated by mechanisms other than just trafficking (Fig 1). Though many aspects of ATF6 activation remain to be discovered, the sensitivity of the pathway to a particular PDI family protein in the ER shows that there is no glory in racing unregulated to the Golgi.
Figure 1. ERp18 promotes regulated trafficking of ATF6 to the Golgi and productive cleavage.
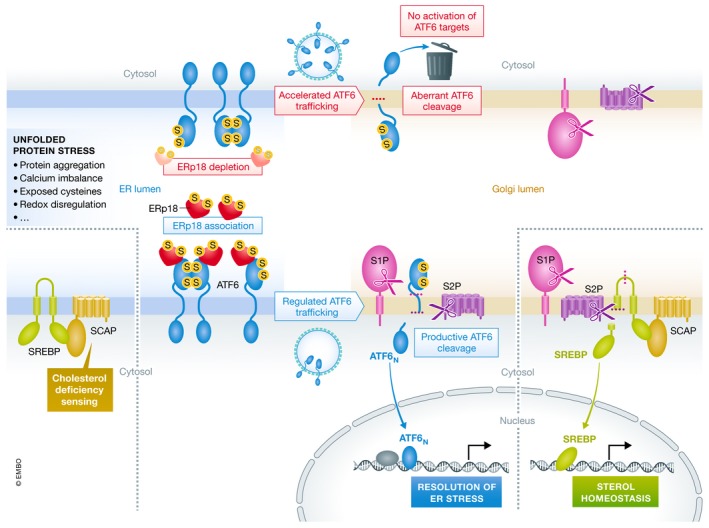
ERp18 (red) associates with the ATF6 unfolded protein stress sensor (blue) in the ER lumen, promoting regulated trafficking to the Golgi apparatus. In the Golgi, productive cleavage of ATF6 by the two proteases S1P and S2P (magenta and purple, respectively) produces the active transcription factor fragment, which turns on the program to resolve ER stress. The cholesterol‐sensing mechanism of SREBP/SCAP responds in a different manner to imbalances in the ER but converges on the same mechanism of trafficking to the Golgi and two‐site cleavage to produce the appropriate transcription factor to restore lipid homeostasis.
The EMBO Journal (2019) 38: e102743
See also: OBV Oka et al (August 2019)
References
- Alanen HI, Williamson RA, Howard MJ, Lappi AK, Jäntti HP, Rautio SM, Kellokumpu S, Ruddock LW (2003) Functional characterization of ERp18, a new endoplasmic reticulum‐located thioredoxin superfamily member. J Biol Chem 278: 28912–28920 [DOI] [PubMed] [Google Scholar]
- Brown MS, Radhakrishnan A, Goldstein JL (2018) Retrospective on cholesterol homeostasis: the Central Role of Scap. Annu Rev Biochem 87: 783–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng JR, Lau KS, Lan YW, Horng JT (2018) A novel role of ER stress signal transducer ATF6 in regulating enterovirus A71 viral protein stability. J Biomed Sci 25: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H et al (2012) A thrombospondin‐dependent pathway for a protective ER stress response. Cell 149: 1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M, Obacz J, Avril T, Ding YP, Papadodima O, Treton X, Daniel F, Pilalis E, Hörberg J, Hou W et al (2019) Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med 11: e10120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mügge FLB, Silva AM (2017) Aspirin metabolite sodium salicylate selectively inhibits transcriptional activity of ATF6α and downstream target genes. Sci Rep 7: 9190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K (2007) Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27: 1027–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka OBV, van Lith M, Rudolf J, Tungkum W, Pringle M‐A, Bulleid NJ (2019) ERp18 regulates the activation of ATF6α during the unfolded protein response. EMBO J 38: e100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3: 99–111 [DOI] [PubMed] [Google Scholar]
- Tam AB, Roberts LS, Chandra V, Rivera IG, Nomura DK, Forbes DJ, Niwa M (2018) The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev Cell 46: 327–343 [DOI] [PMC free article] [PubMed] [Google Scholar]


