Abstract
Background
Cholesterol efflux plays an important role in preventing atherosclerosis progression. Vegetable oils with varying unsaturated fatty acid profiles favorably affect multiple cardiovascular disease risk factors; however, their effects on cholesterol efflux remain unclear.
Objective
The objectives of this study were to examine the effects of diets low in saturated fatty acids (SFAs) with varying unsaturated fatty acid profiles on serum-mediated cholesterol efflux and its association with the plasma lipophilic index and central obesity.
Methods
The present study is a randomized, crossover, controlled-feeding study. Participants [men: n = 50; women: n = 51; mean ± SE age: 49.5 ± 1.2 y; body mass index (in kg/m2): 29.4 ± 0.4] at risk for or with metabolic syndrome (MetS) were randomly assigned to 5 isocaloric diets containing the treatment oils: canola oil, high oleic acid–canola oil, DHA-enriched high oleic acid–canola oil, corn oil and safflower oil blend, and flax oil and safflower oil blend. These treatment oils were incorporated into smoothies that participants consumed 2 times/d. For a 3000-kcal diet, 60 g of treatment oil was required to provide 18% of total energy per day. Each diet period was 4 wk followed by a 2- to 4-wk washout period. We quantified cholesterol efflux capacity with a validated ex vivo high-throughput cholesterol efflux assay. Statistical analyses were performed with the use of the SAS mixed-model procedure.
Results
The 5 diets increased serum-mediated cholesterol efflux capacity from THP-1 macrophages similarly by 39%, 34%, 55%, 49% and 51%, respectively, compared with baseline (P < 0.05 for all). Waist circumference and abdominal adiposity were negatively correlated with serum-mediated cholesterol efflux capacity (r = −0.25, P = 0.01, r = −0.33, P = 0.02, respectively).
Conclusion
Diets low in SFAs with different monounsaturated fatty acid and polyunsaturated fatty acid profiles improved serum-mediated cholesterol efflux capacity in individuals with or at risk for MetS. This mechanism may account, in part, for the cardiovascular disease benefits of diets low in SFAs and high in unsaturated fatty acids. Importantly, central obesity is inversely associated with cholesterol efflux capacity. This trial was registered at www.clinicaltrials.gov as NCT01351012.
Keywords: cholesterol efflux, HDL function, diet, unsaturated fatty acids, metabolic syndrome
Introduction
Metabolic syndrome (MetS) is associated with several major cardiovascular disease risk factors that contribute to the progression of atherosclerosis. Cellular cholesterol efflux from lipid-laden macrophages toward extracellular acceptors (referred to as the cholesterol efflux capacity) is inversely associated with coronary artery disease risk, which illustrates the anti-atherogenic role of the cholesterol efflux process (1–3). There is growing interest in better understanding how cellular cholesterol efflux is regulated and the role of dietary fatty acids in this process.
A limited number of studies have evaluated the effects of dietary fatty acids on HDL cholesterol efflux capacity (4, 5). In healthy participants (n = 26), consumption of extra-virgin olive oil (25 mL/d) for 12 wk increased HDL cholesterol efflux capacity by 25% compared with baseline (4). In participants with elevated LDL cholesterol (≥2.86 mmol/L), a positive dose-response relation was observed between pistachio consumption and HDL cholesterol efflux capacity (5). Virtually nothing is known about the effects of dietary fatty acids on cholesterol efflux capacity in individuals with MetS. In addition, central obesity, the key criteria for MetS, is associated with increased inflammation and oxidative stress, which have been shown to decrease the protective function of HDL, thereby favoring the development of atherosclerosis (6). However, the associations between central obesity, dyslipidemia, and in vitro cholesterol efflux remain to be studied.
Recent studies have reported that microRNA (MiR) plays an important role in regulating cholesterol and lipid metabolism via the following mechanisms: 1) miR-33a/b regulates ATP-binding cassette transporter (ABCA1) transporter-targeted gene (7); 2) miR-106 regulates ABCA1 expression (8, 9); 3) miR-30c reduces lipid synthesis (10); and 4) miR-181a increases gene expression involved in β-oxidation and decreases gene expression involved in lipid synthesis (11). There is limited clinical evidence about the effects of dietary fatty acids in the regulation of MiR.
The Canola Oil Multicentre Intervention Trial (COMIT I) was a randomized, crossover, controlled-feeding trial designed to examine the effects of 5 diets low in SFAs with varying unsaturated fatty acid profiles on endothelial function in a population with or at risk for MetS. The present study was conducted as part of the COMIT I study to investigate the effects of these 5 diets on the following: 1) the serum-mediated cholesterol efflux capacity; 2) the association between serum-mediated cholesterol efflux capacity and central obesity; and 3) the plausible molecular mechanisms of how dietary fatty acids may affect membrane fluidity measured by plasma lipophilic index (LI) and the effect of varying fatty acids on MiR expression that regulate cholesterol efflux capacity in participants from the COMIT I study.
Methods
Study design
The COMIT I was a double-blind, randomized, crossover, controlled-feeding trial. Detailed methods have been published elsewhere (12, 13). In brief, the study included 5 dietary periods. Participants were fed an isocaloric diet with varying fatty acid profiles. Weight was measured daily (Monday through Friday) when meals were distributed at each diet center. Each of the 5 treatment periods lasted 4 wk and was separated by a 2- to 4-wk washout period and compliance break. The primary outcome for this trial was endothelial function.
Participants
One hundred and thirty participants were randomly assigned at 3 research centers across Canada (University of Manitoba and Laval University) and the United States (Penn State University). Recruitment criteria were based on the International Diabetes Federation criteria for metabolic syndrome (14). Inclusion criteria were: men and women aged 20–65 y, BMI (in kg/m2) 22–40 with central obesity (men: waist circumference ≥94 cm; women: waist circumference ≥80 cm) plus ≥1 other MetS criterion. Criteria included elevated fasting blood glucose (≥5.6 mmol/L), decreased HDL cholesterol (men ≤1.0 mmol/L, women ≤1.3 mmol/L), increased TGs (≥1.7 mmol/L) and elevated blood pressure (systolic ≥130 mm Hg or diastolic ≥85 mm Hg). One hundred and one participants completed all 5 controlled diet periods and were included in the analysis. Two participants were excluded from the analysis because their baseline sample was not available. The study was approved by the ethics committees of University Manitoba, Laval University, and Penn State University and carried out in accordance with the Helsinki Declaration.
Sample collection
Fasting blood samples were collected on days 1 and 2 (baseline) as well as on days 29 and 30 (endpoint) for all diet periods. Serum samples from all clinical sites were frozen and shipped to the J Alick Little Lipid Research Laboratory (University of Toronto) for analysis of the primary biochemical endpoints except cholesterol efflux. Cholesterol efflux was measured by taking samples collected at study initiation (day 1 of the first diet period) and at the end of each treatment period (day 29). For the MiR analysis, 2 of the study diets—one with high oleic acid–canola oil (CanolaOleic), which had the highest n–9 MUFA content, and one with DHA-enriched high oleic acid–canola oil (CanolaDHA), which had the highest long-chain n–3 PUFA content—were selected for MiR assessment.
Dietary intervention
Test diets were created using Food Processor SQL software, version 10.8 (ESHA Research, Salem, OR). Participants were fed an isocaloric diet (calculated by the Harris-Benedict equation) and advised to follow their routine physical activity practices. Weight was measured daily Monday through Friday at each diet center. All of participants’ food (3 meals and a snack daily) was provided during the feeding periods. The food preparation was done in the metabolic kitchen at each research center. The macronutrient profile of the study diets was based on a typical American diet with 50% of the energy from carbohydrate, 35% of energy from fat (18% from treatment oils), and 15% of energy from protein. Five oil combinations were studied: conventional canola oil, CanolaOleic, CanolaDHA, corn and safflower oil blend (Corn/Saff), and flax and safflower oil blend (Flax/Saff). These oils were incorporated into smoothies that participants consumed 2 times/d. The smoothie contained the treatment oil, frozen unsweetened strawberry, orange sherbet, and nonfat milk. The quantity of oil was calculated based on the diet calorie level. For a 3000-kcal diet, 60 g treatment oil/d was required to contribute the 18% of total energy in the diet. This amount was split between 2 shakes. Each shake contained 100 g orange sherbet, 100 g nonfat milk, 100 g frozen unsweetened strawberries, and 30 g oil. The fatty acid profiles of treatment oils are presented in Supplemental Table 1. The mean energy intake for all participants was the same between treatments (Supplemental Table 2).
DXA measurements
Body composition was assessed at baseline and at the end of each diet period by DXA (Lunar Prodigy Advance, GE Healthcare; QDR-4500W; Hologic Corp). The DXA method used has been described previously (15). Baseline body composition was assessed in a subset of the study population (n = 54). All participants (n = 101) had DXA scans at the end of each diet period.
THP-1 human monocytes
The THP-1 (Homo sapiens, monocyte) cell line was obtained from the American Type Culture Collection (Rockville, MD) and was cultured in Roswell Park Memorial Institute 1640 with 10% heat-inactivated FBS, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, and antibiotics. To differentiate THP-1 monocytes to macrophages, cells were incubated in growth medium minus 2-mercaptoethanol with addition of 100 nM phorbol myristate acetate for 48 h (16).
High throughput cholesterol efflux assay
The cholesterol efflux assay used has been described previously (16). THP-1 human monocytes were plated in 96-well plates at a density of 1 × 105 cells/well and differentiated into macrophages by incubating with 100 nM phorbol myristate acetate for 48 h. After differentiation, cells were washed twice with PBS and cultured in the growth medium overnight. Cells were loaded with 50 μg/mL of oxidized LDL and 1 μg/mL of 3-nitrobenzoxadiazole cholesterol for 24 h to induce foam cell formation and label the intracellular cholesterol pool. Medium was then discarded and the cells were incubated in 1% FBS for 10–12 h. This allowed the labeled cholesterol to be distributed to various intracellular compartments. After 24 h, cells were washed twice with PBS and incubated with human sera from the study participants (10%, v/v) for 1 h to induce serum-mediated cholesterol efflux. After incubation, 100 μL of the medium from each well was collected and transferred to a new 96-well plate. Residual liquid in each well was removed. Cells were treated with 100 μL lysis buffer and homogenized on an orbital shaker for 10 min. Fluorescence from the PBS fraction and the cell lysate fraction was measured at excitation and emission wavelengths of 485 and 535 nm, respectively. Cholesterol efflux was calculated by dividing the fluorescence intensity of the media by the total fluorescence intensity of the cell lysate and media. This value was multiplied by 100 to obtain percentage of cholesterol efflux. The relative cholesterol efflux was calculated as percentage of cholesterol efflux at the end of each diet period divided by the baseline percentage of cholesterol efflux. To reduce variability, all samples from one individual were analyzed on the same plate (Supplemental Methods).
Plasma fatty acid measurement and LI calculation
The calculation of the plasma LI has been described by Wu et al. (17). The LI for plasma fatty acid composition was calculated at the end of each diet period.
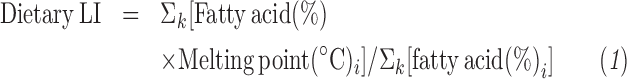 |
The melting points for selected fatty acids have been reported by Senanayake et al. (12).
RNA extraction, reverse transcription, real-time PCR
RNA was isolated from serum samples using a kit from AMRESCO LLC, a simple spin-column based method used for siRNAs and MiR in serum samples. A qScript microRNA cDNA Synthesis Kit (Quanta Biosciences Inc.) was used to convert MiRs into cDNA starting from total RNA. Individual MiR was quantified in real-time qRT-PCR amplification reactions with the use of PerfeCTa MiR assays along with PerfeCTa Universal PCR Primer and PerfeCTa SYBR Green SuperMix according to the manufacturer's protocol and amplified on an ABI Prism 7000 Sequence Detection System (Supplemental Methods).
Statistical analysis
Variables were reported as the mean ± SE. The normality of data was assessed before analysis through the use of skewness and the Shapiro-Wilk test and graphically by evaluating histograms. We applied log transformation on serum-mediated cholesterol efflux and changes of serum-mediated cholesterol efflux to approximate a normal distribution. Models included the diet, visit, age, gender, obesity status (normal weight: BMI ≤24.9; overweight: BMI 24.9–30; obese: BMI ≥30) as fixed effects. Center was included as a random effect to adjust for potential confounders. We applied an unstructured correlation matrix to account for within-individual repeated measure. Of primary interest was comparing the effects of the 5 diets on cholesterol efflux after treatment (endpoint comparisons). We also compared the effects of the diets on changes in cholesterol efflux from baseline (day 1 of the first diet period). Tukey-Kramer adjusted P values were reported for post-hoc comparisons among the 5 diets. For endpoints that were measured in duplicate (lipids and lipoprotein profiles), average values were used for analysis. One-way ANOVA was performed on the miRs expressed. Statistical analyses were performed with the use of the mixed model procedure in the SAS statistical software package, version 9.2 (SAS Institute). Changes from baseline were calculated by subtracting the day 1 measurements from the day 29 measurements. Pearson correlation analysis was used to assess the correlation between cholesterol efflux and waist circumference and abdominal adiposity.
Results
Baseline characteristics
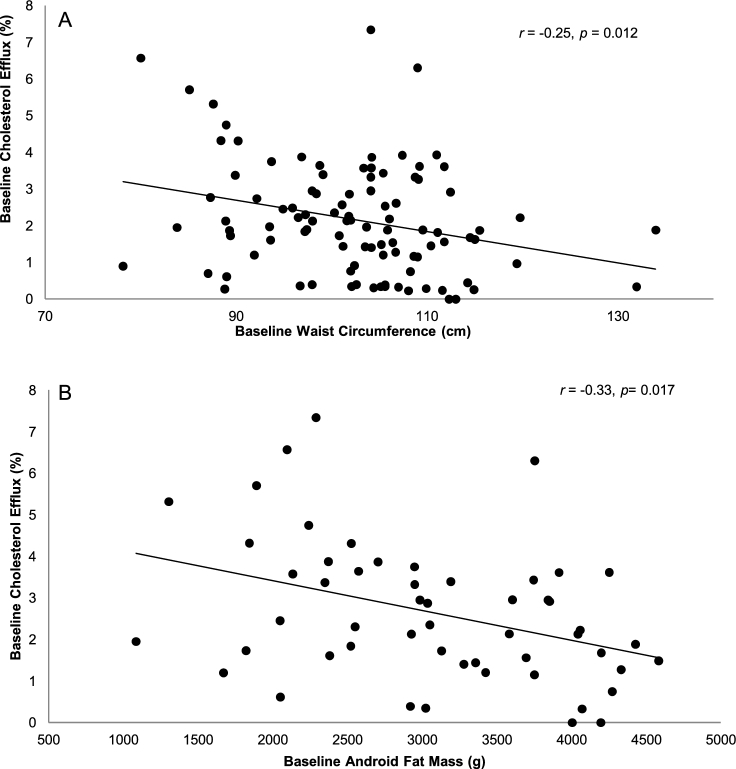
Baseline anthropometric and metabolic characteristics of participants are summarized in Table 1. Baseline body composition was conducted in a subgroup of participants (n = 54) (Supplemental Table 3). Baseline correlations between waist circumference, abdominal fat mass, and cholesterol efflux capacity are presented in Figure 1A, B. Serum-mediated cholesterol efflux capacity was negatively correlated with waist circumference (n = 101, r = −0.25, P = 0.012) and abdominal fat mass (n = 54, r = −0.33, P = 0.017).
TABLE 1.
Baseline anthropometric and cardiometabolic characteristics1
| Characteristic | Participants |
|---|---|
| Anthropometric measurements | |
| Age, y | 49.5 ± 1.2 |
| Body mass, kg | 85.8 ± 1.5 |
| Height, m | 1.7 ± 0.01 |
| Body mass index, kg/m2 | 29.4 ± 0.4 |
| Waist circumference, cm | 101.9 ± 1.1 |
| Metabolic syndrome risk factors | |
| Glucose,2 mmol/L | 5.24 ± 0.11 |
| HDL cholesterol,2 mmol/L | 1.25 ± 0.03 |
| TGs,2 mmol/L | 1.84 ± 0.09 |
| Systolic blood pressure, mm Hg | 121 ± 1 |
| Diastolic blood pressure, mm Hg | 76 ± 1 |
1Values are means ± SEMs, n = 101.
2Glucose, HDL cholesterol, and TGs were measured in serum samples.
FIGURE 1.
Correlation between waist circumference (n = 99) and serum-mediated cholesterol efflux capacity (A), and between abdominal fat mass (n = 54) and serum-mediated cholesterol efflux capacity (B) for participants with or at risk for metabolic syndrome at baseline.
Diet-specific effects on the lipid/lipoprotein profiles and LI
After 4 wk, all diets decreased total cholesterol (TC), LDL cholesterol, and TG concentrations (P < 0.05 for all) from baseline (Table 2). HDL cholesterol was increased from baseline in response to the CanolaDHA diet (P < 0.0001). The canola oil (P = 0.037), CanolaOleic (P = 0.015), and Flax/Saff (P < 0.0001) diets decreased HDL cholesterol from baseline. The Corn/Saff diet also tended to decrease HDL cholesterol (P = 0.072) from baseline. ApoA1 was decreased on the Flax/Saff diet compared with the other test diets (P < 0.05 for all). There were no associations between HDL cholesterol and cholesterol efflux at baseline or between the changes in HDL cholesterol and the changes in cholesterol efflux in response to the treatment diets (Supplemental Table 4). The LI in response to each diet was 22.5 ± 0.14 for canola oil, 22.6 ± 0.14 for CanolaOleic, 21.2 ± 0.14 for CanolaDHA, 22.7 ± 0.16 for Corn/Saff, and 22.7 ± 0.16 for Flax/Saff. Participants had the lowest LI after the CanolaDHA diet compared with the other diets (P < 0.05 for all) (Supplemental Table 5).
TABLE 2.
Baseline and changes in serum lipid/lipoprotein profiles in response to diets low in SFAs with varying unsaturated fatty acid profiles for 28 d in participants with or at risk for MetS1
| Baseline2 | Canola oil | CanolaOleic | CanolaDHA | Corn/Saff | Flax/Saff | |
|---|---|---|---|---|---|---|
| Total cholesterol, mmol/L | 5.48 ± 0.10 | −0.58 ± 0.0513,a,b | −0.61 ± 0.063,a,b | −0.54 ± 0.063,a | −0.62 ± 0.063,b | −0.68 ± 0.043,b |
| HDL cholesterol, mmol/L | 1.22 ± 0.29 | −0.04 ± 0.023,a | −0.02 ± 0.023,a | 0.07 ± 0.023,b | −0.01 ± 0.02a | −0.04 ± 0.023,a |
| LDL cholesterol, mmol/L | 4.26 ± 0.11 | −0.54 ± 0.053 | −0.55 ± 0.053 | −0.58 ± 0.0543 | −0.59 ± 0.053 | −0.60 ± 0.0483 |
| TG, mmol/L | 1.75 ± 0.08 | −0.14 ± 0.053,a | −0.07 ± 0.043,a | −0.50 ± 0.043,b | −0.19 ± 0.053,a | −0.17 ± 0.043,a |
| ApoA1, mmol/L | 1.47 ± 0.02 | −0.06 ± 0.01a | −0.05 ± 0.01a | −0.04 ± 0.01a | −0.07 ± 0.01a | −0.10 ± 0.013,b |
| ApoB, mmol/L | 1.06 ± 0.03 | −0.11 ± 0.013,a,b | −0.11 ± 0.013,a,b | −0.10 ± 0.013,a | −0.13 ± 0.013,b | −0.12 ± 0.0113,b |
1Values are means ± SEMs, n = 101. Means in a row without a common superscript letter differ, P < 0.05 (mixed-model post-hoc analysis). CanolaDHA, DHA-enriched high oleic acid–canola oil; CanolaOleic, high oleic acidcanola oil; Corn/Saff, corn oil and safflower oil blend; Flax/Saff, flax oil and safflower oil blend; MetS, metabolic syndrome.
2Baseline data were collected on day 1 when participants were first enrolled in the study prior to diet intervention.
3Different from baseline, P < 0.05.
Diet effects on serum-mediated cholesterol efflux from THP-macrophages
There was no significant difference in changes in serum-mediated cholesterol efflux capacity among the 5 diets. After 4 wk, all diets increased serum-mediated cholesterol efflux similarly when compared with baseline (Figure 2). Serum-mediated cholesterol efflux from THP-1 was increased by 39.1% (P = 0.021), 33.6% (P = 0.047), 55.3% (P = 0.0096), 49.2% (P = 0.014), and 50.7% (P = 0.012) for the canola oil, CanolaOleic, CanolaDHA, Corn/Saff, and Flax/Saff oil diets, respectively. There was an effect of BMI status on cholesterol efflux across the 3 BMI categories compared with baseline (normal BMI, n = 13, P = 0.0003; overweight, n = 33, P = 0.03; obese, n = 53; P = 0.04) (Figure 3). Participants with a normal BMI had a greater increase in cholesterol efflux capacity (93%) compared with overweight (67%, P = 0.04) and obese participants (25%, P = 0.03) after diet intervention (all diets combined). Cholesterol efflux capacity did not differ between overweight and obese participants.
FIGURE 2.
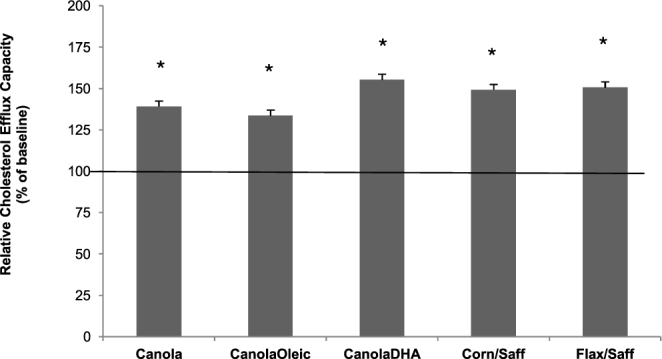
Relative cholesterol efflux capacity in response to diets low in SFAs with varying unsaturated fatty acid profiles after 28 d in participants with or at risk for metabolic syndrome. *Means are significantly different from baseline, P < 0.05 (n = 101). Values are means + SEMs. Canola, canola oil; CanolaDHA, DHA-enriched high oleic acid–canola oil; CanolaOleic; high oleic acid–canola oil; Corn/Saff, corn oil and safflower oil blend; Flax/Saff, flax oil and safflower oil blend.
FIGURE 3.
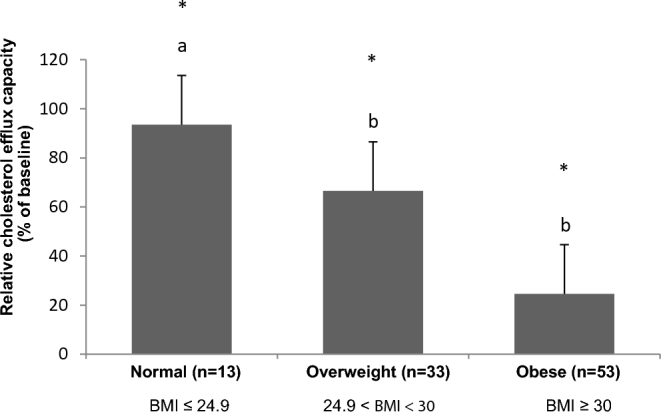
Relative cholesterol efflux capacity as a function of BMI status (all diets combined). Means without a common letter differ, P < 0.05 (n = 99) (mixed-model post-hoc analysis). *Means are significantly different from baseline, P < 0.05.
Diet regulates MiR expression
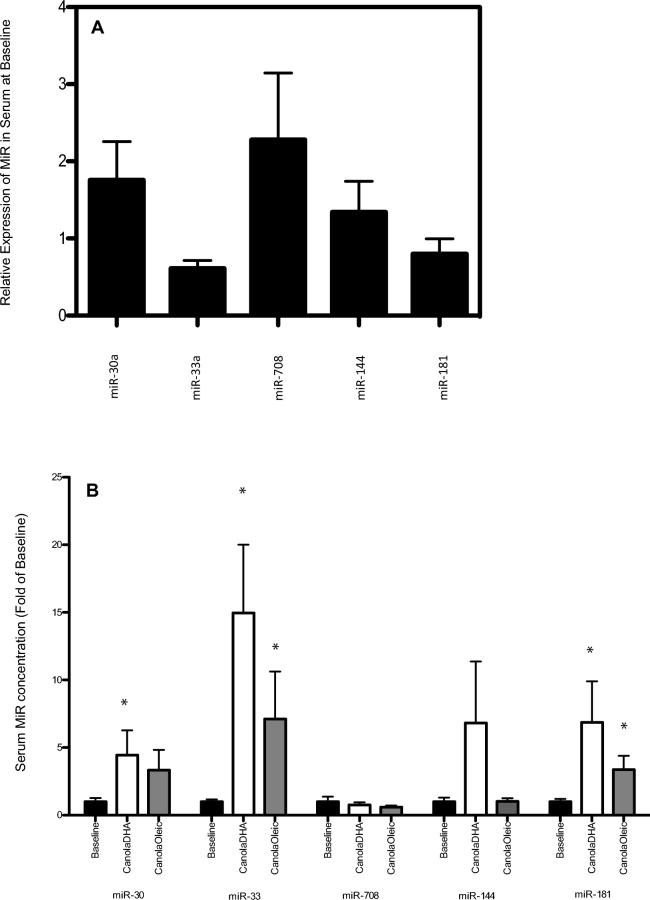
We further investigated the effect of 2 diets: CanolaDHA (highest DHA content), which increased cholesterol efflux the most (from baseline); and CanolaOleic (highest MUFA content), which had the smallest effect on cholesterol efflux on plasma MiR expression and their regulation of cholesterol efflux. Five MiRs (miR-181c-5p, miR-30c-5p, miR-33, miR-144, and miR-708) known for their roles in cholesterol metabolism were quantified at baseline and after consumption of the 2 diets (Figure 4). CanolaOleic and CanolaDHA significantly increased the expression of circulating miR-181a and miR-33 in serum when compared with baseline. CanolaDHA increased the expression of miR-30. Statistical significance was determined by one-way ANOVA within each group (P < 0.05). There were no significant differences in the expression of the miR-708 or miR-144 among the 2 treatment diets tested.
FIGURE 4.
MiR expression in sera from participants with or at risk for metabolic syndrome at baseline (n = 99) (A). Relative expression is MiR expression after normalization by RNU6 control gene. (B) MiR expression in sera in response to the CanolaDHA and CanolaOleic diets after 28 d in participants with or at risk for metabolic syndrome (n = 99). Values are means + SEMs. *Different from baseline, P < 0.05. CanolaDHA, DHA-enriched high oleic acid–canola oil; CanolaOleic, high oleic acid–canola oil; MiR, microRNA.
Discussion
In this randomized, crossover, 5 diet-period, controlled-feeding trial, we found that there was an inverse association between central obesity and serum-mediated cholesterol efflux capacity. In addition, we found that consumption of diets low in SFAs and high in unsaturated fatty acids increase serum-mediated cholesterol efflux capacity in participants with or at risk for MetS. The similar increase in serum-mediated cholesterol efflux in response to all diets suggests that HDL function is independent of HDL cholesterol concentrations.
A novel aspect of our study is the systematic comparison of serum-mediated cholesterol efflux among diets that differed in unsaturated fatty acid profiles in a population with or at risk for MetS. Our findings add to the evidence base that diets high in unsaturated fatty acids improve cholesterol efflux capacity. Kasbi Chadli et al. (18) reported that hamsters fed an n–3 fatty acid–enriched diet (21% w/w fat with 8.6% SFAs, 8.6% MUFAs, 3.5% n–6 PUFAs and 1% of energy as n–3 PUFAs) for 20 wk had a higher cholesterol efflux (31.9%) than did those fed a diet lower in both n–3 and n–6 PUFAs (21% w/w fat including 9.3% SFAs, 9.8% MUFAs, 1.9% n–6 PUFAs) and the control diet (5% w/w fat including 2% SFAs, 1.8% MUFAs, 1.2% n–6 PUFAs). Our results demonstrate that participants had a 55.3% increase in serum-mediated cholesterol efflux capacity after consumption of the CanolaDHA diet (DHA, 1.1% of energy) compared with baseline. A potential mechanism to explain this could be that serum-mediated cholesterol efflux is affected by HDL phospholipid fatty acid composition in response to the diet. Diets enriched with long-chain PUFAs may alter HDL phospholipid fatty acid composition and improve the fluidity of particle membranes (19, 20); HDL particles with the greater fluid surface then accept free cholesterol at a faster rate (21). In the present study, the lowest LI was observed after consumption of the CanolaDHA diet, which is indicative of greater membrane fluidity. Despite having the lowest LI, the cholesterol efflux capacity of the CanolaDHA diet did not differ statistically from the other experimental diets, although it was numerically higher.
The 2 diets enriched with MUFA also increased serum-mediated cholesterol efflux (canola oil, 39.1%; CanolaOleic, 33.6%), which agrees with a study conducted by Sola et al. (22), who reported that consumption of a diet enriched with olive oil (15.6% of total energy) for 7 wk increased the capacity of HDL3 to promote cholesterol efflux. Helal et al. (4) also found that consumption of extra-virgin olive oil (71% MUFA) for 12 wk increased the capacity of serum to mediate cholesterol efflux from THP-1 macrophages by 9.8% (P < 0.01), and this was associated with a 13% increase in the fluidity of the HDL phospholipid layer. It is possible that the improvement in serum-mediated cholesterol efflux capacity in the present study was a result of diet-induced physiochemical changes in HDL via greater fluidity or a smaller particle size, or an upregulation of transport protein (ABCA1/G1) gene expression resulting in an increased efficiency in accepting free cholesterol (4, 23, 24). Importantly, the lack of an association between serum-mediated cholesterol efflux and HDL cholesterol concentrations reported herein illustrates the independent relation between HDL function and HDL cholesterol concentrations.
We observed a greater increase in serum-mediated cholesterol efflux in participants (n = 99) with a normal BMI (but with an increased waist circumference) compared with overweight and obese participants. Obesity adversely affects HDL composition, which has been shown to impair HDL function (25). Indeed, obesity is associated with increased inflammation and oxidative stress, both of which could contribute to an impaired HDL function and consequent diminution of cholesterol efflux (26, 27). In addition, we observed an inverse association between waist circumference and serum-mediated cholesterol efflux capacity, and also between abdominal fat mass and serum-mediated cholesterol efflux capacity. Thus, evidence is accumulating that an increase in visceral adipose tissue mass impairs HDL function.
The exploratory analysis of MiR showed that diets low in SFAs and high in MUFAs and DHA may affect MiR in a way that could increase cholesterol efflux capacity. miR-181 expression inhibits NF-κB activity and target gene expression, which has been shown to decrease inflammation and atherosclerosis in an animal model (28). The increase of miR-181 expression in response to the CanolaDHA and the CanolaOleic diets may protect the HDL particles from inflammation, thus perhaps explaining, in part, the increase in serum-mediated cholesterol efflux. miR-144 suppresses the ABCA1 expression, thereby blunting HDL cholesterol efflux (29). In the present study both CanolaOleic and CanolaDHA did not affect miR-144 gene expression. In contrast with previous findings, we found an increase in the miR-33 for both diets, which previously has been reported to be associated with a decrease in cholesterol efflux in animal studies (30). Further studies are required to understand how diet-induced changes in MiR regulate cholesterol efflux.
Strengths of the present study include the controlled-feeding study design and the precise delivery of the treatment oils. In addition, the crossover study design minimized within-individual variation (31). A limitation of our study is that we compared the treatment diets with the participants’ habitual baseline diet, which we considered to be representative of a Western diet, and for which there was no diet control. Another limitation is that we used whole serum as an acceptor for free cholesterol, which measures the transfer of cellular cholesterol to either HDL or apoB-containing lipoproteins (32). Thus the proportion of efflux contributed by each active transporter is unknown. On the other hand, recent evidence has shown that in addition to HDL particles, apoB-containing lipoproteins also contribute to cholesterol efflux from macrophages by being the potential cellular cholesterol acceptors for both the scavenger receptor class B type 1 (SR-BI)- and the ATP-binding cassette sub-family G member 1 (ABCG1)-mediated cholesterol efflux (33, 34). The question has been raised about the impact of storage and freezing of serum on structural and functional properties of lipoproteins and their effect on the cholesterol efflux assay (35, 36). However, Sankaranarayanan et al. (36) compared cholesterol efflux in fresh and frozen serum and reported similar results.
In summary, consumption of diets low in SFAs and high in MUFAs or PUFAs (n–6 fatty acids or n–3 fatty acids—both plant and marine derived) increased serum-mediated cholesterol efflux in participants with or at risk for MetS. The present study underscores the adverse effects of obesity on cholesterol efflux. Importantly, our findings add to the evidence in support of the beneficial effect of diets in which unsaturated fats replace SFAs for reducing cardiometabolic risk that we suggest are mediated, in part, by an increase in HDL function as measured by cholesterol efflux. Overweight and obese individuals will benefit from a heart-healthy diet low in SFAs and high in unsaturated fatty acids that increases cholesterol efflux, and with weight loss, cholesterol efflux would be expected to improve further.
Supplementary Material
Acknowledgments
We thank the clinical coordinators and nurses Tracy Allen, Cyndi Flanagan, Laurie Aquilino for their assistance. The authors’ responsibilities were as follows: PJJ, BL, DJJ, JAF, SGW, and PMK-E: designed the study; XL, PJJ, BL, DJJ, PWC, PC, SP, JAF, SGW, and PMK-E: conducted the research; JG and JVH: conducted the cholesterol efflux assays; XL and JVH: performed the statistical analysis; XL and PMK-E: wrote the article; PJJ, BL, DJJ, PWC, and PC: reviewed the manuscript; and all authors: read and approved the final manuscript.
Notes
XL and JG contributed equally to this manuscript.
Supported by Agriculture and Agri Food Canada, Canola Council of Canada, Dow Agrosciences and Flax Council of Canada. The project described was supported by the National Center for Research Resources, Grant UL1 RR033184, and is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127. This project was also supported by NIH Grant 2T32DK007703. DSM Company (Dr Norm Salem) provided a gift in support of the cholesterol efflux assay. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Methods and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Author disclosures: PJHJ received grants from Advanced Foods and Materials Network, Danone, Enzymotec, Unilever, the Canadian Institutes of Health Research (CIHR) and Canada Research Chair Endowment of the Federal Government of Canada. PJHJ also serves as President of Nutritional Fundamentals for Health Inc. BL and PC received research grants from CIHR, Agrifood and Agriculture Canada, the Dairy Farmers of Canada, Dairy Australia. BL received research funding from Atrium Innovations and is Chair of Nutrition, supported by endowments from Provigo/Loblaws, Pfizer and Royal Bank of Canada. DJAJ has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd, Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council (CCC), the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund. He has received in-kind supplies as research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), FareWell, Verywell, True Health Initiative, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca-Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 WO Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, ALJ, is a director and partner of Glycemic Index Laboratories, Inc., and his sister received funding through a grant from the St Michael's Hospital Foundation to develop a cookbook for one of his studies. SGW has received research funding and consulting and travel fees from the Canola Council of Canada and Flax Canada. PMK-E serves on the California Walnut Commission Scientific Advisory Council, the Avocado Nutrition Science Advisory, and the Seafood Nutrition Partnership Scientific & Nutrition Advisory Council. XL, JG, JVH, PWC, SP, and JAF, no conflicts of interest.
Abbreviations used: ABCA1, ATP-binding cassette transporter gene; CanolaOleic, high oleic acid–canola oil; CanolaDHA, DHA-enriched high oleic acid–canola oil; COMIT I, Canola Oil Multicentre Intervention Trial; Corn/Saff, corn and safflower oil blend; Flax/Saff, flax and safflower oil blend; LI, lipophilic index; MetS, metabolic syndrome; MiR, microRNA.
References
- 1. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012;125:1905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brousseau ME. Emerging role of high-density lipoprotein in the prevention of cardiovascular disease. Drug Discov Today 2005;10:1095–101. [DOI] [PubMed] [Google Scholar]
- 4. Helal O, Berrougui H, Loued S, Khalil A. Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br J Nutr 2012;109:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Holligan SD, West SG, Gebauer SK, Kay CD, Kris-Etherton PM. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br J Nutr 2014;112:744–52. [DOI] [PubMed] [Google Scholar]
- 6. Efrat M, Aviram M. Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis—a protective role for HDL phospholipids. Adv Exp Med Biol 2010;660:153–66. [DOI] [PubMed] [Google Scholar]
- 7. Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem 2010;285(44):33652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramirez CM, Davalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suarez Y, Fernandez-Hernando C. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol 2011;31:2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C. MiR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Exp Neurol 2012;235:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soh J, Iqbal J, Queiroz J, Fernandez-Hernando C, Hussain MM. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med 2013;19:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 2014;24(3):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Senanayake VK, Pu S, Jenkins DA, Lamarche B, Kris-Etherton PM, West SG, Fleming JA, Liu X, McCrea CE, Jones PJ. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT). Trials 2014;15:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, Couture P, Charest A, Baril-Gravel L, West SG et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr 2014;100:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care 2005;28:2745–9. [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, McCrea CE, Pu S, Couture P, Connelly PW et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring) 2016;24:2261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Cai S, Peterson BR, Kris-Etherton PM, Heuvel JP. Development of a cell-based, high-throughput screening assay for cholesterol efflux using a fluorescent mimic of cholesterol. Assay Drug Dev Technol 2011;9(2):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu H, Ding EL, Toledo ET, Campos H, Baylin A, Hu FB, Sun Q. A novel fatty acid lipophilic index and risk of CHD in US men: the health professionals follow-up study. Br J Nutr 2013;110:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasbi Chadli F, Nazih H, Krempf M, Nguyen P, Ouguerram K. Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS One 2013;8:e61109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fournier N, Atger V, Cogny A, Vedie B, Giral P, Simon A, Moatti N, Paul JL. Analysis of the relationship between triglyceridemia and HDL-phospholipid concentrations: consequences on the efflux capacity of serum in the Fu5AH system. Atherosclerosis 2001;157:315–23. [DOI] [PubMed] [Google Scholar]
- 20. Ottestad I, Hassani S, Borge GI, Kohler A, Vogt G, Hyotylainen T, Oresic M, Bronner KW, Holven KB, Ulven SM et al. Fish oil supplementation alters the plasma lipidomic profile and increases long-chain PUFAs of phospholipids and triglycerides in healthy subjects. PLoS One 2012;7:e42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davidson WS, Gillotte KL, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem 1995;270:5882–90. [DOI] [PubMed] [Google Scholar]
- 22. Sola R, Motta C, Maille M, Bargallo MT, Boisnier C, Richard JL, Jacotot B. Dietary monounsaturated fatty acids enhance cholesterol efflux from human fibroblasts. Relation to fluidity, phospholipid fatty acid composition, overall composition, and size of HDL3. Arterioscler Thromb 1993;13:958–66. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Grieger JA, Kris-Etherton PM, Thompson JT, Gillies PJ, Fleming JA, Vanden Heuvel JP. Walnut oil increases cholesterol efflux through inhibition of stearoyl CoA desaturase 1 in THP-1 macrophage-derived foam cells. Nutr Metab 2011;8:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Kris-Etherton PM, Thompson JT, Hannon DB, Gillies PJ, Vanden Heuvel JP. Alpha-linolenic acid increases cholesterol efflux in macrophage-derived foam cells by decreasing stearoyl CoA desaturase 1 expression: evidence for a farnesoid-X-receptor mechanism of action. J Nutr Biochem 2012;23:400–9. [DOI] [PubMed] [Google Scholar]
- 25. Aicher BO, Haser EK, Freeman LA, Carnie AV, Stonik JA, Wang X, Remaley AT, Kato GJ, Cannon RO III. Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring) 2012;20:2057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009;119:1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, Tabita-Martinez J, Wolfe ML, Badellino K, Pruscino L et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis 2012;222:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK et al. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res 2014;114:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang MH, Zhang LH, Wijesekara N, de Haan W, Butland S, Bhattacharjee A, Hayden MR. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol 2013;33:2724–32. [DOI] [PubMed] [Google Scholar]
- 30. Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, Ford DA, Baldan A. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med 2012;4:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. AbuMweis SS. Optimizing clinical trial design for assessing the efficacy of functional foods. Nutr Rev 2010;68:485–99. [DOI] [PubMed] [Google Scholar]
- 32. Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 2014;289:24020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res 2009;50:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Treguier M, Moreau M, Sposito A, Chapman MJ, Huby T. LDL particle subspecies are distinct in their capacity to mediate free cholesterol efflux via the SR-BI/Cla-1 receptor. Biochim Biophys Acta 2007;1771:129–38. [DOI] [PubMed] [Google Scholar]
- 35. Holzer M, Kern S, Trieb M, Trakaki A, Marsche G. HDL structure and function is profoundly affected when stored frozen in the absence of cryoprotectants. J Lipid Res 2017;58:2220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, Rothblat GH. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res 2011;52:2332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.