ABSTRACT
Background
The relation between subclinical hemoglobinopathies and concentrations of the iron-regulatory hormone hepcidin is not well characterized.
Objective
We investigated the relation of hepcidin concentration with hemoglobinopathies among young children in Kenya.
Methods
We quantified serum hepcidin and ferritin in 435 Kenyan children aged 14–20 mo in a subsample of the Water, Sanitation, and Handwashing (WASH) Benefits Trial. Blood samples were genotyped for α+-thalassemia and for sickle cell disorder. Hepcidin was compared across sickle cell and α+-thalassemia genotypes separately by using generalized linear models, and children who were normozygous for both conditions were also compared with those who had either of these conditions. In the association between hepcidin and ferritin, we assessed effect modification by genotype.
Results
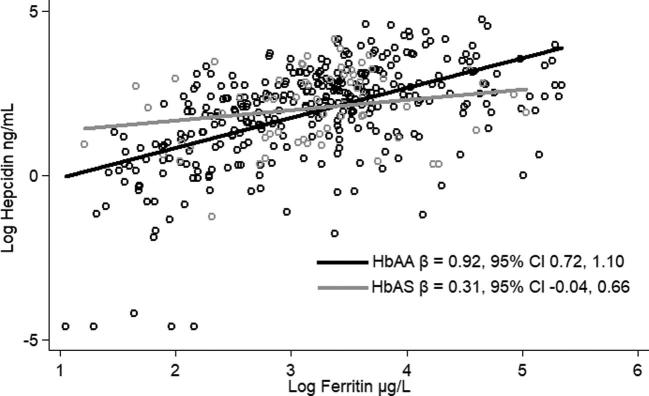
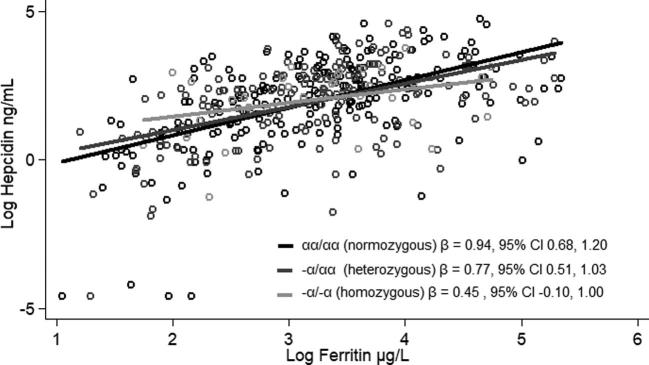
In this population, we found that 16.2% had sickle cell trait and 0.2% had sickle cell disorder, whereas 40.0% were heterozygous for α+-thalassemia and 8.2% were homozygous. Hepcidin concentration did not differ by genotype, but effect modification was found by genotype in the association between hepcidin and ferritin (P < 0.1). Among normozygous sickle cell children (HbAA), there was an association between hepcidin and ferritin (β = 0.92; 95% CI: 0.72, 1.10). However, among those with sickle cell trait (HbAS), the association was no longer significant (β = 0.31; 95% CI: −0.04, 0.66). Similarly, among children who were normozygous (αα/αα) or heterozygous (−α/αα) for α+-thalassemia, hepcidin and ferritin were significantly associated [β = 0.94 (95% CI: 0.68, 1.20) and β = 0.77 (95% CI: 0.51, 1.03), respectively]; however, in children who were homozygous for α+-thalassemia (−α/−α), there was no longer a significant association (β = 0.45; 95% CI: −0.10, 1.00).
Conclusion
Hepcidin was not associated with hemoglobin genotype, but there may be a difference in the way hepcidin responds to iron status among those with either sickle cell trait or homozygous α+-thalassemia in young Kenyan children. This trial was registered at clinicaltrials.gov as NCT01704105.
Keywords: sickle cell, α+-thalassemia, hepcidin, iron status, ferritin
Introduction
Hemoglobinopathies are common in malaria-endemic regions around the world, and are thought to have co-evolved with the presence of malaria parasites, because hemoglobinopathies provide protection from severe and fatal malaria (1–4). Of the 300,000–400,000 infants born with a serious hemoglobinopathy each year, 90% are in low-income countries (1), in which malaria infection is also common. The βs mutation in the HBB gene, which causes both sickle cell disorder (HbSS) and sickle cell trait (HbAS), and α+-thalassemia are 2 hemoglobinopathies that are found at a high prevalence in Kenya. In a study from western Kenya, 1.6% of children had HbSS and 17.1% had HbAS, whereas 39.6% had heterozygous (−α/αα) and 9.7% had homozygous (−α/−α) α+-thalassemia (5).
The presence of a hemoglobinopathy commonly manifests as anemia, ranging in severity from mild to severe (6). However, in countries with a prevalence of anemia >40%, iron supplementation programs are recommended for children aged 6–23 mo (7), despite the fact that the anemia may not be caused by iron deficiency. Ferritin concentration, corrected for inflammation (8), is needed to detect a true iron deficiency, and reference ranges have been established for children (9). Soluble transferrin receptor (sTfR) can also indicate an iron deficiency when concentrations are increased, although an increase in sTfR may also reflect an increase in erythrocyte turnover (10).
Hepcidin is a small, 25-amino-acid peptide and an iron-regulatory hormone that has been documented as a key regulator in iron homeostasis (11–13). It effectively blocks iron absorption in the duodenum and prevents the release of iron from the spleen and liver through binding and degrading the iron export protein ferroportin (13). Hepcidin expression is increased when body iron concentrations increase (14). When hepcidin is suppressed by ineffective erythropoiesis (IE), iron absorption is usually increased regardless of the iron concentration present in the plasma and other tissues (13).
IE is characterized by both RBC precursors, which do not fully differentiate, and early apoptosis of those precursors. IE leads to an overall decrease in the concentration of erythrocytes (15), which may result in anemia. IE occurs in individuals with all thalassemia types to various degrees (16) and suppresses hepcidin, most likely via the hormone erythroferrone (17). However, to our knowledge, hepcidin suppression in those with thalassemia traits has been documented previously in only one study in 15 adults in Brazil (18). With regard to sickle cell traits, the association between HbAS and IE has not been studied. However, there is some evidence that erythropoiesis is increased, if not ineffective, in the presence of HbAS (19) because the body compensates for erythrocytes that have a shorter life span. The relation between IE and hemoglobinopathy traits warrants further investigation, as does the relation between hemoglobinopathy traits and hepcidin.
Our primary objective for this analysis was to investigate the relations between both sickle cell and α+-thalassemia genotypes and serum hepcidin concentrations. Our second objective was to evaluate whether the association between hepcidin and ferritin was altered among children afflicted with these same genetic polymorphisms.
Methods
Study overview and participants
This study was nested within the Water, Sanitation, and Handwashing (WASH) Benefits Trial, which was conducted in rural, western Kenya and designed to investigate the impact of scalable water, sanitation, handwashing (WASH) and nutrition interventions on child growth and development. Details of the WASH Benefits trial have been published elsewhere (20, 21). The WASH Benefits trial took place from September 2012 to August 2016. Measurements for this substudy, which are tertiary outcomes of the main randomized controlled trial, were collected from November 2012 to July 2014.
Women and their households were eligible for enrollment if they were in their second or third trimester of pregnancy, if the family owned their house and were not planning to move within the next 12 mo, and if the family spoke English, Kiswahili, or Kiluyha. The children born to enrolled women were defined as index children.
Eligible households in the main trial were cluster-randomized to 1 of 8 treatment arms: a water arm, a sanitation arm, a handwashing arm, a combination of the 3 interventions (WSH), the nutrition-only arm (N), the WSH plus N arm (WSH+N), a passive control arm, and a double-sized active control arm. Clusters were defined as 1–3 villages with ≥6 women/cluster. In brief, households in the water arms were provided with chlorine for the treatment of drinking water; in the sanitation arms, households were provided with tools to clean up children's feces in the compound and were given pit latrines or pit latrine upgrades, as needed; in the handwashing arms, handwashing stations were built near the kitchen and latrine of every household; and in the N arms, children aged 6–24 mo were given 10 g of small-quantity lipid-based nutrient supplements to take twice daily, which, among other nutrients, contained encapsulated ferrous sulfate, yielding 4.5 mg Fe/packet for a daily dose of 9 mg. With each intervention, community-based health promoters communicated behavior-change messages adapted for the local culture. Supplements and interventions have been previously described in detail (20, 21). The study promoter did not visit participants in the passive control arm; in the active control arm, the study promoter visited once monthly to take a midupper arm circumference measurement. This substudy utilized 4 out of the 8 arms from the main study—WSH, N, WSH+N, and the active control arm—which were selected for the environmental enteric dysfunction (EED) substudy, details of which have been published elsewhere (20).
Sample size
The sample size of the main trial was 8000 families, and the EED substudy targeted a sample size of 1500 children, sampled proportionally from 4 arms of the main trial (WSH, N, WSH+N, and active control) and who were visited at 6 mo, 1 y, and 2 y after the start of the intervention. Genotyping was conducted at year 1, whereas the iron and inflammatory biomarker measurements were conducted at year 2. Assuming a sample size of 1500 infants, power (1-β) = 0.80, α = 0.05, an intracluster correlation coefficient of 0.1, a prevalence of HbAS of 17%, and 20% loss to follow-up, we calculated the minimum detectable difference in hepcidin concentrations to be 0.9 ng/mL comparing children with HbAS to normozygous (HbAA) children. Using the same sample size parameters for investigating α+-thalassemia, and assuming a prevalence of −α/αα of 37% and −α/−α of 9%, we calculated the minimum detectable difference of 1.2 ng/mL, comparing those with either type of α+-thalassemia to those who were normozygous.
Procedures
Families for this substudy were visited at ∼3 mo postpartum, at year 1, and at year 2 after the start of intervention activities. Trained enumerators visited families to conduct surveys using electronic forms; at the end of each day, the surveys were uploaded to a server (SurveyCTO; Dobility).
Phlebotomists collected a nonfasted morning blood sample from the target child. A maximum of 5.0 mL venous blood was collected using an Sarstedt monovette 2.6-mL serum collection tube and an Sarstedt monovette 2.6-mL lithium heparin plasma collection tube. Blood collection was restricted to children who were free from reported or visible illnesses and did not show any signs of dehydration. Samples were centrifuged in the field for 15 min at 3500 rpm, separated in aliquots into serum or plasma tubes, and immediately placed in a cooler box. Packed cells were separately placed into aliquots. Upon returning to the laboratory, the samples were placed in a −20°C freezer, until they could be transferred on dry ice to a −80°C freezer in Nairobi.
Field and laboratory methods
Tests for Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae were conducted immediately after the blood draw by using rapid diagnostic kits (Malaria Ag, P.f, P.f, P.v, Alere; SD Bioline). Any child found to be positive for any type of malaria, combined with an axillary temperature of ≥37.5°C, was referred to the nearest clinic. A drop of blood from the venous blood samples was used to measure hemoglobin concentrations (Hemocue Hb 301). Anemia among the children was defined as a hemoglobin concentration <11 g/dL (22).
Frozen serum samples were sent to the VitMin Laboratory (Willstaett, Germany) and analyzed for ferritin, sTfR, C-reactive protein (CRP), and α-1-acid glycoprotein (AGP) with the use of a sandwich ELISA (23). In determining iron deficiency, ferritin was corrected for inflammation using the method described by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) group (8, 24). We then applied a cutoff of ferritin <12 µg/L to determine iron deficiency. Aliquots of frozen plasma packed cells were sent to the Kenya Medical Research Institute (KEMRI)/Wellcome Trust Research Program in Kilifi where sickle cell and α+-thalassemia genotyping was conducted using PCR, as described in detail previously (25, 26). Children with a single α-globin deletion (−α/−α) were defined as heterozygotes for α+-thalassemia, whereas those with 2 α-globin deletions (−α/−α) were defined as homozygotes.
Serum hepcidin-25 was quantified by using a competitive ELISA kit (PenLabs) per the manufacturer's protocol. Samples were diluted 1:5 in standard diluent (peptide-cleared human serum) and analyzed using a serial dilution curve, with a maximum concentration of 25 ng/mL. Concentration values were interpolated from the standard curve by using a logistic 4-point-parameter nonlinear curve fitted by using Gen5 software (Biotek). The lower limit of detection was estimated to be 0.04 ng/mL on the basis of the mean blank reading at the optical density at 450 nm. Samples below the limit of detection were reported at the limit of detection/4 = 0.01. The intra-assay CV was a mean of 11.5%. The difference between 2 duplicate hepcidin measurements was, on average, 0.291 ng/mL, except for 7 samples in which the difference was >5 ng/mL (representing 0.01% of the 435 samples). We conducted a sensitivity analysis and, when excluding these 7 samples, the β-coefficients did not change. Thus, the 7 samples were left in the analysis.
Statistical methods
Statistical analyses used Stata 14 software (StataCorp). Hepcidin, ferritin, sTfR, CRP, and AGP were log-transformed to achieve a normal distribution.
A generalized linear model was used to examine the association between hepcidin concentrations at year 2 and sickle cell genotype, with robust SEs at the study block level (independent units in the trial). The same model was used to examine the association between hepcidin and the α+-thalassemia genotype. In addition, indicator variables were included in the model to control for intervention arm. Potential covariates for inclusion in the final adjusted models were based on current literature and biological plausibility and included child age, child sex, season, and the presence of the hemoglobinopathy, which was not the exposure variable of interest in a given analysis. Season was defined as the month in which the measurement was taken. In addition, in this investigation, we did not control for iron or inflammatory biomarkers when determining the relation between the hemoglobinopathy traits and hepcidin, because these variables are known influencers of hepcidin concentration, and therefore on the causal pathway. We included season for consideration in the model for its potential to be associated with hepcidin concentration.
Covariates were assessed by using a bivariate generalized linear model with robust SEs with hepcidin as the outcome. Those that were significant at the level of P < 0.10 for hepcidin were included in the adjusted model.
In exploratory analyses, interactions considered included serum ferritin as well as age and sex. Because serum ferritin has been shown to be influenced by hemoglobinopathy traits (27), we hypothesized that the relation between hepcidin and genotype may differ by ferritin concentration. Sex (28) and age (29) have both been shown to affect hepcidin concentrations, and thus may modify the relation between the presence of a hemoglobinopathy and hepcidin. Interactions were considered significant at the P < 0.10 level, and if significant interactions were found, we presented stratified estimates.
Ethics
Ethical approval for the study protocol and consent procedure was provided by the institutional review boards of the Kenya Medical Research Institute (protocol SSC-2271) and the University of California, Berkeley (protocol 2011-09-3654). This trial was registered at clinicaltrials.gov (NCT01704105). Innovations for Poverty Action implemented the intervention delivery and collected the data. Mothers provided written informed consent for themselves and their children.
Results
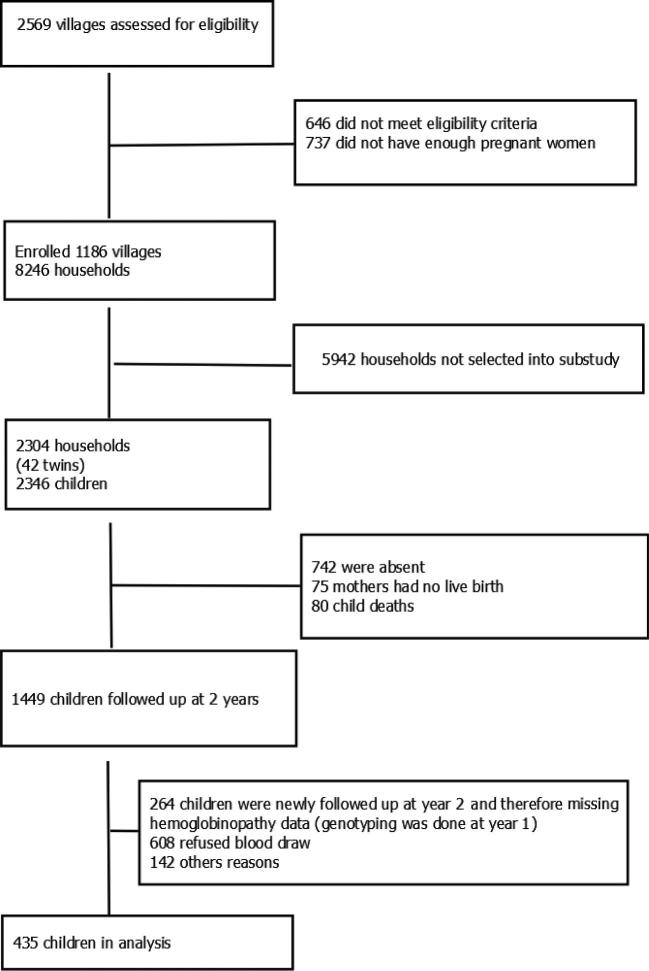
Of the 2304 families enrolled in the EED substudy, 1449 children were included in the year 2 follow-up, 48% (n = 699) provided a sufficient blood sample, and 30% (n = 435) had data available on both hemoglobinopathy traits and hepcidin (Figure 1). The decrease in study population from 1499 to 699 children was mainly attributable to a low rate of caretaker consent for the blood draw at year 2. In addition, there was relatively little overlap in children between the year 1 follow-up, which is when the genotyping was done, and the year 2 follow-up, which is when we collected the sample to analyze hepcidin (Figure 1). This lack of overlap between year 1 and year 2 was the factor that decreased our sample size from 699 to 435. However, there were no significant differences in baseline characteristics between the households that did not contribute to the analysis and the households that did (Supplemental Table 1). Of all 435 children, 55.3% had a hemoglobinopathy trait. The prevalence of HbAS in the study was 16.2%; one child was found to have HbSS, representing 0.2%; and the rest (84.5%) were HbAA. For the α+-thalassemia genotype, 40% were heterozygous (−α/αα), 8.2% were homozygous (−α/−α), and 51.8% of the children were normozygous (αα/αα). Thirty-one of the children had both HbAS and −α/αα (7.1%), and 4 had HbAS and −α/−α (1%). The HbSS participant was also homozygous for α+-thalassemia (−α/−α).
FIGURE 1.
Study flow diagram for the inclusion of children aged 14–26 mo in the final analysis.
Characteristics of the study population, reported by genotype, are shown in Table 1. There were no differences in the characteristics of hemoglobinopathy trait groups when compared with the normozygous children in terms of their iron or inflammatory biomarkers. There were significant differences in the hemoglobin concentrations of the α+-thalassemia trait groups but not in the sickle cell genotypes. Overall, the median (Quartile 1, Q3) of CRP was 0.97 mg/L (0.34, 3.51 mg/L) and for AGP was 0.93 g/L (0.68, 1.53 g/L). At the time of the blood draw, 49% of the children sampled had inflammation (CRP ≥5 mg/L or AGP ≥1 g/L) and 17% had a positive malaria rapid test. The median (Quartile 1, Q3) of serum ferritin concentrations was 14.74 µg/L (8.25, 23.18 µg/L) and for sTfR was 10.07 mg/L (7.82, 14.85 mg/L). A total of 22.1% children were iron deficient (ferritin <12 µg/L) without correcting for inflammation; with the use of the regression correction method proposed by the BRINDA group (8), the percentage with iron deficiency increased to 37.4%. Anemia was found in 32.7% of children, and of those, 16.7% were anemic due to iron deficiency (hemoglobin <110 g/L and inflammation-corrected ferritin <12 µg/L).
TABLE 1.
Characteristics of rural Kenyan children aged 14–26 mo by genotype1
| Sickle cell genotype | α+-Thalassemia genotype | |||||||
|---|---|---|---|---|---|---|---|---|
| Normozygous | Sickle cell trait | Normozygous | Heterozygous | Homozygous | ||||
| Characteristics | (HbAA) (n = 337) | (HbAS) (n = 64) | P | (αα/αα) (n = 210) | (–α/αα) (n = 64) | P | (−α/−α) (n = 34) | P |
| Infant age, mo | 22.2 ± 1.8 | 22.3 ± 1.9 | 0.78 | 22.3 ± 1.8 | 22.0 ± 1.8 | 0.13 | 22.3 ± 1.5 | 0.81 |
| Male sex, % | 48.7 | 38.5 | 0.07 | 45.8 | 52.7 | 0.37 | 55.9 | 0.40 |
| Primiparous mother, % | 12.5 | 15.0 | 0.53 | 12.7 | 14.6 | 0.62 | 10.0 | 0.70 |
| sTfR, mg/L | 10.4 (8.0, 15.2) | 11.0 (7.6, 17.0) | 0.92 | 10.1 (7.8, 14.5) | 11.2 (8.1, 16.5) | 0.17 | 10.6 (8.2, 15.4) | 0.81 |
| Ferritin, µg/L | 26.3 (12.6, 42.8) | 26.2 (16.6, 37.3) | 0.58 | 26.0 (12.9, 40.3) | 27.3 (13.0, 43.0) | 0.96 | 29.7 (13.7, 44.8) | 0.74 |
| CRP, mg/L | 1.2 (0.3, 4.6) | 0.8 (0.3, 2.7) | 0.13 | 1.0 (0.3, 4.2) | 1.2 (0.3, 3.5) | 0.97 | 2.0 (0.6, 4.6) | 0.23 |
| AGP, g/L | 1.0 (0.7, 1.7) | 0.9 (0.6, 1.1) | 0.05 | 1.0 (0.7, 1.6) | 0.9 (0.7, 1.6) | 0.71 | 1.0 (0.7, 1.8) | 0.76 |
| Any inflammation (CRP ≥5 mg/L or AGP ≥1 g/L), % | 52.0 | 38.5 | 0.06 | 52.3 | 45.5 | 0.13 | 58.8 | 0.50 |
| Any malaria (positive RDT), % | 17.1 | 14.8 | 0.62 | 14.1 | 20.7 | 0.10 | 13.8 | 0.89 |
| Iron deficiency (ferritin <12 µg/L), % | 22.6 | 17.2 | 0.24 | 21.8 | 21.5 | 0.93 | 20.6 | 0.89 |
| Iron deficient with inflammation correction,2 % | 41.7 | 33.3 | 0.11 | 42.6 | 37.6 | 0.27 | 33.3 | 0.31 |
| Hemoglobin concentration, g/dL | 11.3 ± 1.3 | 11.5 ± 1.3 | 0.43 | 11.6 ± 1.3 | 11.2 ± 1.3 | <0.01 | 10.8 ± 1.2 | 0.003 |
| Anemia (hemoglobin <11 g/dL), % | 32.8 | 34.4 | 0.72 | 28.9 | 37.3 | 0.11 | 43.3 | 0.11 |
| Iron deficiency anemia, % | 12.5 | 7.9 | 0.74 | 11.4 | 11.5 | 0.97 | 16.1 | 0.49 |
| Iron deficiency anemia with inflammation correction,2 % | 16.7 | 15.0 | 0.27 | 16.5 | 16.1 | 0.60 | 16.7 | 0.84 |
1Values are means ± SDs or medians (Quartile 1, Quartile 3) unless otherwise indicated; n = 435. Means were compared between each genotype separately using linear regression, adjusting for cluster and block of randomization. In the α+-thalassemia group, each trait condition was compared with the normozygous condition. Groups of genotypes are not mutually exclusive. All biomarkers were assessed in serum, except for hemoglobin and malaria, which were detected in whole blood using the Hemocue (Hemocue Hb 301) and RDTs SD Bioline (Alere), respectively. AGP, α1-acid glycoprotein; CRP, C-reactive protein; RDT, rapid diagnostic test; sTfR, soluble transferrin receptor.
2Ferritin corrected by using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) regression method (8).
The geometric mean (95% CI) of hepcidin concentration across the entire sample was 7.3 (6.5, 8.2) ng/mL. Neither age nor sex was associated with hepcidin concentration, but season was, and therefore was included in the adjusted model (Table 2). When we compared hepcidin concentration across Sickle cell or α+-thalassemia genotype groups, we found no significant differences in either the unadjusted or adjusted models (Table 2). However, only one child in the sample was found to have HbSS; therefore, neither the unadjusted or adjusted models were run with HbSS as the outcome. Similarly, there was no difference in median hepcidin concentration when we stratified children by the presence of a hemoglobinopathy trait. All models had robust SEs and controlled for the treatment arm.
TABLE 2.
Hepcidin concentration by genotype in rural Kenyan children aged 14–26 mo1
| Hepcidin | Unadjusted ratio vs. | Adjusted ratio vs. | ||
|---|---|---|---|---|
| n | concentration,2 ng/mL | reference (95% CI) | reference (95% CI)3 | |
| Sickle cell | ||||
| HbAA (normozygous) | 337 | 7.5 (6.5, 8.7) | Reference | Reference |
| HbAS (sickle cell trait) | 64 | 8.2 (6.2, 10.8) | 1.03 (0.79,1.35) | 1.24 (0.90,1.74) |
| HbSS (sickle cell disorder) | 1 | 22.4 | —4 | —4 |
| α+-Thalassemia | ||||
| αα/αα (Normozygous) | 210 | 7.8 (6.5, 9.0) | Reference | Reference |
| –α/αα (Heterozygous) | 164 | 7.4 (6.1, 9.0) | 0.92 (0.70, 1.20) | 0.95 (0.71,1.26) |
| –α/–α (Homozygous) | 34 | 7.9 (5.4,11.6) | 0.83 (0.58, 1.17) | 1.01 (0.71, 1.43) |
| By either hemoglobinopathy | ||||
| Normozygous | 180 | 7.7 (6.5, 9.1) | Reference | Reference |
| Any trait | 226 | 7.6 (6.2, 9.3) | 1.02 (0.76, 1.36) | 1.16 (0.85, 1.59) |
1 n = 435. Differences in the log-hepcidin concentration were estimated by using generalized linear models with robust SEs and controlling for the treatment arm. Genotyping was conducted using PCR on samples of plasma packed cells.
2Values are geometric means (95% CIs).
3Adjusted models include season as a covariate.
4Only one child in the sample was found to have HbSS; therefore, neither the unadjusted or adjusted models were conducted with HbSS as the outcome.
There was significant effect modification of both sickle cell and α+-thalassemia genotype on the association between hepcidin and ferritin concentrations (P < 0.1). In models with robust SEs and controlling for treatment we found that log-hepcidin and log-ferritin were positively associated in the HbAA children (Figure 2). With the use of the same model, we found that among those with HbAS, the magnitude of the association between log-hepcidin and log-ferritin concentrations was attenuated and the relation was no longer significant (Figure 2). Similarly, for α+-thalassemia, log-hepcidin and log-ferritin were associated in the normozygous (αα/αα; Figure 3) and heterozygous (−α/αα; Figure 3) children, but in the homozygotes (−α/−α) the association was attenuated and no longer significant (Figure 3).
FIGURE 2.
Relation between hepcidin and ferritin by sickle cell genotype in rural Kenyan children aged 14–26 mo. Hepcidin and ferritin were measured in serum, and the genotyping was conducted in plasma packed cell samples. Hepcidin and ferritin were log-transformed to achieve a normal distribution. Associations were modeled using robust SEs and controlling for treatment arm. HbAA, normozygous condition; HbAS, sickle cell trait condition.
FIGURE 3.
Relation between hepcidin and ferritin by α+-thalassemia genotype in rural Kenyan children aged 14–26 mo. Hepcidin and ferritin were measured in serum, and the genotyping was conducted in plasma packed cell samples. Hepcidin and ferritin were log-transformed to achieve a normal distribution. Associations were modeled using robust SEs and controlling for treatment arm.
Discussion
To our knowledge, this is the first study to show that sickle cell trait and α+-thalassemia may alter hepcidin concentrations in children. We found that genotype modified the association between ferritin and hepcidin; the association was attenuated and not found among children with either HbAS or homozygous α+-thalassemia (−α/−α). However, hepcidin concentrations did not differ by genotype when comparing across sickle cell or α+-thalassemia genotypic groups. Similarly, when we combined both groups, we did not observe an association with hepcidin concentration among children with any hemoglobinopathy trait compared with those with no trait.
Although hepcidin has been measured in children in both low-income and high-income countries, normal ranges for young, healthy children are yet to be established. Overall, the geometric mean of hepcidin of 7.3 ng/mL in our population of young children was slightly higher than in children residing along the coastal region of Kenya, where a geometric mean of 6.0 ng/mL was reported for children aged 5–7 mo (28), 8.4 ng/mL for those aged 0–12 mo, and 2.5 ng/mL for those aged 1–3 y, with the latter being the age range most comparable to ours (30). The median concentration of hepcidin in healthy children in the Netherlands was found to be 17.3 ng/mL in children aged 12–34 mo (31), which is higher than the median concentration in our population (9.4 ng/mL). Both the study in coastal Kenya and the study in the Netherlands used the Bachem Hepcidin ELISA assay, which is the parent company of the PenLabs Hepcidin ELISA used in this study.
Few studies have looked previously at the relation between sickle cell trait or α+-thalassemia and hepcidin, and none of these studies have looked at the relation between ferritin and hepcidin by genotype. In the comparison of HbAS children with HbAA children, our finding that hepcidin was not associated with the HbAS genotype is consistent with a study in preschoolers in Brazil (19). Similarly, 2 studies conducted in children on the coast of Kenya found no association between α+-thalassemia (30) or HbAS (32) and hepcidin concentrations, although, on average, the children included were slightly older than those in our sample. In addition to our study and the study conducted in Kenyan children, only one other study has looked at the relation between hepcidin and α+-thalassemia, which was conducted in adults in Brazil (18). In contrast to our findings, the study conducted in adults in Brazil found significantly decreased mean hepcidin concentrations in those with heterozygous α+-thalassemia (−α/αα) (18). However, the study did have a much smaller sample size, with 28 adults in the normozygous group and 14 in the α+-thalassemia group. The hepcidin concentrations were much higher in the Brazilian adults, with a mean of 63.2 ng/mL in their normozygous group compared with a geometric mean of 7.5 ng/mL in our normozygous group. The Brazilian adults had an average hemoglobin concentration of 13.8 g/dL in their heterozygous α+-thalassemia carriers compared with 11.2 g/dL in our children. Given that both groups exhibited evidence of a mild anemia for their age groups, the severity of anemias in the respective populations is not likely to account for the discrepancy in our findings. Both studies used a similar ELISA assay; however, the higher concentrations of hepcidin in the adult population may have allowed the suppression of hepcidin by α+-thalassemia to be seen more clearly. Because there is evidence that IE occurs in α+-thalassemia, we had suspected that in our population α+-thalassemia may behave similarly to β-thalassemia (15, 33–36), albeit with a lesser degree of suppressed hepcidin. However, we only saw evidence of this when we looked at the effect modification by genotype on the association between ferritin and hepcidin because we found evidence of hepcidin suppression in hemoglobinopathies at higher ferritin concentrations.
In the presence of HbAS or homozygous α+-thalassemia (−α/−α), the association between ferritin and hepcidin was attenuated: as ferritin concentrations increased, reflecting an increase in iron stores, hepcidin concentrations did not increase as expected. One explanation for this attenuated association is that IE is occurring in those with HbAS or homozygous α+-thalassemia (−α/−α). Because IE is thought to be a potent inhibitor of hepcidin expression (37), it is plausible that IE could override the stimulation of hepcidin expression that is induced by high iron concentrations. Our finding is important, because it suggests that children with subclinical hemoglobinopathies may be more susceptible to the negative side effects of prolonged iron supplementation.
We did find evidence of increased, if not ineffective, erythropoiesis because the median concentration of sTfR was 10.1 mg/L, which is higher than the reference range for healthy children of 1.5–3.3 mg/L (38). However, the concentrations were not significantly different between genotype groups, despite a trend toward an increase in those with a hemoglobinopathy. Future studies could measure erythroferrone to determine if subclinical hemoglobinopathies are associated with the increased erythropoietic drive due to IE, a potent inhibitor of hepcidin (39).
After correcting for inflammation, more than one-third of children had ferritin concentrations that classified them as iron deficient. The burden of inflammation was also quite high, with approximately half suffering from recent or latent infections, which can be partially explained by the high malaria prevalence. Given that hepcidin also responds to inflammatory signals, and that ferritin, hepcidin, and inflammatory biomarkers commonly increase together (40), the evidence that hepcidin is suppressed in the presence of increased inflammation among those with a hemoglobinopathy trait is salient.
In agreement with the study in coastal Kenyan children (32), we found that season was significantly associated with hepcidin, although controlling for season did not change our findings. The association between season and hepcidin may be driven by a change in the rate of illnesses, leading to a change in inflammation, or to a change in diet, leading to a change in iron status (40). Further research could focus on whether season modifies the relation between hemoglobinopathies and hepcidin, because both inflammation and nonclinical malaria have been associated with increased hepcidin concentrations (32) and may obfuscate the hepcidin-suppressing effect of IE. This would allow us to more closely investigate if the erythropoiesis that occurs in an individual with a hemoglobinopathy responds to iron status differently when inflammatory signals are not present.
Strengths and limitations
Strengths of this study include the measurement of genetic traits using PCR and the measurement of many biomarkers, which provides a more complete picture of the health of the participants. This is also the first study, to our knowledge, to look at the association of ferritin and hepcidin by subclinical hemoglobinopathy.
A primary limitation of this study is that the ferritin and hepcidin concentrations were measured cross-sectionally. Factors such as intra- and extracellular iron concentrations and inflammatory biomarkers influence both biomarkers. Therefore, a longitudinal study should be conducted to more closely examine the association between hepcidin and ferritin when a hemoglobinopathy is present. We were also limited by the smaller than anticipated sample size and a high rate of attrition between those who were enrolled and those who consented for the final blood draw. However, we did not see differences in characteristics between the 2 groups (Supplementary Table 1).
Conclusions
We did not find a relation between hepcidin concentrations and clinically silent hemoglobinopathies in this setting. However, our data suggest that hepcidin and ferritin are not associated in children with a subclinical hemoglobinopathy, indicating that a hemoglobinopathy trait may lead to suppressed hepcidin concentrations in the context of high ferritin concentrations. This is the first study, to our knowledge, to show that sickle cell trait and α+-thalassemia may alter hepcidin concentrations in young Kenyan children.
Supplementary Material
Acknowledgments
We thank Benard Chieng of KEMRI Nairobi for the completion of the hepcidin assays and Alex Macharia, Metrine Tendwa, Emily Nyatichi, and Johnston Makale of KEMRI Kilifi for sample genotyping. The authors’ responsibilities were as follows—KAB: drafted the research protocol and manuscript with input from TNW, AL, AJP, BFA, CDA, MK, HND, SMN, GR, JMC, CN, and CPS; developed the analytical approach; conducted the statistical analysis with input from CPS and CDA; constructed the tables and figures, and has primary responsibility for the final content; GR, AL, MK, and HND: assisted with sample and survey collection; TNW: oversaw the genotyping and contributed to the interpretation of results; AJP, AL, CPS, and CN: oversaw the main trial, gave feedback on the study protocol, and contributed to the interpretation of results; KAB and CPS: secured funding for the substudy; CPS and CN: secured the funding for the main trial in which this substudy was nested; and all authors: read, contributed to, and approved the final manuscript.
Notes
Supported in part by Global Development grant OPPGD759 from the Bill & Melinda Gates Foundation to the University of California, Berkeley, and grant AID-OAA-F-13-00040 from the US Agency for International Development (USAID) to Innovations for Poverty Action. This project was also supported in part by NIH Research Training grant R25 TW009343, funded by the Fogarty International Center, as well as the University of California Global Health Institute. TNW is supported by a fellowship from the Wellcome Trust (202800).
The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, the US government, the NIH, or the University of California Global Health Institute.
This research was published with permission from the Director of the Kenya Medical Research Institute (KEMRI).
Author disclosures: KAB, TNW, AL, AJP, BFA, CDA, MK, HND, SMN, GR, JMC, CN, and CPS, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- AGP
α1-acid glycoprotein
- CRP
C-reactive protein
- EED
environmental enteric dysfunction
- HbAA
normozygous
- HbAS
sickle cell trait
- HbSS
sickle cell disorder
- IE
ineffective erythropoiesis
- N
nutrition (arm)
- sTfR
soluble transferrin receptor
- WASH
Water, Sanitation, and Hygiene
- WSH
water, sanitation, and handwashing (arm)
- WSH+N
combined water, sanitation, handwashing, and nutrition (arm).
References
- 1. Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 2012;2(9):a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atkinson SH, Mwangi TW, Uyoga SM, Ogada E, Macharia AW, Marsh K, Prentice AM, Williams TN. The haptoglobin 2-2 genotype is associated with a reduced incidence of Plasmodium Falciparum malaria in children on the coast of Kenya. Clin Infect Dis 2007;44(6):802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet 2012;12:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams TN. How do hemoglobins S and C result in malaria protection? J Infect Dis 2011;204(11):1651–3. [DOI] [PubMed] [Google Scholar]
- 5. Foote EM, Sullivan KM, Ruth LJ, Oremo J, Sadumah I, Williams TN, Suchdev PS. Determinants of anemia among preschool children in rural, western Kenya. Am J Trop Med Hyg 2013;88(4):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorup OA. Fundamentals of clinical hematology. Chicago, Illinois: WB Saunders Company; 1987. [Google Scholar]
- 7. WHO Daily iron supplementation in infants and children. Geneva (Switzerland): WHO; 2016. [PubMed] [Google Scholar]
- 8. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl 1):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO Assessing iron status of populations. 2nd ed Geneva (Switzerland): WHO;2007. [Google Scholar]
- 10. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 2003;329(1–2):9–22. [DOI] [PubMed] [Google Scholar]
- 11. Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011;117(17):4425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 2012;2(5):a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93:1721–41. [DOI] [PubMed] [Google Scholar]
- 14. Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal 2013;18(18):2473–507. [DOI] [PubMed] [Google Scholar]
- 15. Ginzburg Y, Rivella S. Beta-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood 2011;118(16):4321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmermann MB, Fucharoen S, Winichagoon P, Sirankapracha P, Zeder C, Gowachirapant S, Judprasong K, Tanno T, Miller JL, Hurrell RF. Iron metabolism in heterozygotes for hemoglobin E (HbE), α-thalassemia 1, or β-thalassemia and in compound heterozygotes for HbE/β-thalassemia. Am J Clin Nutr 2008;88(4):1026–31. [DOI] [PubMed] [Google Scholar]
- 17. Pasricha SR, McHugh K, Drakesmith H. Regulation of hepcidin by erythropoiesis: the story so far. Ann Rev Nutr 2016;36:417–34. [DOI] [PubMed] [Google Scholar]
- 18. Guimarães JS, Cominal JG, Silva-Pinto AC, Olbina G, Ginzburg YZ, Nandi V, Westerman M, Rivella S, de Souza AM. Altered erythropoiesis and iron metabolism in carriers of thalassemia. Eur J Hematol 2015;94(6):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibson RS, Bailey KB, Williams S, Houghton L, Costa-Ribeiro HC, Mattos AP, Barreto DL, Lander RL. Tissue iron deficiency and adiposity-related inflammation in disadvantaged preschoolers from NE Brazil. Eur J Clin Nutr 2014;68(8):887–91. [DOI] [PubMed] [Google Scholar]
- 20. Arnold BF, Null C, Luby SP, Unicomb L, Stewart CP, Dewey KG, Ahmed T, Ashraf S, Christensen G, Clasen T et al.. Cluster-randomized controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 2013;3:e003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, Benjamin-Chung J, Clasen T, Dewey KG, Fernald LCH et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 2018;6(3):PE316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva (Switzerland): WHO;2011. [Google Scholar]
- 23. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004;134(11):3127–32. [DOI] [PubMed] [Google Scholar]
- 24. Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R, Group BW. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv Nutr 2016;7(2):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chong SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 2000;95(1):360–2. [PubMed] [Google Scholar]
- 26. Waterfall CM, Cobb BD. Single tube genotyping of sickle cell anaemia using PCR-based SNP analysis. Nucleic Acids Res 2001;29(23):E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomez S, Diawara A, Gbeha E, Awadalla P, Sanni A, Idaghdour Y, Rahimy MC. Comparative analysis of iron homeostasis in sub-Saharan african children with sickle cell disease and their unaffected siblings. Front Pediatr 2016;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Tjalsma H, Kortman GA, Joosten I, Mwangi A, Zimmermann MB. Iron status and systemic inflammation, but not gut inflammation, strongly predict gender-specific concentrations of serum hepcidin in infants in rural Kenya. PloS One 2013;8(2):e57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mupfudze TG, Stoltzfus RJ, Rukobo S, Moulton LH, Humphrey JH, Prendergast AJ; The Shine Project Team Hepcidin decreases over the first year of life in healthy African infants. Br J Haematol 2014;164(1):150–3. [DOI] [PubMed] [Google Scholar]
- 30. Atkinson SH, Uyoga SM, Armitage AE, Khandwala S, Mugyenyi CK, Bejon P, Marsh K, Beeson JG, Prentice AM, Drakesmith H et al.. Malaria and age variably but critically control hepcidin throughout childhood in Kenya. EBioMedicine 2015;2(10):1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uijterschout L, Swinkels DW, Domellof M, Lagerqvist C, Hudig C, Tjalsma H, Vos R, van Goudoever JB, Brus F. Serum hepcidin measured by immunochemical and mass-spectrometric methods and their correlation with iron status indicators in healthy children aged 0.5-3 y. Pediatr Res 2014;76(4):409–14. [DOI] [PubMed] [Google Scholar]
- 32. Atkinson S, Armitage AE, Khandwala S, Mwangi TW, Uyoga SM, Bejon PA, Williams TN, Prentice AM, Drakesmith H. Combinatorial effects of malaria season, iron deficiency, and inflammation determine plasma hepcidin concentration in African children. Blood 2014;123(21):3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones E, Pasricha S-R, Allen A, Evans P, Fisher CA, Wray K, Premawardhena A, Bandara D, Perera A, Webster C. Hepcidin is suppressed by erythropoiesis in hemoglobin E β-thalassemia and β-thalassemia trait. Blood 2015;125(5):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol 2016;172(4):512–23. [DOI] [PubMed] [Google Scholar]
- 35. Gardenghi S, Grady RW, Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol Oncol Clin North Am 2010;24(6):1089–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rivella S. The role of ineffective erythropoiesis in non-transfusion-dependent thalassemia. Blood Rev 2012;26:S12–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karafin MS, Koch KL, Rankin AB, Nischik D, Rahhal G, Simpson P, Field JJ. Erythropoietic drive is the strongest predictor of hepcidin level in adults with sickle cell disease. Blood Cells Mol Dis 2015;55(4):304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suominen P, Virtanen A, Lehtonen-Veromaa M, Heinonen OJ, Salmi TT, Alanen M, Möttönen T, Rajamäki A, Irjala K. Regression-based reference limits for serum transferrin receptor in children 6 months to 16 years of age. Clin Chem 2001;47(5):935–7. [PubMed] [Google Scholar]
- 39. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genetics 2014;46(7):678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338(6108):768–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.