Abstract
Background
Celecoxib and low-dose aspirin might decrease risk of breast cancer recurrence.
Methods
In the Canadian Cancer Trials Group MA.27, postmenopausal hormone receptor–positive breast cancer patients were randomly assigned (2 × 2) to adjuvant exemestane or anastrozole, and celecoxib or placebo. Low-dose aspirin of 81 mg or less was a stratification factor. Due to concerns about cardiac toxicity, celecoxib use was stopped in December 2004, while stratification by aspirin use was removed through protocol amendment. We examined the effects of celecoxib and low-dose aspirin on event-free survival (EFS), defined as time from random assignment to time of locoregional or distant disease recurrence, new primary breast cancer, or death from any cause; distant disease–free survival (DDFS); and overall survival (OS). All statistical tests were two-sided.
Results
Random assignment to celecoxib (n = 811, 50.0%) or placebo (n = 811, 50.0%) was discontinued after 18 months (n = 1622). At a median of 4.1 years’ follow-up, among 1622 patients, 186 (11.5%) patients had an EFS event: 80 (4.9%) had distant relapse, and 125 (7.7%) died from any cause. Celecoxib did not statistically significantly impact EFS, DDFS, or OS in univariate analysis (respectively, P = .92, P = .55, and P = .56) or multivariable analysis (respectively, P = .74, P = .60, and P = .76). Low-dose aspirin use (aspirin users n = 476, 21.5%; non–aspirin users n = 1733, 78.5%) was associated in univariate analyses with worse EFS (hazard ratio [HR] = 1.48, 95% confidence interval [CI] = 1.12 to 1.96, P = 0.006) and worse OS (HR = 1.87, 95% CI = 1.35 to 2.61, P < .001). After adjusting for baseline characteristics and treatment arm, aspirin use showed no statistical association with EFS (P = .08) and DDFS (P = .82), but was associated with statistically worse OS (HR = 1.67, 95% CI = 1.13 to 2.49, P = .01).
Conclusion
Random assignment to short-term (≤18 months) celecoxib as well as use of low-dose aspirin showed no effect on DDFS and EFS in multivariable analysis. Low-dose aspirin increased “all-cause” mortality, presumably because of higher preexisting cardiovascular risks.
Cyclooxygenase (COX)-2 overexpression has been reported in many epithelial tumors, including human breast cancer (1, 2), where it is associated with higher grade, estrogen receptor negativity, HER-2/neu overexpression, increased proliferation, lower apoptosis, increased blood vessel formation, and increased aromatase activity (1–7). In 1576 patients with invasive breast cancer, high levels of COX-2 expression were associated with decreased disease-free survival (8). In preclinical studies, celecoxib, a potent COX-2 inhibitor (9–12), has prevented the occurrence and growth of both estrogen receptor (ER)–positive and ER-negative breast cancers (13–18). The combination of celecoxib and an aromatase inhibitor (exemestane) has been shown in a preclinical animal model to be synergistic (19), while clinical trial data in patients with metastatic breast cancer suggested that the combination of celecoxib (400 mg b.i.d.) and exemestane resulted in a trend to longer duration of clinical benefit in the celecoxib arm (20). The antitumorigenic mechanism of the selective COX-2 inhibitor celecoxib is unclear and likely extends beyond its role in the formation of prostaglandins (21–25).
Taken together, these data provided a strong rationale for evaluating the effects of celecoxib in combination with aromatase inhibitors in the adjuvant setting of ER-positive breast cancer. The Canadian Cancer Trials Group (CCTG) MA.27 trial was a phase III trial with a 2×2 factorial design, comparing exemestane with anastrozole with or without celecoxib in postmenopausal women with hormone receptor–positive early breast cancer. In addition, in view of some data supporting the possible benefit of low-dose aspirin in cancer survival (26,27), we examined the effects of the use of low-dose aspirin, which was a stratification factor in MA.27 during celecoxib random assignment. Here we examine the effects of celecoxib and aspirin on outcomes.
Methods
Study Design
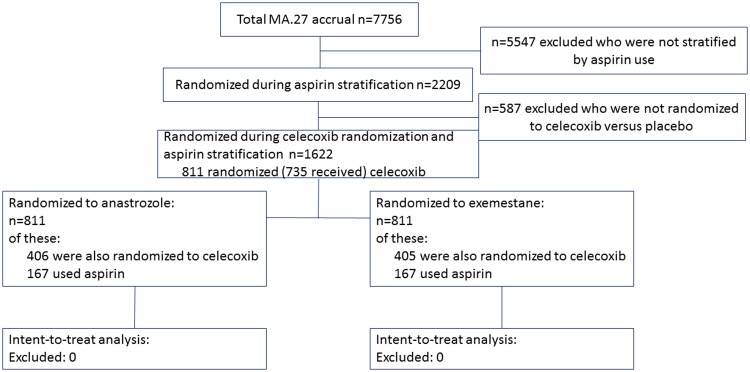
CCTG MA.27 (ClinicalTrials.gov identifier: NCT00066573) was an open-label, randomly assigned phase III multinational trial approved by health regulatory authorities and the institutional review boards of participating centers; patients provided informed consent. Beginning in June 2003, MA.27 postmenopausal hormone receptor–positive breast cancer patients were randomly assigned in a 2×2 factorial design to standard-dose adjuvant exemestane or anastrozole, and to 400 mg of celecoxib or placebo. Baseline concomitant prophylactic low-dose aspirin use (≤81 mg/d) was a stratification factor during celecoxib random assignment (Figure 1, CONSORT Diagram). Patients taking more than 81 mg of aspirin daily at baseline were ineligible for random assignment, and use of more than 81 mg of aspirin daily was not allowed during the study.
Figure 1.
CONSORT diagram for Canadian Cancer Trials Group MA.27.
Random assignment to celecoxib was discontinued on December 17, 2004, based on a joint decision by the Data and Safety Monitoring Committee (DSMC) trials and principal investigators, as well as the Cancer Treatment Evaluation Program (CTEP) following a National Cancer Institute alert on an increase in cardiovascular events on COX-2 inhibitors in the Adenoma Prevention with Celecoxib trial. The corresponding data were published in full soon after (28,29). Allocation to celecoxib was stopped in MA.27 after random assignment of 811 patients to celecoxib, of which 76 patients had not yet received celecoxib, and 735 patients had received celecoxib for a median of 4.6 months (range = 0.03–17.5 months). All patients remained in the study and were followed according to study protocol with inclusion in the comparison of anastrozole vs exemestane. Stratification by aspirin use was removed later through protocol amendment after accrual of 2209 patients.
Trial stratification factors during celecoxib random assignment were use of aspirin (yes, no), lymph node status (negative, positive, or unknown), and prior adjuvant chemotherapy (yes, no). Analyses reported here used the final analysis data, with a median of 4.1 years of follow-up.
Study Population
Briefly, women were recruited to MA.27 with locally excised, hormone receptor–positive primary breast cancer (30). Patients had to have been postmenopausal, defined as older than age 55 years and no menses for 12 or more months; age 55 years or younger and no menses within 12 months and postmenopausal follicle-stimulating hormone; or bilateral oophorectomy. Eligibility criteria also included Eastern Cooperative Oncology Group performance status 0–2, no local or metastatic breast cancer, and no prior aromatase inhibitor or concurrent hormone therapies. Raloxifene was allowed if it had been discontinued three or more weeks before random assignment.
Primary End Point
The primary end point for MA.27 and this investigation was event-free survival (EFS), defined as time from random assignment to loco-regional or distant disease recurrence, new primary breast cancer, or death from any cause. Secondary end points included distant disease–free survival (DDFS), defined as time to distant recurrence, censored at non–breast cancer death, but including time to breast cancer death if no prior recurrence had been recorded, and overall survival (OS). Patients were censored at death or longest follow-up.
Statistical Analyses
The univariate and multivariable effects of celecoxib and low-dose aspirin use on EFS, DDFS, and OS were assessed. Stratification factors utilized for these investigations were lymph node status and receipt of adjuvant chemotherapy, with values at random assignment to trial therapy applied by intent to treat. Univariate and multivariable hazard ratios (HRs) and associated 95% confidence intervals (CIs) are reported. Each statistical test was two-sided and considered statistically significant with an unadjusted P value of .05 or less. Univariate assessments with stratified log-rank tests utilized the maximum available evidence for celecoxib use (n = 1622) and for aspirin use (n = 2209). Graphical depiction was with Kaplan-Meier plots. Exploratory multivariable assessments were with stratified Cox regression restricted to the patients for whom both celecoxib and aspirin use was known: patients accrued during celecoxib random assignment (n = 1622). Stepwise forward selection was used to adjust the observed treatment effect for the influence of potential baseline prognostic factors and identify factors associated with survival outcomes. Baseline factors examined were age (<60, 60–69, ≥70 years), race (white, black/Hawaiian/Asian, American Indian/unknown), estrogen and progesterone receptor (ER/PR) status (ER+/PR+, other), T stage (T1, T2, T3/T4/Tx/missing), N stage (negative, positive, unknown), prior adjuvant chemotherapy (yes, no), adjuvant radiotherapy (yes, no/unknown). The predictive effects of celecoxib and aspirin use were tested with inclusion of interaction terms with exemestane and anastrozole.
Results
The baseline characteristics of the patients included in this investigation are presented in Table 1. The numbers of patients were well balanced between those assigned exemestane (n = 811, 50.0%) and anastrozole (n = 811, 50.0%) and with respect to age, race, performance status, and breast cancer stage. As low-dose aspirin use was a stratification factor until the protocol was amended following cessation of celecoxib, the use of low-dose aspirin (n = 2209) was also well-balanced between patients randomly assigned to exemestane (n = 238, 21.5%, of 1105 patients) and anastrozole (n =238, 21.6%, of 1104), for total aspirin users (n = 476, 21.5%) and nonaspirin users (n = 1733, 78.5%).
Table 1.
Baseline patient characteristics
| Characteristics | Exemestane | Anastrozole | Total |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Total during celecoxib random assignment | 811 (100.0) | 811 (100.0) | 1622 (100.0) |
| Celecoxib use | 405 (49.9) | 406 (50.1) | 811 (50.0) |
| Age, y | |||
| Median | 63.5 | 64.1 | 63.8 |
| <50 | 27 (3.3) | 28 (3.5) | 55 (3.4) |
| 50–59 | 253 (31.2) | 251 (30.9) | 504 (31.1) |
| 60–69 | 299 (36.9) | 307 (37.9) | 606 (37.4) |
| ≥70–79 | 232 (28.6) | 225 (27.7) | 457 (28.2) |
| Race | |||
| White | 770 (94.9) | 758 (93.5) | 1528 (94.2) |
| Black | 24 (3.0) | 31 (3.8) | 55 (3.4) |
| Asian | 11 (1.4) | 15 (1.8) | 26 (1.6) |
| American Indian | 4 (0.5) | 4 (0.5) | 8 (0.5) |
| Unknown | 2 (0.2) | 3 (0.4) | 5 (0.3) |
| ER receptor status | |||
| Negative | 10 (1.2) | 7 (0.9) | 17 (1.0) |
| Positive | 801 (98.8) | 804 (99.1) | 1605 (99.0) |
| PgR receptor status | |||
| Missing | 1 (0.1) | 0(0.0) | 1 (0.1) |
| Negative | 144 (17.8) | 178 (21.9) | 322 (19.9) |
| Positive | 636 (78.4) | 612 (75.5) | 1248 (76.9) |
| Unknown | 30 (3.7) | 21 (2.6) | 51 (3.1) |
| T stage | |||
| T1 | 585 (72.1) | 589 (72.6) | 1174 (72.4) |
| T2 | 198 (24.4) | 201 (24.8) | 399 (24.6) |
| T3 | 24 (3.0) | 18 (2.2) | 42 (2.6) |
| TX | 4 (0.5) | 3 (0.4) | 7 (0.4) |
| N stage | |||
| N0 | 577 (71.1) | 572 (70.5) | 1149 (70.8) |
| N1 | 195 (24.0) | 195 (24.0) | 390 (24.0) |
| N2 | 25 (3.1) | 24 (3.0) | 49 (3.0) |
| N3 | 5 (0.6) | 8 (1.0) | 13 (0.8) |
| NX | 9 (1.1) | 12 (1.5) | 21 (1.3) |
| Prior adjuvant chemotherapy | |||
| No | 558 (68.8) | 560 (69.1) | 1118 (68.9) |
| Yes | 253 (31.2) | 251 (30.9) | 504 (31.1) |
| Radiotherapy (prior or concurrent) | |||
| Unknown/missing | 2 (0.2) | 0 (0.0) | 2 (0.1) |
| Yes | 573 (70.7) | 554 (68.3) | 1127 (69.5) |
| No | 236 (29.1) | 257 (31.7) | 493 (30.4) |
| Total during aspirin stratification* | 1105 (100.0) | 1104 (100.0) | 2209 (100.0) |
| Use of low-dose aspirin* | 238 (21.5) | 238 (21.6) | 476 (21.5) |
Aspirin use was stratified for 2209 women. ER = estrogen receptor; PgR = progesterone receptor.
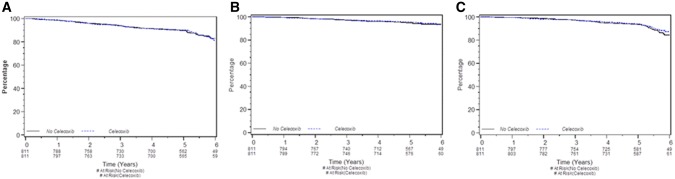
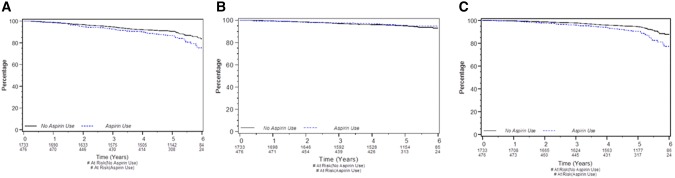
At a median follow-up of 4.1 years, 186/1622 (11.5%) patients (Figure 2A) had an EFS event during celecoxib allocation, 93 (11.5%) of 811 patients accrued to each of celecoxib and placebo, and 125 (7.7%) died from any cause. Of patients taking low-dose aspirin, 72 (15.1%) of 476 had an EFS event (Figure 3A). A DDFS event was experienced by 80 (4.9%) of patients: 38 (4.7%) on celecoxib (Figure 2B) and 21 (4.4%) using aspirin (Figure 3B). Meanwhile, there were 125 (7.7%) deaths from any cause: 60 (7.4%) among celecoxib patients (Figure 2C) and 56 (11.8%) among aspirin users (Figure 3C).
Figure 2.
Survival by celecoxib use. A) Event-free survival. B) Distant disease–free survival. C) Overall survival. Each graphic is a Kaplan-Meier survival plot for 811 women who were allocated celecoxib and 811 women not allocated celecoxib. At a median follow-up of 4.1 years, both the 811 patients allocated and those not allocated celecoxib had 93 (11.5%) event-free survival events (stratified log-rank P = .92); those allocated celecoxib experienced 38 (4.7%) distant disease–free survival events, while those not had 42 (5.2%) events (P = .55), and those allocated or not allocated celecoxib had, respectively, 60 (7.4%) and 65 (8.0%) deaths from any cause (P = .56).
Figure 3.
Survival by aspirin use. A) Event-free survival. B) Distant disease–free survival. C) Overall survival. Each graphic is a Kaplan-Meier survival plot for 476 women who used low-dose aspirin and 1733 women who did not. At a median follow-up of 4.1 years, the 476 aspirin users had 72 (15.1%) event-free survival events, while the nonusers had 177 (10.2%) events (stratified log-rank P = .006); those who used aspirin experienced 21 (4.4%) distant disease–free survival events, while those who did not had 87 (5.0%) events (P = .72), and those who used or did not use aspirin had, respectively, 56 (11.8%) and 110 (6.4%) deaths from any cause (P < .001).
Celecoxib did not have a univariate prognostic impact on EFS (P = .92) (Figure 2A), DDFS (P = .55) (Figure 2B), or OS (P = .56) (Figure 2C). Use of low-dose aspirin was associated with worse EFS (hazard ratio [HR] = 1.48, 95% confidence interval [CI] = 1.12 to 1.96, P = .006) (Figure 3A) and worse OS (HR = 1.87, 95% CI = 1.35 to 2.61, P < .001) (Figure 3C) in univariate analysis. Aspirin use was not statistically significantly associated with DDFS (HR = 1.09, 95% CI = 0.67 to 1.78, P = .72) (Figure 3B).
In multivariable analyses, treatment with celecoxib did not impact EFS (P = .74), DDFS (P = .60), or OS (P = .76). Low-dose aspirin use had no statistically significant association with EFS (P = .08) and DDFS (P = .82), although users of low-dose aspirin had worse OS than nonusers (HR = 1.67, 95% CI = 1.13 to 2.49, P = .01). Worse EFS and worse OS were experienced by those age 70 years or older (P = .01); those with bilateral oophorectomy or who were postmenopausal for less than 12 months at age 45 to 59 years (P = .002 and P = .01, respectively); and who were not fully active by Eastern Cooperative Oncology Group performance status (PS > 0, P = .002 and P < .001, respectively). Greater than T1 tumor was associated with worse EFS (P = .01), DDFS (P = .01), and OS (P < .001). Neither treatment with celecoxib nor use of aspirin had statistically significant interactions with trial treatment for EFS (respectively, P = .79, P = .78), DDFS (respectively, P = .72, P = .26), and OS (respectively, P = .75, P = .44).
Discussion
Adding the COX-2 inhibitor celecoxib to standard aromatase inhibitor therapy in the adjuvant treatment of early breast cancer did not have any effect on EFS, DFS, or OS. The fact that users of low-dose aspirin had higher all-cause mortality but no increased risk regarding breast cancer–associated end points (EFS, DDFS) in multivariable analyses means that these patients had a higher risk for non–breast cancer–associated—presumably cardiovascular—mortality (31). In this context, it seems important to point out that not “use of low-dose aspirin” (indicating a causative role) was associated with increased mortality, but that “users of low-dose aspirin” (where aspirin is a likely surrogate marker for higher cardiovascular risk) were at a higher risk of all-cause mortality. This differentiation seems important as previous observational studies have discussed an association of aspirin with improved survival in breast (32) or colorectal (33) cancer patients “after, but not before cancer diagnosis,” neglecting the fact that the reasons for aspirin use before (cardiovascular risk) and after (including an expectation of a cancer protective effect) diagnosis might differ.
Our study is therefore in line with a meta-analysis on observational studies including more than 700 000 patients that found that aspirin use is likely to have no effect on breast cancer outcomes (34). However, it somewhat contradicts several epidemiological studies indicating the potential of aspirin to prevent recurrence and death among breast cancer survivors (35–37). In most of these studies, the detailed dose and duration of aspirin use were not available, which might explain the differing results. It is possible that higher doses of aspirin than those allowed in MA.27 (>81 mg per day) are required to see cancer preventive effects. The contradictory nature of our findings in comparison with previous data therefore underlines the importance of two large, ongoing trials (38,39) that will likely be able to definitively clarify whether higher doses of aspirin have a positive effect on outcome in early breast cancer. In the UK Add Aspirin Trial, 3100 women are being randomly assigned to 100 or 300 mg of aspirin or placebo for five years. Likewise, in the US Aspirin for Breast Cancer (ABC) study, starting in December of 2016, 3000 women with node-positive human epidermal growth factor receptor 2–negative early breast cancer are being randomly assigned to 300 mg of aspirin or placebo for five years.
In our group of patients with a median age of 64 years, 21.5% used low-dose aspirin at study entry, and these patients had a 67.3% increased risk of death compared with women not using low-dose aspirin. This large effect was seen although by not allowing the use of higher doses of aspirin in MA.27, we likely excluded patients with more serious cardiovascular conditions. Our data therefore emphasize the importance of considering competing causes of mortality in early breast cancer study cohorts (31), even if serious competing morbidities have been ruled out. In particular, when the hazard of event-free survival (or recurrence-free survival, both including death from any cause as an event) is described over long periods of time, non–breast cancer–associated mortality becomes a considerable factor. For the MA.27 cohort, we have indeed previously shown that 56.7% of all deaths were non–breast cancer related, and 15.3% were attributed to cardiovascular disease (a detailed breakdown indicated only seven cardiovascular deaths among both celecoxib and aspirin users, which is too few to pursue further here) (31), As pointed out, it might therefore be more accurate to exclude non–breast cancer–associated mortality when describing the long-term risk of recurrence of early breast cancer (ie, censoring patients at non–breast cancer–associated deaths).
A limitation of this study is its lower-than-planned power in terms of numbers of patients and length of exposure. Therefore, we cannot rule out that an effect of celecoxib on prognosis would have been seen if more patients had been followed for a longer period of time. The equally negative effect on DDFS (which was censored at non–breast cancer deaths) and overall survival also excludes the possibility that 400 mg of celecoxib had a positive effect on breast cancer recurrence that was reversed by a cardiovascular negative effect on all-cause mortality. The UK REACT trial, randomly assigning early breast cancer patients to celecoxib vs placebo for two years, will shortly provide more evidence about celecoxib (40). However, for aspirin, we cannot exclude the possibility that more than 81 mg per day has an effect on breast cancer–specific outcomes, because our study excluded such patients. Multivariable analyses indicating associations of factors such as age or postmenopausal status with EFS and OS need to be interpreted with caution due to the low number of patients involved.
Overall, our study does not support a role for COX2 inhibitors in the treatment of early HR+ breast cancer. Furthermore, our data suggest that the use of low-dose aspirin does not have an effect on breast cancer recurrence. The results of ongoing trials (38,39) clarifying the role of higher doses of aspirin and of celecoxib (40) in preventing breast cancer recurrence are awaited with interest.
Funding
MA.27 was funded by the Canadian Cancer Society Research Institute, US National Cancer Institute, and Pfizer.
Notes
Affiliations of authors: Center for Oncology, Hematology and Palliative Care, Wilhelminen Hospital, Vienna, Austria (KSW); Mater Misericordiae University Hospital, Dublin, Ireland (MJH); Canadian Cancer Trials Group, Queen’s University, Kingston, ON, Canada (JAWC, LH, CE, LES); Division of Medical Oncology, Mayo Clinic, Rochester, MN (JNI); Stanford University Medical Center, Stanford, CA (GWS); Taussig Cancer Center, Cleveland Clinic, Cleveland, OH (GTB); Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, TX (MJE); Sunnybrook Odette Cancer Centre, University of Toronto, Toronto, ON, Canada (KIP); Division of Medical Oncology, Department of Medicine, University of Ottawa, Ottawa, ON, Canada (MJC); Hoffman-La Roche, Basel, Switzerland (TBC); British Columbia Cancer Agency, Vancouver, BC, Canada (KAG); International Breast Cancer Study Group Coordinating Center, Inselspital, Berne, Switzerland (MR); Massachusetts General Hospital, Harvard Medical School, Boston, MA (PEG).
Data were collected, managed, and analyzed by the Canadian Cancer Trials Group. The authors vouch for the integrity of the data and are the sole writers of the article. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
This study was presented in poster form at the 35th Annual San Antonio Breast Cancer Symposium, December 2012, San Antonio, Texas.
The authors have no disclosures.
Clinical Trial information: NCT00066573.
References
- 1. Half E, Tang XM, Gwyn K, et al. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;626:1676–1681. [PubMed] [Google Scholar]
- 2. Boland GP, Butt IS, Prasad R, et al. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;902:423–429. 10.1038/sj.bjc.6601534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan KB, Yong WP, Putti TC.. Cyclooxygenase-2 expression: A potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology. 2004;441:24–28. 10.1111/j.1365-2559.2004.01774.x [DOI] [PubMed] [Google Scholar]
- 4. Zhao Y, Agarwal VR, Mendelson CR, et al. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;13712:5739–5742. 10.1210/endo.137.12.8940410 [DOI] [PubMed] [Google Scholar]
- 5. Richards JA, Petrel TA, Brueggemeier RW.. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002;802:203–212. 10.1016/S0960-0760(01)00187-X [DOI] [PubMed] [Google Scholar]
- 6. Brueggemeier RW, Quinn AL, Parrett ML, et al. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140(1–2):27–35. [DOI] [PubMed] [Google Scholar]
- 7. Brueggemeier RW, Richards JA, Joomprabutra S, et al. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J Steroid Biochem Mol Biol. 2001;79(1–5):75–84. [DOI] [PubMed] [Google Scholar]
- 8. Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;623:632–635. [PubMed] [Google Scholar]
- 9. Schonthal AH. Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. Br J Cancer. 2007;9711:1465–1468. 10.1038/sj.bjc.6604049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schonthal AH. Induction of apoptosis by celecoxib in cell culture: An uncertain role for cyclooxygenase-2. Cancer Res. 2007;6711:5575–5576; author reply 5576. [DOI] [PubMed] [Google Scholar]
- 11. Grosch S, Maier TJ, Schiffmann S, et al. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;9811:736–747. 10.1093/jnci/djj206 [DOI] [PubMed] [Google Scholar]
- 12. Kashfi K, Rigas B.. Non-COX-2 targets and cancer: Expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol. 2005;707:969–986. 10.1016/j.bcp.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 13. Wong TY, Li F, Lin SM, et al. Celecoxib increases miR-222 while deterring aromatase-expressing breast tumor growth in mice. BMC Cancer. 2014;14:426. 10.1186/1471-2407-14-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai ZJ, Ma XB, Kang HF, et al. Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in vitro and in vivo. Cancer Cell Int. 2012;121:53. 10.1186/1475-2867-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnes NL, Warnberg F, Farnie G, et al. Cyclooxygenase-2 inhibition: Effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;964:575–582. 10.1038/sj.bjc.6603593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;605:1306–1311. [PubMed] [Google Scholar]
- 17. Fu SL, Wu YL, Zhang YP, et al. Anti-cancer effects of COX-2 inhibitors and their correlation with angiogenesis and invasion in gastric cancer. World J Gastroenterol. 2004;1013:1971–1974. 10.3748/wjg.v10.i13.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnes NLP, Warnberg F, White D, et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. Breast Cancer Res Treat. 2003;82(suppl 1):abstr 667. [Google Scholar]
- 19. Pesenti E, Masferrer JL, Di Salle E.. Effect of exemestane and celecoxib alone or in combination on DMBA-induced mammary carcinoma in rats. Breast Cancer Res Treat. 2003;693:288. [Google Scholar]
- 20. Dirix LY, Ignacio J, Nag S, et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. J Clin Oncol. 2008;268:1253–1259. 10.1200/JCO.2007.13.3744 [DOI] [PubMed] [Google Scholar]
- 21. Hla T, Bishop-Bailey D, Liu CH, et al. Cyclooxygenase-1 and -2 isoenzymes. Int J Biochem Cell Biol. 1999;315:551–557. 10.1016/S1357-2725(98)00152-6 [DOI] [PubMed] [Google Scholar]
- 22. Bhattacharya A, Li Y, Shi Y, et al. Enhanced inhibition of urinary bladder cancer growth and muscle invasion by allyl isothiocyanate and celecoxib in combination. Carcinogenesis. 2013;3411:2593–2599. 10.1093/carcin/bgt280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramer R, Walther U, Borchert P, et al. Induction but not inhibition of COX-2 confers human lung cancer cell apoptosis by celecoxib. J Lipid Res. 2013;5411:3116–3129. 10.1194/jlr.M042283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pagliarulo V, Ancona P, Niso M, et al. The interaction of celecoxib with MDR transporters enhances the activity of mitomycin C in a bladder cancer cell line. Mol Cancer. 2013;12:47. 10.1186/1476-4598-12-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katkoori VR, Manne K, Vital-Reyes VS, et al. Selective COX-2 inhibitor (celecoxib) decreases cellular growth in prostate cancer cell lines independent of p53. Biotech Histochem. 2013;881:38–46. 10.3109/10520295.2012.724713 [DOI] [PubMed] [Google Scholar]
- 26. Mills EJ, Wu P, Alberton M, et al. Low-dose aspirin and cancer mortality: A meta-analysis of randomized trials. Am J Med. 2012;1256:560–567. 10.1016/j.amjmed.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 27. Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;3799826:1591–1601. 10.1016/S0140-6736(12)60209-8 [DOI] [PubMed] [Google Scholar]
- 28. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;35211:1071–1080. 10.1056/NEJMoa050405 [DOI] [PubMed] [Google Scholar]
- 29. Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: The cross trial safety analysis. Circulation. 2008;11716:2104–2113. 10.1161/CIRCULATIONAHA.108.764530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol. 2013;3111:1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;1004:252–260. 10.1093/jnci/djn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang XZ, Gao P, Sun JX, et al. Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: A meta-analysis. Cancer Causes Control. 2015;264:589–600. 10.1007/s10552-015-0539-y [DOI] [PubMed] [Google Scholar]
- 33. Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta-analysis. Gut. 2015;649:1419–1425. 10.1136/gutjnl-2014-308260 [DOI] [PubMed] [Google Scholar]
- 34. Zhong S, Zhang X, Chen L, et al. Association between aspirin use and mortality in breast cancer patients: A meta-analysis of observational studies. Breast Cancer Res Treat. 2015;1501:199–207. 10.1007/s10549-015-3300-z [DOI] [PubMed] [Google Scholar]
- 35. Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;289:1467–1472. 10.1200/JCO.2009.22.7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: Past present, and future. Steroids. 2010;75(4–5):297–306. 10.1016/j.steroids.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fraser DM, Sullivan FM, Thompson AM, et al. Aspirin use and survival after the diagnosis of breast cancer: A population-based cohort study. Br J Cancer. 2014;1113:623–627. 10.1038/bjc.2014.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cancer Research UK. Add Aspirin TRIAL. http://www.addaspirintrial.org/. Accessed February 10, 2018.
- 39.Alliance for Clinical Trials in Oncology. Alliance researchers to explore use of aspirin to treat breast cancer. https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=/Public/News-ABC-Trial-Nov2015. Accessed February 10, 2018.
- 40. Cancer Research UK. A trial looking at celecoxib for women with breast cancer (REACT). http://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-looking-at-celecoxib-for-women-with-breast-cancer#undefined. Accessed February 10, 2018.