Abstract
Background
Consumption of a high-iron diet causes copper deficiency in weanling rodents; however, the minimum amount of dietary iron that disrupts copper homeostasis has not been established.
Objective
We tested the hypothesis that dietary iron at only several-fold above physiologic requirements would cause copper depletion.
Methods
Weanling male Sprague-Dawley rats (n = 6/group) were fed AIN-93G-based diets with adequate (88 µg Fe/g = 1×), or excessive (4×, 9.5×, 18.5×, 38×, or 110×) iron content for 7 wk (110× group, due to notable morbidity) or 8 wk (all other groups). Copper-related physiologic parameters were then assessed.
Results
A hierarchy of copper-related, pathologic symptoms was noted as dietary iron concentrations increased. All statistical comparisons reported here refer to differences from the 1× (i.e., control) group. The highest iron concentration (110×) impaired growth (final body weights decreased ∼40%; P < 0.0001), and caused anemia (blood hemoglobin and hematocrit decreased ∼65%; P < 0.0001) and hepatic copper depletion (>85% reduction; P < 0.01). Cardiac hypertrophy occurred in the 110× (∼130% increase in mass; P < 0.0001) and 38× (∼25% increase; P < 0.05) groups, whereas cardiac copper content was lower in the 110× (P < 0.01), 38× (P < 0.01), and 18.5× (P < 0.05) groups (∼70% reductions). Splenic copper was also depleted in the 110× (>90% reduction; P < 0.0001), and in the 38× (P < 0.001) and 18.5× (P < 0.01) groups (∼70% reductions). Moreover, serum ceruloplasmin activity was decreased in the 110× and 38× (>90% reductions; P < 0.0001), and 18.5× (P < 0.001) and 9.5× (P < 0.05) (∼50% reductions) groups, typifying moderate to severe copper deficiency.
Conclusions
Increasing dietary iron intakes to ∼9.5-fold above dietary recommendations caused copper deficiency. Importantly, human iron supplementation is common, and recommended intakes for at-risk individuals may be ≤10-fold above the RDA. Whether these iron intakes perturb copper metabolism is worth considering, especially since copper defi-ciency can impair iron utilization (e.g., by decreasing the ferroxidase activity of ceruloplasmin).
Keywords: anemia, cardiac hypertrophy, copper deficiency, ceruloplasmin, iron supplementation
Introduction
Iron and copper are essential dietary components for humans and other mammals. Excess or deficient intakes of either of these trace minerals can result in significant morbidity and mortality. Moreover, physiologically relevant interactions between iron and copper have been frequently observed (1). In previous dietary iron-overload experiments, we fed growing rats and mice diets with high iron (∼110-fold in excess of requirements) in combination with adequate or high copper (∼20-fold excess). Unexpectedly, rats and mice consuming the high-iron diet with adequate copper developed severe physiologic perturbations, including premature mortality, growth impairment, severe anemia, cardiac hypertrophy, tissue copper depletion, and decreased serum ceruloplasmin (Cp) activity. Many of these pathological symptoms typify severe copper deficiency (2, 3). Notably, these abnormalities did not occur in animals consuming the high-iron diet with excess copper (4, 5), essentially proving that these pathologies were caused by copper depletion. The threshold of dietary iron necessary to disrupt copper metabolism has not, however, been experimentally determined. The purpose of the current study was thus to determine the lowest amount of dietary iron that disrupts copper homeostasis.
Defining the minimum dietary iron concentration that antagonizes copper is important, given that many humans consume supplemental iron (6, 7). Individuals most likely to require iron supplements include infants and children, adolescents, and menstruating and pregnant women, particularly amongst those of modest economic means (8–12). Moreover, some pathologic situations may impair intestinal iron absorption, thus increasing the risk for iron depletion and the likelihood that iron supplementation may be recommended. These include gastritis and achlorhydria (13), gastric bypass surgery (14), chronic acid suppression therapy (15), and malabsorptive disorders (16). Dosing varies, but in some cases, supplemental iron at 4- to 10-fold in excess of the RDA may be necessary to correct the iron deficiency (10–12). Given this background, the current investigation was undertaken to test the hypothesis that supplemental iron, at concentrations similar to what humans may consume, will disrupt copper homeostasis. To test this postulate, we fed rapidly growing rats diets with adequate or excessive iron concentrations (with adequate copper) and then assessed the impact on copper homeostasis. Notably, disturbances of copper metabolism were observed in rats consuming iron at only 9.5-fold in excess of requirements.
Methods
Animal experiments
All animal studies were approved by the University of Florida Institutional Animal Care and Use Committee. Three-week-old, weanling, male Sprague-Dawley rats (Envigo) were housed in stainless steel overhanging, wire mesh-bottom cages to minimize coprophagia for 7–8 wk until they were killed. Weanling rats were utilized for this investigation since iron demand is higher during periods of rapid growth, and we sought to model situations in which iron supplementation in humans is more likely. The rats were randomly assigned to the different dietary groups (n = 6/group) and had ad libitum access to diet and purified water. A single, large batch of basal AIN-93G diet (17) was first produced, containing all ingredients except for iron (Dyets Inc.). The background iron concentration was then measured by inductively-coupled plasma MS (ICP-MS), followed by adjusting the iron content up to 80 µg/g with ferric citrate. This basal diet was then split into 6 equal parts and carbonyl iron was added to 5 of these to achieve the desired final iron concentrations, which were 80, 320, 760, 1520, 3040, and 8800 µg/g, respectively. All diets contained adequate copper (6–7 µg/g), and were otherwise almost identical (Supplemental Table 1). At the low end, 80 µg/g was considered adequate for rapidly growing rats, and at the high end, 8880 µg/g was used since this was the iron concentration used in our previous dietary iron-overload studies (4, 5). Carbonyl iron was used to increase iron content since it is more stable and less prone to cause lipid peroxidation compared to iron salts (such as ferric citrate). Given the differing amounts of carbonyl iron added to each diet, extra corn starch was added to diets with less iron (except the 80 µg Fe/g diet); nevertheless, the energy content of the diets varied by <1% (Supplemental Table 1). Rats were weighed weekly and food consumption was estimated by weighing the amount of food provided daily to each cage of rats. After the dietary treatments, animals were killed by thoracotomy after CO2 narcosis.
Hematologic parameters, tissue iron concentrations, and Cp activity
Blood hemoglobin (Hb) and hematocrit (Hct) concentrations were assessed using standard assays, as described previously (18). Serum and tissue nonheme iron concentrations, and total iron-binding capacity (TIBC), were determined using standard colorimetric assays (4). Percent transferrin saturation (TSAT) was calculated as: (serum nonheme iron concentration/TIBC) × 100. Serum Cp activity was quantified using a well-established amine oxidase (para-phenylenediamine) assay (19–22).
Quantification of mineral concentrations in diets and tissues
Ten pellets of each diet were randomly selected and ground with an acid-washed mortar and pestle. Diet samples and tissues were digested with HNO3/H2O2 on a hot block. Digested samples were filtered (0.45 µm) and analyzed by ICP-MS (NexIon 300, Perkin-Elmer Corp.). Iron concentrations in diets, and iron and copper concentrations in tissues, were normalized by premeasured weights.
qRT-PCR
Total RNA was isolated from kidney [for erythropoietin (Epo) expression] and liver [for hepcidin (Hepc) expression] with RNAzol RT reagent (Molecular Research Center, Inc.) and relative mRNA expression was determined with a well-established SYBR Green qRT-PCR technique. To minimize amplification of genomic DNA, oligonucleotide primers were designed to span large introns. Expression of each experimental gene was normalized to the expression of cyclophilin, which did not vary significantly among groups. Average fold changes in mRNA expression were calculated by the 2−ΔΔCt analysis method. The following primers were utilized (sequences are 5′ to 3′): cyclophilin, forward CTTGCTGCAATGGTCAACC, reverse TGCTGTCTTTGGAACTTTGTCTGC; Epo, forward AGTCGCGTTCTGGAGAGGTA, reverse ACTTTGGTATCTGGGACGGTAA; Hepc, forward GGCAGAAAGCAAGACTGATGAC, reverse ACAGGAATAAATAATGGGGCG.
Statistical analysis
The methods used to analyze data were selected after consultation with a biostatistician in the Institute of Food and Agricultural Sciences at the Unversity of Florida. To evaluate the physiologic and pathophysiologic effects of consuming diets with varying iron concentrations, data were first tested for equal variance using Levene's test. Since data sets were not equally distributed, data were log10 transformed, and subsequently analyzed by the nonparametric Kruskal-Wallis rank-sum test (Jmp Pro, version 12.2.0). When statistically significant differences were noted with the use of this test, the Wilcoxon post-hoc test was utilized to make comparisons between individual data sets. Some results are depicted as box-and-whisker plots, displaying the minimum, the lower (25th percentile), the median (50th percentile), the upper (75th percentile), and the maximum ranked sample. The mean value is indicated by a “+” sign. Note that for ease of interpretation, the nontransformed data are presented in the figures. The Pearson product-moment correlation coefficient (r) was calculated to determine selected relations between 2 variables (GraphPad Prism, version 7.0).
Results
Diet analysis
ICP-MS analysis of the diets revealed the following iron concentrations: 88, 320, 756, 1475, 3021, and 8834 μg/g. For ease of interpretation, the adequate-iron diet (with 88 μg Fe/g) is designated as 1×, the diet with 320 μg Fe/g as 4× (indicating 4-fold above requirements), and so forth.
Deviations from the experimental plan
The feeding trial was planned to end after 8 wk; however, rats consuming the highest-iron (i.e., the 110×) diet were hypophagic by 6 wk of age (data not shown) and by 7 wk of age were in obvious distress, and were therefore euthanized a week early (note that blood and tissues were harvested normally from these animals). As a result, data from these animals were compared to rats in the other groups after 8 wk on the diets. Moreover, 2 rats in the 38× group died 1–2 d before the trial was scheduled to end; nonetheless, body weights were recorded, and tissues (but not blood) were successfully harvested from these 2 animals.
Growth rates and organ weights
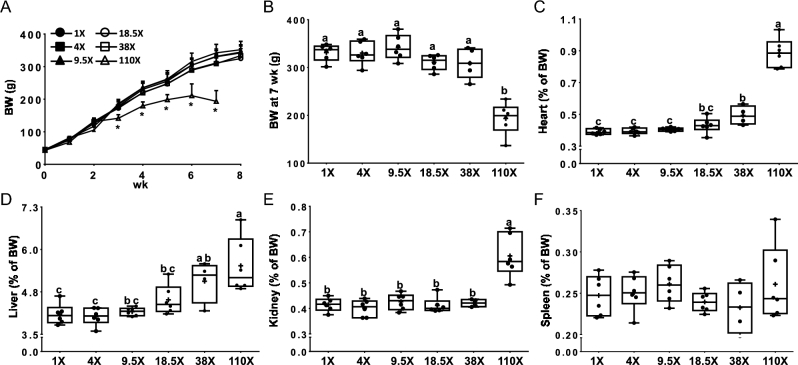
The growth rate was slower after 3 wk (Figure 1A) and final body weights were decreased (∼40%) at 7 wk (Figure 1B) in rats consuming the 110× diet (in comparison to all other groups). Cardiac hypertrophy was noted in the 110× (heart mass increased ∼130%) and 38× (∼25% increase) groups (Figure 1C). Hepatomegaly was also noted in the 110× (increase in mass ∼35%) and 38× (∼25% increase) groups (Figure 1D). Enlargement of the kidneys was observed in the 110× group (∼45% increase in mass) compared to all other groups (Figure 1E). Spleen weights were not different between any dietary groups (Figure 1F).
FIGURE 1.
Growth curves (A), BW at 7 wk (B), and relative heart (C), liver (D), kidney (E), and spleen (F) weights of male rats fed diets varying in iron concentration for 7–8 wk. All data points are shown, n = 4–6. *Different from the other dietary groups at that age, P < 0.05 (A). Labeled means without a common letter differ, P < 0.05 (B–F). BW, body weight.
Hematologic and iron-related parameters, and Epo mRNA expression
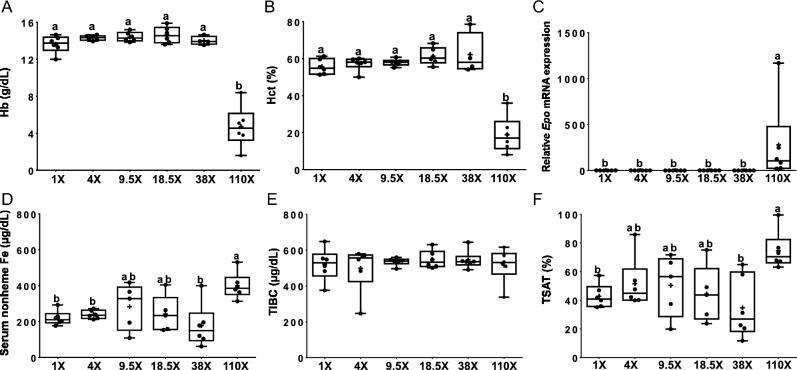
Blood Hb (Figure 2A) and Hct (Figure 2B) concentrations were reduced by ∼65% in the 110× group, compared to all others. Renal Epo expression, an established biomarker of erythroid demand (23–25), increased dramatically (∼28-fold) (Figure 2C) in rats consuming the 110× diet. Dietary iron consumption >38-fold above requirements is thus necessary to induce anemia in growing rats. Moroever, serum nonheme iron concentrations increased by ∼80–90% in the 110× group (Figure 2D) compared to the 38×, 4× and 1× groups. TIBC was similar in all dietary groups (Figure 2E). TSAT increased (by ∼75%) in the 110× group (compared to the 38× and 1× groups (Figure 2F).
FIGURE 2.
Hb (A), Hct (B), renal Epo mRNA expression (C), serum nonheme iron (D), total-iron-binding capacity (E) and transferrin saturation (F) of male rats fed diets varying in iron concentration for 7–8 wk. All data points are shown, n = 4–6. Labelled means without a common letter differ, P < 0.05. Epo, erythropoietin; Hb, hemoglobin; Hct, hematocrit; TIBC, total-iron-binding capacity; TSAT, transferrin saturation.
Tissue iron concentrations and hepatic Hepc mRNA expression
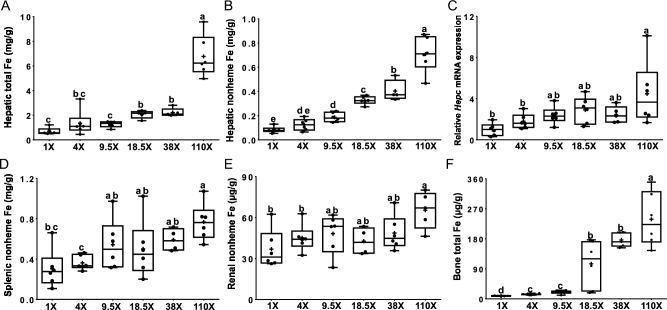
Total iron in the liver was higher in rats consuming the 110× (9.7-fold), and 38× and 18.5× (∼3-fold) diets (Figure 3A). Hepatic nonheme iron concentrations also increased in the 110× (∼8.5-fold), 38× (∼5-fold), 18.5× (∼4-fold), and 9.5× (∼2-fold) groups (compared to the 1× group) (Figure 3B). Hepatic Hepc mRNA levels were significantly elevated only in the 110× group (∼4-fold) compared to the 4× and 1× groups (Figure 3C). Splenic nonheme iron concentrations increased (by ∼2.5-fold) in the 110× group compared to the 4× and 1× groups (Figure 3D). Total iron concentrations in the spleen also showed a significant increase (by ∼3-fold), but only in the 110× and 18.5× groups (compared to all others) (Table 1). Renal nonheme iron concentrations increased in the 110× group (by ∼75%), compared to the 4× and 1× groups (Figure 3E). Total iron concentrations in the kidney, however, were not different between dietary groups (Table 1). Total iron concentrations in bone progressively increased as dietary iron concentrations went up (∼27-fold in the 110× group; 16-fold in the 38× and 18.5× groups; ∼2-fold in the 9.5× and 4× groups), compared to the 1× group (Figure 3F). Total iron in the heart was not affected by changes in dietary iron (Table 1). Overall, these data demonstrate that progressive increases in dietary iron are associated with increases in tissue iron concentrations, which is particularly striking in liver and bone.
FIGURE 3.
Hepatic total iron (A), hepatic nonheme iron (B), hepatic Hepc mRNA expression (C), splenic nonheme iron (D), renal nonheme iron (E), and bone total iron (F) of male rats fed diets varying in iron concentration for 7–8 wk. All data points are shown, n = 4–6. Labelled means without a common letter differ, P < 0.05 Hepc, hepcidin.
TABLE 1.
Bone and kidney copper concentrations, and heart, kidney and spleen total iron concentrations of male rats fed diets varying in iron concentration for 7–8 wk1
| Organ | 1 × Fe | 4 × Fe | 9.5 × Fe | 18.5 × Fe | 38 × Fe | 110 × Fe |
|---|---|---|---|---|---|---|
| Bone Cu, μg/g | 0.44 ± 0.05 | 0.53 ± 0.20 | 0.65 ± 0.27 | 0.70 ± 0.35 | 0.52 ± 0.29 | 0.52 ± 0.15 |
| Kidney Cu, μg/g | 78 ± 16ac | 84 ± 34a | 56 ± 11bc | 53 ± 22ac | 70 ± 26ac | 41 ± 45c |
| Heart Fe, μg/g | 573 ± 203 | 720 ± 470 | 642 ± 394 | 703 ± 416 | 463 ± 228 | 471 ± 186 |
| Kidney Fe, μg/g | 880 ± 146 | 1120 ± 512 | 1080 ± 340 | 1480 ± 1160 | 2390 ± 1350 | 3650 ± 660 |
| Spleen Fe, μg/g | 2680 ± 1130bc | 2790 ± 446c | 5510 ± 2290a | 2920 ± 766bc | 3560 ± 610b | 10,200 ± 5520a |
1Tissue mineral concentrations are shown. Values are means ± SDs, n = 5–6. Labeled means without a common superscript letter differ, P < 0.05.
Tissue copper concentrations and serum Cp activity
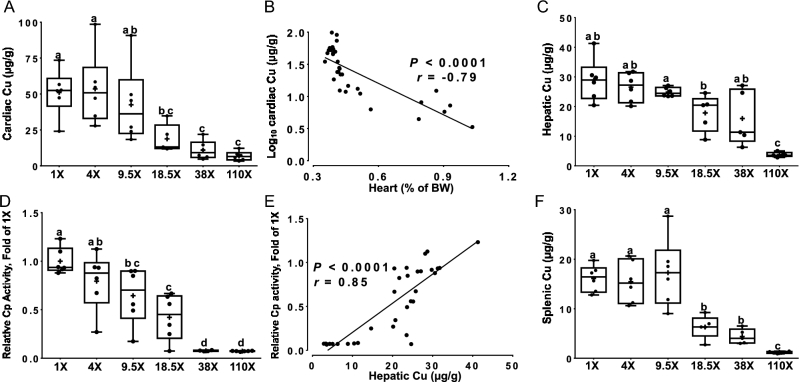
Decrements in copper concentrations in cardiac tissue were noted in the 110× and 38× (∼80% reductions), and 18.5× (∼60% reduction) groups, compared to the 4× and 1× groups (Figure 4A). Cardiac copper concentrations inversely correlated with heart weights (Figure 4B). Heart enlargement (i.e., hypertrophy) is a well-established, pathophysiologic effect of severe copper deficiency in rodents (3, 26, 27). Hepatic copper concentrations were significantly decreased (<90%) in the 110× group (Figure 4C). Serum Cp activity was lower in the 110× and 38× (<90% reductions), and 18.5× (∼60%) and 9.5× (∼35%) groups, in comparison to the 1× group (Figure 4D). Decrements in serum Cp activity are diagnostic for moderate to severe copper deficiency (1, 2, 28, 29). Cp activity correlated with hepatic copper concentrations (Figure 4E) (19). Cp activity also inversely correlated with log10 hepatic nonheme iron concentrations (data not shown) (r = −0.88; P < 0.0001); that is, as hepatic nonheme iron concentrations increased, serum Cp activity decreased. Furthermore, splenic copper concentrations were lower in the 110× (>90% reduction), 38×, and 18.5× (∼65% reductions for both) groups, compared to all others (Figure 4F) In sum, these data confirm earlier studies showing that high-iron intake leads to copper deficiency. Importantly, the current studies extend previous investigations and establish that copper metabolism was disrupted in growing rats when dietary iron intakes were <10-fold above requirements.
FIGURE 4.
Cardiac copper concentrations (A), correlation between cardiac copper concentrations and heart mass (B), hepatic copper concentrations (C), serum Cp activity (D), correlation between serum Cp activity and hepatic copper concentrations (E), and splenic copper concentrations (F) of male rats fed diets varying in iron concentration for 7–8 wk. All data points are shown, n = 4–6. For correlation analyses, the line of best fit is shown along with the correlation coefficient (r), P < 0.0001 (B and E). Labelled means without a common letter differ, P < 0.05 (A, C, D, F). BW, body weight; Cp, ceruloplasmin.
Discussion
Dietary iron loading of rodents is a commonly used intervention to model iron-overload disorders in humans (30, 31). We previously noted that mice and rats fed high-iron diets developed severe copper deficiency (4, 5). These experimental diets contained iron at concentrations far above adequate intake levels (110-fold in excess), but we hypothesized that lower, closer to physiologic amounts of dietary iron would also antagonize copper homeostasis. This is an important consideration since many humans consume supplemental iron (in addition to dietary iron), and it is conceivable that this could lead to copper depletion (32). Notably, some of the experimental diets used here, containing iron at 4- and 9.5-fold above requirements, are within the range of iron intake that humans could achieve when consuming iron supplements (6–8, 13–16).
Consumption of excess dietary iron led to accumulation of nonheme iron in serum, liver, spleen, and bone, as expected. These increases in tissue iron occurred simultaneously with decrements of tissue copper concentrations. In some tissues, however, copper was depleted even though iron concentrations did not increase. For example, cardiac iron concentrations were unaltered by the dietary treatments, yet copper was progressively depleted from the heart as dietary iron increased. Consumption of the diet with ∼110-fold excess iron impaired growth and caused anemia and enlargement of the heart, liver, and kidneys, as previously noted (4, 5). Cardiac hypertrophy and hepatomegaly were also observed when dietary iron concentrations were ∼38-fold in excess. Tissue copper depletion and depression of serum Cp activity occurred when dietary iron was at even lower concentrations (∼18.5- and ∼9.5-fold above requirements, respectively). Growth retardation, anemia in the setting of normal serum iron concentrations and TSAT, cardiac hypertrophy, hepatomegaly, tissue copper depletion, and decrements in serum Cp activity are indicative of copper deficiency in rodents (33).
The current observations are consistent with previous reports demonstrating that high dietary iron can antagonize copper homeostasis (34). For example, high iron intake perturbed copper absorption in infants and adults (32). Also, iron overload was postulated to interfere with copper utilization in humans with aceruloplasminemia (caused by mutation of the Cp gene) (35), and in those with acquired copper-deficiency myelopathy (36). Cp, an established biomarker of copper status, was also decreased in individuals with the genetic iron-loading disorder hereditary hemochromatosis (37), supporting the postulate that high iron impairs copper metabolism. Based upon these observations, it was previously suggested that iron supplements should contain copper (32, 34).
In conclusion, this investigation has demonstrated that dietary iron intakes at <10-fold above the adequate level caused moderate to severe copper deficiency in growing rodents, as indicated by suppression of serum Cp activity. Since Cp ferroxidase activity facilitates iron release from stores in the liver (i.e., hepatocytes) and reticuloendothelial macrophages of the spleen, bone marrow, and liver (to support erythropoiesis), suppression of Cp activity could at least partially counteract any positive outcomes from consuming supplemental iron. Since the iron intakes that depressed serum Cp activity are within the range that may be consumed by individuals at risk for iron deficiency, this investigation could have important implications for iron supplementation in humans.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—J-HH and JFC: conceived the investigation and designed the experimental approach; J-HH, CD, SRLF, and TW: performed the experiments; J-HH: analyzed the data and prepared the figures; J-HH and JFC: interpreted the data and drafted the paper; and all authors: reviewed and approved the final version of this manuscript.
Notes
Supported by NIH grants R01 DK074867 and R01 DK 109717 (to J.F.C.)
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Cp; ceruloplasmin; Epo, erythropoietin; Hb, hemoglobin; Hct, hematocrit; Hepc, hepcidin; ICP-MS, inductively coupled plasma MS; TIBC, total-iron-binding capacity; TSAT, transferrin saturation.
References
- 1. Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 2003;16:9–40. [DOI] [PubMed] [Google Scholar]
- 2. Prohaska JR. Impact of copper deficiency in humans. Ann N Y Acad Sci 2014;1314:1–5. [DOI] [PubMed] [Google Scholar]
- 3. Prohaska JR, Heller LJ. Mechanical properties of the copper-deficient rat heart. J Nutr 1982;112:2142–50. [DOI] [PubMed] [Google Scholar]
- 4. Ha JH, Doguer C, Wang X, Flores SR, Collins JF. High-iron consumption impairs growth and causes copper-deficiency anemia in weanling Sprague-Dawley Rats. PLoS One 2016;11:e0161033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ha JH, Doguer C, Collins JF. Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am J Physiol Gastrointest Liver Physiol 2017; 313:G353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2016;387(10021):907–16. [DOI] [PubMed] [Google Scholar]
- 7. Low MS, Speedy J, Styles CE, De-Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev 2016;4:CD009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daru J, Cooper NA, Khan KS. Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy. Acta Obstet Gynecol Scand 2016;95:270–9. [DOI] [PubMed] [Google Scholar]
- 9. Betesh AL, Santa Ana CA, Cole JA, Fordtran JS. Is achlorhydria a cause of iron deficiency anemia? Am J Clin Nutr 2015;102:9–19. [DOI] [PubMed] [Google Scholar]
- 10. Weng TC, Chang CH, Dong YH, Chang YC, Chuang LM. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open 2015;5:e006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol 2013;6:443–51. [DOI] [PubMed] [Google Scholar]
- 12. Martin J, Radeke HH, Dignass A, Stein J. Current evaluation and management of anemia in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2017;11(1):19–32. [DOI] [PubMed] [Google Scholar]
- 13. Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 15. Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012;12:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: recommendation statement. Am Fam Physician 2016;93:133–6. [PubMed] [Google Scholar]
- 17. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 18. Gulec S, Collins JF. Investigation of iron metabolism in mice expressing a mutant Menke's copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y)). PLoS One 2013;8:e66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 2011;118:3146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antonov MP, Antonova LA, Laputina TV. [Determination of ceruloplasmin activity using p-phenylenediamine as substrate]. Lab Delo 1985(6):335–8. [PubMed] [Google Scholar]
- 21. Rice EW. Standardization of ceruloplasmin activity in terms of International Enzyme Units. Oxidative formation of “Bandrowski's base” from p-phenylenediamine by ceruloplasmin. Anal Biochem 1962;3:452–6. [DOI] [PubMed] [Google Scholar]
- 22. Walaas E, Walaas O. Oxidation of reduced phosphopyridine nucleotides by p-phenylenediamines, catecholamines and serotonin in the presence of ceruloplasmin. Arch Biochem Biophys 1961;95:151–62. [DOI] [PubMed] [Google Scholar]
- 23. Rishi G, Subramaniam VN. The relationship between systemic iron homeostasis and erythropoiesis. Biosci Rep 2017;37(6):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moura IC, Hermine O, Lacombe C, Mayeux P. Erythropoiesis and transferrin receptors. Curr Opin Hematol 2015;22:193–8. [DOI] [PubMed] [Google Scholar]
- 25. Cavill I. Iron and erythropoietin in renal disease. Nephrol Dial Transplant 2002;17(Suppl 5):19–23. [DOI] [PubMed] [Google Scholar]
- 26. Dawson R, Milne G, Williams RB. Changes in the collagen of rat heart in copper-deficiency-induced cardiac hypertrophy. Cardiovasc Res 1982;16(10):559–65. [DOI] [PubMed] [Google Scholar]
- 27. Goodman JR, Warshaw JB, Dallman PR. Cardiac hypertrophy in rats with iron and copper deficiency: quantitative contribution of mitochondrial enlargement. Pediatr Res 1970;4(3):244–56. [DOI] [PubMed] [Google Scholar]
- 28. Gulec S, Collins JF. Molecular mediators governing iron-copper interactions. Annu Rev Nutr 2014;34:95–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams DM. Copper deficiency in humans. Semin Hematol 1983;20(2):118–28. [PubMed] [Google Scholar]
- 30. Park CH, Bacon BR, Brittenham GM, Tavill AS. Pathology of dietary carbonyl iron overload in rats. Lab Invest 1987;57(5):555–63. [PubMed] [Google Scholar]
- 31. Wu WH, Meydani M, Meydani SN, Burklund PM, Blumberg JB, Munro HN. Effect of dietary iron overload on lipid peroxidation, prostaglandin synthesis and lymphocyte proliferation in young and old rats. J Nutr 1990;120(3):280–9. [DOI] [PubMed] [Google Scholar]
- 32. Klevay LM. IHD from copper deficiency: a unified theory. Nutr Res Rev 2016;29(2):172–9. [DOI] [PubMed] [Google Scholar]
- 33. Scheiber I, Dringen R, Mercer JF. Copper: effects of deficiency and overload. Met Ions Life Sci 2013;13:359–87. [DOI] [PubMed] [Google Scholar]
- 34. Klevay LM. Iron overload can induce mild copper deficiency. J Trace Elem Med Biol 2001;14(4):237–40. [DOI] [PubMed] [Google Scholar]
- 35. Videt-Gibou D, Belliard S, Bardou-Jacquet E, Troadec MB, Le Lan C, Jouanolle AM, Loreal O, Rivalan J, Brissot P. Iron excess treatable by copper supplementation in acquired aceruloplasminemia: a new form of secondary human iron overload? Blood 2009;114(11):2360–1. [DOI] [PubMed] [Google Scholar]
- 36. Videt-Gibou D, Belliard S, Rivalan J, Menard D, Edan G. [Acquired copper deficiency myelopathy]. Rev Neurol (Paris) 2010;166(6-7):639–43. [DOI] [PubMed] [Google Scholar]
- 37. Cairo G, Conte D, Bianchi L, Fraquelli M, Recalcati S. Reduced serum ceruloplasmin levels in hereditary haemochromatosis. Br J Haematol 2001;114(1):226–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.