Abstract
Background
The endogenous production of arginine relies on the synthesis of citrulline by enteral ornithine transcarbamylase (OTC). Mutations in the gene coding for this enzyme are the most frequent cause of urea cycle disorders. There is a lack of correlation between in vivo metabolic function and DNA sequence, transcript abundance, or in vitro enzyme activity.
Objective
The goal of the present work was to test the hypothesis that enteroids, a novel ex vivo model, are able to recapitulate the in vivo citrulline production of wild-type (WT) and mutant mice.
Methods
Six-week-old male WT and OTC-deficient mice [sparse fur and abnormal skin (spf-ash) mutation] were studied. Urea and citrulline fluxes were determined in vivo, and OTC abundance was measured in liver and gut tissue. Intestinal crypts were isolated and cultured to develop enteroids. Ex vivo citrulline production and OTC abundance were determined in these enteroids.
Results
Liver OTC abundance was lower (mean ± SE: 0.16 ± 0.01 compared with 1.85 ± 0.18 arbitrary units; P < 0.001) in spf-ash mice than in WT mice, but there was no difference in urea production. In gut tissue, OTC was barely detectable in mutant mice; despite this, a lower but substantial citrulline production (67 ± 3 compared with 167 ± 8 µmol · kg−1 · h−1; P < 0.001) was shown in the mutant mice. Enteroids recapitulated the in vivo findings of a very low OTC content accompanied by a reduced citrulline production (1.07 ± 0.20 compared with 4.64 ± 0.44 nmol · µg DNA−1 · d−1; P < 0.001).
Conclusions
Enteroids recapitulate in vivo citrulline production and offer the opportunity to study the regulation of citrulline production in a highly manipulable system.
Keywords: arginine, citrulline, enteroid, spf-ash, urea cycle disorders
Introduction
Although the urea cycle and the pathway for the endogenous synthesis of arginine share many enzymes and transporters, they have different functional goals. Although the urea cycle functions to detoxify ammonia with all its components occupying a well-defined compartment spanning the mitochondrial membrane (1), the pathway for arginine synthesis is an interorgan process geared toward the production of this semi-indispensable amino acid (2). Both pathways start with the production of citrulline by carbamylation of ornithine by ornithine transcarbamylase (OTC), which is only expressed in hepatocytes and enterocytes (3, 4). In hepatocytes, the citrulline produced stays within the cycle to yield urea and regenerate ornithine. In contrast, citrulline synthesized in enterocytes is exported into portal blood and is available to tissues and organs (mainly the kidney) for arginine synthesis (2).
Mutations of the OTC gene are the most common urea disorder, with hundreds of distinct mutations reported (5) that result in a wide range of clinical presentations (6). Furthermore, because the OTC gene is located in the X chromosome, females are usually carriers and manifest the disorder later in life (7). The devastating consequences of impaired ammonia detoxification and hyperammonemia have made the restoration of the urea cycle the primary therapeutic target for urea cycle disorders (8). However, the increased survival of children with urea cycle disorders has created a new need for transitional care from infancy to adulthood (9). Among those needs, the maintenance of arginine supply is crucial to meet the multiple fates of this amino acid (10). Although the determination of citrulline production by the gut and de novo arginine synthesis is a straightforward procedure (11), the manipulation of enteral citrulline production by metabolic or genetic means becomes rather complex in a whole organism.
In vitro studies are an attractive alternative to test different interventions, because many manipulations can be easily performed. However, transformed intestinal epithelial cell lines may not represent normal in vivo metabolism and primary cell cultures have a short life that prevents some interventions. In addition, these cell cultures rarely represent the whole population of epithelial cells that constitute the intestinal lining. In contrast, enteroids, a novel ex vivo model, are a nontransformed tissue culture system containing intestinal stem cells and differentiated intestinal epithelial cells within a 3-dimensional structure containing crypt-, villus-, and lumen-like domains that recapitulate the in vivo architecture of the small intestinal epithelium (12). However, little is known about the metabolism of enteroids (12, 13) and even less about citrulline production. In addition, because citrulline has been proposed as a marker of gut mass and function in vivo (14), it may serve the same role in this novel ex vivo system. Therefore, the goal of the present work was to test the hypothesis that ex vivo enteroids recapitulate the in vivo citrulline production of wild-type (WT) and sparse fur and abnormal skin (spf-ash) mice, a hypomorphic model of OTC deficiency.
Methods
Animals and housing
ICRspf-ash mice were obtained by back-crossing B6EiC3Sn a/A-Otcspf-ash/J mice into an Institute of Cancer Research (ICR) background (15) for >30 generations. Mutant mice (spf-ash) on the ICR background have higher viability, growth rate, and fertility than mice on the original background and are able to maintain ureagenesis despite a nitrogen-load challenge (15). Six- to eight-week-old male WT and spf-ash littermate mice were studied. Mice had free access to an autoclaved pelleted feed (LabDiet 5V5R chow: fat, 59 g/kg; fiber, 24 g/kg; carbohydrate, 570 g/kg; crude protein, 180 g/kg; arginine, 7.5 g/kg; ash, 50 g/kg), and autoclaved reverse osmosis water was available at all times. Mice were under a 12-h light cycle (0600–1800) in a temperature-controlled (22° ± 2°C) and humidity-controlled (55% ± 5%) environment. All animal procedures were authorized by the Baylor College of Medicine Institutional Animal Care and Use Committee.
In vivo urea and citrulline production
After removing the feed at 0700 and a 3-h feed-deprivation period, mice (WT: n = 11; weight: 32.7 ± 0.5 g; spf-ash: n = 9; 27.5 ± 1.6 g) were fitted with tail-vein catheters and primed-continuously infused for 4 h with (guanidino)[15N2]arginine (prime: 45 µmol/kg; continuous: 45 µmol · kg−1 · h−1), 5-[13C]3,3,4,4[2H4]citrulline (prime: 8 µmol/kg; continuous: 8 µmol · kg−1 · h−1), 3,3[2H2]tyrosine (prime: 10 µmol/kg; continuous: 10 µmol · kg−1 · h−1), (ring)[2H5]phenylalanine (prime: 16 µmol/kg; continuous: 16 µmol · kg−1 · h−1), and [13C18O]urea (prime: 100 µmol/kg; continuous: 100 µmol · kg−1 · h−1). A blood sample was obtained from the submandibular bundle at the end of the infusion. We have shown that isotopic plateau enrichment of the metabolites of interest is achieved within the 4 h infusion (16, 17). Gut (proximal jejunum) and liver tissue were collected, and a random subset of these samples (4 for gut and 5 for liver) was analyzed to determine OTC abundance and localization.
Ex vivo citrulline production
Intestinal crypts from the proximal jejunum were collected from WT (n = 4) and spf-ash (n = 4) mice after euthanasia as described by others (18). Crypts were cultured in Matrigel (Becton Dickinson) and complete medium with growth factors (CMGF+; Digestive Disease Center, Baylor College of Medicine, Houston, Texas) at 37°C and 5% CO2 in 6-well plates (passage zero). CMGF+ media is composed of DMEM/Ham's F-12 supplemented with alanyl-glutamine, N-acetylcysteine, Spondin, Noggin, epithelial growth factor, and B-27 and N-2 Supplement (ThermoFisher) (19). Enteroids were passaged twice after 6 d in culture to obtain the passage 2 experimental enteroids. These enteroids were cultured in 24-well plates for 120 h in triplicate per mouse; media were changed at 48 and 96 h, and the 96–120 h media were saved for citrulline concentration analysis. Enteroids were collected at this time for DNA and OTC abundance determinations. Additional enteroids were cultured in a 4-well chamber slide system (ThermoFisher) under similar conditions for immunohistochemistry (18).
Analytical analysis
For OTC abundance, 10 mg homogenized frozen tissue or enteroids from 1 well were lysed in 300 μL radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.5% Na-deoxycholate; 0.1% sodium dodecyl sulfate; 150 mM NaCl; 2 mM EDTA; and 50 mM NaF) containing protease inhibitor (P3100-005; GenDEPOT) and phosphatase inhibitor (04906837001; Roche). Samples were centrifuged at 13,000 × g for 15 min after incubation for 20 min on ice at 4°C. Bicinchoninic acid protein assay was used for quantitation of protein, and 20 µg protein/sample (except for liver, 10 µg) was loaded in 4–20% sodium dodecyl sulfate polyacrylamide gel for electrophoresis and transferred to polyvinylidene difluoride membrane (Bio-Rad). After 1 h of blocking in 5% nonfat milk at room temperature, membranes were incubated with antibodies (OTC: GTX105140, dilution 1:6000; GeneTex, Inc.; β-actin: AM4302, dilution 1:5000; ThermoFisher Scientific) overnight at 4°C. Due to the low abundance of OTC in mutant spf-ash mice, gel overloading (60 µg protein) and a second OTC antibody (AV41766; Sigma-Aldrich) were also used. Membranes were incubated with secondary antibodies (sc-2305 and sc-2031; Santa Cruz Biotechnology) for 1 h at room temperature. After incubation with West-Q Pico ECL solution (dilution 1:2000; GenDEPOT) for 3 min, signals were detected by Quantity One (Bio-Rad), and data were normalized with β-actin and analyzed by ImageJ software (NIH) (20).
For immunohistochemistry, formalin-fixed samples were paraffin-embedded and 5-µm-thick sections were dewaxed; antigen retrieval was performed with sodium citrate (pH 6.0) for 20 min at 95°C. Sections were incubated in blocking buffer (5% horse serum in PBS with 0.5% Tween-20) for 30 min. OTC primary antibody (GTX105140) was applied to sections overnight at 4°C (dilution: 1:100). After washing with 1× PBS with Tween-20 for 3 × 10 min, secondary antibodies were applied to sections at room temperature for 1 h (Alexa Fluor 488 donkey anti-goat, A-11055, dilution of 1:1000; and Alexa Fluor 555 donkey anti-rabbit, A-31572, dilution of 1:1000; ThermoFisher Scientific). Sections were mounted with Vectashield mounting medium with 4', 6-diamidino-2-phenylindole (H-1200; Vector Laboratories, Inc.). Signal was detected by using a deconvolution microscope (DVLive fluorescence microscope; GE Healthcare). Enteroids were immunostained as described by Debnath et al. (21).
The amount of DNA per well of enteroids was assessed with Hoechst 33258 dye (ThermoFisher) and measured by fluorescence spectrophotometry (excitation: 350 nm; emission: 473 nm). In addition, 5-ethynyl-2′-deoxyuridine (ThermoFisher) staining to determine DNA synthesis was used to visualize proliferating cells. Isotopic enrichments and concentrations of citrulline and other amino acids were determined by heated electrospray ionization–LC–tandem MS as previously described (22).
Calculations
Citrulline, urea, and other amino acid fluxes were determined by the isotopic dilution of the respective infused tracers as previously reported (23). Enteroid citrulline production was determined by the accumulation of citrulline in the medium in a 24-h period and expressed on a per-microgram DNA basis.
Data analysis
Data were analyzed by utilizing the Proc Mixed procedure of SAS (version 9.2; SAS Institute), with genotype as the fixed effect of the model. All of the data are reported as means ± SEMs and differences were considered significant at P < 0.05.
Results
In vivo urea and citrulline production
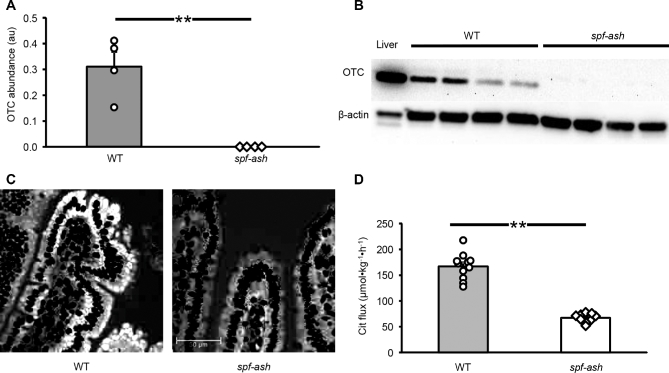
A lower OTC abundance in the liver of spf-ash mice (P < 0.001; Figure 1A) measured by Western blot (Figure 1B) was observed, with only ∼9% of residual abundance compared with WT control mice. Despite this finding, no differences (P = 0.32) in urea production were determined between the 2 genotypes. OTC abundance in intestinal tissue was barely detectable in spf-ash mice (Figure 2A, B). Increasing the protein load in the Western blot analysis (Supplemental Figure 1) or the use of a second OTC antibody against a different region of the OTC protein (Supplemental Figure 2) did not improve detection. Immunostaining showed faint basolateral staining in enterocytes of spf-ash mice (Figure 2C, Supplemental Figure 3); however, in tissue from WT mice, a stronger apical and basolateral staining was observed. A smaller citrulline flux was measured in spf-ash mice (P < 0.001; Figure 2D) accounting for ∼40% of the citrulline produced by the WT mice. Plasma citrulline concentration was also reduced in mutant mice compared with WT controls (25 ± 2 compared with 92 ±9 µmol/L, respectively; P < 0.001). Both arginine flux (320 ± 24 compared with 465 ± 26 µmol · kg−1 · h−1; P < 0.001) and plasma concentrations (63 ± 9 compared with 132 ± 3 µmol/L; P < 0.001) were lower in spf-ash mice than in their littermate WT controls. No differences (P > 0.45) in phenylalanine and tyrosine fluxes and plasma concentrations were observed between the 2 genotypes.
FIGURE 1.
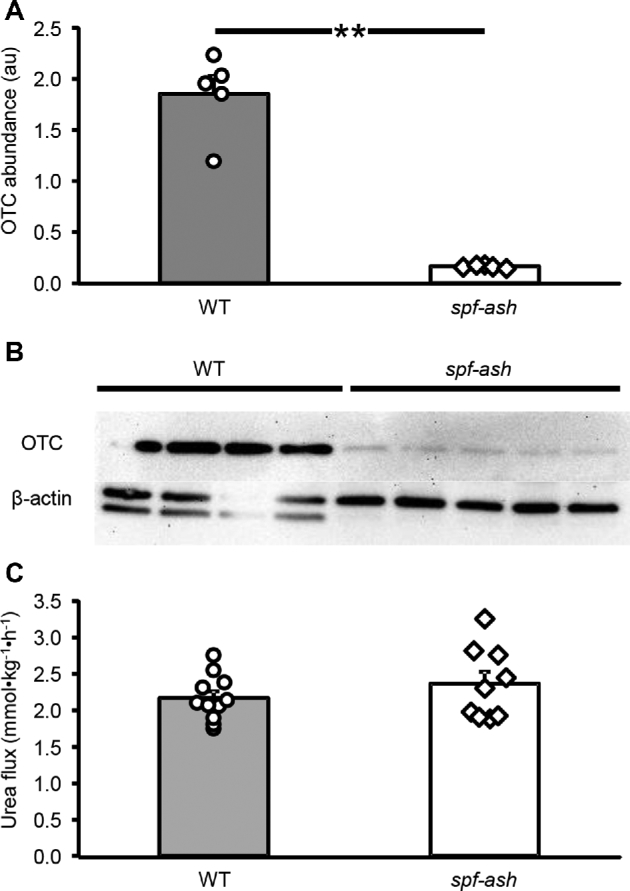
Liver OTC abundance (A), Western blot (B), and urea production (C) in ICR WT and ICRspf-ash mice. Values are means ± SEs; n = 5 for WT and spf-ash OTC abundance, n = 11 for WT, and n = 9 for spf-ash for urea flux. Individual values are also shown. **P < 0.001. au, arbitrary units; ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase; spf-ash, sparse fur and abnormal skin; WT, wild-type.
FIGURE 2.
Small intestine OTC abundance (A), Western blot (B), immunohistochemistry (C), and citrulline flux (D) in ICR WT and ICRspf-ash mice. A representative immunochemistry figure, presented here in black and white, has been manipulated to increase the contrast between the nuclei (in black) and the OTC protein (white). (A color figure is available as Supplemental Figure 3.) Values are means ± SEs; n = 4 for WT and spf-ash OTC abundance, n = 11 for WT, and n = 9 for spf-ash for citrulline flux. Individual values are also shown. **P < 0.001. au, arbitrary units; Cit, citrulline; ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase; spf-ash, sparse fur and abnormal skin; WT, wild-type.
Ex vivo citrulline production
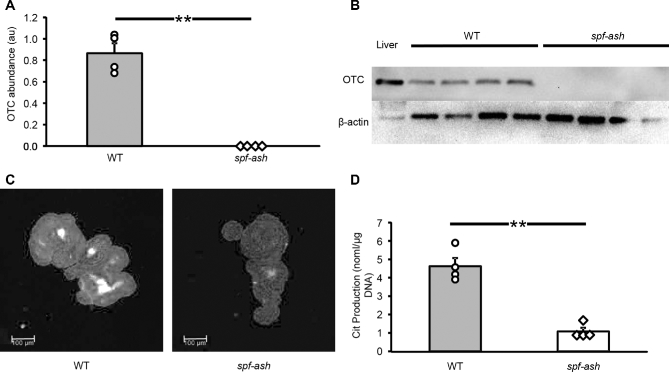
Enteroids derived from both WT and spf-ash mice developed at a similar rate and had budding crypt-like structures (Figure 3A, B), which showed proliferating cells (Figure 3C, D; Supplemental Figure 4). OTC abundance was barely distinguishable in enteroids derived from spf-ash mice (Figure 4A, B). Immunostaining also showed a faint staining in the mutant enteroids (Figure 4C; Supplemental Figure 5), and citrulline production was lower (P < 0.001) in enteroids derived from spf-ash mice than those derived from WT mice (Figure 4D). The citrulline produced by the mutant enteroids was ∼23% of that produced by the WT enteroids.
FIGURE 3.
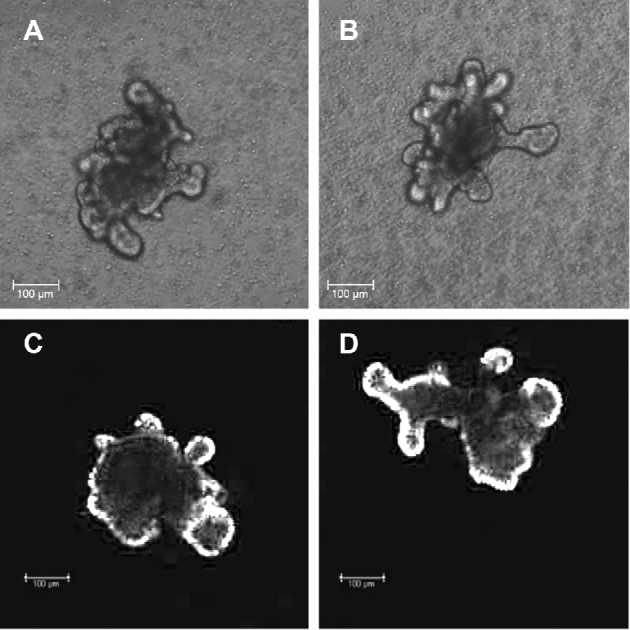
Enteroids derived from ICR WT (A, C) and ICRspf-ash (B, D) mice. Representative enteroid samples are shown using bright-field (A and B) and fluorescence (C and D) microscopy. The fluorescence microscopy figure, presented here in black and white, has been manipulated to increase the contrast of proliferating cells (in white). (A color figure is available as Supplemental Figure 4.) ICR, Institute of Cancer Research; spf-ash, sparse fur and abnormal skin; WT, wild-type.
FIGURE 4.
OTC abundance (A), Western blot (B), immunohistochemistry (C), and 24-h citrulline production (D) in enteroids derived from the small intestine of ICR WT and ICRspf-ash mice. A representative immunochemistry figure, presented here in black and white, has been manipulated to increase the contrast of the OTC protein (white). (A color figure is available as Supplemental Figure 5.) Values are means ± SEs, n = 4 mice. Individual mouse values (mean of 3 wells/mouse) are also shown. **P < 0.001. au, arbitrary units; Cit, citrulline; ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase; spf-ash, sparse fur and abnormal skin; WT, wild-type.
Discussion
The spf-ash mutation is a single nucleotide polymorphism that results in differential splicing of the mRNA, yielding 2 products: one that translates into an enzyme with normal function and another that produces an elongated nonfunctional protein that is immediately degraded (24). Human patients carrying the same nucleotide change have been identified, and this mutation has been associated both with early and late onset of the disease (25).
Despite the reduction in hepatic OTC abundance and only 5–15% residual in vitro enzyme activity compared with WT control mice (26, 27), urea production was not different between the mutant and control mice during feed deprivation. We have shown that B6EiC3Sn spf-ash mice are able to sustain ureagenesis despite a large amino acid load (28) and that mutant ICR mice were even more resilient and able to tolerate an unbalanced amino acid load (15). However, a large hepatic capacity to detoxify ammonia exists and thus urea production does not reflect the extent of the OTC deficiency; a high-protein diet (26) or the infusion of an unbalanced amino acid mixture (15, 28) is needed to overwhelm the deficient urea cycle and produce a hyperammonemic phenotype.
Patients carrying a mutation equivalent to the spf-ash mice had very low (<3%), but detectable, OTC activity measured in jejunal biopsy samples when compared with control subjects (29). This is similar to the ∼6% residual activity measured in the intestine of spf-ash mice (27). Here we have shown that the OTC protein in small intestinal tissue was barely detectable; however, citrulline flux in mutant mice was a substantial ∼40% of that measured in WT mice. This agrees with previous determinations in both B6EiC3Sn and ICR mutant mice (15). In humans, patients with nonsymptomatic OTC disorder carrying different mutations had circulating citrulline concentrations no different than those in healthy control subjects, although null-OTC patients had very low plasma citrulline (30). Here we have shown a reduced plasma citrulline concentration in mutant mice, which translated into reduced arginine fluxes and plasma concentrations. However, whole-body protein metabolism as measured by phenylalanine-tyrosine did not seem to be affected by the mutation. This highlights the importance of endogenous citrulline-arginine production in the maintenance of arginine homeostasis.
Whole-body citrulline flux includes not only the citrulline produced and released by the gut but also citrulline produced by NO synthase and in the recycling of post-translational modified arginine. However, these last 2 sources account for ∼5–8% of the total flux (23), and thus >90% of the citrulline produced is of enteral origin. For this reason, plasma citrulline concentration has been used as a marker for gut mass and function (31) and functional tests proposed to determine gut function (32). In addition, the lack of correlation between enzyme abundance and in vitro activity with in vivo fluxes (in vivo enzyme activity) shows the metabolic resilience of certain pathways and indicates that metabolic flux control is not only given by enzyme abundances but also by the metabolites involved in the pathway (33).
Enteroids derived from spf-ash mice had a normal development, with budding crypt-like structures that showed proliferating cells. Mutant and WT enteroids recapitulated the in vivo findings: a barely detectable OTC enzyme in enteroids carrying the spf-ash mutation but still able to produce ∼25% of the citrulline synthesized by the WT controls. Thus, enteroids may offer the possibility to study different interventions to increase citrulline ex vivo, before attempting in vivo procedures. This is particularly attractive for the study of different OTC mutations in humans, because in vivo metabolic function does not always correlate with DNA sequence, transcript abundance, or in vitro enzyme activity (34). A limitation of this approach is the need for a biopsy to isolate crypt stem cells; however, the advent of techniques that produce inducible intestinal organoids from pluripotent stem cells (35) will overcome this limitation, and the effect of OTC human mutations on citrulline production can be easily determined.
The advent of novel ex vivo technologies (e.g., “organ on a chip”) allows mimicking organ-level complexity by progressively integrating different cell types (including microbes) and studying the system at varying levels of complexity (36, 37). This is particularly attractive for those pathways, such as arginine synthesis, that rely on the cooperation of multiple organs. However, organoids need to be characterized and able to replicate the in vivo metabolic phenotype before they can be incorporated into more complex systems.
In conclusion, enteroids are able to recapitulate in vivo citrulline production and offer the opportunity to study the regulation of citrulline production in a highly manipulable system. In addition, citrulline ex vivo may play the same role as that in vivo as a marker of metabolic function and thus be a valuable variable to monitor in enteroid metabolic studies.
Supplementary Material
Acknowledgments
We thank Mary Estes, Director of the Texas Medical Center Digestive Disease Center (NIH P30DK056338), and Shaji Chacko, Manager of the Stable Isotope Laboratory at the Children's Nutrition Research Center at Baylor, for their assistance with sample processing and analysis. We also thank Doug Burrin for useful comments on the manuscript. The authors’ responsibilities were as follows—JCM: conceived of and designed the research; XW, YY, ICD, MAM, and JCM: performed experiments; YY and MAM: analyzed samples; JCM: analyzed the data, interpreted the results of experiments, prepared the figures, and drafted the manuscript; JCM and MAM: edited and revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by federal funds from the USDA, Agricultural Research Service, under cooperative agreement 58-6250-6-001 and the NIH (R01 GM108940).
Author disclosures: XW, YY, ICD, MAM, and JCM, no conflicts of interest.
Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase; spf-ash, sparse fur and abnormal skin; WT, wild-type.
References
- 1. Meijer AJ, Lamers WH, Chamuleau R. Nitrogen metabolism and ornithine cycle function. Physiol Rev 1990;70:701–48. [DOI] [PubMed] [Google Scholar]
- 2. Marini JC, Agarwal U, Robinson JL, Yuan Y, Didelija IC, Stoll B, Burrin DG. The intestinal-renal axis for arginine synthesis is present and functional in the neonatal pig. Am J Physiol 2017;313:E233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wraight C, Lingelbach K, Hoogenraad N. Comparison of ornithine transcarbamylase from rat-liver and intestine—evidence for differential regulation of enzyme levels. Eur J Biochem 1985;153:239–42. [DOI] [PubMed] [Google Scholar]
- 4. Genotype-Tissue-Expression (GTEx) Portal Accession no. phs000424.v7.p2. [cited 2018 Jan 1]. Available from: https://www.gtexportal.org/home/gene/OTC. [Google Scholar]
- 5. Arranz JA, Riudor E, Marco-Marin C, Rubio V. Estimation of the total number of disease-causing mutations in ornithine transcarbamylase (OTC) deficiency: value of the OTC structure in predicting a mutation pathogenic potential. J Inherit Metab Dis 2007;30:217–26. [DOI] [PubMed] [Google Scholar]
- 6. Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr Int J Paediatr 2008;97:1420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brassier A, Gobin S, Arnoux JB, Valayannopoulos V, Habarou F, Kossorotoff M, Servais A, Barbier V, Dubois S, Touati G et al. Long-term outcomes in ornithine transcarbamylase deficiency: a series of 90 patients. Orphanet J Rare Dis 2015;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilcken B. Problems in the management of urea cycle disorders. Mol Genet Metab 2004;81:S86–91. [DOI] [PubMed] [Google Scholar]
- 9. Maillot F, Crenn P. Les deficits du cycle de l'uree chez les patients adultes. [Urea cycle disorders in adult patients.] Rev Neurol 2007;163:897–903 (in French). [DOI] [PubMed] [Google Scholar]
- 10. Morris SM., Jr. Arginine metabolism revisited. J Nutr 2016;146(Suppl):2579S–86S. [DOI] [PubMed] [Google Scholar]
- 11. Marini JC, Agarwal U, Didelija IC, Azamian M, Stoll B, Nagamani SCS. Plasma glutamine is a minor precursor for the synthesis of citrulline: a multispecies study. J Nutr 2017;147:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore SR, Guedes MM, Costa TB, Vallance J, Maier EA, Betz KJ, Aihara E, Mahe MM, Lima AAM, Oriá RB et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am J Physiol 2015;308:G831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jattan J, Rodia C, Li D, Diakhate A, Dong H, Bataille A, Shroyer NF, Kohan AB. Using primary murine intestinal enteroids to study dietary TAG absorption, lipoprotein synthesis, and the role of apoC-III in the intestine. J Lipid Res 2017;58:853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008;27:328–39. [DOI] [PubMed] [Google Scholar]
- 15. Marini JC, Erez A, Castillo L, Lee B. Interaction between murine spf-ash mutation and genetic background yields different metabolic phenotypes. Am J Physiol 2007;293:E1764–71. [DOI] [PubMed] [Google Scholar]
- 16. Marini JC, Lee B, Garlick PJ. In vivo urea kinetic studies in conscious mice. J Nutr 2006;136:202–6. [DOI] [PubMed] [Google Scholar]
- 17. Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol 2010;299:E69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 2013;3:217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 2016;90:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rasband WS. ImageJ. Bethesda (MD): NIH; 1997–2016 Available from: http://imagej.nih.gov/ij/, accessed Jan 2016. [Google Scholar]
- 21. Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003;30:256–68. [DOI] [PubMed] [Google Scholar]
- 22. Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 2011;25:1291–6. [DOI] [PubMed] [Google Scholar]
- 23. Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 2012;142:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges PE, Rosenberg LE. The spf(ash) mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci USA 1989;86:4142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rivera-Barahona A, Sánchez-Alcudia R, Viecelli HM, Rüfenacht V, Pérez B, Ugarte M, Häberle J, Thöny B, Desviat LR. Functional characterization of the spf/ash splicing variation in OTC deficiency of mice and man. PLoS One 2015;10(4):e0122966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, Yu H, Xu C, Morizono H, Musunuru K et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol 2016;34:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saheki T, Mori K, Kobayashi K, Horiuchi M, Shige T, Obara T, Suzuki S, Mori M, Yamamura K. Importance of ornithine transcarbamylase (OTC) deficiency in small-intestine for urinary orotic acid excretion—analysis of OTC-deficient spf-ash mice with OTC transgene. BBA Mol Basis Dis 1995;1270:87–93. [DOI] [PubMed] [Google Scholar]
- 28. Marini JC, Lee B, Garlick PJ. Reduced ornithine transcarbamylase activity does not impair ureagenesis in OTC(spf-ash) mice. J Nutr 2006;136:1017–20. [DOI] [PubMed] [Google Scholar]
- 29. García-Pérez MA, Sanjurjo P, Rubio V. Demonstration of the spf-ash mutation in Spanish patients with ornithine transcarbamylase deficiency of moderate severity. Hum Genet 1995;95:183–6. [DOI] [PubMed] [Google Scholar]
- 30. Marini JC, Lanpher BC, Scaglia F, O'Brien WE, Sun Q, Garlick PJ, Jahoor F, Lee B. Phenylbutyrate improves nitrogen disposal via an alternative pathway without eliciting an increase in protein breakdown and catabolism in control and ornithine transcarbamylase-deficient patients. Am J Clin Nutr 2011;93:1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000;119:1496–505. [DOI] [PubMed] [Google Scholar]
- 32. Peters JHC, Wierdsma NJ, Teerlink T, Van Leeuwen PAM, Mulder CJJ, Van Bodegraven AA. The citrulline generation test: proposal for a new enterocyte function test. Aliment Pharmacol Ther 2008;27:1300–10. [DOI] [PubMed] [Google Scholar]
- 33. Wegner A, Meiser J, Weindl D, Hiller K. How metabolites modulate metabolic flux. Curr Opin Biotechnol 2015;34:16–22. [DOI] [PubMed] [Google Scholar]
- 34. Scaglia F, Zheng QP, O'Brien WE, Henry J, Rosenberger J, Reeds P, Lee B. An integrated approach to the diagnosis and prospective management of partial ornithine transcarbamylase deficiency. Pediatrics 2002;109:150–2. [DOI] [PubMed] [Google Scholar]
- 35. Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, De Jonge HR, Estes MK, Donowitz M. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem 2016;291:3759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 2018;8https://www.nature.com/articles/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang TH, Kim HJ. Farewell to animal testing: innovations on human intestinal microphysiological systems. Micromachines 2016;7(7):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.