Abstract
Background
Feeding stimulates protein synthesis in skeletal muscle of neonates and this response is regulated through activation of mechanistic target of rapamycin complex 1 (mTORC1). The identity of signaling components that regulate mTORC1 activation in neonatal muscle has not been fully elucidated.
Objective
We investigated the independent effects of the rise in amino acids (AAs) and insulin after a meal on the abundance and activation of potential regulators of mTORC1 in muscle and whether the responses are modified by development.
Methods
Overnight-fasted 6- and 26-d-old pigs were infused for 2 h with saline (control group) or with a balanced AA mixture (AA group) or insulin (INS group) to achieve fed levels while insulin or AAs, respectively, and glucose were maintained at fasting levels. Muscles were analyzed for potential mTORC1 regulatory mechanisms and results were analyzed by 2-factor ANOVA followed by Tukey's post hoc test.
Results
The abundances of DEP domain-containing mTOR-interacting protein (DEPTOR), growth factor receptor bound protein 10 (GRB10), and regulated in development and DNA damage response 2 (REDD2) were lower (65%, 73%, and 53%, respectively; P < 0.05) and late endosomal/lysosomal adaptor, MAPK and mTOR activator 1/2 (LAMTOR1/2), vacuolar H+-ATPase (V-ATPase), and Sestrin2 were higher (94%, 141%, 145%, and 127%, respectively; P < 0.05) in 6- than in 26-d-old pigs. Both AA and INS groups increased phosphorylation of GRB10 (P < 0.05) compared with control in 26- but not in 6-d-old pigs. Formation of Ras-related GTP-binding protein A (RagA)-mTOR, RagC-mTOR, and Ras homolog enriched in brain (RHEB)-mTOR complexes was increased (P < 0.05) and Sestrin2-GTPase activating protein activity towards Rags 2 (GATOR2) complex was decreased (P < 0.05) by both AA and INS groups and these responses were greater (P < 0.05) in 6- than in 26-d-old pigs.
Conclusion
The results suggest that formation of RagA-mTOR, RagC-mTOR, RHEB-mTOR, and Sestrin2-GATOR2 complexes may be involved in the AA- and INS-induced activation of mTORC1 in skeletal muscle of neonates after a meal and that enhanced activation of the mTORC1 signaling pathway in neonatal muscle is in part due to regulation by DEPTOR, GRB10, REDD2, LAMTOR1/2, V-ATPase, and Sestrin2.
Keywords: translation initiation, protein synthesis, REDD, growth, newborn
Introduction
The neonatal period is characterized by rapid growth and development, especially of skeletal muscle. Our studies in the neonatal piglet model have shown that during this period, the feeding-induced stimulation of protein synthesis is an important contributor to skeletal muscle growth (1). The rise in amino acids (AAs) and in insulin after a meal independently mediate this stimulation of protein synthesis in muscle (1) and the response decreases with development (2, 3).
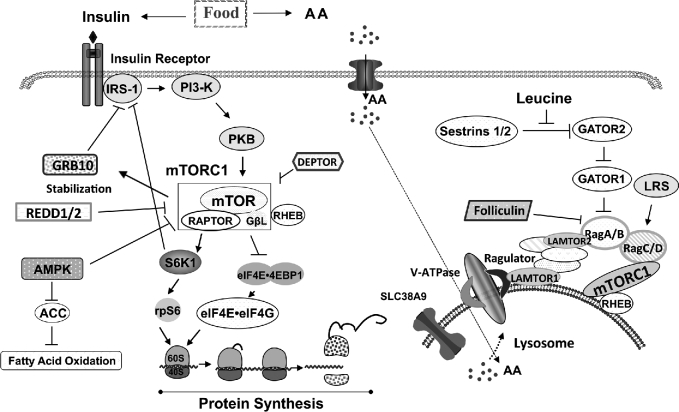
Skeletal muscle exhibits intricate ways of responding to AAs and insulin, and the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) and downstream translation initiation factors are the prominent targets (4, 5). We have shown that the enhanced response of protein synthesis in neonatal muscle is largely due to the enhanced sensitivity of mTORC1 signaling to AAs and insulin (2). The postprandial rise of insulin activates signaling cascades leading to the activation of mTORC1 (6) and this process is governed by positive and negative regulators (Figure 1). Negative regulation that dampens mTORC1 activation occurs in response to excess nutrients or stress conditions. Components that can act as negative regulators include insulin receptor substrate 1 (IRS-1, when phosphorylated on Ser636/639) (7), growth factor receptor bound protein 10 (GRB10) (8–10), DEP domain-containing mTOR-interacting protein (DEPTOR), AMP-activated protein kinase (AMPK), and regulated in development and DNA damage response 1/2 (REDD1/2) (11–18) (Figure 1). Their role in the inhibition of mTORC1 in neonatal muscle has not been fully determined.
FIGURE 1.
Current concepts of the regulation of mTORC1 activation by AAs and insulin. AA, amino acids; ACC, acetyl CoA carboxylase; AMPK, AMP-activated protein kinase; DEPTOR, DEP domain-containing mechanistic target of rapamycin-interacting protein; eIF4E, eukaryotic translation initiation factor 4E; eIF4G, eukaryotic translation initiation factor 4G; GATOR1/2, GAP activity toward Rags 1/2; GβL, G protein beta protein subunit-like; GRB10, growth factor receptor bound protein 10; IRS-1, insulin receptor substrate 1; LAMTOR1/2, late endosomal/lysosomal adaptor, MAPK and mechanistic target of rapamycin activator 1/2; LRS, leucyl-tRNA synthetase; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; PI3-K, phosphatidylinositol-3-kinase; PKB, protein kinase B; Rag A/B C/D, RAS-related GTP-binding protein A/B C/D; RAPTOR, regulatory associated protein of mechanistic target of rapamycin complex 1; REDD1/2, regulated in development and DNA damage response 1/2; RHEB, Ras homolog enriched in brain; rpS6, ribosomal protein S6; Sestrin1/2, stress response protein 1/2; SLC38A9, Solute Carrier Family 38 Member 9; S6K1, p70 ribosomal protein S6 kinase 1; V-ATPase, vacuolar H+-ATPase; 4EBP1, eukaryotic translation initiation factor 4E binding protein 1.
The mechanisms that govern AA-induced mTORC1 activation are beginning to be elucidated (19, 20). Current in vitro studies suggest that, after the uptake of AAs into cells by AA transporters (21), the AAs enter the lysosome compartment where AA sensing components reside [Ras homolog enriched in brain (RHEB), RAS-related GTP-binding protein (Rag) A/B and C/D, Ragulator (p14, MP1, HBXIP, C7orf59, and p18), Solute Carrier Family 38 Member 9 (SLC38A9), and vacuolar H+-ATPase (V-ATPase)] (19). Under a rich AA environment, these sensing components form an active complex that recruits an inactive form of mTORC1 to the lysosome, thereby allowing RHEB to bind to and activate mTORC1 (22–24). Folicullin also may activate mTORC1 by directly binding to Rag C/D and facilitate Rag-mTOR complex formation (25, 26). In the last few years, 2 leucine sensors have been identified: stress response protein 2 (Sestrin2) (27) and leucyl-tRNA synthetase (LRS) (28). Leucine has been reported to modulate Sestrin2–GTPase-activating protein activity toward Rags (GATOR) 2 and LRS-Rag GTPase interactions leading to mTORC1 activation (27, 28).
Most of the aforementioned studies were conducted in in vitro conditions and with the use of cell culture models. Therefore, the accuracy and the physiologic relevance of this signaling model in metabolically important tissues, such as skeletal muscle, under in vivo conditions that arise during fasting and feeding cycles are yet to be fully explored. Therefore, in this study, we examined the independent effects of the rise in AAs and insulin, similar to that which occurs after a meal, on the activation of these potential regulators of mTORC1 and whether their abundance and activation change with development in skeletal muscle of neonatal pigs.
Methods
Animals and housing
Pregnant sows (multiparous cross-bred: Landrace × Yorkshire × Duroc × Hampshire) (Agriculture Headquarters, Texas Dept. of Criminal Justice, Huntsville, TX) were housed in lactation crates in environmentally controlled rooms prior to farrowing. Sows were fed a commercial diet (no. 5084; PMI Feeds) and provided water ad libitum. After farrowing, piglets remained with the sow with no access to the sow's diet. At the age of 2–3 d, sterile catheters were inserted into the jugular vein and carotid artery, as previously described in Suryawan et al. (2). The protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council guidelines.
Experimental design
After an 8-h fast, piglets (6 d of age, mean ± SD weight: 1.9 ± 0.3 kg; 26 d of age: 5.2 ± 0.8 kg) were randomly assigned to one of the following treatment groups (n = 4–6 per treatment group): 1) euinsulinemic-euglycemic-euaminoacidemic conditions (control), 2) euinsulinemic-euglycemic-hyperaminoacidemic clamps, and 3) hyperinsulinemic-euglycemic-euaminoacidemic clamps, as previously described (2). Briefly, during the experiment, blood samples were collected and analyzed for glucose (YSI 2300 STAT Plus; Yellow Springs Instruments) and total BCAAs by rapid enzymatic kinetic assay (29). Clamps were initiated with a primed, constant (12 mL/h) infusion of insulin (Eli Lilly) at 0 or 100 ng · kg−0.66· min−1 given to attain plasma insulin concentrations of 3 (fasting insulin level) or 30 μU/mL (fed insulin level) and sustained for a period of 2 h. To ensure that glucose and AAs were clamped at fasting levels, venous blood samples were acquired every 5 min and immediately analyzed for glucose and BCAA concentrations. Euglycemia and euaminoacidemia were obtained by infusing dextrose (Baxter Healthcare) and a balanced AA mixture to maintain blood glucose and BCAA within 10% of fasting levels. Hyperaminoacidemia was achieved by infusing a balanced AA mixture (30) to raise plasma BCAA concentrations by 2-fold of the fasting level to reproduce the fed-state AA levels. Circulating insulin concentrations were analyzed with the use of a radioimmunoassay kit (EMD Millipore) (31).
Immunoblotting and immunoprecipitation
Skeletal muscle (longissimus dorsi) samples were homogenized in cold HEPES buffer followed by centrifugation at 10,000 × g for 10 min at 4°C (2). Equal amounts (10–50 μg) of proteins were electrophoretically separated in polyacrylamide gels, transferred to polyvinylidene difluoride membranes (Bio-Rad), and incubated with indicated primary antibodies followed by appropriate secondary antibodies. Blots were developed through the use of an enhanced chemiluminescence kit (GE Healthcare Bio-Sciences), then imaged and analyzed with the use of a ChemiDoc-It Imaging System (Ultra-Violet Products Ltd). The amount of β-actin in the samples was used to normalize the protein abundance of each signaling component. The mTOR-RagA, mTOR-RagC, mTOR-RHEB, and Sestrin2-GATOR2 complex abundance was determined by immunoprecipitation as previously described (32). Briefly, muscle samples were homogenized in (3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate) buffer (32). Protein homogenates (500 μg) containing 2 µL of mTOR or Sestrin2 antibody were incubated overnight at 4°C. The next day, 200 μg of BioMag goat anti-rabbit IgG beads (Qiagen) were added to the sample followed by incubation for 2 h at 4°C and washing procedures (32). The samples were subjected to SDS-PAGE followed by immunoblotting with anti-RagA, anti-RagC, anti-RHEB, or anti-Mios (GATOR2 subunit) antibody. The protein complexes were normalized by the amount of total mTOR or Sestrin2 in the precipitates. Primary antibodies that were used in the analyses were from Bethyl Laboratories (β-actin, #A300-491A; LRS, #A304-316A-T), Cell Signaling Technology [IRS-1, #3407; p-IRS1 Ser636/639, #2388; acetyl CoA carboxylase (ACC), #3676; p-ACC Ser79, #3661; Folliculin, #3697; RHEB, #13879; RagA, #4357; RagC, #9480; late endosomal/lysosomal adaptor, MAPK and mTOR activator (LAMTOR) 1/C11orf59, #9875; LAMTOR2/Roadblock domain containing 3, #8145; GATOR1/Nitrogen permease regulator 2-like, #37344; GATOR2/Mios, #13557; Sestrin2, #8487; Folliculin, #3697; mTOR, #2972], GeneTex (V-ATPase/ATP6V0D1, #GTX111027), Novus Biologicals (DEPTOR, #NBP1-49674SS; Sestrin1, #NBP1-68677), Bioss (GRB10, #bs-2769R), Millipore (GRB10 Ser501/503, #07-1520), and ProteinTech (REDD1, #10638-1-AP; REDD2/DNA damage inducible transcript 4-like, #12094-1-AP).
Statistical analysis
Values are presented as least square means ± SEMs (n = 4–6). All of the data were analyzed through SAS (version 9.4; SAS Institute). Differences were determined by 2-factor ANOVA. Comparisons between multiple groups were analyzed by Tukey's post hoc test. Differences were considered significant at P < 0.05.
Results
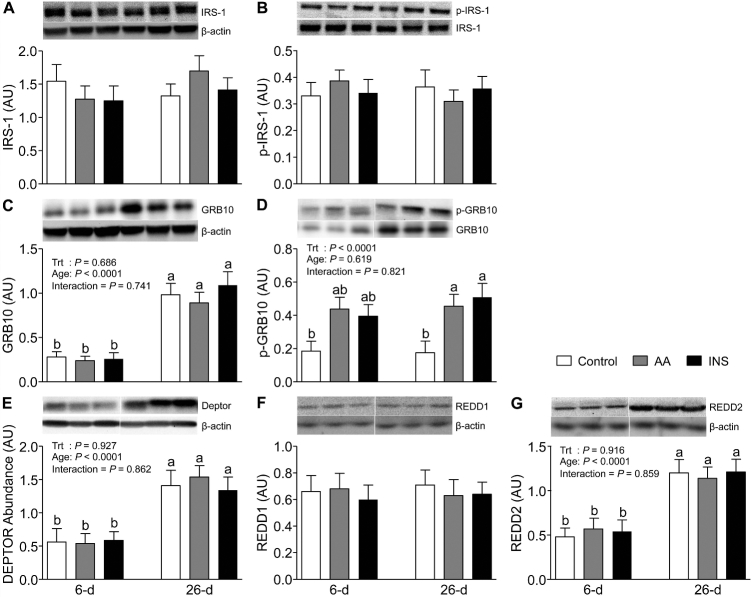
We determined the abundance and phosphorylation of IRS-1 on Ser636/639, a negative regulator of the insulin signaling pathway. Neither the abundance nor the phosphorylation of IRS-1 was affected by the rise in AAs or insulin, and they did not change with development (Figure 2A, B). To investigate a newly identified mTORC1 target that regulates feedback inhibition of the insulin signaling pathway toward activation of mTORC1, we measured the levels of GRB10 abundance and phosphorylation (Figure 2C, D). The GRB10 abundance was lower in 6- than in 26-d-old pigs (P < 0.05). Short-term AA and insulin infusions to mimic the rise after a meal had no effect on the abundance of GRB10. In skeletal muscle of 26-d-old pigs but not in younger counterparts, the phosphorylation of GRB10 was increased independently by both AAs and insulin (P < 0.05). Interestingly, AA- and insulin-induced GRB10 phosphorylation was not affected by age.
FIGURE 2.
The abundance of IRS-1 (A), phosphorylation of IRS-1 at Ser636/639 (B), abundance of GRB10 (C), phosphorylation of GRB10 at Ser501/503 (D), and abundance of DEPTOR (E), REDD1 (F), and REDD2 (G) in longissimus dorsi muscle of 6- and 26-d-old pigs after 2-h infusion of saline (control), amino acids, or insulin. The values for protein abundance were normalized by β-actin abundance. The phosphorylation values were corrected by their abundance in the samples. White lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Values are least square means ± SEM; n = 4–6. Means with uncommon letters are significantly different: a > b. P < 0.05 was considered significant. AA, amino acid group; AU, arbitrary unit; DEPTOR, DEP domain-containing mechanistic target of rapamycin-interacting protein; GRB10, growth factor receptor bound protein 10; INS, insulin group; IRS-1, insulin receptor substrate 1; p, phosphorylated; REDD, regulated in development and DNA damage response; Trt, treatment.
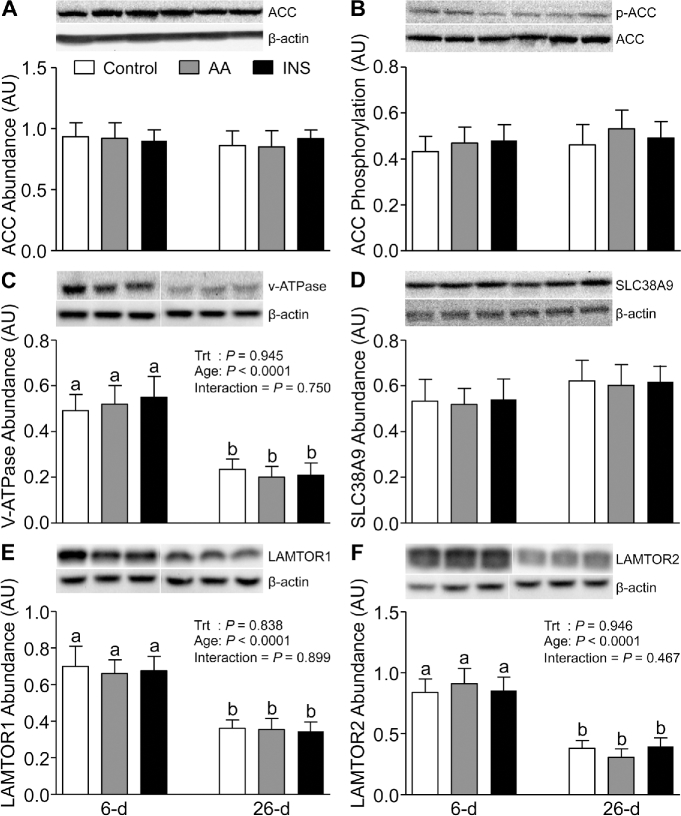
In our previous studies, we showed that the activation of mTORC1 is higher in skeletal muscle of neonates compared with slightly older pigs (32). Since the activation of mTORC1 is governed by the balance between positive and negative regulators, we analyzed the abundance of DEPTOR, REDD1, and REDD2, potent inhibitors of mTORC1. Skeletal muscle of 6-d-old pigs showed lower DEPTOR and REDD2 (P < 0.05), but not lower REDD1, abundance than that of 26-d-old pigs (Figure 2E–G). However, AAs and insulin had no effect on DEPTOR or REDD2 abundance. When we analyzed ACC, a downstream substrate of AMPK, we found that neither AAs nor insulin had an effect on the abundance or phosphorylation of ACC and there was no change with age (Figure 3A, B).
FIGURE 3.
The abundance of ACC (A), phosphorylation of ACC at Ser79 (B), and the abundance of V-ATPase (C), SLC38A9 (D), LAMTOR1 (E), and LAMTOR2 (F) in longissimus dorsi muscle of 6- and 26-d-old pigs after 2-h infusion of saline (control), amino acids, or insulin. The values for protein abundance were normalized by β-actin abundance. The phosphorylation values of ACC were corrected by the ACC abundance in the samples. White lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Values are least square means ± SEM; n = 4–6. Means with uncommon letters are significantly different: a > b. P < 0.05 was considered significant. AA, amino acid group; ACC, acetyl CoA carboxylase; AU, arbitrary unit; INS, insulin group; LAMTOR, late endosomal/lysosomal adaptor, MAPK and mechanistic target of rapamycin actvator; p, phosphorylated; SLC38A9, Solute Carrier Family 38 Member 9; Trt, treatment; V-ATPase, vacuolar H+-ATPase.
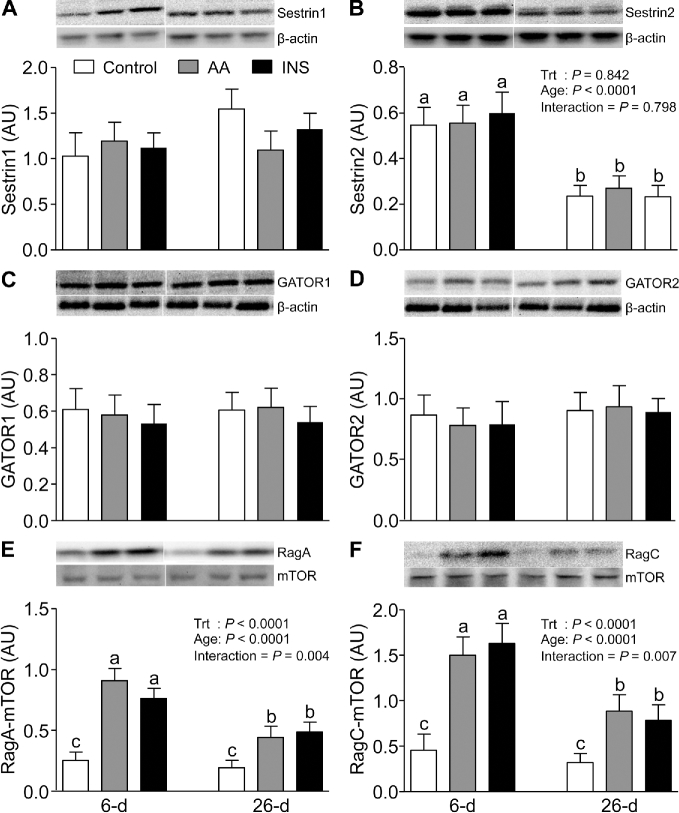
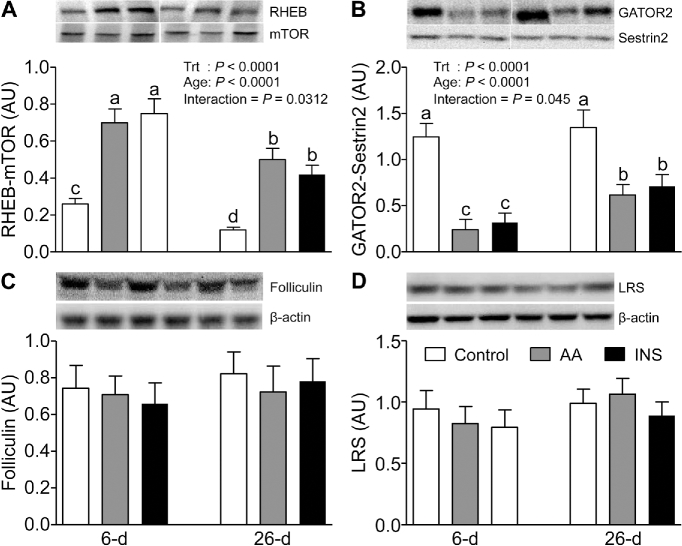
To explore the molecular mechanisms by which mTORC1 activation in the lysosomal compartment is regulated, we examined the abundance of the newly discovered members of this signaling component, i.e., SLC38A9, V-ATPase, LAMTOR1, LAMTOR2, Sestrin1, Sestrin2, GATOR1, GATOR2, LRS, and Folliculin, as well as the Sestrin2-GATOR2, mTOR-RagC, mTOR-RagA, and mTOR-RHEB complexes. The abundances of V-ATPase, LAMTOR1, LAMTOR2 (Figure 3C, E, and F), and Sestrin2 (Figure 4B) were higher in 6- than in 26-d-old pigs (P < 0.05). However, SLC38A9 (Figure 3D), Sestrin1, GATOR1, and GATOR2 (Figure 4A, C, and D) were unaffected by age. Neither AAs nor insulin had an effect on the abundance of these signaling components. The interactions between mTOR and RagA, mTOR and RagC, and mTOR and RHEB were increased (P < 0.05) (Figure 4E, F, and Figure 5A) and the abundance of the Sestrin2-GATOR2 complex was reduced (P < 0.05) (Figure 5B) by both AAs and insulin; these effects were significantly greater in muscle of 6- than of 26-d-old pigs (P < 0.05). The abundances of Folliculin, an inhibitor of Rag complex activation, and LRS, an activator of Rag GTPase, were not affected by AAs, insulin, or development (Figure 5C, D).
FIGURE 4.
The abundance of Sestrin1 (A), Sestrin2 (B), GATOR1 (C), GATOR2 (D), RagA-mTOR complex (E), and RagC-mTOR complex (F) in longissimus dorsi muscle of 6- and 26-d-old pigs after 2-h infusion of saline (control), amino acids, or insulin. The values were normalized by β-actin abundance. White lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Values are least square means ± SEM; n = 4–6. Means with uncommon letters are significantly different: a > b > c. P < 0.05 was considered significant. AA, amino acid group; AU, arbitrary unit; GATOR, GAP activity toward Rags; INS, insulin group; mTOR, mechanistic target of rapamycin; Rag, RAS-related GTP-binding protein; Sestrin, stress response protein; Trt, treatment.
FIGURE 5.
The abundance of RHEB-mTOR complex (A), GATOR2-Sestrin2 complex (B), Folliculin (C), and LRS (D) in longissimus dorsi muscle of 6- and 26-d-old pigs after 2-h infusion of saline (control), amino acids, or insulin. The values were normalized by β-actin abundance. White lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Values are least square means ± SEM; n = 4–6. Means with uncommon letters are significantly different: a > b > c. P < 0.05 was considered significant. AA, amino acid group; AU, arbitrary unit; GATOR2, GAP activity toward Rags 2; INS, insulin group; LRS, leucyl-tRNA synthetase; mTOR, mechanistic target of rapamycin; RHEB, Ras homolog enriched in brain; Sestrin2, stress response protein 2; Trt, treatment.
Discussion
The feeding-induced rise in AAs and insulin induces the stimulation of muscle protein synthesis through activation of mTORC1 via the AA and insulin signaling pathways. In agreement with the enhanced sensitivity of muscle protein synthesis to feeding in skeletal muscle of neonates, the sensitivity of mTORC1 signaling to activation by AAs and insulin is enhanced in neonatal muscle. In this study, we analyzed in muscle of neonatal pigs the abundance and the activation of signaling components that recently have been suggested, principally based on in vitro studies, to be involved in the regulation of AA- and insulin-induced stimulation of protein synthesis. We demonstrated that the abundances of a majority of the negative and positive regulators in the mTORC1 pathway were affected by development, whereas the activation of many of these components was positively regulated by both AAs and insulin, as well as upregulated in early life. The results of this study suggest that, during the neonatal period, a set of regulatory components in skeletal muscle are likely involved in ensuring enhanced activation of mTORC1 toward protein synthesis to support lean growth.
Although the mechanism of IRS protein activation is very complex, it plays a central role in the regulation of the insulin signaling pathway (33). Here we analyzed the mTOR/S6K1 (p70 ribosomal protein S6 kinase 1)-dependent phosphorylation of IRS-1 (Ser636/639). Unlike tyrosine phosphorylation of IRS-1 (32) which positively controls insulin signaling and is upregulated after a meal in neonatal muscle (34), serine phosphorylation of IRS-1 (Ser636/639) was not affected by insulin, AAs, or development, suggesting that this postprandial feedback inhibition mechanism may not play a significant role in neonates.
The phosphorylation of GRB10 at Ser501/503 has been reported from in vitro studies to destabilize IRS function and inhibit the mTORC1 signaling pathway (9, 10). Leucine, a critical nutrient signal that stimulates protein synthesis (35), may attenuate the activation of GRB10 through a PI3-K/AKT (phosphatidylinositol-3-kinase)-independent mechanism (36). Deletion of GRB10 in mice has been reported to enhance muscle hypertrophy (37). Our results showed that the GRB10 abundance was lower in younger than in older pigs, suggesting less inhibition of mTORC1 activation in early postnatal life. In older pigs, both insulin and AAs induced the phosphorylation of GRB10 at Ser501/503. Interestingly, the activation of GRB10 was not influenced by age. These results suggest that in older pigs, AA- and insulin-induced mTORC1 activation stimulates GRB10 phosphorylation, resulting in the stability of this protein and feedback inhibition of the insulin signaling pathway.
DEPTOR acts as a crucial inhibitor of mTORC1 and mTORC2 (13, 38). A 50% reduction of DEPTOR in mouse gastrocnemius muscle has been demonstrated to prevent disuse atrophy, in part due to increased protein synthesis (39). Our results showed a lower abundance of DEPTOR in skeletal muscle of 6- compared with 26-d-old pigs, consistent with the enhanced activation of mTORC1 in neonatal muscle (2). Another inhibitory event, suppression of mTORC1 activation by AMPK, can be monitored by measuring the activation of ACC, a bona fide readout of AMPK activity (17). In the current study we found that both the abundance and phosphorylation of ACC were not affected by AAs, insulin, or age, suggesting that AMPK does not play an important role in the feeding-induced activation of the mTORC1 pathway in neonatal muscle.
The observations which indicate that REDD1/2-dependent mTOR regulation contributes to cell growth (11) suggest that these proteins may play a role in the regulation of muscle protein synthesis in neonates. Indeed, we found that the abundance of REDD2, but not REDD1, was lower in muscle of younger pigs, consistent with higher mTOR activation. Our findings are in agreement with Miyazaki and Esser (12) who found that overexpression of REDD2 in skeletal muscle blunted the leucine-induced mTOR activation.
Components of AA sensing machinery in the lysosome have been implicated in the regulation of mTORC1 activation (Figure 1) (19). In the presence of AAs, the heterodimeric Rag GTPases interact with mTORC1 and localize it to the lysosome. RHEB, which also resides in the lysosome, interacts with mTOR and activates mTORC1 (40). Consistent with a previous in vitro report (41), our results showed that both AAs and insulin independently induced RHEB-mTOR interaction and this effect dampened with development. Similar results were found for the interactions between mTOR and RagA and between mTOR and RagC, consistent with the finding that leucine stimulates RagA-mTOR and RagC-mTOR associations (42).
SLC38A9, a putative arginine sensor, regulates the activation of mTORC1 through the Rag-Ragulator complex (22–24). Our data indicate that the abundance of SLC38A9 was not affected either by short-term AA or insulin treatments or by development. No other data are available regarding the function of this AA transporter in vivo in skeletal muscle, and thus, the notion that SLC38A9 may act as a lysosomal AA sensor needs further study.
V-ATPase has been implicated as an essential component of several growth signaling pathways, including mTORC1 (43, 44). In a current model, AAs signal to the V-ATPase-Ragulator complex, resulting in activation of Rag GTPases, followed by recruitment of mTORC1 to the lysosomal surface where RHEB activates mTORC1. In this study, we found that V-ATPase abundance was higher in muscle of younger pigs than in their older counterparts. It is noteworthy that a human skeletal muscle genetic disease which decreases V-ATPase abundance induces myopathy and reduces mTORC1 activation (45).
The Ragulator complex [LAMTOR1 (p18), LAMTOR2 (p14), MP1, HBXIP, and C7orf59] is crucial for the activation of mTORC1 (19). LAMTOR1 appears to be an essential anchor of a scaffolding complex that is involved in the activation of the mTORC1 pathway (46). LAMTOR2 is also important for both mTOR and MAPK signaling (47, 48). Our results showed that the abundances of LAMTOR1 and LAMTOR2 were higher in muscle of 6- compared with 26-d-old pigs, consistent with the higher mTORC1 activation in muscle of young pigs (32). In agreement with the lack of effect of leucine administration (49), our data showed that both AAs and insulin had no effect on the abundances of these adaptor proteins.
Folliculin has been reported to act as an inhibitor of the lysosomal nutrient apparatus that activates mTORC1 (26). Loss of Folliculin can cause severe cardiac hypertrophy (50) but the role of Folliculin in the mTORC1 pathway in skeletal muscle has been unstudied. We found no effect of age on Folliculin abundance, suggesting that Folliculin may not play a crucial role in the developmental changes in protein synthesis in skeletal muscle.
LRS, a purported leucine sensor, can positively affect Rag GTPase in cell culture, resulting in the activation of mTORC1 (28). However, the role of LRS in mTORC1 activation in vivo is unclear. An in vivo study with human subjects showed that essential AA consumption stimulated muscle protein synthesis and mTOR activation but had no effect on LRS mRNA or protein abundance (51). We also found that there was no effect of AAs, as well as insulin or development, on LRS protein abundance.
A recent model of AA sensing proposes that Sestrin2 binds to and inhibits GATOR2, leading to inhibition of the mTORC1 pathway (Figure 1) (27). Leucine directly binds to Sestrin2, causing the dissociation of the Sestrin2-GATOR2 complex and relieving mTORC1 from GATOR2 inhibition (27). Our results showed that AAs reduced the abundance of the Sestrin2-GATOR2 complex and this effect was greater in muscle of younger pigs. Our finding that insulin affected Sestrin2-GATOR2 association may suggest that insulin indirectly caused this event by inducing the transport of AAs, including leucine, into the cells (52). In younger pigs, the greater abundance of Sestrin2, but not Sestrin1, GATOR1, or GATOR2, may indicate enhanced sensitivity to leucine stimulation.
In conclusion, in vitro studies with cell lines have resulted in the identification of signaling components that positively and negatively regulate the activation of mTORC1. However, due to the nature of the in vitro studies, it is necessary to delineate their biological functions through the use of transgenic mice, knockout mice, and intact animals. Here we report that the activation of the majority of signaling components we studied is in agreement with previous in vitro studies. In regard to protein abundance, it is important to note that the lack of response to AA or insulin treatment may be due to the transient condition of the experiment. Nonetheless, the results of the current study suggest that the formation of the Rag-mTOR, RHEB-mTOR, and Sestrin2-GATOR2 complexes may play an important role in the feeding-induced stimulation of mTORC1 and that this enhanced responsiveness of mTORC1 in neonatal muscles is in part due to developmental changes in the abundance of GRB10, DEPTOR, REDD2, LAMTOR1/2, V-ATPase, and Sestrin2.
Acknowledgments
We thank Rose Parada and Hanh Nguyen for technical assistance and Marko Rudar for statistical analysis. The authors’ contributions were as follows—AS: conducted the research, analyzed the data, and wrote the article; TAD: edited the manuscript: and all authors: designed the research and read and approved the final manuscript.
Notes
Supported by NIH grants AR44474, HD085573, and HD072891, USDA NIFA grant 2013-67015-20438, and USDA/ARS grant 6250-51000-055.
Author disclosures: AS and TAD, no conflicts of interest.
Abbreviations used: AA, amino acids; ACC, acetyl CoA carboxylase; AMPK, AMP-activated protein kinase; DEPTOR, DEP domain-containing mTOR-interacting protein; GATOR, GTPase activating protein activity toward Rags; GRB10, growth factor receptor bound protein 10; IRS-1, insulin receptor substrate 1; LAMTOR, late endosomal/lysosomal adaptor, MAPK and mTOR activator; LRS, leucyl-tRNA synthetase; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; Rag, Ras-related GTP-binding protein; REDD, regulated in development and DNA damage response; RHEB, Ras homolog enriched in brain; Sestrin, stress response protein; SLC38A9, Solute Carrier Family 38 Member 9; V-ATPase, vacuolar H+-ATPase.
References
- 1. Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 2009;12:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 2007;293:E1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis TA, Suryawan A, Orellana RA, Fiorotto ML, Burrin DG. Amino acids and insulin are regulators of muscle protein synthesis in neonatal pigs. Animal 2010;4:1790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 2006;34:213–16. [DOI] [PubMed] [Google Scholar]
- 5. Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr 2014;99:237S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. AJP Endocrinol Metab 2009;296:E581–91. [DOI] [PubMed] [Google Scholar]
- 7. Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol 2006;26:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith FM, Holt LJ, Garfield AS, Charalambous M, Koumanov F, Perry M, Bazzani R, Sheardown SA, Hegarty BD, Lyons RJ et al. Mice with a disruption of the imprinted GRB10 gene exhibit altered body composition, glucose homeostasis, and insulin signaling during postnatal life. Mol Cell Biol 2007;27:5871–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011;332:1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu Y, Yoon S-O, Poulogiannis G, Yang Q, Ma XM, Villén J, Kubica N, Hoffman GR, Cantley LC, Gygi SP et al. Phosphoproteomic analysis identifies GRB10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 2011;332:1322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellisen LW. Growth control under stress: mTOR regulation through the REDD1-TSC pathway. Cell Cycle 2005;4:1500–2. [DOI] [PubMed] [Google Scholar]
- 12. Miyazaki M, Esser KA. REDD2 is enriched in skeletal muscle and inhibits mTOR signaling in response to leucine and stretch. Am J Physiol Cell Physiol 2009;296:C583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009;137:873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanchez AMJ, Candau RB, Csibi A, Pagano AF, Raibon A, Bernardi H. The role of AMP-activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. AJP Cell Physiol 2012;303:C475–85. [DOI] [PubMed] [Google Scholar]
- 15. Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol 2009;196:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 2013;126:1713–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomson DM, Winder WW. AMP-activated protein kinase control of fat metabolism in skeletal muscle. Acta Physiol (Oxf) 2009;196:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rehman G, Shehzad A, Khan AL, Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Arch Pharm (Weinheim) 2014;347:457–68. [DOI] [PubMed] [Google Scholar]
- 19. Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014;24:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 2012;18:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 2009;296:E603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jung J, Genau HM, Behrends C. Amino acid-dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol Cell Biol 2015;35:2479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rebsamen M, Pochini L, Stasyk T, de Araújo MEG, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015;519:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015;347:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol 2013;202:1107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini D. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell 2013;52:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016;351:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–24. [DOI] [PubMed] [Google Scholar]
- 29. Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 2001;280:E770–9. [DOI] [PubMed] [Google Scholar]
- 30. Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 2002;282:E880–90. [DOI] [PubMed] [Google Scholar]
- 31. Suryawan A, O'Connor PMJ, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 2009;37:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol 2010;109:1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitz-Peiffer C, Whitehead JP. IRS-1 regulation in health and disease. IUBMB Life 2003;55:367–74. [DOI] [PubMed] [Google Scholar]
- 34. Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 2001;281:E908–15. [DOI] [PubMed] [Google Scholar]
- 35. Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015;47:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu M, Bai J, He S, Villarreal R, Hu D, Zhang C, Yang X, Liang H, Slaga TJ, Yu Y et al. GRB10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab 2014;19:967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holt LJ, Turner N, Mokbel N, Trefely S, Kanzleiter T, Kaplan W, Ormandy CJ, Daly RJ, Cooney GJ. GRB10 regulates the development of fiber number in skeletal muscle. FASEB J 2012;26:3658–69. [DOI] [PubMed] [Google Scholar]
- 38. Gao D, Inuzuka H, Tan MKM, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S et al. MTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell 2011;44:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kazi AA, Hong-Brown L, Lang SM, Lang CH. Deptor knockdown enhances mTOR activity and protein synthesis in myocytes and ameliorates disuse muscle atrophy. Mol Med 2011;17:925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Groenewoud MJ, Zwartkruis FJT. RHEB and Rags come together at the lysosome to activate mTORC1. Biochem Soc Trans 2013;41:951–5. [DOI] [PubMed] [Google Scholar]
- 41. Long X, Ortiz-Vega S, Lin Y, Avruch J. RHEB binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 2005;280:23433–6. [DOI] [PubMed] [Google Scholar]
- 42. Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/RHEB mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. AJP Cell Physiol 2012;302:C1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun-Wada GH, Wada Y. Role of vacuolar-type proton ATPase in signal transduction. Biochim Biophys Acta 2015;1847:1166–72. [DOI] [PubMed] [Google Scholar]
- 44. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 2011;334:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dowling JJ, Moore SA, Kalimo H, Minassian BA. X-linked myopathy with excessive autophagy: a failure of self-eating. Acta Neuropathol 2015;129:383–90. [DOI] [PubMed] [Google Scholar]
- 46. Soma-Nagae T, Nada S, Kitagawa M, Takahashi Y, Mori S, Oneyama C, Okada M. The lysosomal signaling anchor p18/LAMTOR1 controls epidermal development by regulating lysosome-mediated catabolic processes. J Cell Sci 2013;126:3575–84. [DOI] [PubMed] [Google Scholar]
- 47. Sparber F, Scheffler JM, Amberg N, Tripp CH, Heib V, Hermann M, Zahner SP, Clausen BE, Reizis B, Huber LA et al. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of Langerhans cell homeostasis. Blood 2014;123:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogel GF, Ebner HL, de Araujo MEG, Schmiedinger T, Eiter O, Pircher H, Gutleben K, Witting B, Teis D, Huber LA et al. Ultrastructural morphometry points to a new role for LAMTOR2 in regulating the endo/lysosomal system. Traffic 2015;16:617–34. [DOI] [PubMed] [Google Scholar]
- 49. Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 2014;46:2787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hasumi Y, Baba M, Hasumi H, Huang Y, Lang M, Reindorf R, Oh HB, Sciarretta S, Nagashima K, Haines DC et al. Folliculin (Flcn) inactivation leads to murine cardiac hypertrophy through mTORC1 deregulation. Hum Mol Genet 2014;23:5706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carlin MB, Tanner RE, Agergaard J, Jalili T, McClain DA, Drummond MJ. Skeletal muscle Ras-related GTP binding B mRNA and protein expression is increased after essential amino acid ingestion in healthy humans. J Nutr 2014;144:1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elsas LJ, Wheeler FB, Danner DJ, DeHaan RL. Amino acid transport by aggregates of cultured chicken heart cells. Effect of insulin. J Biol Chem 1975;250:9381–90. [PubMed] [Google Scholar]